Novel Nanocomposites of Carbon Nanomaterials and Poly(Neutral Red) Electropolymerized from Reline for DNA Damage Detection and Beverage Antioxidant Influence Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
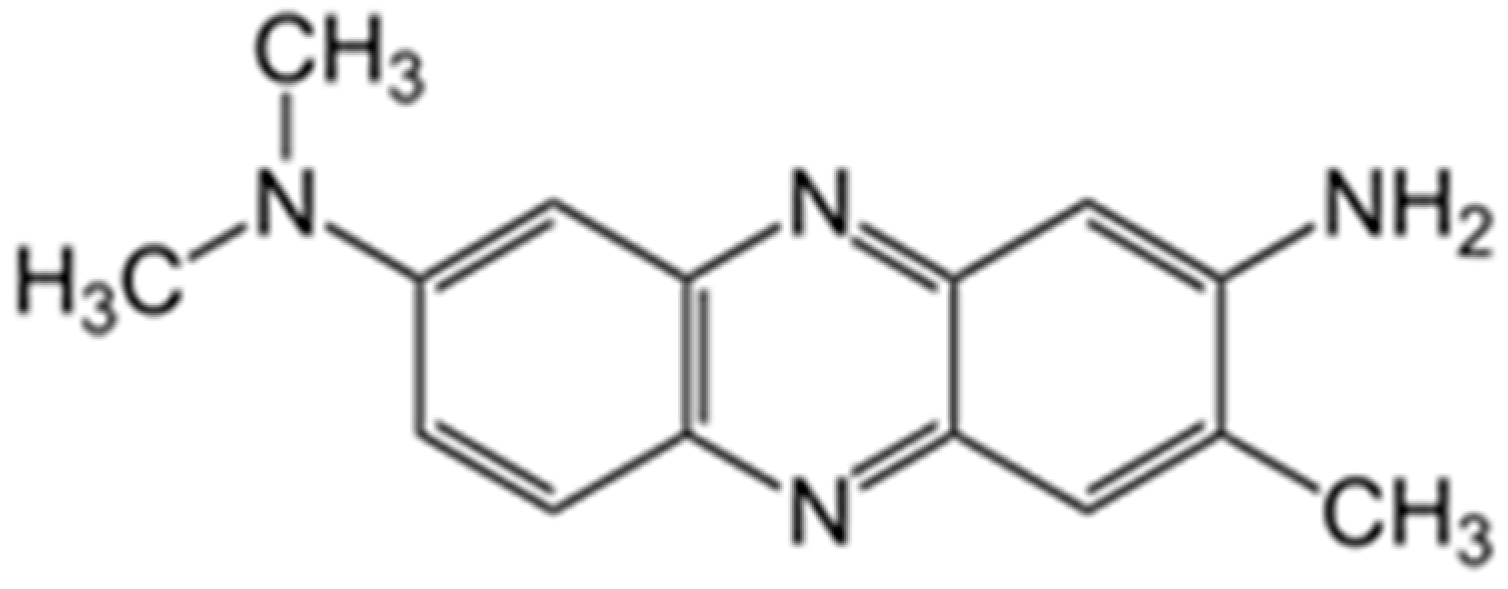
2.3. Neutral Red Electropolymerization and DNA Biosensor Assembling
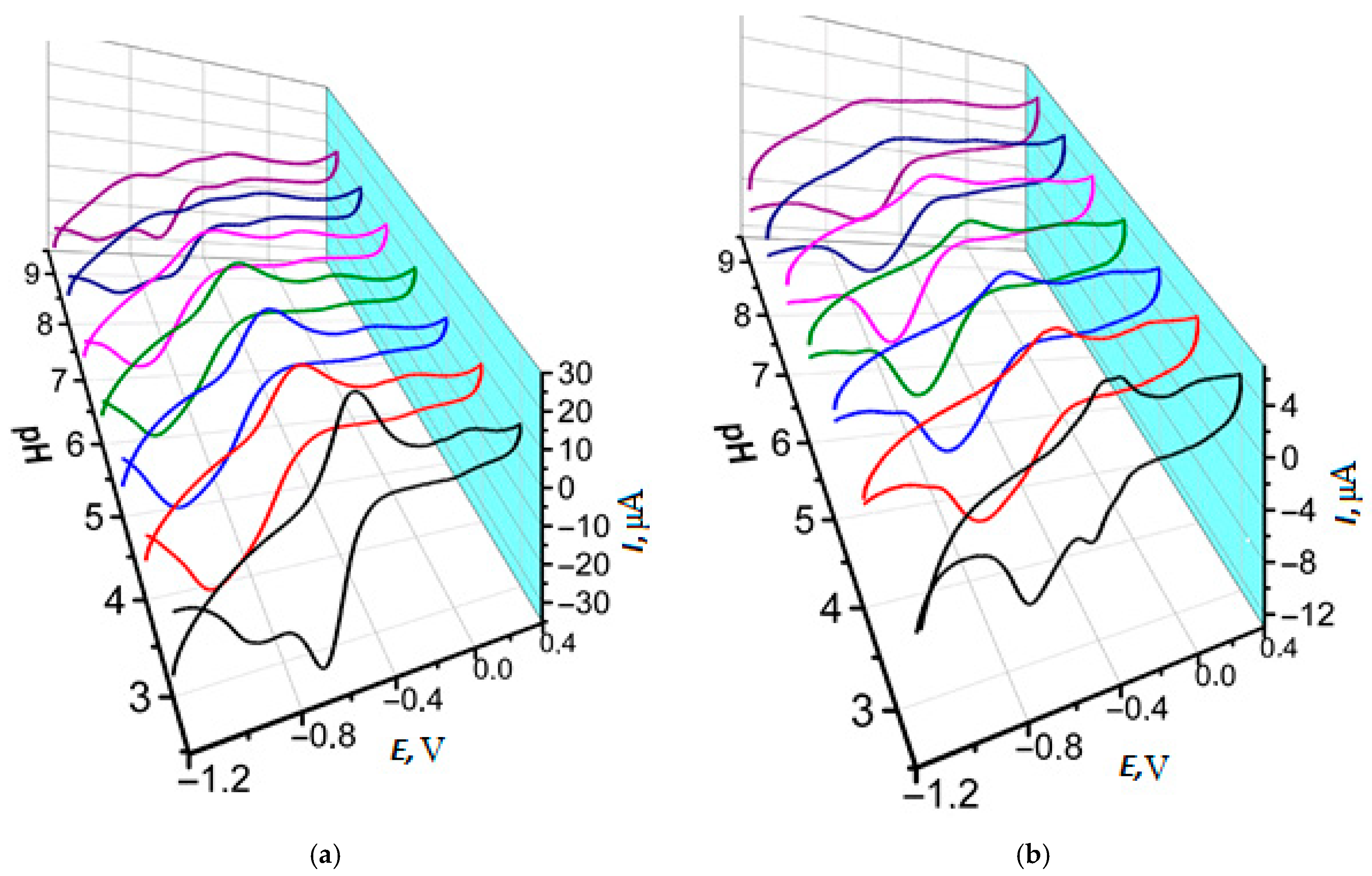
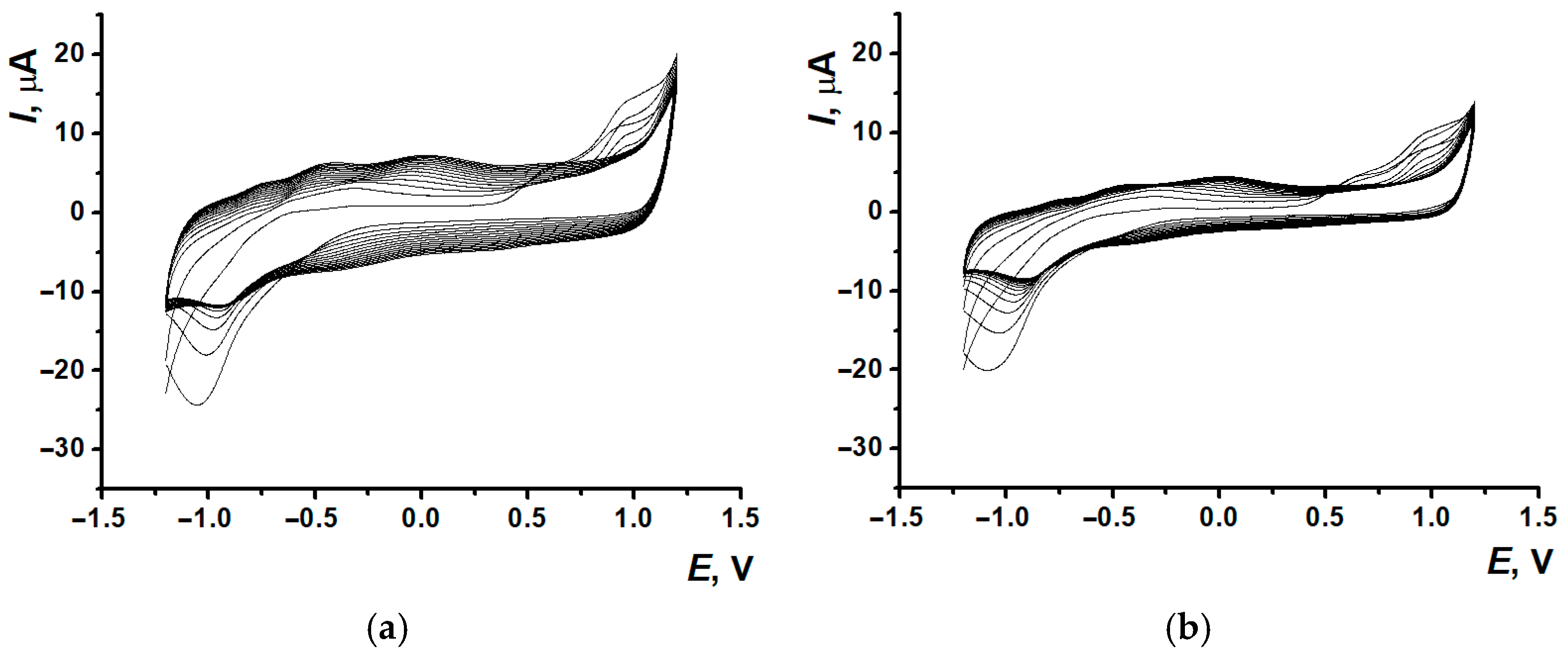
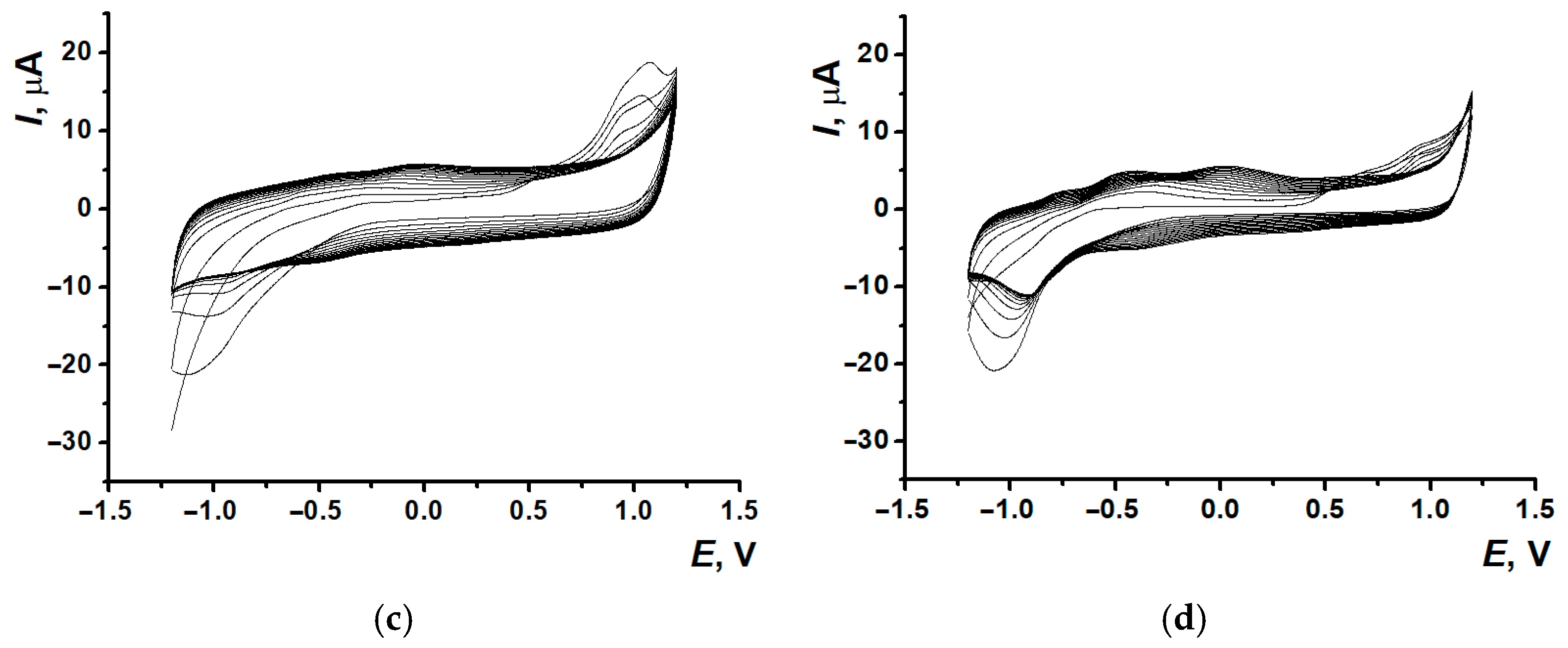
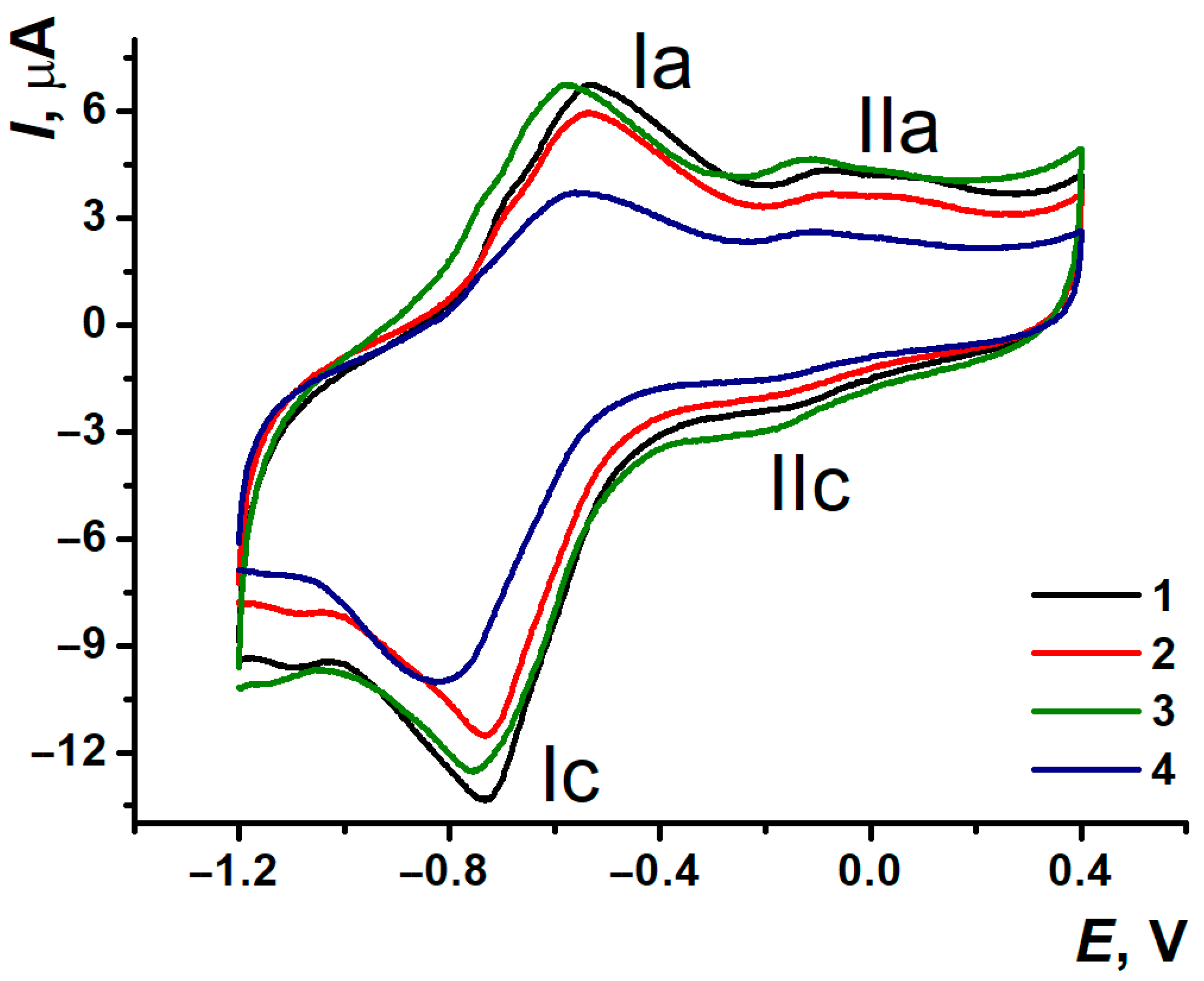
3. Results and Discussion
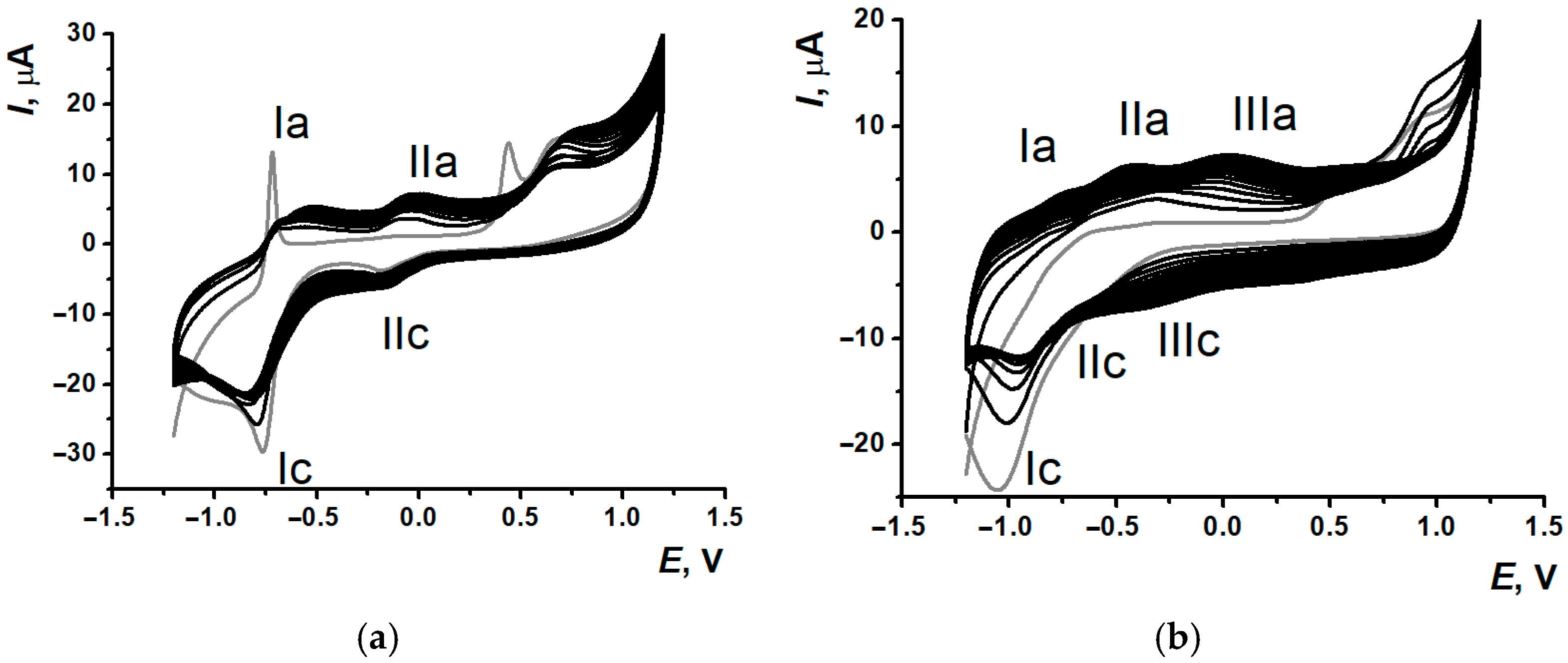
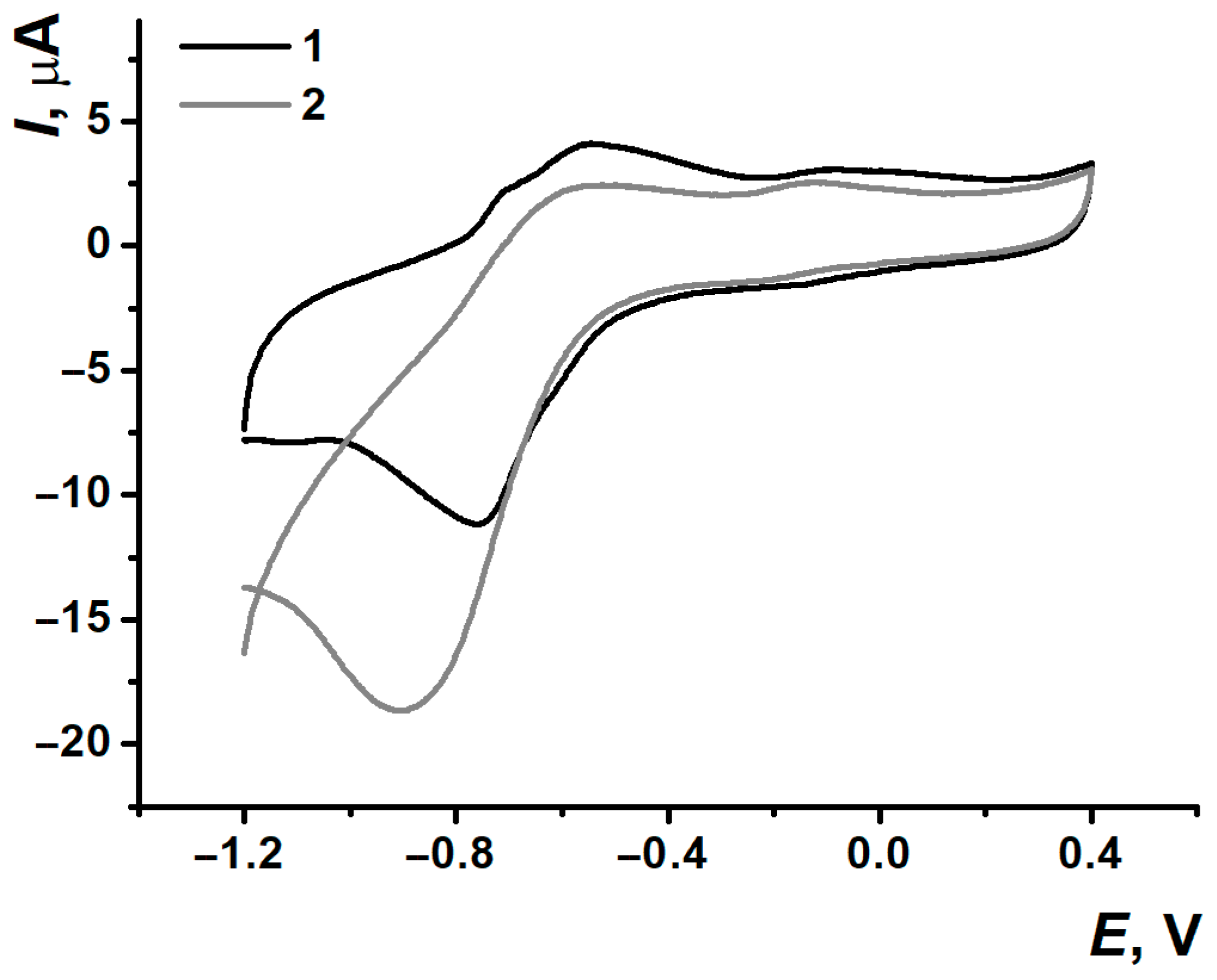
3.1. Electropolymerization of Neutral Red on Screen-Printed Carbon Electrodes from Phosphate Buffer or Reline
3.2. Comparison of Electrochemical Properties of Poly(Neutral Red) from Reline and Phosphate Buffer
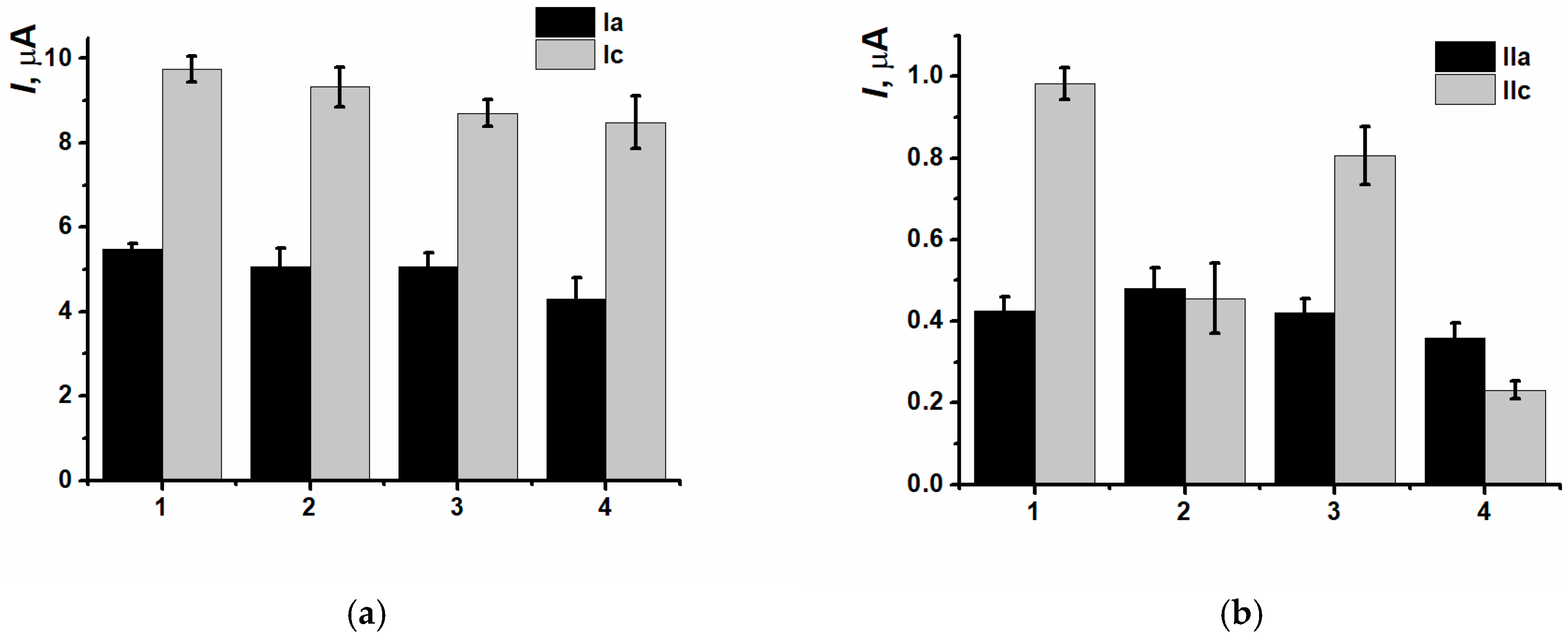
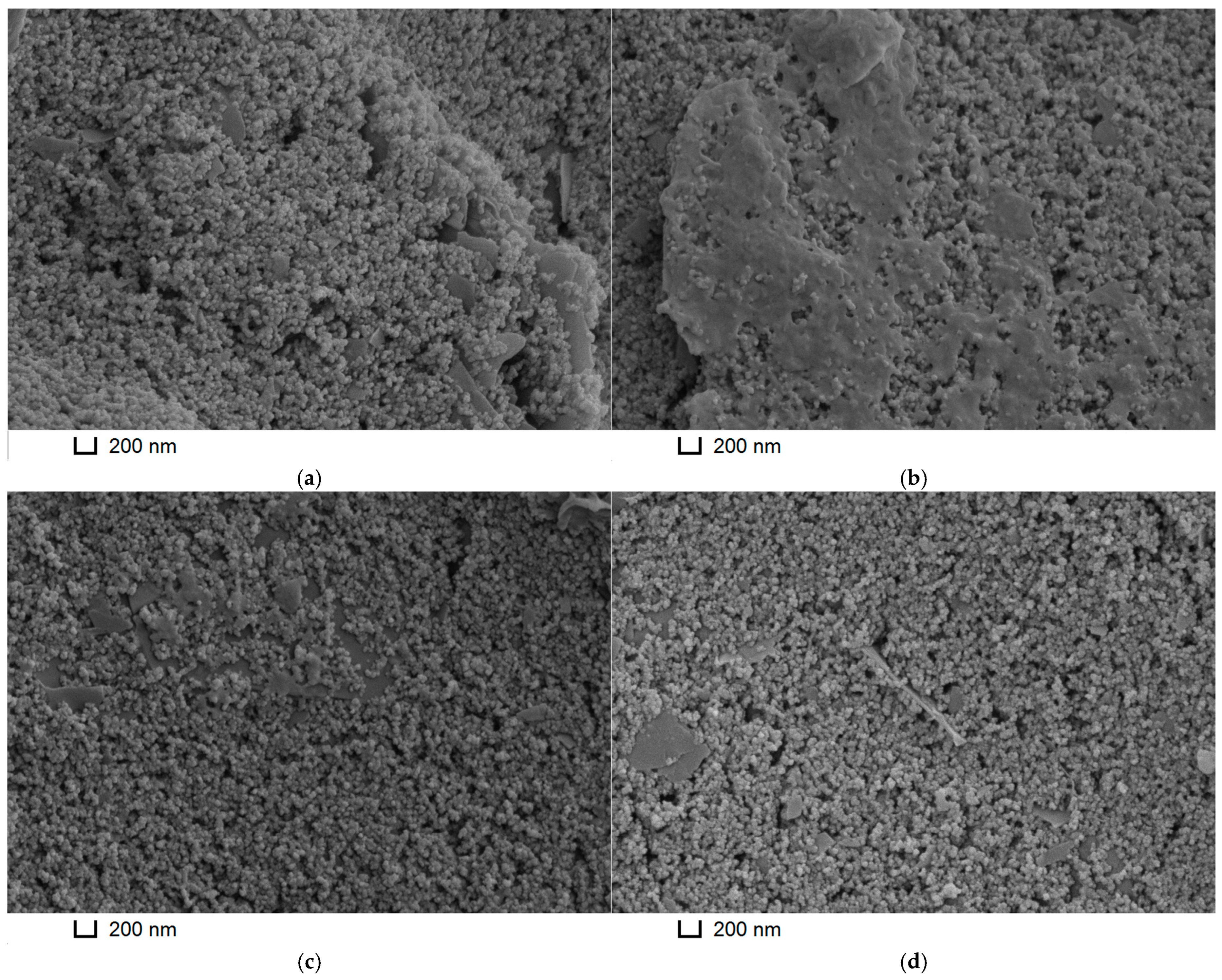
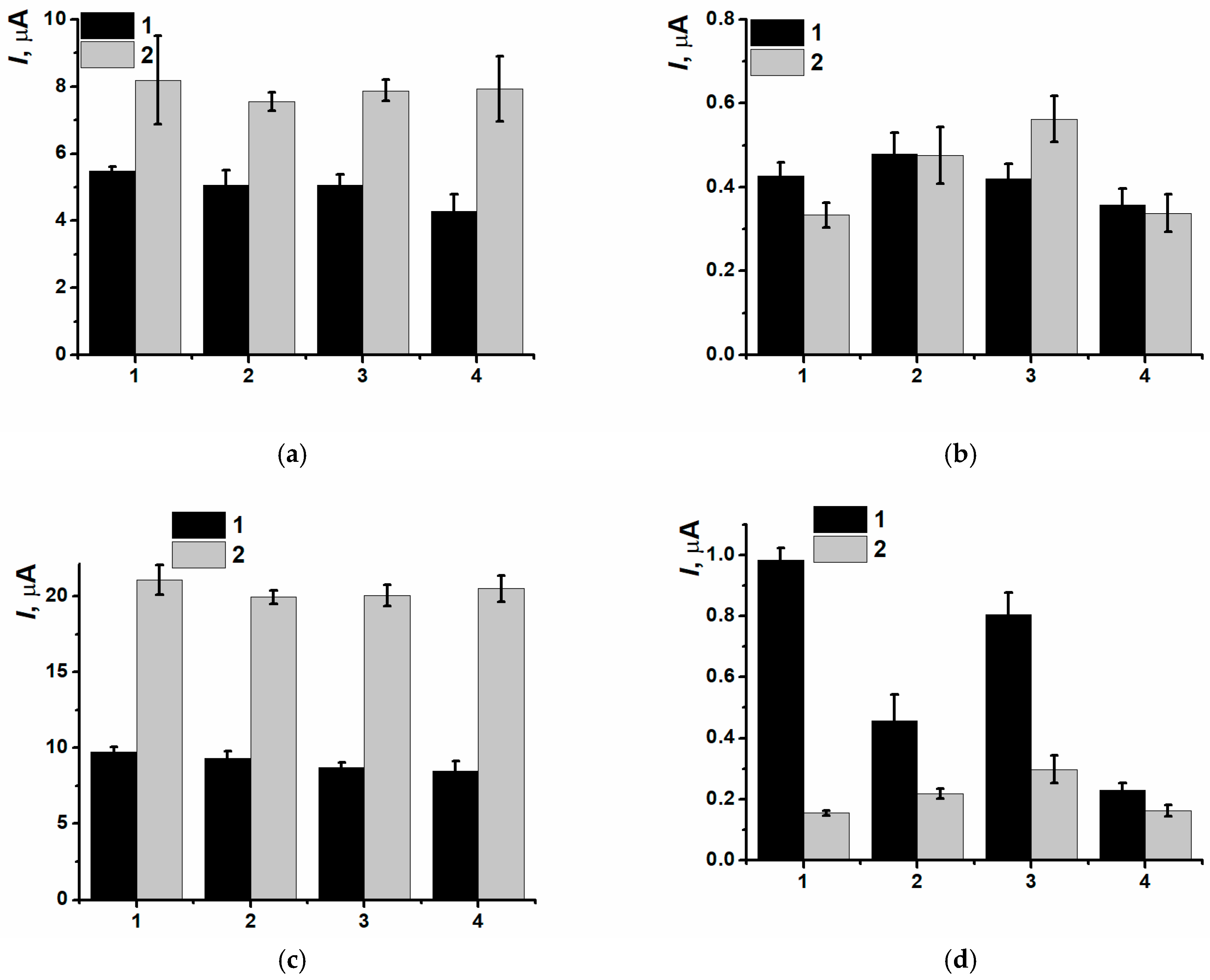
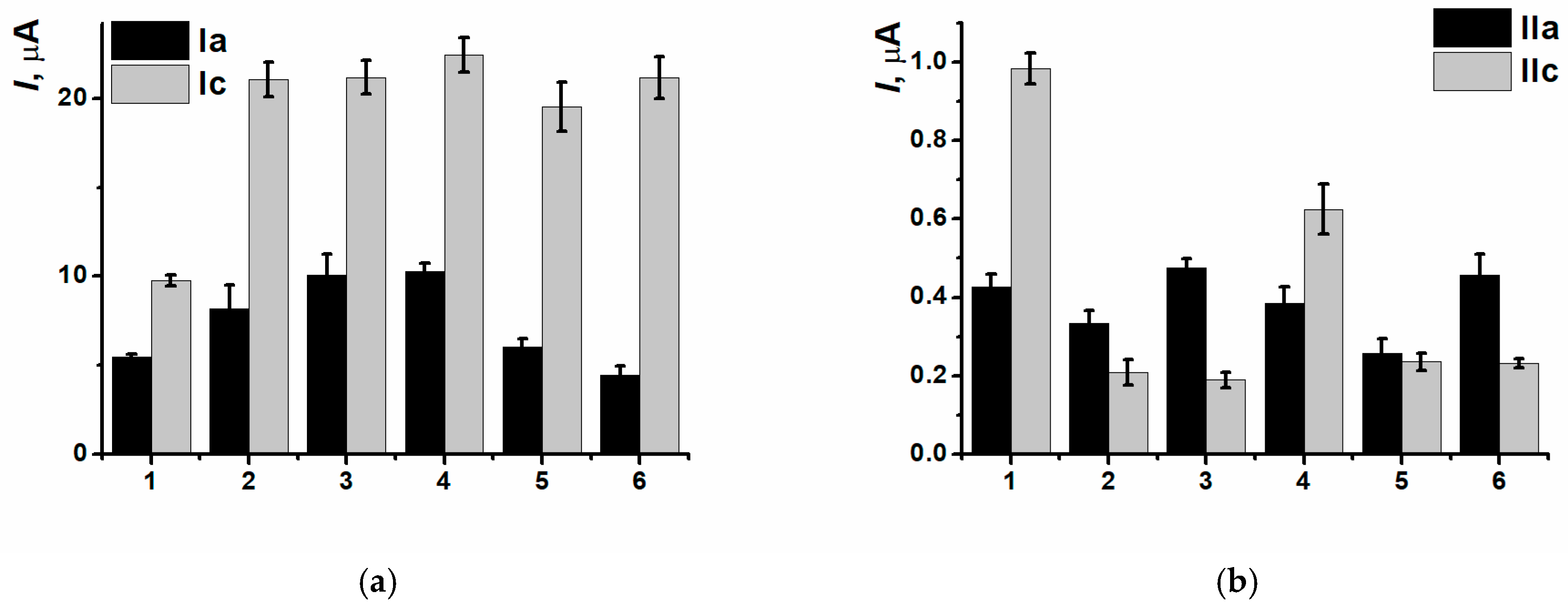
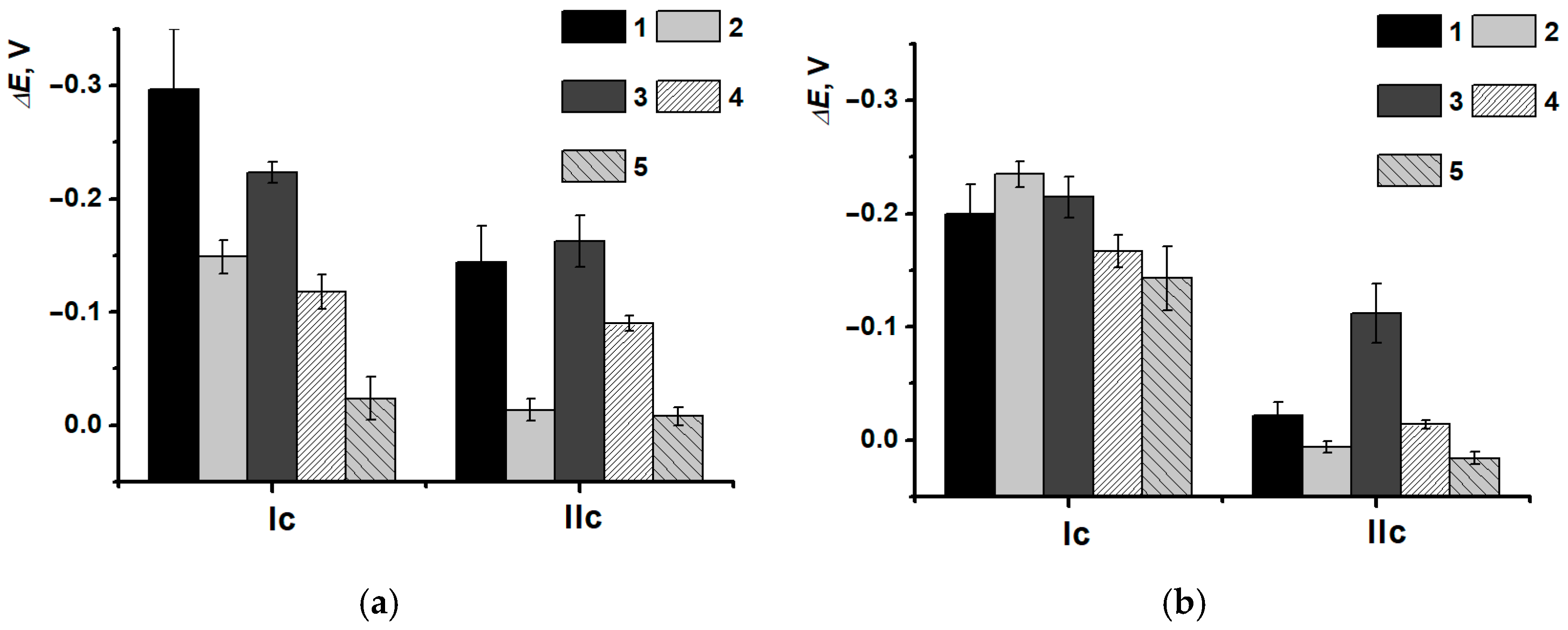
3.3. Electrodeposition of Poly(Neutral Red) from Reline with Carbon Nanomaterials
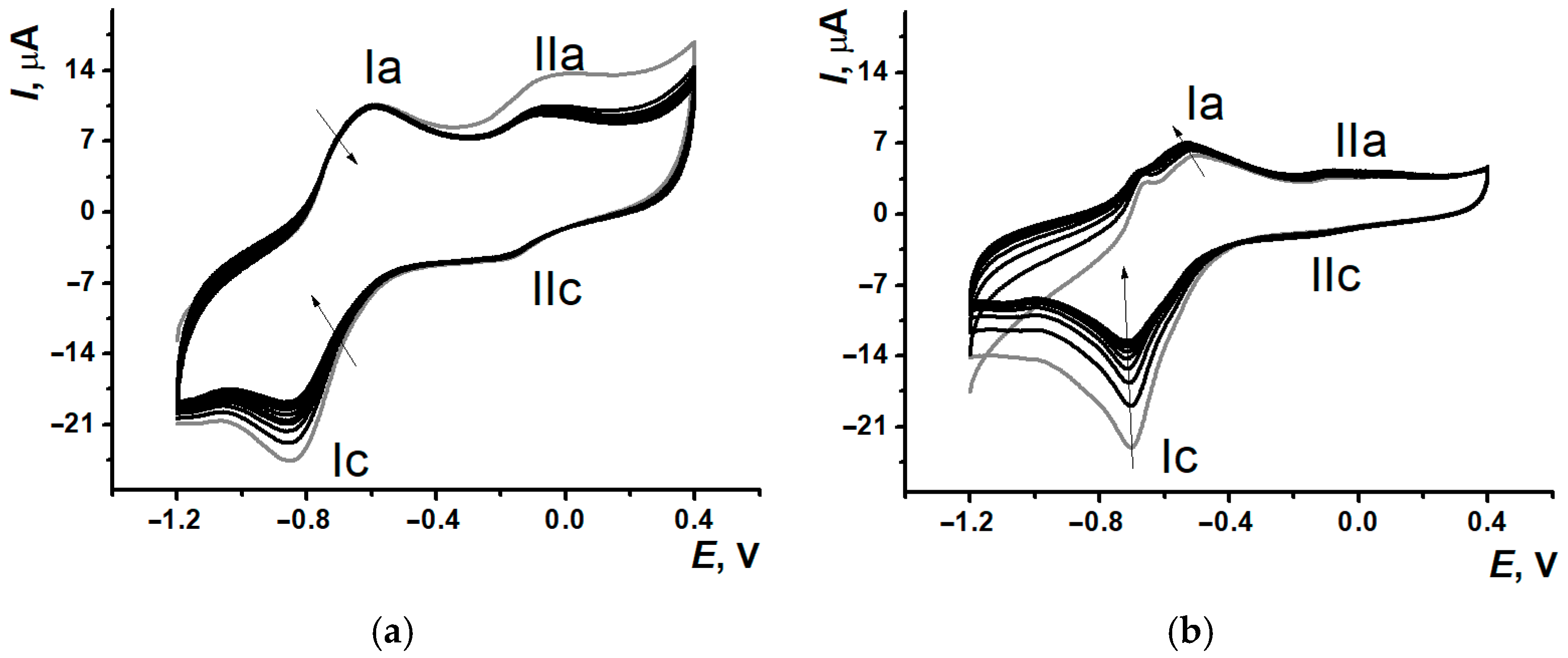
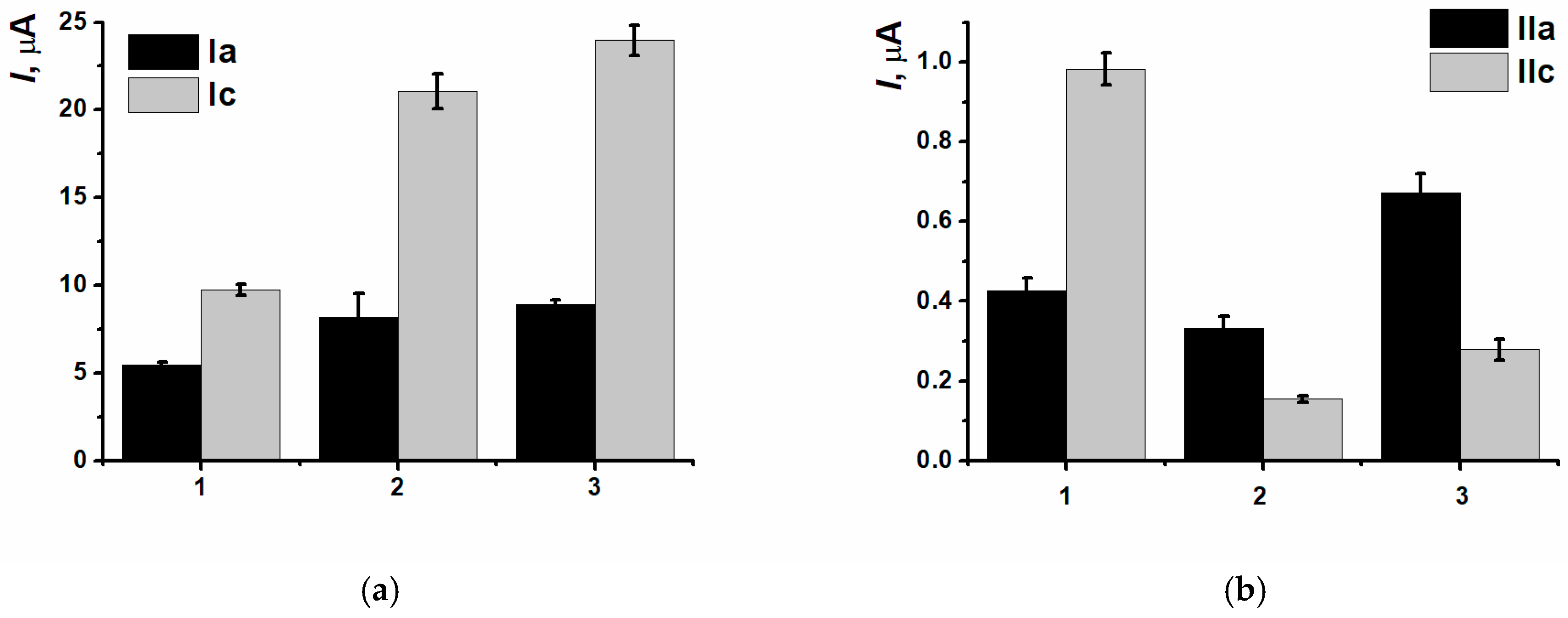
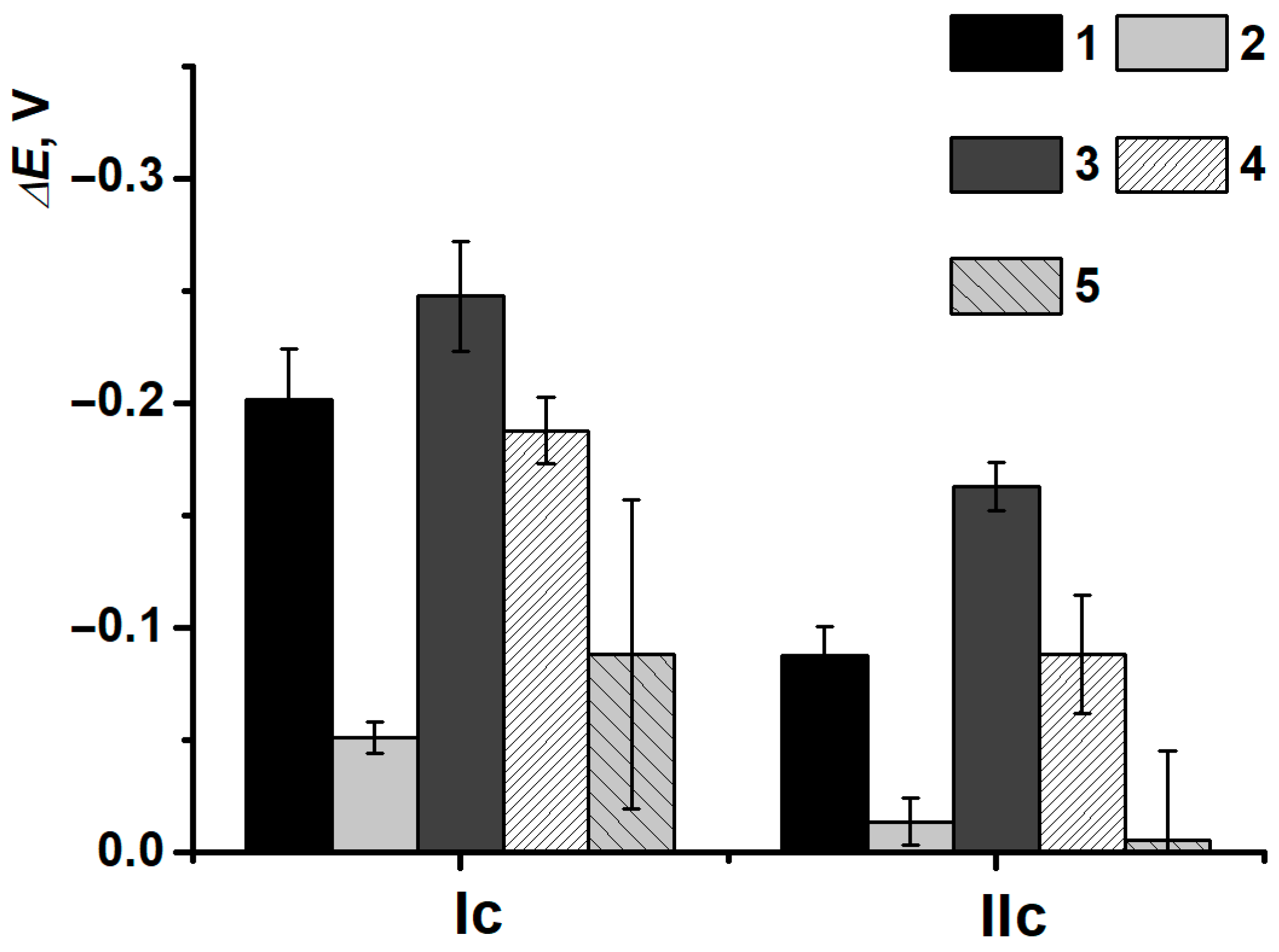
3.4. DNA Implementation Effect
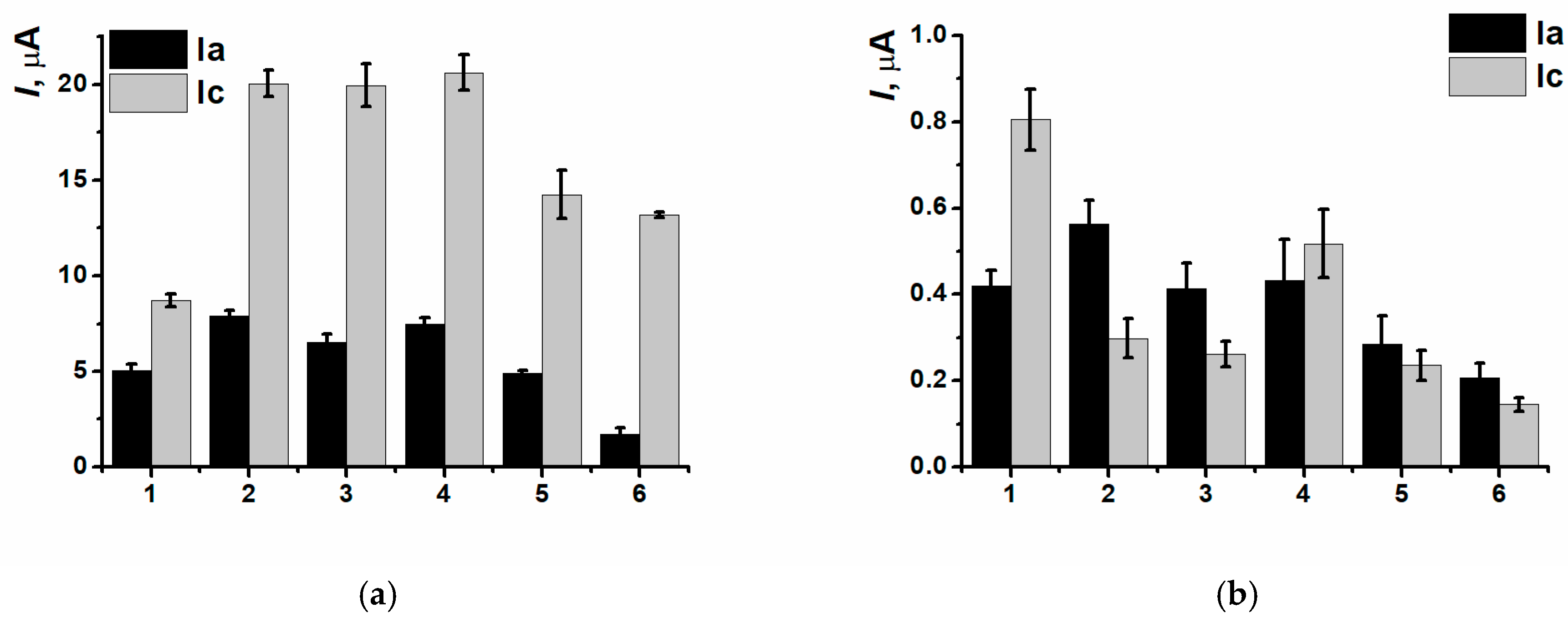
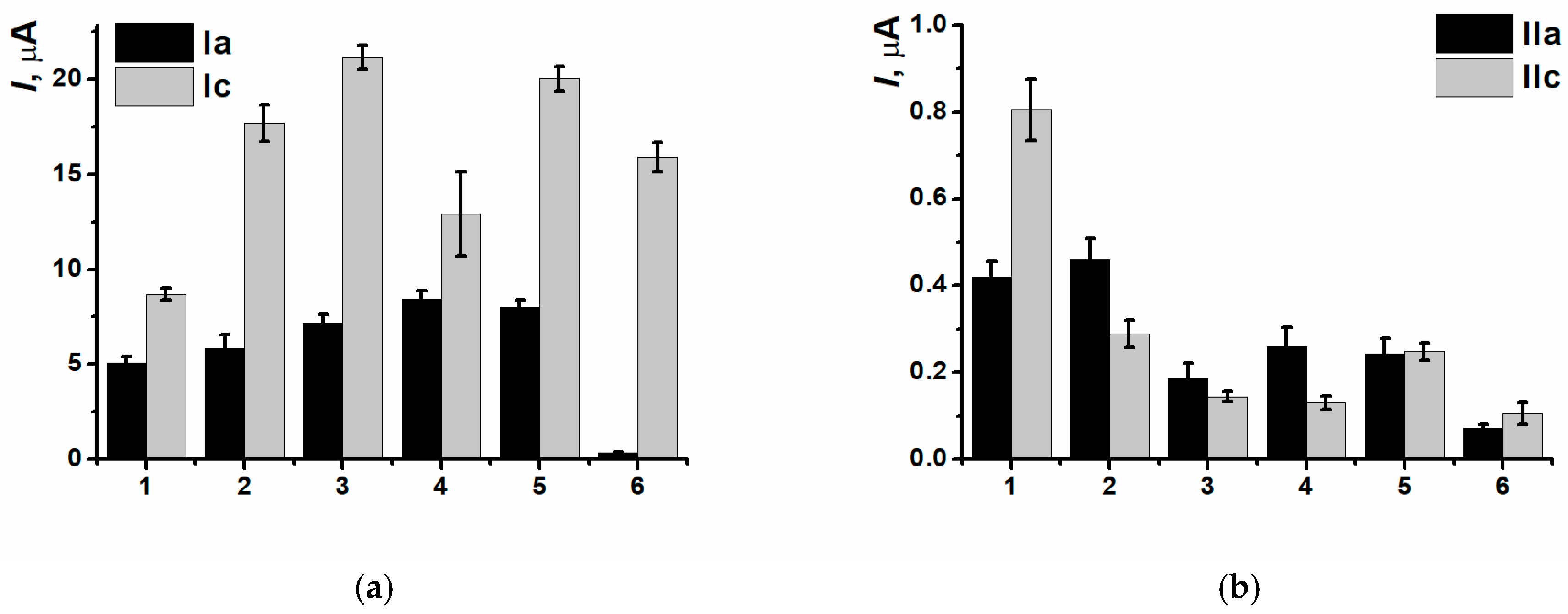
3.5. Detection of DNA Damage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Yin, L.; Dong, Y.; Peng, L.; Liu, G.; Man, S.; Ma, L. CRISPR-Cas13a based bacterial detection platform: Sensing pathogen Staphylococcus aureus in food samples. Anal. Chim. Acta 2020, 1127, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang., T.; Li, H.; Xia, X.; Liu, J.; Lu, Y.; Khan, M.; Deng, S.; Busquets, R.; He, G.; He, Q. Direct detection of foodborne pathogens via a proximal DNA probe-based CRISPR-Cas12 assay. J. Agric. Food Chem. 2021, 69, 12828–12836. [Google Scholar] [CrossRef]
