Aggregation-Induced Emission-Based Lateral Flow Immunoassay for Ultra-Sensitive and On-Site Detection of Porcine Epidemic Diarrhea Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
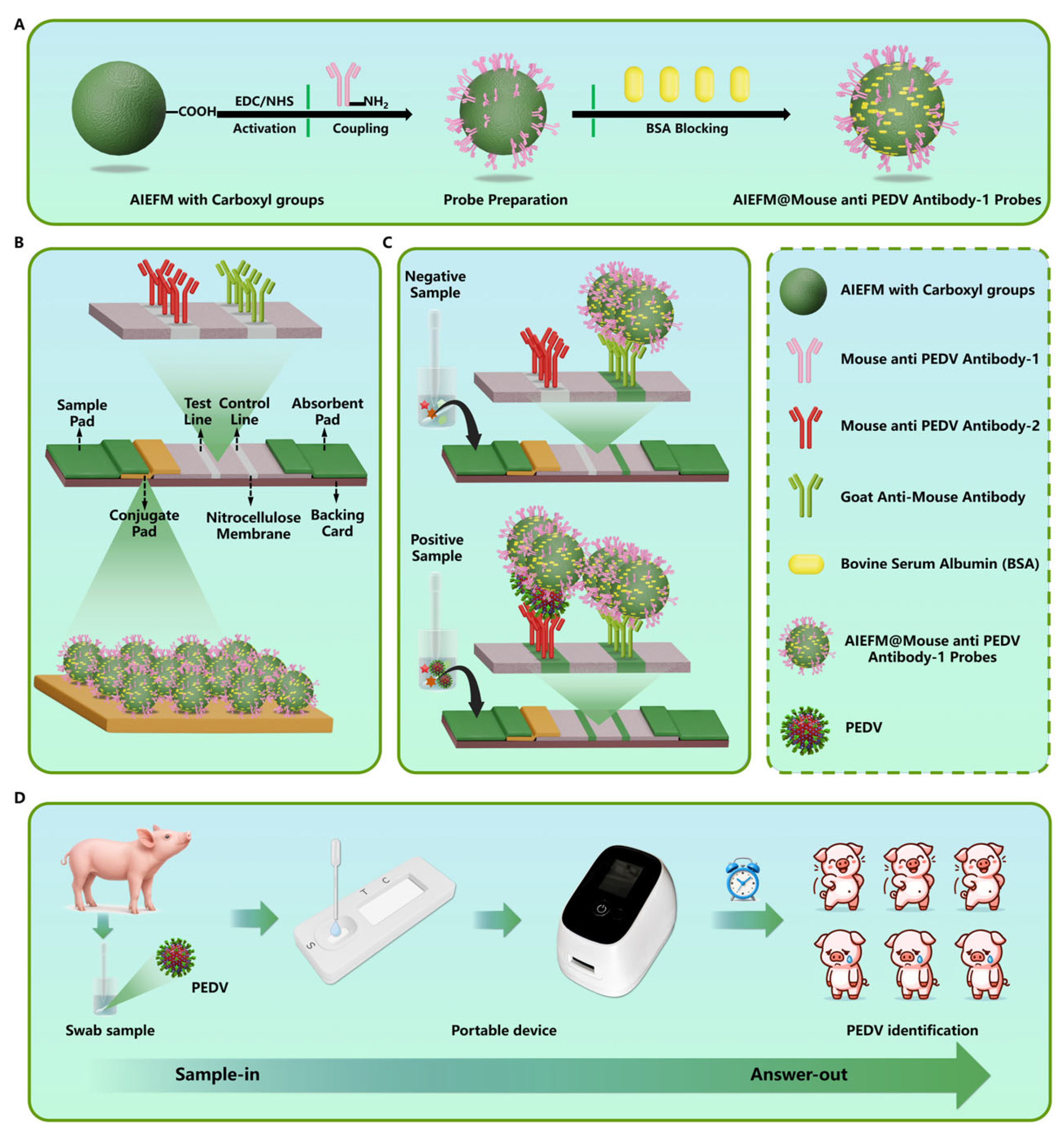
2.2. Preparation of AIEFM@PEDV Detection Antibody Probe
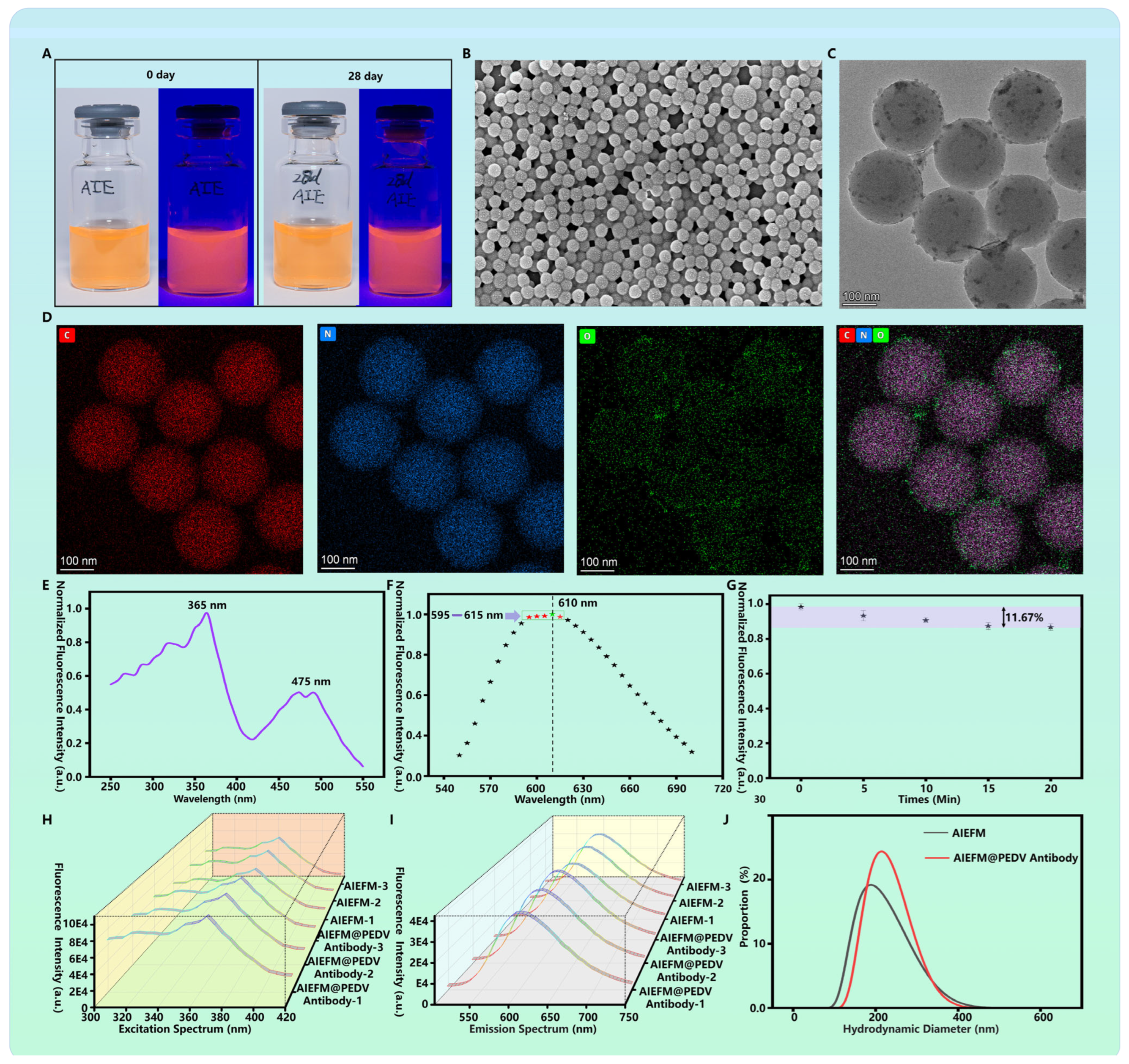
2.3. Characterization of AIEFM@PEDV Detection Antibody Probe
2.4. Fabrication of PED-ALFIA Test Strips
2.5. Establishment of the PED-ALFIA Method
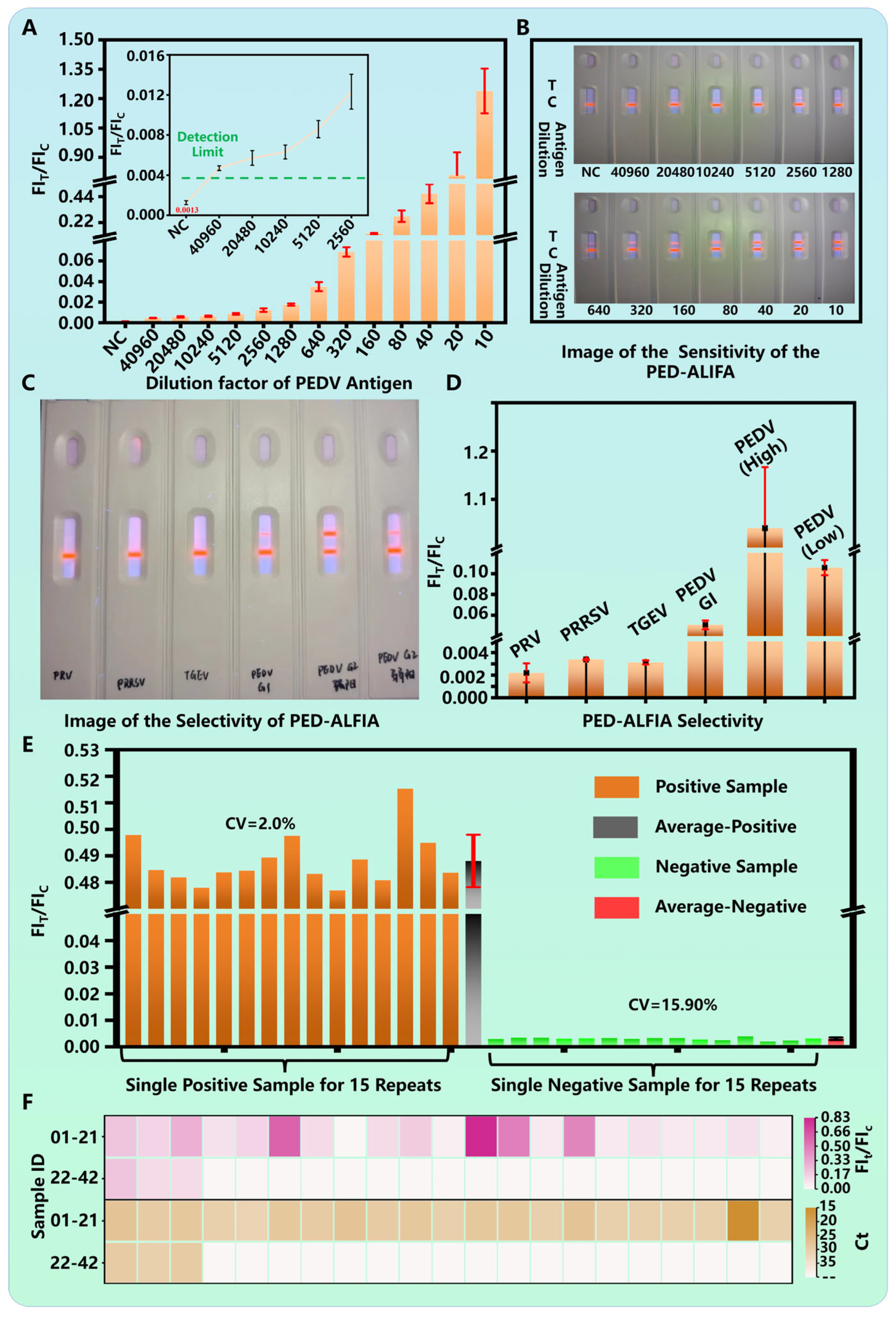
2.6. Evaluation of PED-ALFIA Performance
3. Results
3.1. Characterization of AIEFM and AIEFM@PEDV Detection Antibody Probe
3.2. Optimization of PED-ALFIA
3.3. Analytical Performance and Clinical Validation of PED-ALFIA
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEDV | Porcine epidemic diarrhea virus |
| TGEV | Transmissible gastroenteritis virus |
| LFIA | Lateral flow immunoassays |
| AIE | Aggregation-induced emission |
| PDCoV | Porcine deltacoronavirus |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative real-time PCR |
| ELISA | Enzyme-linked immunosorbent assay |
| CLIA | Chemiluminescent immunoassay |
| POCT | Point-of-care testing |
| ACQ | Aggregation-caused quenching |
| AIEFM | AIE fluorescent microspheres |
| PRV | Pseudorabies virus |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| DLS | Dynamic light scattering |
| EDS | Energy-dispersive X-ray spectroscopy |
| LOD | Limit of detection |
| CV | Coefficients of variation |
References
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 2, 193. [Google Scholar] [CrossRef]
- Kim, D.M.; Moon, S.H.; Kim, S.C.; Cho, H.S.; Tark, D. Genetic and Pathogenic Analysis of a Novel Porcine Epidemic Diarrhea Virus Strain Isolated in the Republic of Korea. Viruses 2024, 16, 1108. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhou, J.; Wang, X.; Ma, L.; Li, J.; Yang, L.; Yuan, H.; Pang, D.; Ouyang, H. Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus-Host Interactions. Viruses 2022, 14, 2434. [Google Scholar] [CrossRef] [PubMed]
