A Review on 3D-Printed Miniaturized Devices for Point-of-Care-Testing Applications
Abstract
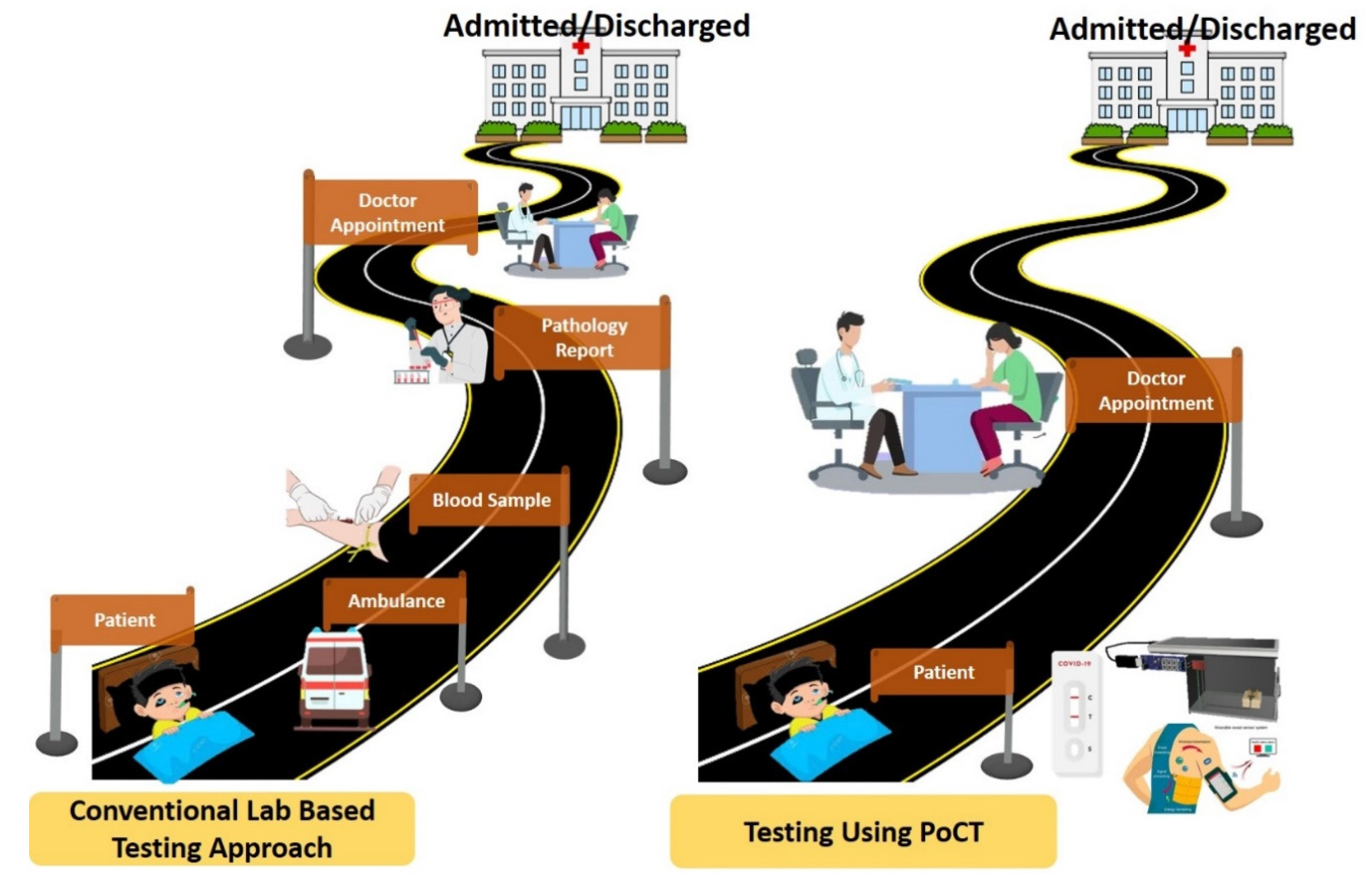
1. Introduction
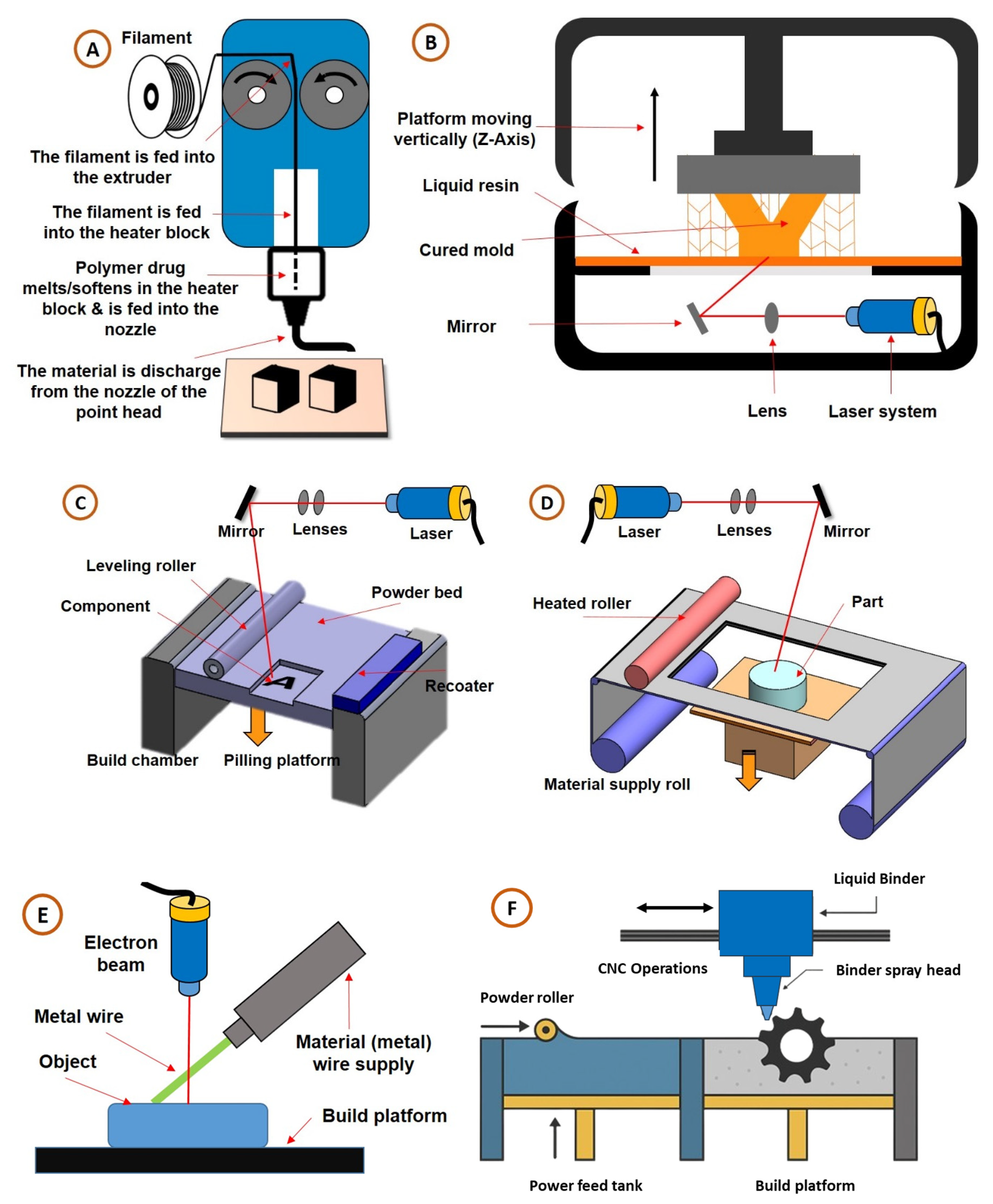
2. Three-Dimensional Printing Technology Classification
2.1. Material Extrusion/Fused Deposition Modelling (FDM)
2.2. Vat Photopolymerization/Stereolithography (SLA)
2.3. Powder Bed Fusion (PBF)
2.4. Sheet Lamination/Laminated Object Manufacturing (LOM)
2.5. Binder Jetting (BJ)
2.6. Direct Energy Deposition (DED)
2.7. Material Considerations in 3DP PoCT Devices
2.7.1. Thermoplastics and Conductive Composites
2.7.2. Photopolymer Resins for Microfluidic Devices
2.7.3. Elastomers for Flexible and Wearable Sensors
2.7.4. Advanced Functional Materials
3. Applications of 3D Printing Technology
3.1. Three-Dimensionally Printed Wearable Devices for PoCT Applications
3.2. Three-Dimensionally Printed Biosensors for PoCT Applications
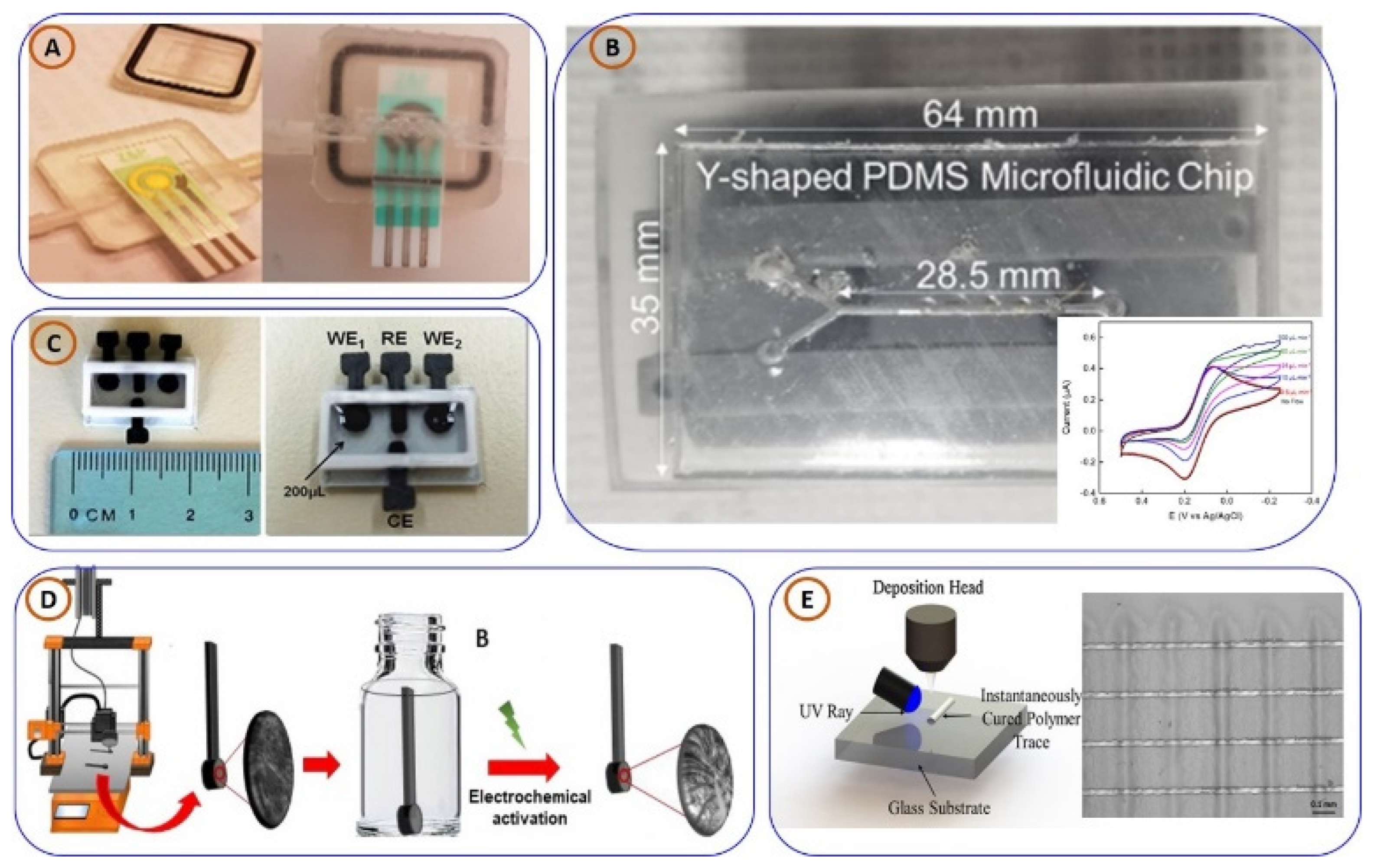
3.2.1. Three-Dimensionally Printed Electrochemical Sensors for PoCT Applications
3.2.2. Three-Dimensionally Printed Electrochemiluminescence (ECL) and Chemiluminescence (CL) Sensors for PoCT Applications
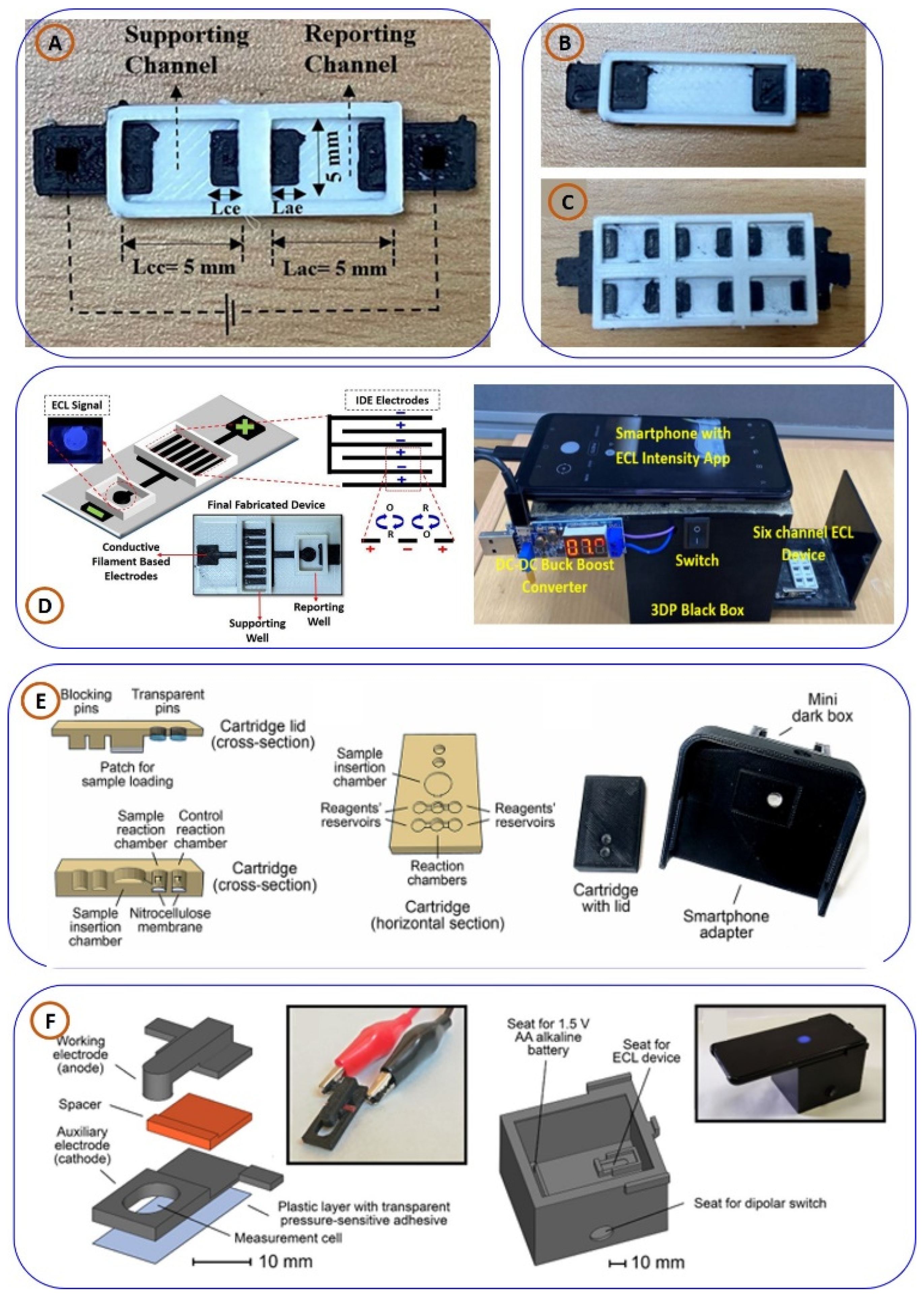
3.3. Three-Dimensionally Printed Lab-on-Chip (LoC) and Microfluidic Devices for PoCT Applications
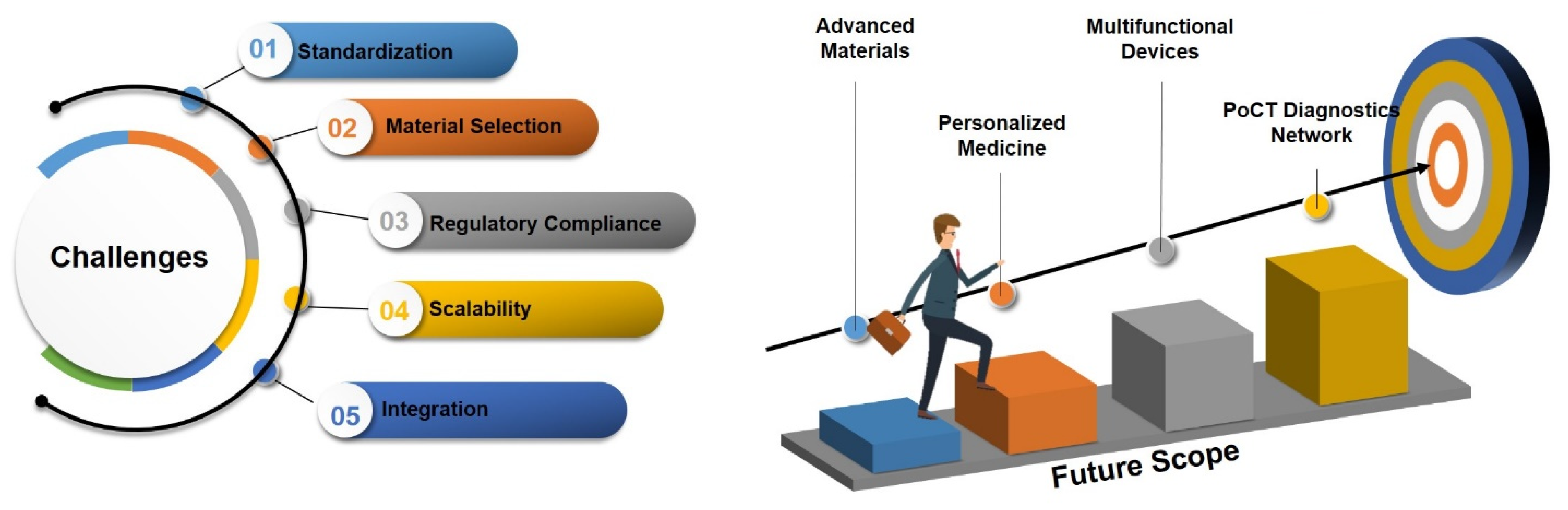
4. Challenges and Future Scope
4.1. Challenges in 3DP PoCT Devices
4.1.1. Standardization
4.1.2. Material Selection
4.1.3. Regulatory Compliance
4.2. Future Opportunities for 3DP-PoCT Devices
4.2.1. Advanced Materials
4.2.2. Multifunctional Devices
4.2.3. Integration of Emerging Technologies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. 3D-Printing Technologies for Electrochemical Applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [PubMed]
- Bhaiyya, M.; Pattnaik, P.K.; Goel, S. Multiplexed and Simultaneous Biosensing in a 3D-Printed Portable Six-Well Smartphone Operated Electrochemiluminescence Standalone Point-of-Care Platform. Microchim. Acta 2022, 189, 79. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.K.; Tappa, K.; Jammalamadaka, U.; Mills, P.A.S.; Alexander, J.S.; Weisman, J.A. Medical Applications for 3D Printing. In Advances in Manufacturing and Processing of Materials and Structures; CRC Press: Boca Raton, FL, USA, 2018; pp. 163–186. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical Applications of 3D Printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Pal, A.; Amreen, K.; Dubey, S.K.; Goel, S. Highly Sensitive and Interference-Free Electrochemical Nitrite Detection in a 3D Printed Miniaturized Device. IEEE Trans. Nanobiosci. 2021, 20, 175–182. [Google Scholar] [CrossRef]
- Dong, C.; Petrovic, M.; Davies, I.J. Applications of 3D Printing in Medicine: A Review. Ann. 3D Print. Med. 2024, 14, 100149. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Kulkarni, M.B.; Pattnaik, P.K.; Goel, S. IoT Enabled PMT and Smartphone Based Electrochemiluminescence Platform to Detect Choline and Dopamine Using 3D-Printed Closed Bipolar Electrodes. Luminescence 2021, 37, 357–365. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar Bhagat, A.; Bhaiyya, M.; Amreen, K.; Kumar Dubey, S.; Goel, S. A Machine Learning Approach for Simultaneous Electrochemical Detection of Dopamine and Serotonin in an Optimized Carbon Thread-Based Miniaturized Device. IEEE Sens. J. 2024, 24, 21378–21385. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Pattnaik, P.K.; Goel, S. ScienceDirect Electrochemistry A Brief Review on Miniaturized Electrochemiluminescence Devices: From Fabrication to Applications. Curr. Opin. Electrochem. 2021, 30, 100800. [Google Scholar] [CrossRef]
- Chan, H.N.; Tan, M.J.A.; Wu, H. Point-of-Care Testing: Applications of 3D Printing. Lab Chip 2017, 17, 2713–2739. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. 3D Printing Applications for Healthcare Research and Development. Glob. Health J. 2022, 6, 217–226. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Panigrahi, D.; Rewatkar, P.; Haick, H. Role of Machine Learning Assisted Biosensors in Point-of-Care-Testing For Clinical Decisions. ACS Sens. 2024, 9, 4495–4519. [Google Scholar] [CrossRef] [PubMed]
- Bastawrous, S.; Wu, L.; Liacouras, P.C.; Levin, D.B.; Ahmed, M.T.; Strzelecki, B.; Amendola, M.F.; Lee, J.T.; Coburn, J.; Ripley, B. Establishing 3D Printing at the Point of Care: Basic Principles and Tools for Success. Radiographics 2022, 42, 451–468. [Google Scholar] [CrossRef]
- Dave, S.; Churi, H.S.; Vishwakarma, P.A.; Krishnamoorthy, A.; Jagtap, U.P. Advancements in Healthcare through 3D-Printed Micro and Nanosensors: Innovation, Application, and Prospects. Hybrid Adv. 2024, 7, 100311. [Google Scholar] [CrossRef]
- Bhaiyya, M.L.; Pattnaik, P.K.; Goel, S. Miniaturized Electrochemiluminescence Platform with Laser-Induced Graphene Based Single Electrode for Interference-Free Sensing of Dopamine, Xanthine and Glucose. IEEE Trans. Instrum. Meas. 2021, 70, 9508108. [Google Scholar] [CrossRef]