- Srisomwat, C.; Teengam, P.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Pop-up paper electrochemical device for label-free hepatitis B virus DNA detection. Sens. Actuators B Chem. 2020, 316, 128077. [Google Scholar] [CrossRef]
- Lomae, A.; Preechakasedkit, P.; Hanpanich, O.; Ozer, T.; Henry, S.; Maruyama, A.; Pasomsub, E.; Phuphuakrat, A.; Rengpipat, S.; Vilaivan, T.; et al. Label free electrochemical DNA biosensor for COVID-19 diagnosis. Talanta 2023, 253, 123992. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Pulsed amperometric detection of DNA with an ssDNA/polypyrrole-modified electrode. Anal. Bioanal. Chem. 2004, 379, 287–293. [Google Scholar] [CrossRef]
- Škugor Rončević, I.; Krivić, D.; Buljac, M.; Vladislavić, N.; Buzuk, M. Polyelectrolytes Assembly: A Powerful Tool for Electrochemical Sensing Application. Sensors 2020, 20, 3211. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Brett, C. Poly(neutral red): Electrosynthesis, characterization, and application as a redox mediator. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 1275–1285. [Google Scholar] [CrossRef]
- Pouget, J.P.; Oblakowski, Z.; Nogami, Y.; Albouy, P.A.; Laridjani, M.; Oh, E.J.; Min, Y.; MacDiarmid, A.G.; Tsukamoto, J.; Ishiguro, T.; et al. Recent structural investigations of metallic polymers. Synth. Met. 1994, 65, 131–140. [Google Scholar] [CrossRef]
- Broncová, G.; Shishkanova, T.V.; Matĕjka, P.; Volf, R.; Král, V. Citrate selectivity of poly(Neutral red) electropolymerized films. Anal. Chim. Acta 2004, 511, 197–205. [Google Scholar] [CrossRef]