- Makau, D.N.; Pamornchainavakul, N.; VanderWaal, K.; Kikuti, M.; Picasso-Risso, C.; Geary, E.; Corzo, C.A. Postepidemic Epidemiology of Porcine Epidemic Diarrhea Virus in the United States. Transbound. Emerg. Dis. 2024, 2024, 5531899. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Wang, X.; Zhou, J.; Ma, L.; Li, J.; Yang, L.; Ouyang, H.; Yuan, H.; Pang, D. Transmissible Gastroenteritis Virus: An Update Review and Perspective. Viruses 2025, 15, 359. [Google Scholar] [CrossRef]
- Shan, X.; Li, R.; Ma, X.; Qiu, G.; Xiang, Y.; Zhang, X.; Wu, D.; Wang, L.; Zhang, J.; Wang, T.; et al. Epidemiology, pathogenesis, immune evasion mechanism and vaccine development of porcine Deltacoronavirus. Funct. Integr. Genomics 2024, 24, 79. [Google Scholar] [CrossRef]
- Papaneri, A.; Cui, G.; Chen, S.H. Next-Generation Nucleic Acid-Based Diagnostics for Viral Pathogens: Lessons Learned from the SARS-CoV-2 Pandemic. Microorganisms 2025, 13, 1905. [Google Scholar] [CrossRef]
- Olech, M. Current State of Molecular and Serological Methods for Detection of Porcine Epidemic Diarrhea Virus. Pathogens 2022, 11, 1074. [Google Scholar] [CrossRef]
- Lazov, C.M.; Papetti, A.; Belsham, G.J.; Bøtner, A.; Rasmussen, T.B.; Boniotti, M.B. Multiplex Real-Time RT-PCR Assays for Detection and Differentiation of Porcine Enteric Coronaviruses. Pathogens 2023, 12, 1040. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Shang, Y.; Wu, J.; Lan, X. A novel and cost-effective real-time RT-PCR targeting 24 nucleotides deletion to differentiate PEDV wild-type and classical attenuated vaccine strains. J. Virol. Methods 2024, 329, 114986. [Google Scholar] [CrossRef]