- Biglino, G.; Hopfner, C.; Lindhardt, J.; Moscato, F.; Munuera, J.; Oberoi, G.; Tel, A.; Esteve, A.V. Perspectives on Medical 3D Printing at the Point-of-Care from the New European 3D Printing Special Interest Group. 3D Print. Med. 2023, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Frisch, P.; Platzman, A.; Booth, P. 3D Printing in a Hospital: Centralized Clinical Implementation and Applications for Comprehensive Care. Digit. Health 2023, 9, 20552076231221900. [Google Scholar] [CrossRef] [PubMed]
- Bhaiyya, M.; Rewatkar, P.; Pattnaik, P.K.; Goel, S. Novel 3D Printed Single Electrode-Based Portable and Miniaturized Electrochemiluminescence Platform to Detect Lactate from Human Serum. J. Micromech. Microeng. 2023, 33, 024001. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Kumar, P.S.; Pattnaik, P.K.; Shankar, K.; Goel, S. Stereolithography 3-D Printed Electrochemiluminescence Platform With Random Grade Graphite Electrodes: Detection of HO and Cholesterol Using a Smartphone. IEEE Sens. J. 2023, 23, 750–757. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, Y.; Gong, X.; Yi, S.; Li, C.W.; Jiang, L.; Yi, C. An Integrative Review on the Applications of 3D Printing in the Field of in Vitro Diagnostics. Chin. Chem. Lett. 2022, 33, 2231–2242. [Google Scholar] [CrossRef]
- Murtezani, I.; Sharma, N.; Thieringer, F.M. Medical 3D Printing with a Focus on Point-of-Care in Cranio- and Maxillofacial Surgery. A Systematic Review of Literature. Ann. 3D Print. Med. 2022, 6, 100059. [Google Scholar] [CrossRef]
- Wang, H.; Enders, A.; Preuss, J.-A.; Bahnemann, J.; Heisterkamp, A.; Torres-Mapa, M.L. 3D Printed Microfluidic Lab-on-a-Chip Device for Fiber-Based Dual Beam Optical Manipulation. Sci. Rep. 2021, 11, 14584. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D Printed Microfluidic Devices: Enablers and Barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; De Oliveira, P.R.; Banks, C.E.; Janegitz, B.C.; Bonacin, J.A. 3D-Printed Immunosensor for the Diagnosis of Parkinson’s Disease. Sens. Actuators B Chem. 2023, 381, 133353. [Google Scholar] [CrossRef]
- Bănică, C.-F.; Sover, A.; Anghel, D.-C. Printing the Future Layer by Layer: A Comprehensive Exploration of Additive Manufacturing in the Era of Industry 4.0. Appl. Sci. 2024, 14, 9919. [Google Scholar] [CrossRef]
- Rodríguez-Espíndola, O.; Chowdhury, S.; Beltagui, A.; Albores, P. The Potential of Emergent Disruptive Technologies for Humanitarian Supply Chains: The Integration of Blockchain, Artificial Intelligence and 3D Printing. Int. J. Prod. Res. 2020, 58, 4610–4630. [Google Scholar] [CrossRef]
- Prakashan, D.; PR, R.; Gandhi, S. A Systematic Review on the Advanced Techniques of Wearable Point-of-Care Devices and Their Futuristic Applications. Diagnostics 2023, 13, 916. [Google Scholar] [CrossRef]
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef]
- Li, Q.; Gao, M.; Sun, X.; Wang, X.; Chu, D.; Cheng, W.; Xi, Y.; Lu, Y. All-in-One Self-Powered Wearable Biosensors Systems. Mater. Sci. Eng. R Rep. 2025, 163, 100934. [Google Scholar] [CrossRef]
- Shakibania, S.; Khakbiz, M.; Bektas, C.K.; Ghazanfari, L.; Banizi, M.T.; Lee, K.B. A Review of 3D Printing Technology for Rapid Medical Diagnostic Tools. Mol. Syst. Des. Eng. 2022, 7, 315–324. [Google Scholar] [CrossRef]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef] [PubMed]
- Manzanares Palenzuela, C.L.; Pumera, M. (Bio)Analytical Chemistry Enabled by 3D Printing: Sensors and Biosensors. TrAC Trends Anal. Chem. 2018, 103, 110–118. [Google Scholar] [CrossRef]
- Su, C.K. Review of 3D-Printed Functionalized Devices for Chemical and Biochemical Analysis. Anal. Chim. Acta 2021, 1158, 338348. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-Printed Biosensor Arrays for Medical Diagnostics. Micromachines 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.J.; Zhu, W.; Zhou, K. Recent Progress on Polymer Materials for Additive Manufacturing. Adv. Funct. Mater. 2020, 30, 2003062. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive Manufacturing: Technology, Applications and Research Needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Jadhav, A.; Jadhav, V.S. A Review on 3D Printing: An Additive Manufacturing Technology. Mater. Today Proc. 2022, 62, 2094–2099. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Ul Haq, M.I. 3D Printing—A Review of Processes, Materials and Applications in Industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive Manufacturing Technologies with Emphasis on Stereolithography 3D Printing in Pharmaceutical and Medical Applications: A Review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef]
- Huang, S.; Wei, H.; Li, D. Additive Manufacturing Technologies in the Oral Implant Clinic: A Review of Current Applications and Progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D Printing of a Bladder Device for Intravesical Drug Delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat Photopolymerization 3D Printing for Advanced Drug Delivery and Medical Device Applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Thakur, V.; Singh, R.; Kumar, R. Chapter Two—Materials for Additive Manufacturing in Clinical Podiatry. In 3D Printing in Podiatric Medicine; Sandhu, K., Singh, S., Prakash, C., Subburaj, K., Ramakrishna, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 35–50. ISBN 978-0-323-91911-1. [Google Scholar]