- Chakkarapani, L.; Arumugam, S.; Brandl, M. Simultaneous electrochemical detection of L-dopa and hydrogen peroxide by poly (amido amine) dendrimer/poly (neutral red) modified sensor. J. Food Compos. Anal. 2024, 126, 105847. [Google Scholar] [CrossRef]
- Kappo, D.; Stoikov, D.I.; Stoikov, D.I.; Karaguzina, K.R.; Shurpik, D.N.; Stoikov, I.I.; Evtugyn, G.A. Voltammetric sensor based on electropolymerized poly(Neutral Red) and pillar[3]arene[2]hydroquinone ammonium derivative for dopamine and ascorbic acid determination. Chim. Techno Acta 2024, 12, 12102. [Google Scholar] [CrossRef]
- Kappo, D.; Kuzin, Y.I.; Shurpik, D.N.; Stoikov, I.I.; Evtyugin, G.A. Voltammetric DNA sensor based on redox-active dyes for determining doxorubicin. J. Anal. Chem. 2022, 77, 94–100. [Google Scholar] [CrossRef]
- Monte, F.; Carriazo, D.; Serrano, M.; Gutiérrez, M.; Ferrer, L. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. ChemSusChem 2013, 7, 999–1009. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, X.; Ouyang, P.; Zhang, R.; Liu, H.; Xu, C.; Liu, Z. Electrochemical behavior and electrodeposition of Fe-Co-Ni thin films in choline chloride/urea deep eutectic solvent. J. Electroanal. Chem. 2022, 919, 116516. [Google Scholar] [CrossRef]
- Kityk, A.; Pavlik, V.; Hnatko, M. Exploring deep eutectic solvents for the electrochemical and chemical synthesis of photo-and electrocatalysts for hydrogen evolution. Int. J. Hydrogen Energy 2023, 48, 39823–39853. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Pekel, A.G.; Toprakçı, İ.; Özçelik, G.; Bilgin, M.; Şahin, S. Hydrophobic carboxylic acid based deep eutectic solvent for the removal of diclofenac. Biomass Convers. Biorefinery 2020, 12, 2219–2227. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Swebocki, T.; Barras, A.; Abderrahmani, A.; Haddadi, K.; Boukherroub, R. Deep Eutectic Solvents Comprising Organic Acids and Their Application in (Bio)Medicine. Int. J. Mol. Sci. 2023, 24, 8492. [Google Scholar] [CrossRef] [PubMed]