- Wang, F.X.; Yuan, D.Y.; Jin, Y.N.; Hu, L.; Sun, Z.Y.; He, Q.; Zhao, S.H.; Zhan, S.B.; Wen, Y.J. Reverse Transcription Cross-Priming Amplification-Nucleic Acid Test Strip for Rapid Detection of Porcine Epidemic Diarrhea Virus. Sci. Rep. 2016, 6, 24072. [Google Scholar] [CrossRef] [PubMed]
- Pewlaoo, S.; Phanthong, S.; Kong-Ngoen, T.; Santajit, S.; Tunyong, W.; Buranasinsup, S.; Kaeoket, K.; Thavorasak, T.; Pumirat, P.; Sookrung, N.; et al. Development of a Rapid Reverse Transcription-Recombinase Polymerase Amplification Couple Nucleic Acid Lateral Flow Method for Detecting Porcine Epidemic Diarrhoea Virus. Biology 2022, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, J.M.; Baek, J.S.; Park, J.; Kim, W.I.; Ku, B.K.; Jeoung, H.Y.; Lee, K.K.; Park, C.K. An Advanced Multiplex Real-Time Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Rapid and Reliable Detection of Porcine Epidemic Diarrhea Virus and Porcine Internal Positive Control. Viruses 2023, 15, 2204. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Huang, N.; Guo, J.; Ge, L.; Yang, X. High-affinity monoclonal antibodies against the porcine epidemic diarrhea virus S1 protein. BMC Vet. Res. 2024, 20, 239. [Google Scholar] [CrossRef]
- Shan, Y.; Gao, Q.; Mao, J.; Zheng, J.; Xu, X.; Zhang, C.; Huang, X.; Xu, J.; Shi, F.; Yue, M.; et al. Establishment of enzyme-linked immunosorbent assays based on recombinant S1 and its truncated proteins for detection of PEDV IgA antibody. BMC Vet. Res. 2022, 18, 154. [Google Scholar] [CrossRef]
- Tao, S.; Duan, Y.; Zha, Y.; Tong, X.; He, Y.; Feng, H.; Shu, J. Development and Evaluation of an Immunochromatographic Strip and a Magnetic Chemiluminescence Immunoassay for Detection of Porcine Circovirus Type 2 Antigen. Vet. Sci. 2025, 12, 40. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Tran, T.; Tellez-Corrales, E.; Madiraju, C. Enzyme-Linked Immunosorbent Assay: Types and Applications. Methods Mol. Biol. 2023, 2612, 1–17. [Google Scholar]