- Abdalla, Y.; Ferianc, M.; Awad, A.; Kim, J.; Elbadawi, M.; Basit, A.W.; Orlu, M.; Rodrigues, M. Smart Laser Sintering: Deep Learning-Powered Powder Bed Fusion 3D Printing in Precision Medicine. Int. J. Pharm. 2024, 661, 124440. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Abbas, N.; Kwon, Y.S.; Kim, D. Transforming Biofabrication with Powder Bed Fusion Additive Manufacturing Technology: From Personalized to Multimaterial Solutions. Prog. Addit. Manuf. 2024. [Google Scholar] [CrossRef]
- Li, M.; Benn, F.; Derra, T.; Kröger, N.; Zinser, M.; Smeets, R.; Molina-Aldareguia, J.M.; Kopp, A.; LLorca, J. Microstructure, Mechanical Properties, Corrosion Resistance and Cytocompatibility of WE43 Mg Alloy Scaffolds Fabricated by Laser Powder Bed Fusion for Biomedical Applications. Mater. Sci. Eng. C 2021, 119, 111623. [Google Scholar] [CrossRef]
- McGregor, M.; Patel, S.; McLachlin, S.; Vlasea, M. Architectural Bone Parameters and the Relationship to Titanium Lattice Design for Powder Bed Fusion Additive Manufacturing. Addit. Manuf. 2021, 47, 102273. [Google Scholar] [CrossRef]
- Schäfer, K.; Lutzi, M.; Khan, M.B.; Schäfer, L.; Dirba, I.; Bruns, S.; Valizadeh, I.; Weeger, O.; Hartmann, C.; Kupnik, M.; et al. Magneto-Active Composites with Locally Tailored Stiffness Produced by Laser Powder Bed Fusion. Addit. Manuf. 2024, 79, 103905. [Google Scholar] [CrossRef]
- Nag, N.; Chandra, M.; Kazmi, K.H.; Shukla, A.; Sharma, S.K. Selection of Suitable Powder Bed Fusion Technique for Medical Applications Using Mcdm Techniques. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Islam, M.K.; Hazell, P.J.; Escobedo, J.P.; Wang, H. Biomimetic Armour Design Strategies for Additive Manufacturing: A Review. Mater. Des. 2021, 205, 109730. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical Characterization of 3D-Printed Polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in Powder Bed Fusion 3D Printing in Drug Delivery and Healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]
- Buchanan, C.; Gardner, L. Metal 3D Printing in Construction: A Review of Methods, Research, Applications, Opportunities and Challenges. Eng. Struct. 2019, 180, 332–348. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D Printing of Polymer Matrix Composites: A Review and Prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. PDMS Lab-on-a-Chip Fabrication Using 3D Printed Templates. Lab Chip 2014, 14, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Radenkovic, D.; Solouk, A.; Seifalian, A. Personalized Development of Human Organs Using 3D Printing Technology. Med. Hypotheses 2016, 87, 30–33. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gonzalez, G.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Current and Emerging Trends in Polymeric 3D Printed Microfluidic Devices. Addit. Manuf. 2022, 55, 102867. [Google Scholar] [CrossRef]
- Roudný, P.; Syrový, T. Thermal Conductive Composites for FDM 3D Printing: A Review, Opportunities and Obstacles, Future Directions. J. Manuf. Process. 2022, 83, 667–677. [Google Scholar] [CrossRef]
- Lazarus, N.; Bedair, S.S. Creating 3D Printed Sensor Systems with Conductive Composites. Smart Mater. Struct. 2020, 30, 15020. [Google Scholar] [CrossRef]
- Tirado-Garcia, I.; Garcia-Gonzalez, D.; Garzon-Hernandez, S.; Rusinek, A.; Robles, G.; Martinez-Tarifa, J.M.; Arias, A. Conductive 3D Printed PLA Composites: On the Interplay of Mechanical, Electrical and Thermal Behaviours. Compos. Struct. 2021, 265, 113744. [Google Scholar] [CrossRef]
- Milton, L.A.; Viglione, M.S.; Ong, L.J.Y.; Nordin, G.P.; Toh, Y.-C. Vat Photopolymerization 3D Printed Microfluidic Devices for Organ-on-a-Chip Applications. Lab Chip 2023, 23, 3537–3560. [Google Scholar] [CrossRef]
- Shahzadi, L.; Li, F.; Alejandro, F.M.; Breadmore, M.C.; Thickett, S.C. Chapter 4 Resin Design in Stereolithography 3D Printing for Microfluidic Applications. In 3D Printing with Light; Xiao, P., Zhang, J., Eds.; De Gruyter: Berlin, Germany, 2021; pp. 135–174. ISBN 9783110570588. [Google Scholar]
- Li, S.; Zhou, X.; Dong, Y.; Li, J. Flexible Self-Repairing Materials for Wearable Sensing Applications: Elastomers and Hydrogels. Macromol. Rapid Commun. 2020, 41, 2000444. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Lee, J.; Kim, J.K.; An, H.K.; Kang, S.-W.; Jung, D. Highly Sensitive and Flexible Wearable Pressure Sensor with Dielectric Elastomer and Carbon Nanotube Electrodes. Sens. Actuators A Phys. 2020, 305, 111941. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene Hydrogels: Fundamentals and Applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Rehman, A.U.; Chen, C.; Zhao, T.; Hayat, A.; Bashir, T.; Shen, L.; Orooji, Y.; Lin, H. Synergistically Self-Assembled in Situ Growth of MXene@ MOF Derived Sodium Alginate Hydrogel 3D Frameworks as Next-Generation Electrocatalysts for Oxygen and Hydrogen Evolution. J. Mater. Chem. A 2025, 13, 4390–4403. [Google Scholar] [CrossRef]
- Abshirini, M.; Charara, M.; Marashizadeh, P.; Saha, M.C.; Altan, M.C.; Liu, Y. Functional Nanocomposites for 3D Printing of Stretchable and Wearable Sensors. Appl. Nanosci. 2019, 9, 2071–2083. [Google Scholar] [CrossRef]
- Guo, S.Z.; Qiu, K.; Meng, F.; Park, S.H.; McAlpine, M.C. 3D Printed Stretchable Tactile Sensors. Adv. Mater. 2017, 29, 1701218. [Google Scholar] [CrossRef]
