Thermal Response Analysis of Phospholipid Bilayers Using Ellipsometric Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Substrate Preparation
2.4. Surfactant Deposition Using Langmuir Blodgett Technique
2.5. SPR/Ellipsometry Set-Up
2.6. Ellipsometric Model and Refractive Indexes
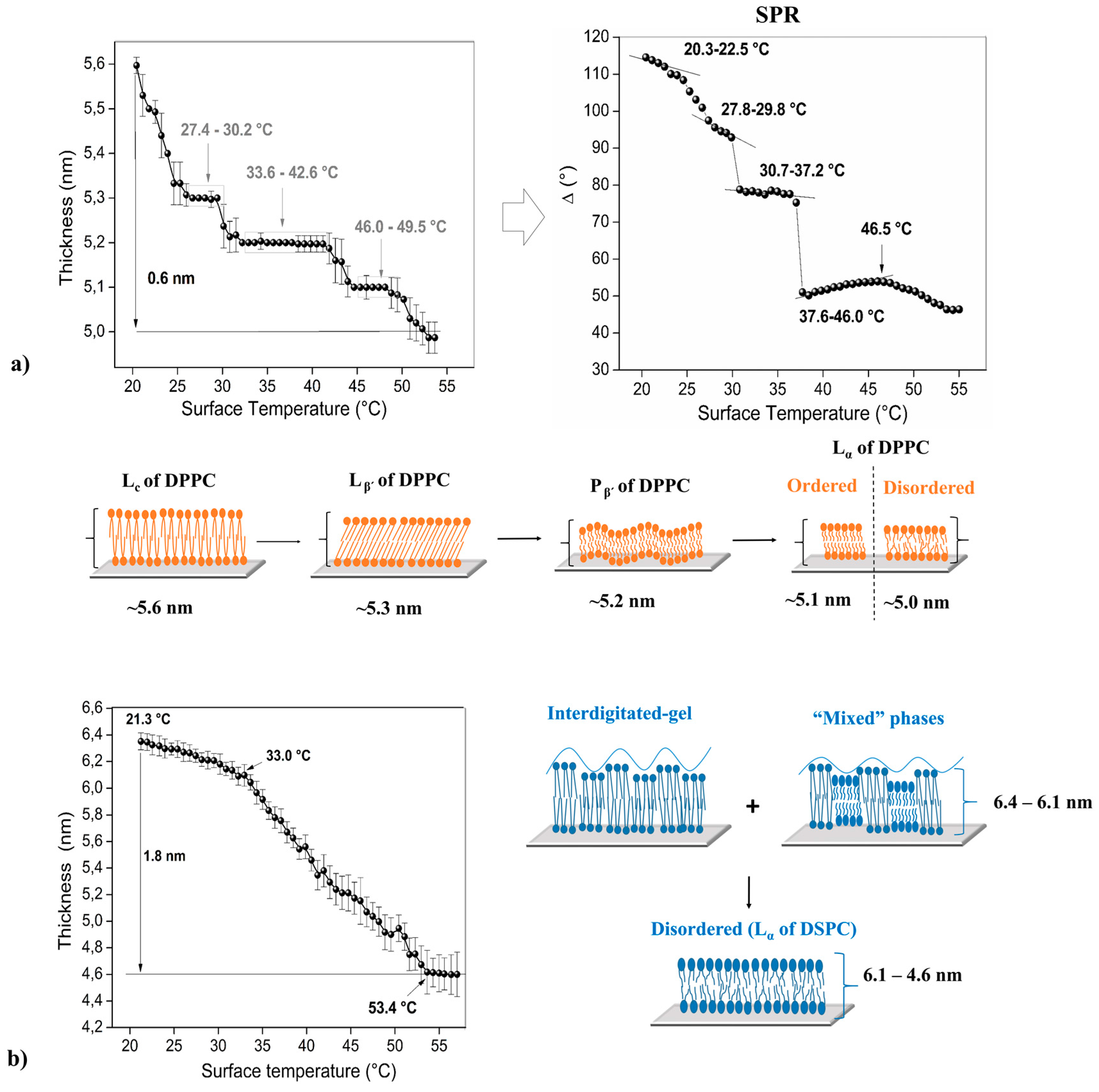
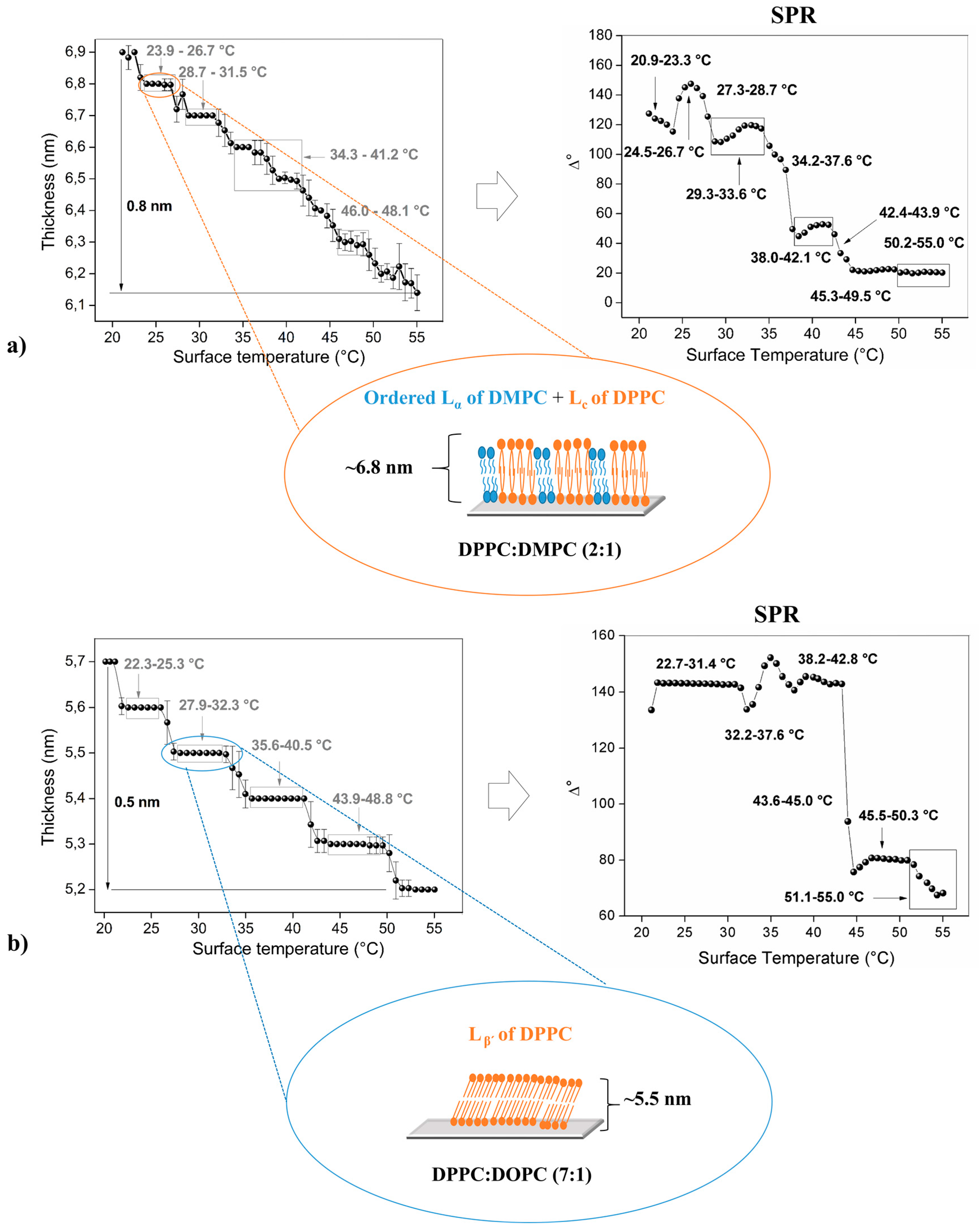
3. Results and Discussion
3.1. Thermal Studies Performed by Ellipsometric and SPR Technique
3.2. Pure Phospholipid Bilayer—Homogeneous Membrane
3.3. Phospholipid Binary Mixture—Heterogeneous Membrane
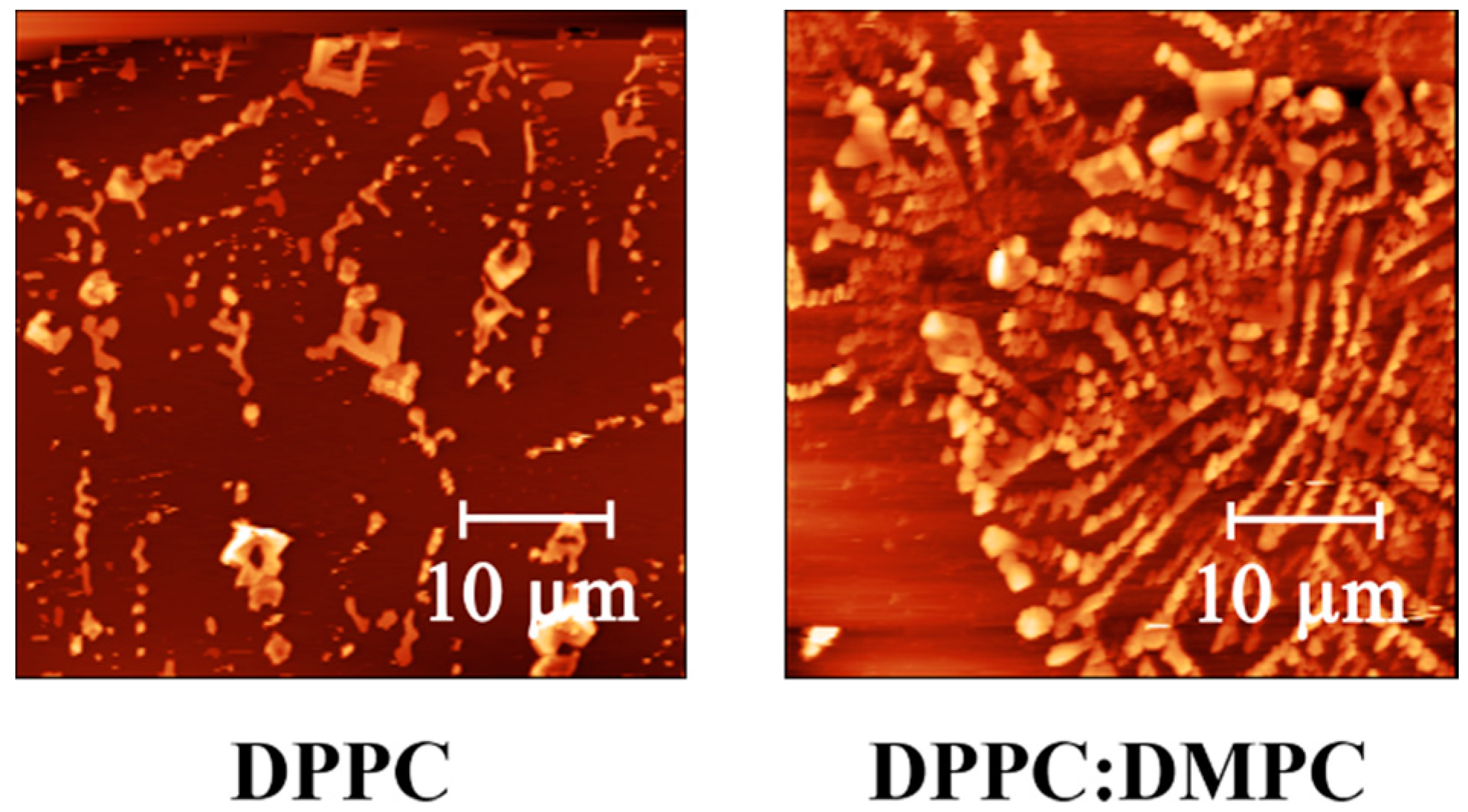
3.4. AFM and Force Spectroscopy Measures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rupert, D.L.M.; Lasser, C.; Eldh, M.; Block, S.; Zhdanov, V.P.; Lotvall, J.O.; Bally, M.; Hook, F. Determination of exosome concentration in solution using surface plasmon resonance spectroscopy. Anal. Chem. 2014, 86, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Andrecka, J.; Spillane, K.M.; Ortega-Arroyo, J.; Kukura, P. Direct Observation and Control of Supported Lipid Bilayer Formation with Interferometric Scattering Microscopy. ACS Nano 2013, 7, 10662–10670. [Google Scholar] [CrossRef] [PubMed]
- Heftberger, P.; Kollmitzer, B.; Rieder, A.A.; Amenitsch, H.; Pabst, G. In Situ Determination of Structure and Fluctuations of Coexisting Fluid Membrane Domains. Biophys. J. 2015, 108, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Hai, P.; Zhou, Y.; Zhang, R.; Ma, J.; Li, Y.; Shao, J.-Y.; Wang, L.V. Label-free high-throughput detection and quantification of circulating melanoma tumor cell clusters by linear-array-based photoacoustic tomography. J. Biomed. Opt. 2016, 22, 41004. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Jackman, J.A.; Ferhan, R.; Cho, N. Nanoplasmonic sensors for biointerfacial science. Chem. Soc. Rev. 2017, 46, 3615–3660. [Google Scholar]
- Mazur, F.; Bally, M.; Städler, B.; Chandrawati, R. Liposomes and lipid bilayers in biosensors. Adv. Colloid Interface Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Jackman, J.A.; Yorulmaz, S.; Zhdanov, V.P.; Lee, H.; Cho, N. Contribution of Temperature to Deformation of Adsorbed Vesicles Studied by Nanoplasmonic Biosensing. Langmuir 2015, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Sobolewska, K.; Flasiński, M.; Wydro, P. Studies on the interactions of bisphenols with anionic phospholipids of decomposer membranes in model systems. Biochim. Biophys. Acta Biomembr. 