- Brett, C. Perspectives for the use of deep eutectic solvents in the preparation of electrochemical sensors and biosensors. Curr. Opin. Electrochem. 2024, 45, 101465. [Google Scholar] [CrossRef]
- Silva, W.; Queiroz, A.C.; Brett, C. Nanostructured poly(Phenazine)/Fe2O3 nanoparticle film modified electrodes formed by electropolymerization in ethaline—Deep eutectic solvent. Microscopic and electrochemical characterization. Electrochim. Acta 2020, 347, 136284. [Google Scholar] [CrossRef]
- Leote, R.; Ghica, M.; Brett, C. Pyruvate oxidase biosensors based on glassy carbon electrodes modified with carbon nanotubes and poly(Neutral Red) synthesized in ethaline deep eutectic solvent. Electroanalysis 2022, 34, 724–734. [Google Scholar] [CrossRef]
- Chuenjitt, S.; Kongsuwan, A.; Phua, C.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Samoson, K.; Wangchuk, S.; Promsuwan, K.; Limbut, W. A poly(neutral red)/porous graphene modified electrode for a voltammetric hydroquinone sensor. Electrochim. Acta 2022, 434, 141272. [Google Scholar] [CrossRef]
- Goida, A.; Krasnova, T.; Shamagsumova, R.; Evtugyn, V.; Saveliev, A.; Porfireva, A. Impedimetric DNA Sensor Based on a Composite of Electrochemically Reduced Graphene Oxide and Polyproflavine Electropolymerized from Natural Deep Eutectic Solvent for Anthracycline Medications Determination. Biosensors 2025, 15, 385. [Google Scholar] [CrossRef]