- Ho, D.H.; Hong, P.; Han, J.T.; Kim, S.Y.; Kwon, S.J.; Cho, J.H. 3D-Printed Sugar Scaffold for High-Precision and Highly Sensitive Active and Passive Wearable Sensors. Adv. Sci. 2020, 7, 1902521. [Google Scholar] [CrossRef]
- Yin, X.Y.; Zhang, Y.; Cai, X.; Guo, Q.; Yang, J.; Wang, Z.L. 3D Printing of Ionic Conductors for High-Sensitivity Wearable Sensors. Mater. Horiz. 2019, 6, 767–780. [Google Scholar] [CrossRef]
- Kim, T.; Yi, Q.; Hoang, E.; Esfandyarpour, R. A 3D Printed Wearable Bioelectronic Patch for Multi-Sensing and In Situ Sweat Electrolyte Monitoring. Adv. Mater. Technol. 2021, 6, 2001021. [Google Scholar] [CrossRef]
- Pal, S.; Su, Y.Z.; Chen, Y.W.; Yu, C.H.; Kung, C.W.; Yu, S.S. 3D Printing of Metal−Organic Framework-Based Ionogels: Wearable Sensors with Colorimetric and Mechanical Responses. ACS Appl. Mater. Interfaces 2022, 14, 28247–28257. [Google Scholar] [CrossRef]
- Nassar, H.; Ntagios, M.; Navaraj, W.T.; Dahiya, R. Multi-Material 3D Printed Bendable Smart Sensing Structures. In Proceedings of the 2018 IEEE Sensors, New Delhi, India, 28–31 October 2018. [Google Scholar]
- Ma, L.; Xia, T.; Yu, R.; Lei, X.; Yuan, J.; Li, X.; Cheng, G.J.; Liu, F. A 3D-Printed, Sensitive, Stable, and Flexible Piezoresistive Sensor for Health Monitoring. Adv. Eng. Mater. 2021, 23, 2100379. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.; Hwang, J.Y.; Chung, J.; Jang, T.M.; Seo, D.G.; Gao, Y.; Lee, J.; Park, H.; Lee, S.; et al. 3D Printed, Customizable, and Multifunctional Smart Electronic Eyeglasses for Wearable Healthcare Systems and Human-Machine Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 21424–21432. [Google Scholar] [CrossRef]
- NajafiKhoshnoo, S.; Kim, T.; Tavares-Negrete, J.A.; Pei, X.; Das, P.; Lee, S.W.; Rajendran, J.; Esfandyarpour, R. A 3D Nanomaterials-Printed Wearable, Battery-Free, Biocompatible, Flexible, and Wireless PH Sensor System for Real-Time Health Monitoring. Adv. Mater. Technol. 2023, 8, 2201655. [Google Scholar] [CrossRef]
- Ota, H.; Chao, M.; Gao, Y.; Wu, E.; Tai, L.C.; Chen, K.; Matsuoka, Y.; Iwai, K.; Fahad, H.M.; Gao, W.; et al. 3D Printed “Earable” Smart Devices for Real-Time Detection of Core Body Temperature. ACS Sens. 2017, 2, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D Printing of a Wearable Personalized Oral Delivery Device: A First-in-Human Study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, S.; Sun, L.; Yang, L.; Zhang, L.; Lou, J.; You, Z. Degradable and Fully Recyclable Dynamic Thermoset Elastomer for 3D-Printed Wearable Electronics. Adv. Funct. Mater. 2021, 31, 2009799. [Google Scholar] [CrossRef]
- Tavares, C.; Leitão, C.; Lo Presti, D.; Domingues, M.F.; Alberto, N.; Silva, H.; Antunes, P. Respiratory and Heart Rate Monitoring Using an FBG 3D-Printed Wearable System. Biomed. Opt. Express 2022, 13, 2299. [Google Scholar] [CrossRef]
- Wei, J.; Xie, J.; Zhang, P.; Zou, Z.; Ping, H.; Wang, W.; Xie, H.; Shen, J.Z.; Lei, L.; Fu, Z. Bioinspired 3D Printable, Self-Healable, and Stretchable Hydrogels with Multiple Conductivities for Skin-like Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, E.; Montazerian, H.; Haghniaz, R.; Rashidi, A.; Ahadian, S.; Sheikhi, A.; Chen, J.; Khademhosseini, A.; Milani, A.S.; Hoorfar, M.; et al. 3D-Printed Ultra-Robust Surface-Doped Porous Silicone Sensors for Wearable Biomonitoring. ACS Nano 2020, 14, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Gowers, S.A.N.; Curto, V.F.; Seneci, C.A.; Wang, C.; Anastasova, S.; Vadgama, P.; Yang, G.Z.; Boutelle, M.G. 3D Printed Microfluidic Device with Integrated Biosensors for Online Analysis of Subcutaneous Human Microdialysate. Anal. Chem. 2015, 87, 7763–7770. [Google Scholar] [CrossRef]
- Cao, W.T.; Ma, C.; Mao, D.S.; Zhang, J.; Ma, M.G.; Chen, F. MXene-Reinforced Cellulose Nanofibril Inks for 3D-Printed Smart Fibres and Textiles. Adv. Funct. Mater. 2019, 29, 1905898. [Google Scholar] [CrossRef]
- Kee, S.; Haque, M.A.; Corzo, D.; Alshareef, H.N.; Baran, D. Self-Healing and Stretchable 3D-Printed Organic Thermoelectrics. Adv. Funct. Mater. 2019, 29, 1905426. [Google Scholar] [CrossRef]
- Miyake, M. Chapter 26—Electrochemical Functions. In Carbon Alloys; Yasuda, E., Inagaki, M., Kaneko, K., Endo, M., Oya, A., Tanabe, Y., Eds.; Elsevier Science: Oxford, UK, 2003; pp. 435–445. ISBN 978-0-08-044163-4. [Google Scholar]
- Mahato, K.; Wang, J. Electrochemical Sensors: From the Bench to the Skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-Based Electrochemical Sensors and Biosensors for the Detection of Pharmaceutical Compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Ramiah Rajasekaran, P.; Chapin, A.A.; Quan, D.N.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. 3D-Printed Electrochemical Sensor-Integrated Transwell Systems. Microsyst. Nanoeng. 2020, 6, 100. [Google Scholar] [CrossRef]
- Rocha Neto, J.B.M.; Soares, J.C.; Longhitano, G.A.; Coatrini-Soares, A.; Carvalho, H.F.; Oliveira, O.N.; Beppu, M.M.; da Silva, J.V.L. Three-Dimensional Printing and Its Potential to Develop Sensors for Cancer with Improved Performance. Biosensors 2022, 12, 685. [Google Scholar] [CrossRef]