- Raafat, N.; Blacksell, S.D.; Maude, R.J. A review of dengue diagnostics and implications for surveillance and control. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 653–660. [Google Scholar] [CrossRef]
- Azizian, R.; Mamishi, S.; Jafari, E.; Mohammadi, M.R.; Heidari Tajabadi, F.; Pourakbari, B. From Conventional Detection to Point-of-care Tests (POCT) Method for Pediatric Respiratory Infections Diagnosis: A Systematic Review. Arch. Iran. Med. 2025, 28, 112–123. [Google Scholar] [CrossRef]
- Seok, Y.; Mauk, M.G.; Li, R.; Qian, C. Trends of respiratory virus detection in point-of-care testing: A review. Anal. Chim. Acta 2023, 1264, 341283. [Google Scholar] [CrossRef]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Lee, Y.C. Current Trends in RNA Virus Detection via Nucleic Acid Isothermal Amplification-Based Platforms. Biosensors 2024, 14, 97. [Google Scholar] [CrossRef]
- Gao, M.; Yang, C.; Si, W.; Xi, X.; Chen, L.; Zeng, Z.; Rong, Y.; Yang, Y.; Wang, F.; Yuan, C. Combining CRISPR-Cas12a with Microsphere Array-Enhanced Fluorescence for Portable Pathogen Nucleic Acid Detection. ACS Appl. Mater. Interfaces 2025, 17, 20932–20942. [Google Scholar] [CrossRef]
- Men, D.; Zhou, J.; Li, W.; Leng, Y.; Chen, X.; Tao, S.; Zhang, X.E. Fluorescent Protein Nanowire-Mediated Protein Microarrays for Multiplexed and Highly Sensitive Pathogen Detection. ACS Appl. Mater. Interfaces 2016, 8, 17472–17477. [Google Scholar] [CrossRef]
- Jeon, M.J.; Kim, S.K.; Hwang, S.H.; Lee, J.U.; Sim, S.J. Lateral flow immunoassay based on surface-enhanced Raman scattering using pH-induced phage-templated hierarchical plasmonic assembly for point-of-care diagnosis of infectious disease. Biosens. Bioelectron. 2024, 250, 116061. [Google Scholar] [CrossRef]