2016, 1858, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Flasiński, M.; Gawryś, M.; Broniatowski, M.; Wydro, P. Studies on the interactions between parabens and lipid membrane components in monolayers at the air/aqueous solution interface. Biochim. Biophys. Acta Biomembr. 2016, 1858, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Domanov, Y.; Pietiäinen, M.; Kontinen, V.P.; Kinnunen, P.K.J. Binding of LL-37 to model biomembranes: Insight into target vs host cell recognition. Biochim. Biophys. Acta Biomembr. 2008, 1778, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Casadó, A.; Giuffrida, M.C.; Sagristá, M.L.; Castelli, F.; Pujol, M.; Alsina, M.A.; Mora, M. Langmuir monolayers and Differential Scanning Calorimetry for the study of the interactions between camptothecin drugs and biomembrane models. Biochim. Biophys. Acta Biomembr. 2016, 1858, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Guidelli, R.; Becucci, L. Mechanism of voltage-gated channel formation in lipid membranes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Equation of State for Phospholipid Self-Assembly. Biophys. J. 2016, 110, 188–196. [Google Scholar] [CrossRef] [PubMed]
- González-Henríquez, C.M.; Pizarro-Guerra, G.C.; Córdova-Alarcón, E.N.; Sarabia-Vallejos, M.A.; Terraza-Inostroza, C.A. Artificial biomembranes stabilized over spin coated hydrogel scaffolds. Crosslinking agent nature induces wrinkled or flat surfaces on the hydrogel. Chem. Phys. Lipids 2016, 196, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Skjevik, Å.A.; Madej, B.D.; Dickson, C.J.; Lin, C.; Teigen, K.; Walker, R.C.; Gould, I.R. Simulation of lipid bilayer self-assembly using all-atom lipid force fields. Phys. Chem. Chem. Phys. 2016, 18, 10573–10584. [Google Scholar] [CrossRef] [PubMed]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A. Electrospinning deposition of hydrogel fibers used as scaffold for biomembranes. Thermal stability of DPPC corroborated by ellipsometry. Chem. Phys. Lipids 2015, 190, 51–60. [Google Scholar] [CrossRef] [PubMed]