- Damiati, S.; Peacock, M.; Leonhardt, S.; Damiati, L.; Baghdadi, M.A.; Becker, H.; Kodzius, R.; Schuster, B. Embedded Disposable Functionalized Electrochemical Biosensor with a 3D-Printed Flow Cell for Detection of Hepatic Oval Cells (HOCs). Genes 2018, 9, 89. [Google Scholar] [CrossRef]
- Belmonte, I.; White, R.J. 3-D Printed Microfluidics for Rapid Prototyping and Testing of Electrochemical, Aptamer-Based Sensor Devices under Flow Conditions. Anal. Chim. Acta 2022, 1192, 339377. [Google Scholar] [CrossRef]
- Koukouviti, E.; Kokkinos, C. 3D Printed Enzymatic Microchip for Multiplexed Electrochemical Biosensing. Anal. Chim. Acta 2021, 1186, 339114. [Google Scholar] [CrossRef] [PubMed]
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-Printed Graphene Direct Electron Transfer Enzyme Biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rahman, M.T.; Du, D.; Panat, R.; Lin, Y. 3-D Printed Adjustable Microelectrode Arrays for Electrochemical Sensing and Biosensing. Sens. Actuators B Chem. 2016, 230, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; Neumsteir, N.V.; Roberto de Oliveira, P.; Janegitz, B.C.; Bonacin, J.A. Sensing of L-Methionine in Biological Samples through Fully 3D-Printed Electrodes. Anal. Chim. Acta 2021, 1142, 135–142. [Google Scholar] [CrossRef]
- Malhotra, S.; Pham, D.S.; Lau, M.P.H.; Nguyen, A.H.; Cao, H. A Low-Cost, 3D-Printed Biosensor for Rapid Detection of Escherichia Coli. Sensors 2022, 22, 2382. [Google Scholar] [CrossRef]
- Silva, L.R.G.; Stefano, J.S.; Orzari, L.O.; Brazaca, L.C.; Carrilho, E.; Marcolino-Junior, L.H.; Bergamini, M.F.; Munoz, R.A.A.; Janegitz, B.C. Electrochemical Biosensor for SARS-CoV-2 CDNA Detection Using AuPs-Modified 3D-Printed Graphene Electrodes. Biosensors 2022, 12, 622. [Google Scholar] [CrossRef]
- Katseli, V.; Economou, A.; Kokkinos, C. Single-Step Fabrication of an Integrated 3D-Printed Device for Electrochemical Sensing Applications. Electrochem. Commun. 2019, 103, 100–103. [Google Scholar] [CrossRef]
- Damiati, S.; Küpcü, S.; Peacock, M.; Eilenberger, C.; Zamzami, M.; Qadri, I.; Choudhry, H.; Sleytr, U.B.; Schuster, B. Acoustic and Hybrid 3D-Printed Electrochemical Biosensors for the Real-Time Immunodetection of Liver Cancer Cells (HepG2). Biosens. Bioelectron. 2017, 94, 500–506. [Google Scholar] [CrossRef]
- Zafir Mohamad Nasir, M.; Novotný, F.; Alduhaish, O.; Pumera, M. 3D-Printed Electrodes for the Detection of Mycotoxins in Food. Electrochem. Commun. 2020, 115, 106735. [Google Scholar] [CrossRef]
- Rocha, D.P.; Squissato, A.L.; da Silva, S.M.; Richter, E.M.; Munoz, R.A.A. Improved Electrochemical Detection of Metals in Biological Samples Using 3D-Printed Electrode: Chemical/Electrochemical Treatment Exposes Carbon-Black Conductive Sites. Electrochim. Acta 2020, 335, 135688. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.; Jiang, H.; Fang, Y.; Jiang, D. A Novel 3D Bio-Printing “Liver Lobule” Microtissue Biosensor for the Detection of AFB1. Food Res. Int. 2023, 168, 112778. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Singh, P.; Halder, S.; Chanda, N.; Mandal, S. 3-D Printed Electrode Integrated Sensing Chip and a PoC Device for Enzyme Free Electrochemical Detection of Blood Urea. Bioelectrochemistry 2021, 142, 107893. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.C.; Baldo, T.A.; Silva-Neto, H.A.; Figueredo, F.; Janegitz, B.C.; Coltro, W.K.T. 3D Printing of Compact Electrochemical Cell for Sequential Analysis of Steroid Hormones. Sens. Actuators B Chem. 2022, 364, 131850. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Pattnaik, P.K.; Goel, S. Smartphone Integrated 3D-Printed Standalone Electrochemiluminescence Platform for Cholesterol Detection. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 4–8. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Bhaiyya, M.; Dudala, S.; Hota, C.; Goel, S. A Machine Learning Approach for Electrochemiluminescence Based Point of Care Testing Device to Detect Multiple Biomarkers. Sens. Actuators A Phys. 2023, 350, 114135. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M.; Calabria, D.; Maddalena Calabretta, M.; Cevenini, L.; Michelini, E. A 3D-Printed Device for a Smartphone-Based Chemiluminescence Biosensor for Lactate in Oral Fluid and Sweat. Analyst 2014, 139, 6494–6501. [Google Scholar] [CrossRef]
- Calabria, D.; Lazzarini, E.; Pace, A.; Trozzi, I.; Zangheri, M.; Cinti, S.; Difonzo, M.; Valenti, G.; Guardigli, M.; Paolucci, F.; et al. Biosensors and Bioelectronics Smartphone-Based 3D-Printed Electrochemiluminescence Enzyme Biosensor for Reagentless Glucose Quantification in Real Matrices. Biosens. Bioelectron. 2023, 227, 115146. [Google Scholar] [CrossRef]
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.K. Electrochemiluminescence Detection of Human Breast Cancer Cells Using Aptamer Modified Bipolar Electrode Mounted into 3D Printed Microchannel. Biosens. Bioelectron. 2018, 118, 217–223. [Google Scholar] [CrossRef]
- Chiadò, A.; Palmara, G.; Chiappone, A.; Tanzanu, C.; Pirri, C.F.; Roppolo, I.; Frascella, F. A Modular 3D Printed Lab-on-a-Chip for Early Cancer Detection. Lab Chip 2020, 20, 665–674. [Google Scholar] [CrossRef]
- Aschenbrenner, D.; Friedrich, O.; Gilbert, D.F. 3D Printed Lab-on-a-Chip Platform for Chemical Stimulation and Parallel Analysis of Ion Channel Function. Micromachines 2019, 10, 548. [Google Scholar] [CrossRef]