- Abbott, A.P.; Al-Murshedi, A.Y.M.; Alshammari, O.A.O.; Harris, R.C.; Kareem, J.H.; Qader, I.B. Thermodynamics of phase transfer for polar molecules from alkanes to deep eutectic solvents. Fluid Phase Equilibria 2017, 448, 99–104. [Google Scholar] [CrossRef]
- Porfireva, A.; Begisheva, E.; Evtugyn, V.; Evtugyn, G. Electrochemical DNA sensor for valrubicin detection based on poly (Azure C) films deposited from deep eutectic solvent. Biosensors 2023, 13, 931. [Google Scholar] [CrossRef]
- Du, P.; Liu, S.; Wu, P.; Cai, C. Single-walled carbon nanotubes functionalized with poly(nile blue A) and their application to dehydrogenase-based biosensors. Electrochim. Acta 2007, 53, 1811–1823. [Google Scholar] [CrossRef]
- Halliday, S.; Matthew, D. Some electrochemical and photoelectrochemical properties of 3-amino-7-dimethylamino-2-methylphenazine (Neutral Red) in aqueous solution. Aust. J. Chem. 1983, 36, 507–516. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2000; p. 833. [Google Scholar]
- Kulikova, T.; Shiabiev, I.; Padnya, P.; Rogov, A.; Evtugyn, G.; Stoikov, I.; Porfireva, A. Impedimetric DNA Sensor Based on Electropolymerized N-Phenylaminophenothiazine and Thiacalix[4]arene Tetraacids for Doxorubicin Determination. Biosensors 2023, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Porfireva, A.; Goida, A.; Evtugyn, V.; Evtugyn, G. Impedimetric sensor based on molecularly imprinted polythionine from deep eutectic solvent for epinephrine determination. Green Anal. Chem. 2024, 9, 100113. [Google Scholar] [CrossRef]
- Fojta, M.; Daňhel, A.; Havran, L.; Vyskočil, V. Recent progress in electrochemical sensors and assays for DNA damage and repair. TrAC Trends Anal. Chem. 2016, 79, 160–167. [Google Scholar] [CrossRef]