- González-Henríquez, C.M.; Pizarro, G.D.C.; Sarabia-Vallejos, M.A.; Terraza, C.A.; Pizarro, C.; Sarabia-Vallejos, M.A.; Terraza, C.A. Thin and ordered hydrogel films deposited through electrospinning technique; a simple and efficient support for organic bilayers. BBA Biomembr. 2015, 1848, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Freed, J. Hydration, structure, and molecular interactions in the headgroup region of dioleoylphosphatidylcholine bilayers: An electron spin resonance study. Biophys. J. 2003, 85, 4023–4040. [Google Scholar] [CrossRef]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta Rev. Biomembr. 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Petrache, H.I.; Tristram-Nagle, S.; Gawrisch, K.; Harries, D.; Parsegian, V.A.; Nagle, J.F. Structure and Fluctuations of Charged Phosphatidylserine Bilayers in the Absence of Salt. Biophys. J. 2004, 86, 1574–1586. [Google Scholar] [CrossRef]
- Brea, R.J.; Rudd, A.K.; Devaraj, N.K. Nonenzymatic biomimetic remodeling of phospholipids in synthetic liposomes. Proc. Natl. Acad. Sci. USA 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ollila, O.H.S.; Pabst, G. Atomistic resolution structure and dynamics of lipid bilayers in simulations and experiments. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2512–2528. [Google Scholar] [CrossRef] [PubMed]
- Bonora, S.; Di Foggia, M.; Markarian, S.A.; Tugnoli, V. Vibrational and calorimetric study on the effect of di-n-propylsulfoxide (DPSO) on DMPC, DPPC and DMPE liposomes. J. Mol. Struct. 2009, 935, 115–122. [Google Scholar] [CrossRef]
- González, C.M.; Pizarro-Guerra, G.; Droguett, F.; Sarabia, M. Artificial biomembrane based on DPPC—Investigation into phase transition and thermal behavior through ellipsometric techniques. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2295–2307. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.A.; Sierra, M.B.; Pedroni, V.I.; Alarcon, L.M.; Appignanesi, G.A.; Disalvo, E.A. Influence of temperature, anions and size distribution on the zeta potential of DMPC, DPPC and DMPE lipid vesicles. Colloids Surf. B Biointerfaces 2015, 131, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Zander, T.; Wieland, D.C.F.; Raj, A.; Wang, M.; Nowak, B.; Krywka, C.; Dėdinaitė, A.; Claesson, P.M.; Garamus, V.M.; Schreyer, A.; et al. The influence of hyaluronan on the structure of a DPPC-bilayer under high pressures. Colloids Surf. B Biointerfaces 2016, 142, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Bilge, D.; Kazanci, N.; Severcan, F. Concentration-dependent effect of melatonin on DSPC membrane. J. Mol. Struct. 2013, 1052, 183–188. [Google Scholar] [CrossRef]