- Santangelo, M.F.; Shtepliuk, I.; Filippini, D.; Puglisi, D.; Vagin, M.; Yakimova, R.; Eriksson, J. Epitaxial Graphene Sensors Combined with 3D-Printed Microfluidic Chip for Heavy Metals Detection. Sensors 2019, 19, 2393. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.N.; Shu, Y.; Xiong, B.; Chen, Y.; Chen, Y.; Tian, Q.; Michael, S.A.; Shen, B.; Wu, H. Simple, Cost-Effective 3D Printed Microfluidic Components for Disposable, Point-of-Care Colorimetric Analysis. ACS Sens. 2016, 1, 227–234. [Google Scholar] [CrossRef]
- Siller, I.G.; Preuss, J.A.; Urmann, K.; Hoffmann, M.R.; Scheper, T.; Bahnemann, J. 3D-printed Flow Cells for Aptamer-based Impedimetric Detection of e. Coli Crooks Strain. Sensors 2020, 20, 4421. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Enders, A.; Ackerman, S.; Bahnemann, J.; Segal, E. 3D-Printed Microfluidics Integrated with Optical Nanostructured Porous Aptasensors for Protein Detection. Microchim. Acta 2021, 188, 67. [Google Scholar] [CrossRef]
- Fraser, L.A.; Kinghorn, A.B.; Dirkzwager, R.M.; Liang, S.; Cheung, Y.W.; Lim, B.; Shiu, S.C.C.; Tang, M.S.L.; Andrew, D.; Manitta, J.; et al. A Portable Microfluidic Aptamer-Tethered Enzyme Capture (APTEC) Biosensor for Malaria Diagnosis. Biosens. Bioelectron. 2018, 100, 591–596. [Google Scholar] [CrossRef]
- Yu, N.; Wu, J. Rapid and Reagentless Detection of Thrombin in Clinic Samples via Microfluidic Aptasensors with Multiple Target-Binding Sites. Biosens. Bioelectron. 2019, 146, 111726. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a Biodegradable Subcutaneous Implant for Prolonged Drug Delivery Using 3D Printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef]
- Bauer, M.; Kulinsky, L. Fabrication of a Lab-on-Chip Device Using Material Extrusion (3D Printing) and Demonstration via Malaria-Ab ELISA. Micromachines 2018, 9, 27. [Google Scholar] [CrossRef]
- Pohanka, M.; Keresteš, O.; Žáková, J. A 3D-Printed Do-It-Yourself ELISA Plate Reader as a Biosensor Tested on TNFα Assay. Biosensors 2024, 14, 331. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Silva, P.R.L.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Muñoz, R.A.A. 3D-Printed Graphene/Polylactic Acid Electrode for Bioanalysis: Biosensing of Glucose and Simultaneous Determination of Uric Acid and Nitrite in Biological Fluids. Sens. Actuators B Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Lopes, C.E.C.; de Faria, L.V.; Araújo, D.A.G.; Richter, E.M.; Paixão, T.R.L.C.; Dantas, L.M.F.; Muñoz, R.A.A.; da Silva, I.S. Lab-Made 3D-Printed Electrochemical Sensors for Tetracycline Determination. Talanta 2023, 259, 124536. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Wang, H.; Qiu, Y.; Luo, H.; He, B.; Wu, B.; Gao, B. 3D Printed Smart Silk Wearable Sensors. Analyst 2021, 146, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Lee-Lewandrowski, E.; Kent, L. Regulatory compliance for point-of-care testing: A per-spective from the United States (circa 2000). Clin. Lab. Med. 2001, 2, 241–254. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Tian, Y.; Zhu, W.; Yan, C.; Shi, Y.; Kong, L.B.; Qi, H.J.; Zhou, K. 3D-Printed Anisotropic Polymer Materials for Functional Applications. Adv. Mater. 2022, 34, 2102877. [Google Scholar] [CrossRef]
- Nizam, M.; Purohit, R.; Taufik, M. 3D Printing in Healthcare: A Review on Drug Printing, Challenges and Future Perspectives. Mater. Today Commun. 2024, 40, 110199. [Google Scholar] [CrossRef]



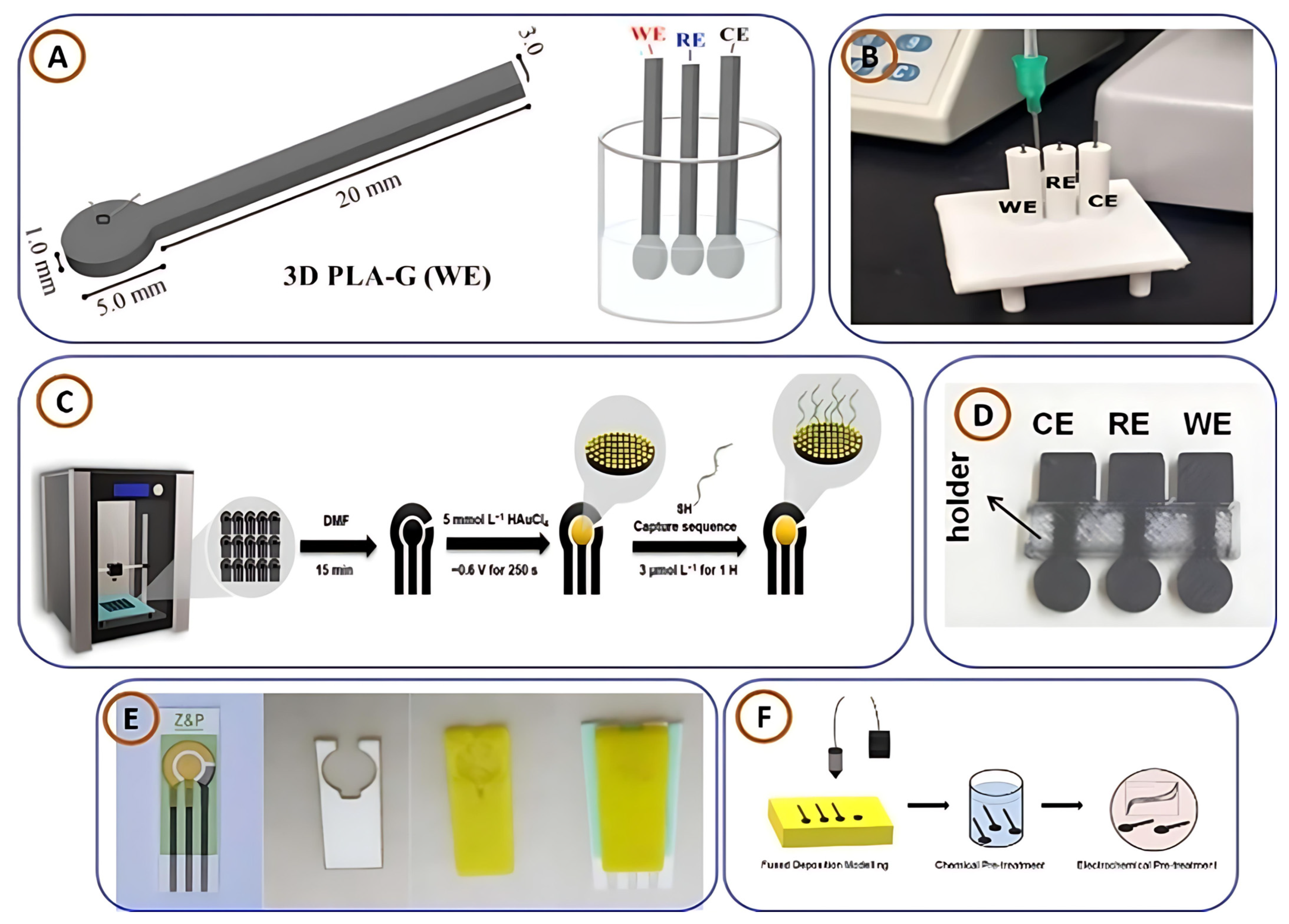

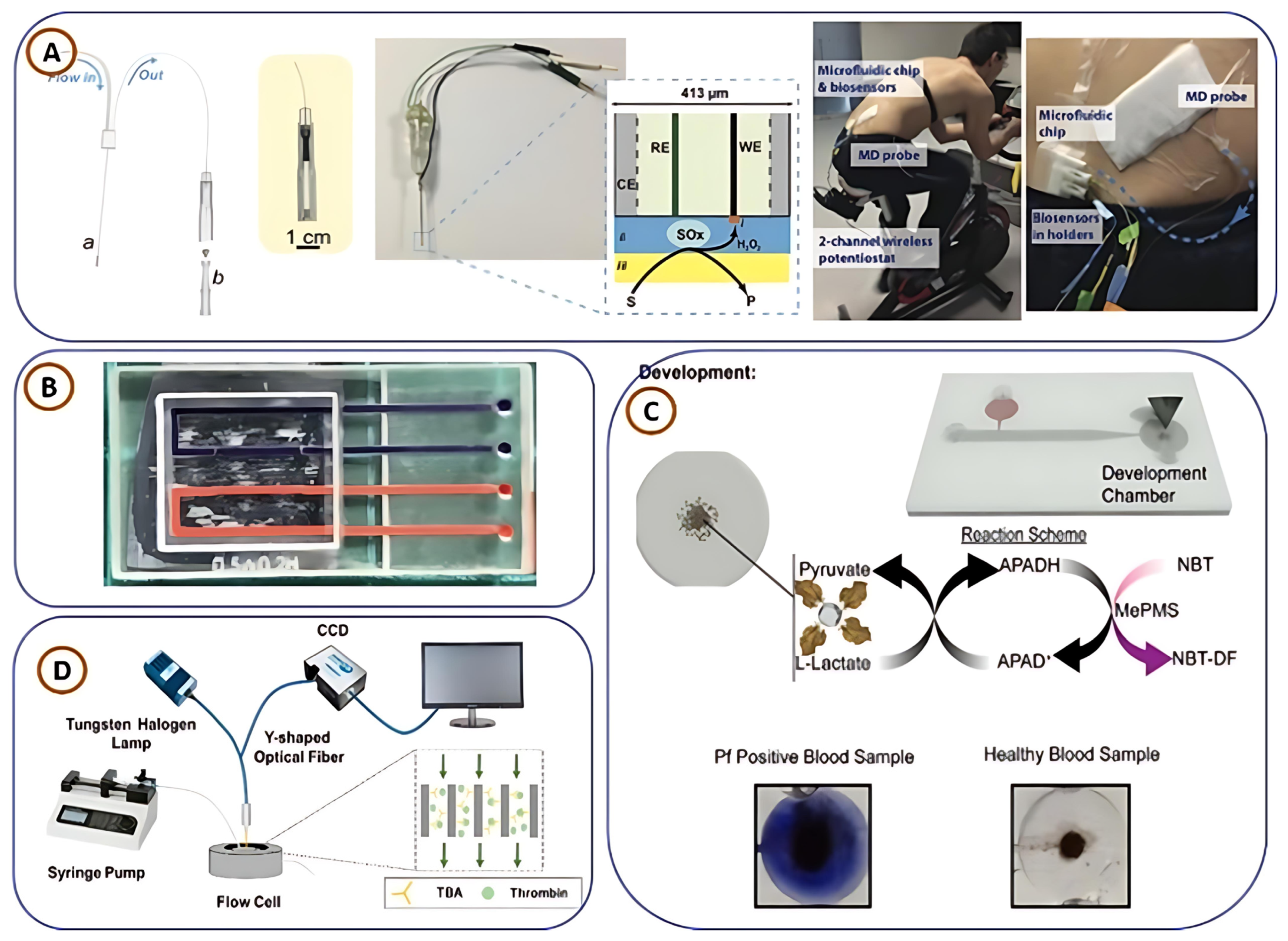
| Attribute | FDM | SLA | PBF | BJ | DED | LOM |
|---|---|---|---|---|---|---|
| Material Used | PLA, ABS, PETG | Photopolymers | Metals, polymers | Metal, ceramic powders | Metals | Paper, plastic, metal |
| Resolution (µm) | 100–300 | <50 | <100 | 100–200 | <50 | 200–400 |
| Speed (mm/s) | 50–150 | 10–100 | 20–80 | 50–200 | 5–20 | 50–100 |
| Energy Consumption (W) | 50–150 | 100–250 | 200–500 | 150–300 | 500–1000 | 30–80 |
| Surface Roughness (Ra, µm) | 10–25 | 1–5 | 5–15 | 15–30 | 5–20 | 25–50 |
| Tensile Strength (MPa) | 20–50 | 40–65 | 100–150 (metal) | 20–40 | 150–300 | 15–30 |
| Typical Applications | Prototypes, medical models, educational aids | Dental models, hearing aids, jewelry | Functional parts, implants, aerospace | Molds, architectural models | Repairs, implants, aerospace components | Packaging, low-cost prototypes |
| Drawbacks | Lower precision, poor surface quality | Resin handling issues, material limitations | Expensive, material-specific, needs inert gas | Weak mechanical strength, post-processing required | High equipment cost, complex process control | Low bonding strength, poor resolution |
| Estimated Cost per Device (USD) | 1–5 | 3–10 | 10–100 | 5–15 | 20–150 | 1–3 |
| Material Type | Key Properties | Impact on PoCT Devices |
|---|---|---|
| Graphene–PLA Composites | High conductivity, biocompatibility | Enhanced electrochemical sensing capabilities |
| Photopolymer Resins | High resolution, optical clarity | Precise microfluidic channel fabrication |
| PDMS | Flexibility, optical transparency | Suitable for microfluidic devices and wearable sensors |
| SEBS | Elasticity, skin compatibility | Ideal for stretchable wearable sensors |