- Ye, J.; Chen, S.; Mao, S.; Jaffrezic-Renault, N.; Guo, Z. Dna Sensor Based on MoS2/MWCNT/AgNPs Nanocomposites for Sensitive Electrochemical Detection of Hydroxyl Radicals. Electroanalysis 2025, 37, e70021. [Google Scholar] [CrossRef]
- Kaya, H.K.; Haghmoradi, N.; Kaplan, B.Y.; Kuralay, F. Platinum nanoparticles loaded carbon black: Reduced graphene oxide hybrid platforms for label-free electrochemical DNA and oxidative DNA damage sensing. J. Electroanal. Chem. 2022, 910, 116180. [Google Scholar] [CrossRef]
- Güngör, M.A.; Alev, O.; Kaya, H.K.; Arslan, L.Ç.; Büyükköse, S.; Öztürk, Z.Z.; Kuralay, F. Atomic layer deposited zinc oxide thin film on pencil graphite for DNA sensor applications. Mater. Today Commun. 2023, 36, 106776. [Google Scholar] [CrossRef]
- Wu, H. Electrochemical evaluation of total antioxidant properties in red wine. Food Meas. 2023, 17, 5344–5351. [Google Scholar] [CrossRef]
- Morais, S.L.; Rede, D.; Ramalhosa, M.J.; Correia, M.; Santos, M.; Delerue-Matos, C.; Moreira, M.M.; Soares, C.; Barroso, M.F. Assessment of the Antioxidant Capacity of Commercial Coffee Using Conventional Optical and Chromatographic Methods and an Innovative Electrochemical DNA-Based Biosensor. Biosensors 2023, 13, 840. [Google Scholar] [CrossRef]
| logIp = a + b × logv | |
|---|---|
| SPCE/PNRPB | log(I(Ia), µA) = −(0.42 ± 0.059) + (0.62 ± 0.032) × log(ν, mV/s) R2 = 0.965 |
| log(I(Ic), µA) = (0.14 ± 0.027) + (0.49 ± 0.015) × log(ν, mV/s) R2 = 0.995 | |
| SPCE/PNRREL | log(I(Ia), µA) = −(0.49 ± 0.029) + (0.56 ± 0.031) × log(ν, mV/s) R2 = 0.992 |
| log(I(Ic), µA) = (0.15 ± 0.006) + (0.50 ± 0.004) × log(ν, mV/s) R2 = 0.997 | |
| logIp = a + b × logv | |
|---|---|
| SPCE/PNRPB | log(I(IIa), µA) = −(1.13 ± 0.014) + (0.68 ± 0.026) × log(ν, mV/s) R2 = 0.991 |