- Morales-Nava, R.; Ortega-Blake, I. Activity of Amphotericin B on the Membrane Structure Presenting Macro, Modulated and Nano Domains in Mixtures of DSPC/DOPC/POPC/CHOL. Biophys. J. 2013, 104, 586a. [Google Scholar] [CrossRef]
- Watanabe, R.; Soga, N.; Yamanaka, T.; Noji, H. High-throughput formation of lipid bilayer membrane arrays with an asymmetric lipid composition. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric lipid membranes: Towards more realistic model systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.A.; Krizhanovsky, V. Physiological and pathological consequences of cellular senescence. Cell. Mol. Life Sci. 2014, 71, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- Allhusen, J.S.; Conboy, J.C. The Ins and Outs of Lipid Flip-Flop. Acc. Chem. Res. 2017, 50, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lebarron, J.; London, E. Effect of lipid composition and amino acid sequence upon transmembrane peptide-accelerated lipid transleaflet diffusion (flip-flop). Biochim. Biophys. Acta Biomembr. 2016, 1858, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; London, E. The Effect of Membrane Lipid Composition on the Formation of Lipid Ultrananodomains. Biophys. J. 2015, 109, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; London, E. Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles. Biophys. J. 2015, 108, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Cholesterol-induced fluid membrane domains: A compendium of lipid-raft ternary phase diagrams. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- Visco, I.; Chiantia, S.; Schwille, P. Asymmetric supported lipid bilayer formation via methyl-β-cyclodextrin mediated lipid exchange: Influence of asymmetry on lipid dynamics and phase behavior. Langmuir 2014, 30, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Tamm, L.K. Fluorescence Microscopy to Study Domains in Supported Lipid Bilayers. In Methods in Membrane Lipids; Humana Press: New York, NY, USA, 2007; pp. 481–488. [Google Scholar]