| Hydrogels | High water content, biocompatibility | Mimic biological tissues for biosensing applications |
| MXenes | Electrical conductivity, flexibility | Development of sensitive and flexible wearable biosensors |
| MOFs | High surface area, porosity | Improved sensitivity and selectivity in analyte detection |
| Ref. No. | Application | 3D Printing Material | Sensing Mechanism | Key Features | Statistical Analysis |
|---|---|---|---|---|---|
| [72] | Stretchable and Wearable Sensors | Multi-Walled Carbon Nanotubes (MWNT) and PDMS | Piezoresistive Sensing | Highly flexible, strain detection up to 146% | Sensitivity (gauge factor = 12.15), tested under cyclic loads |
| [74] | High-Precision Wearable Biosensors | 3D-Printed Sugar Scaffold | Capacitive and Resistive | Personalized, lightweight, and highly sensitive | Statistical validation for EMG, EDA, and EEG sensing |
| [73] | Stretchable Tactile Sensors | Conductive Polymer Composite | Piezoresistive | High sensitivity, used for prosthetics | Response time and mechanical stability validated |
| [75] | Ionic Skin Sensors | Photo-Polymerized Hydrogel | Ionic Conductivity | Skin-like elasticity, high-resolution 3D printing | Linearity and LOD analysis provided |
| [76] | Bioelectronic Sweat Monitoring Patch | 3D-Printed Flexible Sensors | Electrochemical | Detects multiple electrolytes in sweat | Real-time health monitoring, reproducibility tested |
| [79] | Piezoresistive Health Monitoring Sensor | CNT Surface-Filled SEBS Substrate | Piezoresistive | Highly stretchable, stable | 2000+ cycle durability and response time (149 ms) measured |
| [82] | Wearable Smart Device | Liquid Metal and 3D-Printed Polymer | Infrared and Acoustic | Core body temperature and bone conduction | Real-time data accuracy comparison with clinical tools |
| [83] | Personalized Oral Drug Delivery | PLA-PVA 3D-Printed Device | Drug-Release Mechanism | Custom-fit mouthguard with tunable release | First-in-human study, drug diffusion kinetics analyzed |
| [84] | Recyclable Wearable Electronics | Dynamic Thermoset Elastomer | Capacitive and Triboelectric | Fully degradable and recyclable electronics | Mechanical durability and recyclability efficiency evaluated |
| [85] | Respiratory and Heart Rate Monitoring | 3D-Printed FBG-Based Sensor | Optical Strain Sensing | High accuracy HR and RR monitoring | Metrological properties validated |
| [86] | Skin-Like Wearable Strain Sensors | Self-Healable Hydrogel with CNTs | Piezoresistive and Piezoelectric | Multifunctional, real-time response | Sensitivity: GF = 6.29 (resistance), 1.25 kPa−1 (capacitance) |
| [87] | Ultra-Robust Biomonitoring Sensors | Graphene-Doped Porous Silicone | Piezoresistive | Long-term durability (>12 months), stable under 75% compression | 400+ cycle durability, resistance stability validated |
| [88] | Microfluidic Biosensor for Human Tissue | 3D-Printed Electrode Holders | Electrochemical | Continuous glucose and lactate monitoring | Real-time data validation on cyclists |
| [89] | Smart Fibers and Textiles | MXene-Reinforced Cellulose Nanofibrils | Electrical, Mechanical, Photonic | High flexibility, responsive to multiple stimuli | Wearable heating and sensing applications tested |
| [90] | Stretchable Thermoelectric Generators | PEDOT:PSS Composite | Thermoelectric | Self-healing, maintains > 85% power after damage | Power output: 12.2 nW, retained post-cutting |
| Ref. No. | Detection Target | 3D Printing Material | Electrochemical Method | Sensitivity and Detection Limit | Statistical Analysis |
|---|---|---|---|---|---|
| [96] | Hepatic Oval Cells (HOCs) | MWCNTs with Chitosan Film | Cyclic Voltammetry and Square-Wave Voltammetry | Enhanced sensitivity due to MWCNT scaffold | Reproducibility tested; RSD values provided |
| [97] | Insulin and ATP | Epoxy-embedded electrodes with microfluidic devices | Aptamer-based electrochemical detection | Simultaneous detection under flow conditions | Linearity and LOD values evaluated |
| [98] | Cardiac Biomarkers (Cholesterol and Choline) | Enzymatic 3D-printed microchip | Amperometric determination | Low LOD for cardiac biomarkers (3.36 and 0.08 μm) | Multiplexed assay statistical validation |
| [99] | Hydrogen Peroxide (H2O2) | Conductive graphene filaments | Direct Electron Transfer (DET) | No need for mediators, stable response: LOD 11.1 and 9.1 μM for H2O2 | Repeatability tests and comparative performance with traditional methods |
| [25] | Parkinson’s Disease Biomarker (PARK7/DJ-1 Protein) | PLA-based conductive filaments | Impedimetric and Voltammetric Analysis | LOD: 1.01 µg/L (impedimetric), 3.46 µg/L (voltammetric) | Repeatability and reproducibility confirmed |
| [103] | SARS-CoV-2 cDNA and Creatinine | AuPs-modified graphene–PLA electrodes | Square-Wave Voltammetry | LOD for SARS-CoV-2 cDNA: 0.30 µmol/L | RSD = 1.14%, n = 3 |
| [100] | Hydrogen Peroxide and Glucose | Silver microelectrode arrays | Amperometric sensing | LOD: 0.45 µM (H2O2), 1.7 µM (glucose) | Sensitivity analysis and diffusion limitations tested |
| [107] | Cadmium and Lead in Biological Fluids | Carbon black–PLA electrodes | Square-Wave Anodic Stripping Voltammetry (SWASV) | LOD: 2.9 µg/L (Cd2+), 2.6 µg/L (Pb2+) | RSD < 6.5%, high reproducibility |