- Shi, X.; Luo, Y.; Yan, H.; Tian, G.; Yang, S.; He, Z.; Zhang, F.; Wang, Y.; Guo, L.; Chen, H. Gold nanoparticle dimer-based immunochromatography for in situ ultrasensitive detection of porcine epidemic diarrhea virus. Mikrochim. Acta 2023, 190, 430. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Rodríguez, M.; Serrano-Pertierra, E.; García, A.C.; López-Martín, S.; Yañez-Mo, M.; Cernuda-Morollón, E.; Blanco-López, M.C. Point-of-care detection of extracellular vesicles: Sensitivity optimization and multiple-target detection. Biosens. Bioelectron. 2017, 87, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Hui, Y.Y.; Chen, O.Y.; Wang, Y.L.; Chang, H.C. Thermometric lateral flow immunoassay with colored latex beads as reporters for COVID-19 testing. Sci. Rep. 2022, 12, 3905. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, J.; Li, J.; Liu, Q. Dual FITC lateral flow immunoassay for sensitive detection of Escherichia coli O157:H7 in food samples. Biosens. Bioelectron. 2016, 85, 734–739. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Binazadeh, M.; Chiang, W.H. Recent Advances in Quantum Dot-Based Lateral Flow Immunoassays for the Rapid, Point-of-Care Diagnosis of COVID-19. Biosensors 2023, 13, 786. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Liang, R.; Fu, T.; Ji, S.; Li, B.; Zhang, P.; Wu, H.; Wang, W.; Bai, L.; et al. PED-TRFIA: PEDV early on-site detection based on the time-resolved fluorescence ImmunoAssay. Anal. Chim. Acta 2025, 1366, 344270. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, L.; Wang, J.; Zhou, Y.; Hu, H.; Ye, X.; Liu, G.; Xu, Z.; Xu, H.; Yang, W.; et al. Aggregation caused quenching to aggregation induced emission transformation: A precise tuning based on BN-doped polycyclic aromatic hydrocarbons toward subcellular organelle specific imaging. Chem. Sci. 2022, 13, 3129–3139. [Google Scholar] [CrossRef]
- Gouthaman, S.; Jayaraj, A.; Sugunalakshmi, M.; Sivaraman, G.; P, C.A.S. Supramolecular self-assembly mediated aggregation-induced emission of fluorene-derived cyanostilbenes: Multifunctional probes for live cell-imaging. J. Mater. Chem. B 2022, 10, 2238–2250. [Google Scholar] [CrossRef]