| log(I(IIc), µA) = −(1.29 ± 0.014) + (0.73 ± 0.027) × log(ν, mV/s) R2 = 0.987 | |
| SPCE/PNRREL | log(I(IIa), µA) = −(1.88 ± 0.030) + (0.91 ± 0.062) × log(ν, mV/s) R2 = 0.979 |
| log(I(IIc), µA) = −(2.23 ± 0.030) + (1.01 ± 0.059) × log(ν, mV/s) R2 = 0.988 | |
| Em = a + b × pH | |||||
|---|---|---|---|---|---|
| pH Range | a ± Δa | b ± Δb | R2 | ||
| SPCE/PNRPB | (Ia/Ic) | 3.0–5.0 | −0.476 ± 0.015 | −0.056 ± 0.003 | 0.99 |
| 5.0–7.0 | −0.771 ± 0.029 | 0.0036 ± 0.005 | 0.95 | ||
| 7.0–9.0 | −0.433 ± 0.078 | −0.045 ± 0.011 | 0.90 | ||
| (IIa/IIc) | 3.0–9.0 | 0.135 ± 0.02 | −0.048 ± 0.003 | 0.97 | |
| SPCE/PNRREL | (Ia/Ic) | 3.0–5.0 | −0.448 ± 0.051 | −0.033 ± 0.012 | 0.87 |
| 5.0–7.0 | −0.532 ± 0.001 | −0.015 ± 0.001 | 0.99 | ||
| 7.0–9.0 | −0.378 ± 0.088 | −0.037 ± 0.012 | 0.89 | ||
| (IIa/IIc) | 3.0–9.0 | 0.246 ± 0.019 | −0.054 ± 0.003 | 0.98 | |
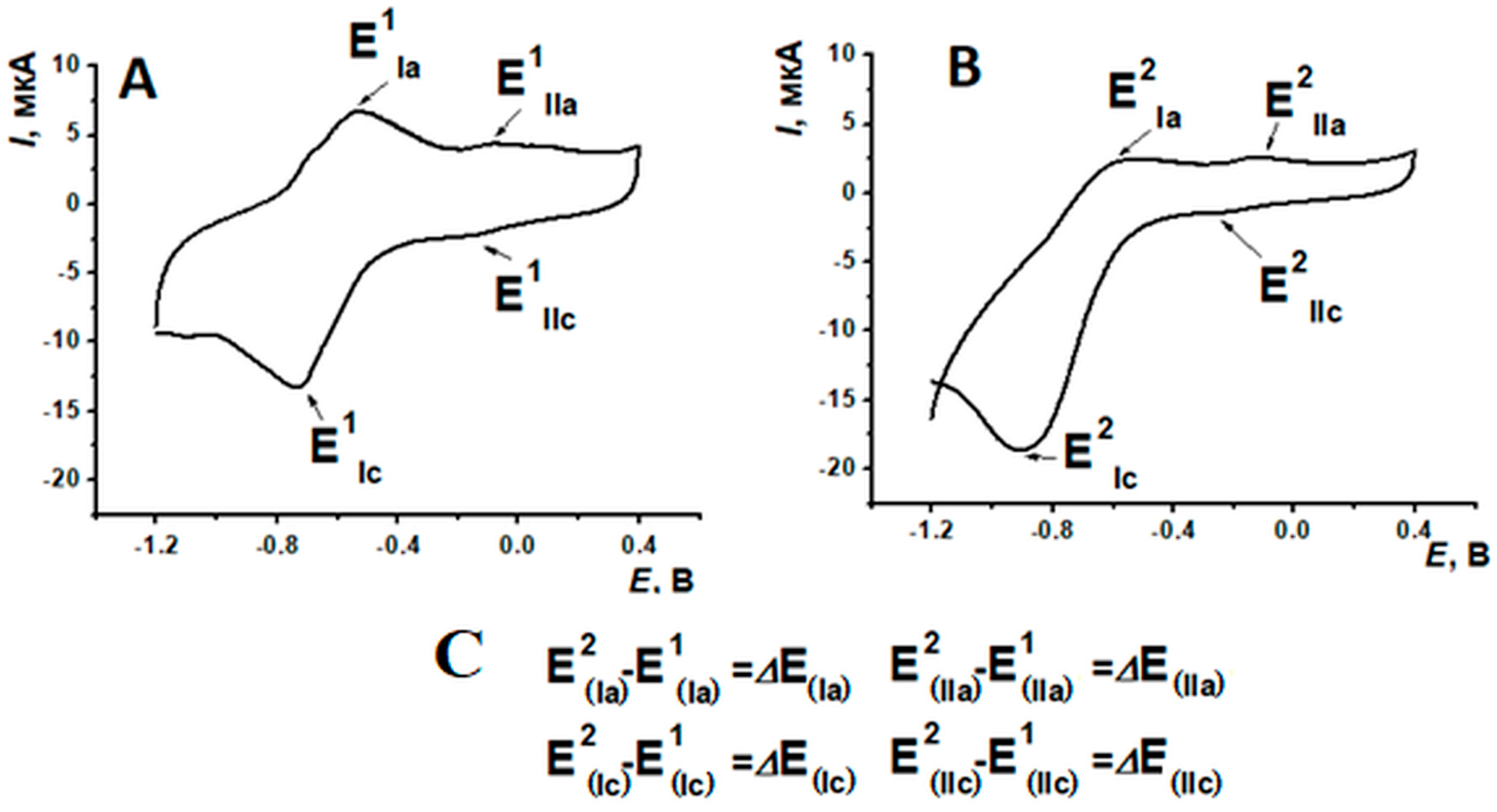
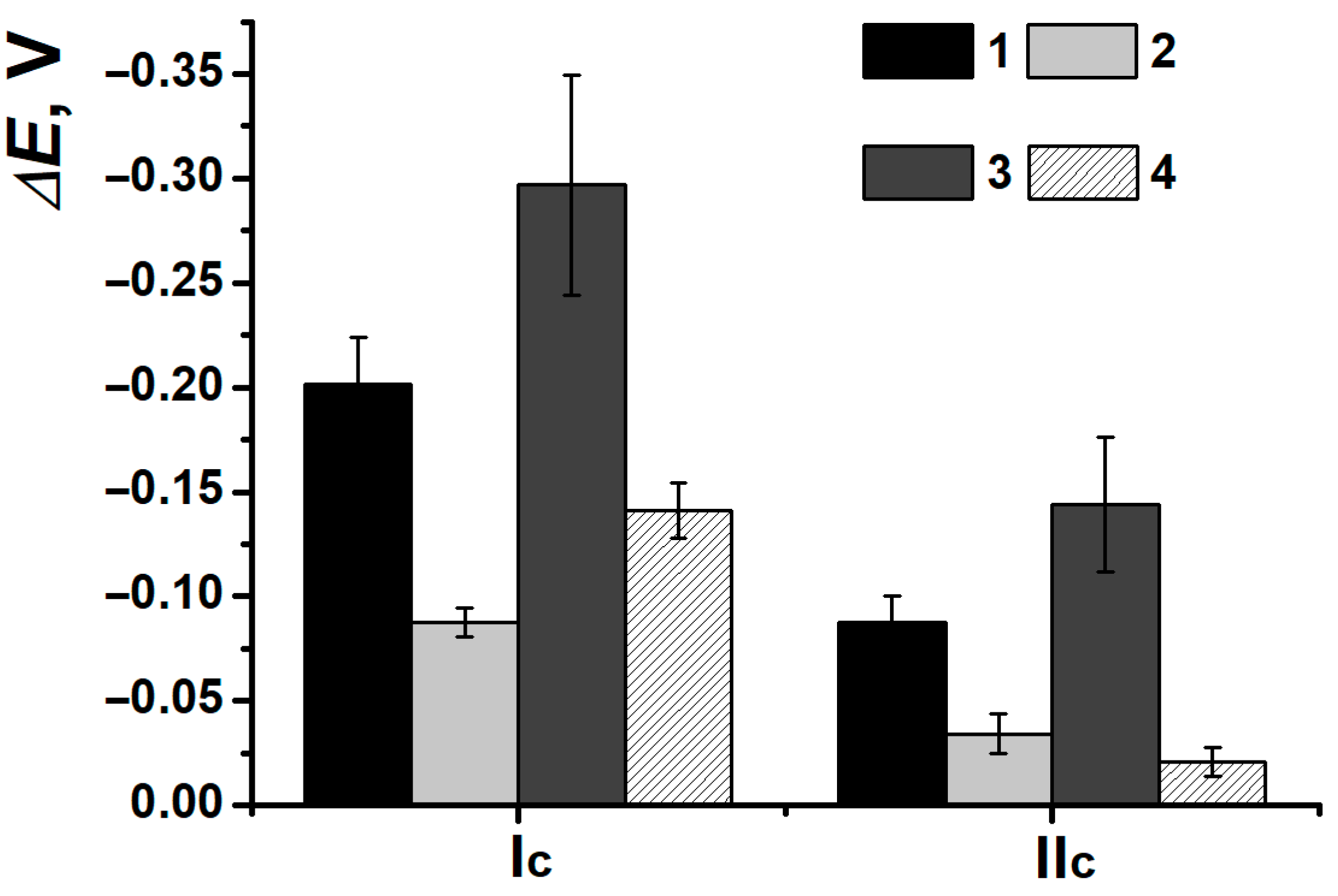
| Layer content | E1(Ia) | E1(Ic) | E1(IIa) | E1(IIc) |
| SPCE/PNRREL | −0.548 ± 0.016 | −0.925 ± 0.020 | −0.108 ± 0.009 | −0.200 ± 0.013 |
| E2(Ia) | E2(Ic) | E2(IIa) | E2(IIc) | |
| SPCE/PNRREL/DNAss | −0.503 ± 0.007 | −0.724 ± 0.014 | −0.069 ± 0.003 | −0.112 ± 0.009 |
| SPCE/PNRREL/PSS | −0.547 ± 0.010 | −0.936 ± 0.015 | −0.134 ± 0.007 | −0.196 ± 0.008 |
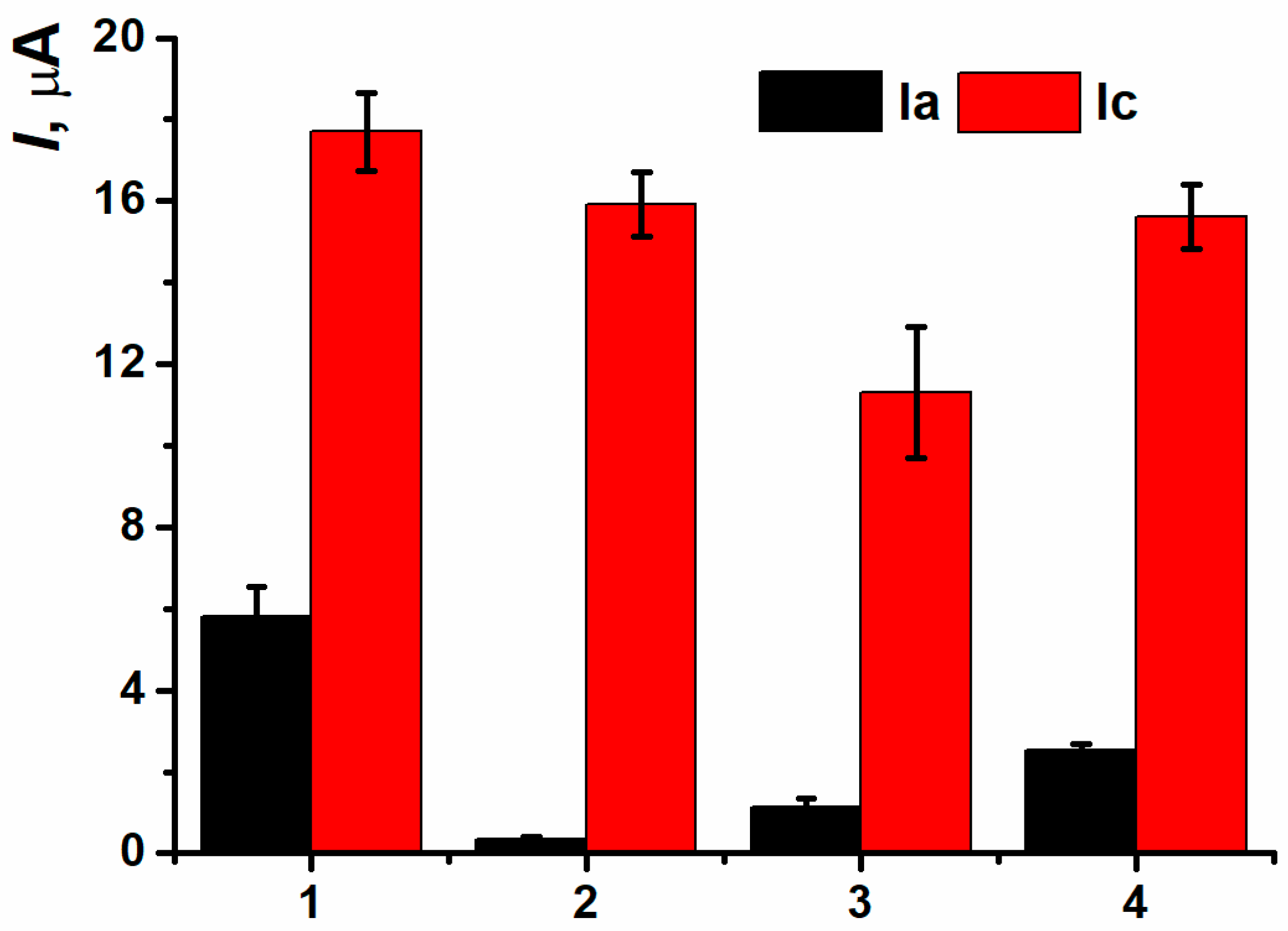
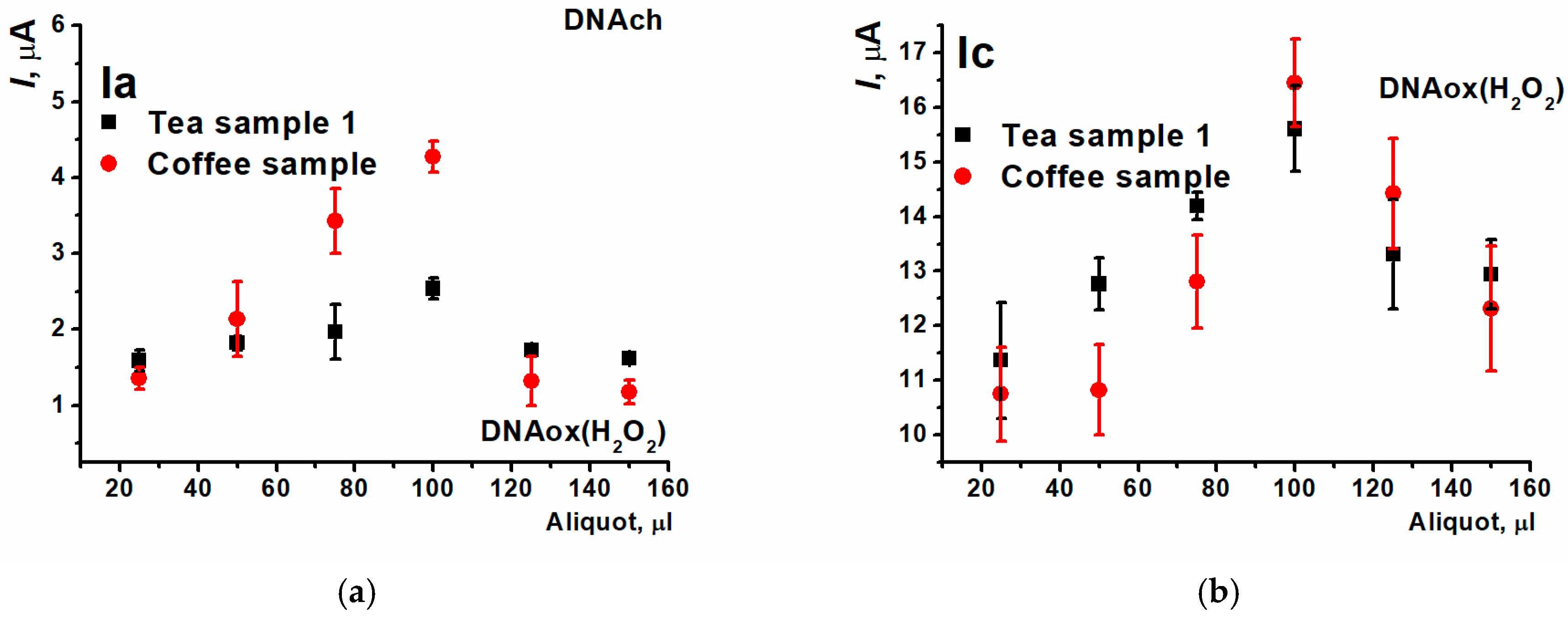
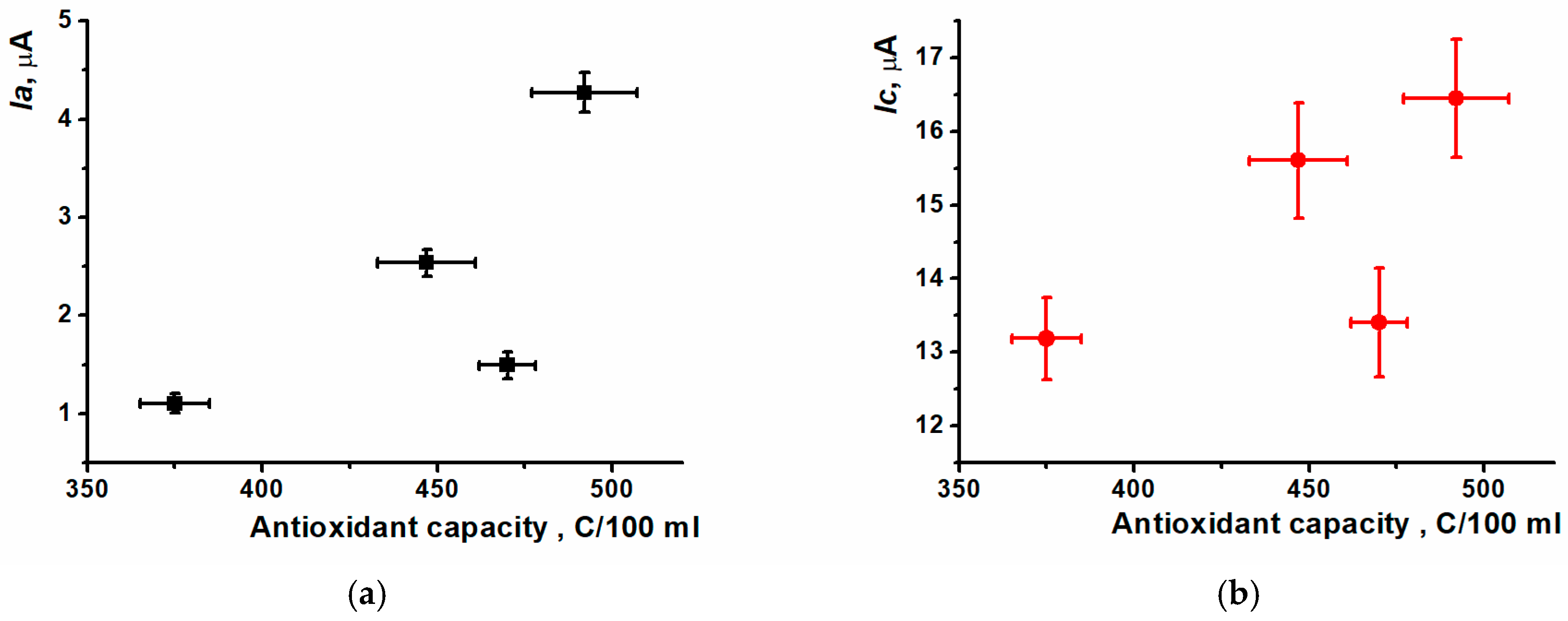
| Ia, µA | Ic, µA | AC, C/100 mL | |
|---|---|---|---|
| Tea sample 1 | 2.54 ± 0.14 | 15.6 ± 0.79 | 447 ± 14, Sr = 0.026 |
| Tea sample 2 | 1.10 ± 0.10 | 13.2 ± 0.55 | 375 ± 10, Sr = 0.011 |
| Tea sample 3 | 1.49 ± 0.14 | 13.4 ± 0.74 | 470 ± 8, Sr = 0.013 |
| Coffee sample | 4.27 ± 0.20 | 16.5 ± 0.81 | 492 ± 15, Sr = 0.025 |