| [105] | Liver Cancer Cells (HepG2) | Hybrid 3D-printed electrochemical sensor | Cyclic Voltammetry and Quartz Crystal Microbalance (QCM-D) | Highly selective for CD133 biomarker | Real-time detection with label-free analysis |
| [108] | Aflatoxin B1 (AFB1) | 3D-bio-printed liver lobule microtissue | Differential Pulse Voltammetry | LOD: 0.039 µg/mL | Stability and reproducibility tested |
| [109] | Blood Urea | Gold nanoparticle-integrated 3D-printed chip | Linear Sweep Voltammetry | LOD: 0.1 µM, Sensitivity: 183 µA mM−1 cm−2 | RSD = 3.63%, shelf life > 6 months |
| [110] | Steroid Hormones (Estradiol and Progesterone) | PLA-CB and ABS electrochemical cell | Differential Pulse Voltammetry | LOD: 0.11 µmol/L (E2), 17.8 µmol/L (P4) | RSD = 3.1% (repeatability), 10.7% (reproducibility) |
| [102] | Escherichia coli | 3D-printed graphite pencil electrode | Cyclic Voltammetry | LOD: 53 CFU/mL, LOQ: 270 CFU/mL | Cost-effective and rapid detection (USD 2.50/test) |
| [104] | Multipurpose Electrochemical Sensing | Carbon-loaded PLA electrodes | Single-Step 3D Printing | Versatile detection with a broad potential range | High precision and reproducibility |
| Ref. No. | Application | 3DP Material | Sensing Mechanism | Key Features | Statistical Analysis |
|---|---|---|---|---|---|
| [124] | Biodegradable Drug Delivery Implants | PLA and PCL | Controlled Drug Release | Personalized, long-duration drug release | Drug diffusion kinetics analyzed |
| [125] | Malaria Detection | FDM 3D-Printed Fluidic Cartridge | Colorimetric ELISA | Portable, automated reagent dispensing | Cost-effective with smartphone-based analysis |
| [117] | Ion Channel Functional Analysis | ABS Microfluidic Chip | Fluorescence-Based Functional Imaging | Low-cost, reproducible, high throughput | Homogeneity of solution exchange validated |
| [118] | Heavy Metal Detection (Pb and Cd) | Epitaxial Graphene | Conductometric | Fast response, real-time detection | Sensitivity (13.90 Ω/µM) tested with Langmuir isotherm correlation |
| [119] | Urinary Protein Quantification | 3DP Microfluidic Components | Colorimetric Analysis | Simple, cost-effective, portable | Smartphone-based quantification tested (LoD: 8.5 μg/mL) |
| [88] | Online Subcutaneous Monitoring | Integrated Microfluidic Biosensors | Electrochemical Detection | Real-time glucose (6.02 ± 1.08 mM) and lactate (1.81 ± 0.33 mM) monitoring | Wireless connectivity tested in athletes |
| [120] | E. coli Detection | 3DP Flow Cells | Impedimetric Aptasensor | High specificity, microfluidic integration | Sensitivity analysis performed |
| [121] | Protein Detection | Polyacrylate-Based Microfluidic Platform | Optical Aptasensor | High selectivity, improved LOD | Comparative performance with PDMS microfluidics |
| [122] | Malaria Diagnosis | 3DP Microfluidic Chambers | Aptamer-Tethered Enzyme Capture (APTEC) | Portable, high sensitivity (90% across all patient samples) | Clinical sample validation conducted |
| [123] | Thrombin Detection | Open-Ended Porous Silicon | Reflective Interferometric Fourier Transform Spectroscopy (RIFTS) | Rapid, reagentless detection | ELISA-based verification tested (LoD: ∼6.70 nM) |
| [98] | Multiplexed Cardiac Biomarker Detection | 3DP Enzymatic Microchip | Amperometric Electrochemical Biosensing | Simultaneous cholesterol and choline detection | LOD 3.36 and 0.08 μm |
| [116] | Modular Early Cancer Detection | Functional Polymeric 3D Device | Immunoassay for Protein Biomarkers | Rapid, cost-effective, scalable | LOD for VEGF: 11 ng/mL, Angiopoietin-2: 0.8 ng/mL |
| [126] | DIY ELISA Plate Reader | 3DP Optical Sensor | Colorimetric Detection | Low-cost alternative to commercial plate readers | LOD: 19 pg/mL for TNFα assay |
| [127] | Glucose, Uric Acid, and Nitrite Detection | Graphene–PLA Electrodes | Differential Pulse Voltammetry | Multi-analyte sensing in biological fluids | LOD: 0.02 µM (uric acid), 0.03 µM (Nitrite), 15 µM (glucose) |
| [128] | Tetracycline Antibiotic Detection | Conductive Graphite–PLA Electrode | Amperometric BIA-AD System | High sensitivity, food and water safety application | LOD: 0.19 µM, recovery: 92–117% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.S.; Khandelwal, S.; Thakre, Y.; Rangole, J.; Kulkarni, M.B.; Bhaiyya, M. A Review on 3D-Printed Miniaturized Devices for Point-of-Care-Testing Applications. Biosensors 2025, 15, 340. https://doi.org/10.3390/bios15060340
Kulkarni AS, Khandelwal S, Thakre Y, Rangole J, Kulkarni MB, Bhaiyya M. A Review on 3D-Printed Miniaturized Devices for Point-of-Care-Testing Applications. Biosensors. 2025; 15(6):340. https://doi.org/10.3390/bios15060340
Chicago/Turabian StyleKulkarni, Amol S., Sarika Khandelwal, Yogesh Thakre, Jyoti Rangole, Madhusudan B. Kulkarni, and Manish Bhaiyya. 2025. "A Review on 3D-Printed Miniaturized Devices for Point-of-Care-Testing Applications" Biosensors 15, no. 6: 340. https://doi.org/10.3390/bios15060340
APA StyleKulkarni, A. S., Khandelwal, S., Thakre, Y., Rangole, J., Kulkarni, M. B., & Bhaiyya, M. (2025). A Review on 3D-Printed Miniaturized Devices for Point-of-Care-Testing Applications. Biosensors, 15(6), 340. https://doi.org/10.3390/bios15060340