- Crane, J.M.; Kiessling, V.; Tamm, L.K. Measuring Lipid Asymmetry in Planar Supported Bilayers by Fluorescence Interference Contrast Microscopy. Langmuir 2005, 21, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, O.R.; Live, L.S.; Masson, J.-F. High-resolution surface plasmon resonance sensors based on a dove prism. Talanta 2009, 77, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Roa, J.J.; Oncins, G.; Díaz, J.; Sanz, F.; Segarra, M. Calculation of Young’s Modulus Value by Means of AFM. Recent Pat. Nanotechnol. 2011, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.K.; Horkay, F.; Maresca, J.; Kachar, B.; Chadwick, R.S. Determination of Elastic Moduli of Thin Layers of Soft Material Using the Atomic Force Microscope. Biophys. J. 2002, 82, 2798–2810. [Google Scholar] [CrossRef]
- Tidswell, I.M.; Ocko, B.M.; Pershan, P.S.; Wasserman, S.R.; Whitesides, G.M.; Axe, J.D. X-ray specular reflection studies of silicon coated by organic monolayers (alkylsiloxanes). Phys. Rev. B 1990, 41, 1111–1128. [Google Scholar] [CrossRef]
- Hughes, A.; Roser, S.; Gerstenberg, M.; Goldar, A.; Stidder, B.; Feidenhans’l, R.; Bradshaw, J. Phase behavior of DMPC free supported bilayers studied by neutron reflectivity. Langmuir 2002, 18, 8161–8171. [Google Scholar] [CrossRef]
- Ramkaran, M.; Badia, A. Gel-to-fluid phase transformations in solid-supported phospholipid bilayers assembled by the Langmuir-Blodgett technique: Effect of the Langmuir monolayer phase state and molecular density. J. Phys. Chem. B 2014, 118, 9708–9721. [Google Scholar] [CrossRef] [PubMed]
- Tien, P.K. Integrated optics and new wave phenomena in optical waveguides. Rev. Mod. Phys. 1977, 49, 361–420. [Google Scholar] [CrossRef]
- Abbas, A.; Linman, M.J.; Cheng, Q. Sensitivity comparison of surface plasmon resonance and plasmon-waveguide resonance biosensors. Sens. Actuators B Chem. 2011, 156, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kretschmann, E. Die Bestimmung optischer Konstanten von Metallen durch Anregung von Oberflächenplasmaschwingungen. Z. Phys. A Hadron. Nucl. 1971, 241, 313–324. [Google Scholar] [CrossRef]
- Moirangthem, R.S.; Chang, Y.C.; Hsu, S.H.; Wei, P.K. Surface plasmon resonance ellipsometry based sensor for studying biomolecular interaction. Biosens. Bioelectron. 2010, 25, 2633–2638. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.E.; Rusell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001; ISBN -13 978-0-87969-577-4. [Google Scholar]
- Yeshchenko, O.A.; Bondarchuk, I.S.; Gurin, V.S.; Dmitruk, I.M.; Kotko, A.V. Temperature dependence of the surface plasmon resonance in gold nanoparticles. Surf. Sci. 2013, 608, 275–281. [Google Scholar] [CrossRef]