| White wine sample | 0.39 ± 0.25 | 5.85 ± 2.6 | 20 ± 1, Sr = 0.046 |
| Cocoa sample | 1.03 ± 0.20 | 10.8 ± 0.51 | 207 ± 7, Sr = 0.037 |
| Effervescent Vitamin C sample | 1.17 ± 0.03 | 4.62 ± 0.77 | 374 ± 4, Sr = 0.023 |
| Fruit-based drink sample | 0.83 ± 0.17 | 13.06 ± 1.5 | 43 ± 1, Sr = 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malanina, A.; Derbisheva, R.; Krasnova, T.; Shamagsumova, R.; Evtugyn, V.; Ivanov, A.; Porfireva, A. Novel Nanocomposites of Carbon Nanomaterials and Poly(Neutral Red) Electropolymerized from Reline for DNA Damage Detection and Beverage Antioxidant Influence Assessment. Biosensors 2025, 15, 735. https://doi.org/10.3390/bios15110735
Malanina A, Derbisheva R, Krasnova T, Shamagsumova R, Evtugyn V, Ivanov A, Porfireva A. Novel Nanocomposites of Carbon Nanomaterials and Poly(Neutral Red) Electropolymerized from Reline for DNA Damage Detection and Beverage Antioxidant Influence Assessment. Biosensors. 2025; 15(11):735. https://doi.org/10.3390/bios15110735
Chicago/Turabian StyleMalanina, Anastasia, Rufiia Derbisheva, Tatiana Krasnova, Rezeda Shamagsumova, Vladimir Evtugyn, Alexey Ivanov, and Anna Porfireva. 2025. "Novel Nanocomposites of Carbon Nanomaterials and Poly(Neutral Red) Electropolymerized from Reline for DNA Damage Detection and Beverage Antioxidant Influence Assessment" Biosensors 15, no. 11: 735. https://doi.org/10.3390/bios15110735
APA StyleMalanina, A., Derbisheva, R., Krasnova, T., Shamagsumova, R., Evtugyn, V., Ivanov, A., & Porfireva, A. (2025). Novel Nanocomposites of Carbon Nanomaterials and Poly(Neutral Red) Electropolymerized from Reline for DNA Damage Detection and Beverage Antioxidant Influence Assessment. Biosensors, 15(11), 735. https://doi.org/10.3390/bios15110735