- Bilge, D.; Sahin, I.; Kazanci, N.; Severcan, F. Interactions of tamoxifen with distearoyl phosphatidylcholine multilamellar vesicles: FTIR and DSC studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bakht, O.; Pathak, P.; London, E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): Identification of multiple raft-stabilization mechanisms. Biophys. J. 2007, 93, 4307–4318. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.S.; Yu, Z.W.; Yu, Y.X. Structural characterization on the gel to liquid-crystal phase transition of fully hydrated DSPC and DSPE bilayers. J. Phys. Chem. B 2009, 113, 8114–8123. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Nieh, M.P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Leonenko, Z.; Finot, E.; Ma, H.; Dahms, T.E.S.; Cramb, D.T. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Grit, M.; de Smidt, J.H.; Struijke, A.; Crommelin, D.J.A. Hydrolysis of phosphatidylcholine in aqueous liposome dispersions. Int. J. Pharm. 1989, 50, 1–6. [Google Scholar] [CrossRef]
- Poghosyan, A.H.; Gharabekyan, H.H.; Shahinyan, A.A. Molecular Dynamics Simulations of DMPC/DPPC Mixed Bilayers. Int. J. Mod. Phys. C 2007, 18, 73–89. [Google Scholar] [CrossRef]
- Woodka, A.C.; Butler, P.D.; Porcar, L.; Farago, B.; Nagao, M. Lipid bilayers and membrane dynamics: Insight into thickness fluctuations. Phys. Rev. Lett. 2012, 109, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lubensky, T.C.; MacKintosh, F.C. Theory of “ripple” Phases of Lipid Bilayers. Phys. Rev. Lett. 1993, 71, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- M’Baye, G.; Mély, Y.; Duportail, G.; Klymchenko, A.S. Liquid ordered and gel phases of lipid bilayers: Fluorescent probes reveal close fluidity but different hydration. Biophys. J. 2008, 95, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Michonova-Alexova, E.I.; Sugár, I.P. Component and state separation in DMPC/DSPC lipid bilayers: A Monte Carlo simulation study. Biophys. J. 2002, 83, 1820–1833. [Google Scholar] [CrossRef]
- Corrales, T.P.; Bai, M.; Homm, P.; Ferrari, P.; Diama, A.; Wagner, C.; Taub, H.; Knorr, K.; Deutsch, M.; Retamal, M.J.; et al. Spontaneous Formation of Nanopatterns in Velocity-Dependent Dip-Coated Organic Films: From Dragon fl ies to Stripes. ACS Nano 2014, 8, 9954–9963. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Lea Sanford, R.; Andersen, O.S.; Scheuring, S. Effect of Statins on the Nanomechanical Properties of Supported Lipid Bilayers. Biophys. J. 2016, 111, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Picas, L.; Rico, F.; Scheuring, S. Direct measurement of the mechanical properties of lipid phases in supported bilayers. Biophys. J. 2012, 102, L01–L03. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.; Mouritsen, O.G. Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys. J. 1995, 69, 942–954. [Google Scholar] [CrossRef]


| Material | Refractive Index (Solution) | Refractive Index (Film) | Extinction Coefficient |
|---|---|---|---|
| Silicon (Si100) | N/A | 3.870 | 0.019 |
| Silicon Dioxide (SiO2) | N/A | 1.462 ± 0.025 | N/A |
| DPPC | 1.489 ± 0.063 | 1.484 ± 0.058 | N/A |
| DSPC | 1.426 ± 0.089 | 1.439 ± 0.086 | N/A |
| DPPC:DMPC (2:1) | 1.461 ± 0.068 | 1.488 ± 0.072 | N/A |
| DPPC:DOPC (7:1) | 1.459 ± 0.078 | 1.481 ± 0.067 | N/A |
| Thickness (nm) | |||
|---|---|---|---|
| 21 °C | 55.0 °C | Difference (Δ) | |
| DPPC | 5.6 ± 0.2 | 5.0 ± 0.3 | ~0.6 |
| DSPC | 6.4 ± 0.3 | 4.6 ± 0.4 | ~1.8 |
| DPPC:DMPC (2:1) | 6.9 ± 0.2 | 6.1 ± 0.5 | ~0.8 |
| DPPC:DOPC (7:1) | 5.7 ± 0.1 | 5.2 ± 0.6 | ~0.5 |
| DSPC:DMPC (2:1) | 4.2 ± 0.3 * | 4.0 ± 0.4 | ~0.2 |
| DSPC:DOPC (7:1) | 5.1 ± 0.2 | 3.8 ± 0.5 | ~1.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Henríquez, C.M.; Villegas-Opazo, V.A.; Sagredo-Oyarce, D.H.; Sarabia-Vallejos, M.A.; Terraza, C.A. Thermal Response Analysis of Phospholipid Bilayers Using Ellipsometric Techniques. Biosensors 2017, 7, 34. https://doi.org/10.3390/bios7030034
González-Henríquez CM, Villegas-Opazo VA, Sagredo-Oyarce DH, Sarabia-Vallejos MA, Terraza CA. Thermal Response Analysis of Phospholipid Bilayers Using Ellipsometric Techniques. Biosensors. 2017; 7(3):34. https://doi.org/10.3390/bios7030034
Chicago/Turabian StyleGonzález-Henríquez, Carmen M., Vanessa A. Villegas-Opazo, Dallits H. Sagredo-Oyarce, Mauricio A. Sarabia-Vallejos, and Claudio A. Terraza. 2017. "Thermal Response Analysis of Phospholipid Bilayers Using Ellipsometric Techniques" Biosensors 7, no. 3: 34. https://doi.org/10.3390/bios7030034
APA StyleGonzález-Henríquez, C. M., Villegas-Opazo, V. A., Sagredo-Oyarce, D. H., Sarabia-Vallejos, M. A., & Terraza, C. A. (2017). Thermal Response Analysis of Phospholipid Bilayers Using Ellipsometric Techniques. Biosensors, 7(3), 34. https://doi.org/10.3390/bios7030034