- Hsiao, W.W.; Pham, U.K.; Le, T.N.; Lam, X.M.; Chiang, W.H. Advances in aggregation-induced emission luminogens for biomedicine: From luminescence mechanisms to diagnostic applications. Biosens. Bioelectron. 2025, 270, 116942. [Google Scholar] [CrossRef]
- Keremane, K.S.; Acharya, M.G.; Naik, P.; Malakar, C.C.; Wang, K.; Poudel, B. Recent Advances in Aggregation-Induced Emission (AIE) Fluorescent Sensors for Biomolecule Detection. Chemosensors 2025, 13, 174. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, L.H.; He, X. Applications of Aggregation-Induced Emission Materials in Immunology: From Diagnostics to Immunotherapy. Chem. Biomed. Imaging 2025, 3, 499–521. [Google Scholar] [CrossRef]
- Su, Y.; Fan, X.J.; Jiang, H.; Li, X.M.; Lai, W.H.; Zheng, L.Y.; Xiong, Y.H.; Tang, B.Z.; Huang, X.L. Aggregation-induced emission sparkles in immunoassay. Coord. Chem. Rev. 2025, 544, 216957. [Google Scholar] [CrossRef]

| Detection Techniques | Cost | Instrument | LOD | Assay Time | On-site Detection | Reference |
|---|---|---|---|---|---|---|
| qPCR | High | PCR Instrument | 7.5 × 102 RNA Copies/Reaction | >1 h | NO | [10] |
| ELISA | High | Microplate Reader | 0.05 ng/mL | >1 h | NO | [15] |
| Conventional Gold-LFIA | Cost-ffective | NO | 6.25 × 104 TCID50/mL | <15 min | YES | [31] |
| PED-TRFIA | Cost-effective | Fluorescence Reader | 9.76 × 102 TCID50/mL | <15 min | YES | [31] |
| PED-ALFIA | Cost-effective | Fluorescence Reader | 2.44 × 102 TCID50/mL | <15 min | YES | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Feng, X.; He, Q.; Shi, H.; Hou, W.; Geng, J.; Wang, H. Aggregation-Induced Emission-Based Lateral Flow Immunoassay for Ultra-Sensitive and On-Site Detection of Porcine Epidemic Diarrhea Virus. Biosensors 2025, 15, 736. https://doi.org/10.3390/bios15110736
Wang B, Feng X, He Q, Shi H, Hou W, Geng J, Wang H. Aggregation-Induced Emission-Based Lateral Flow Immunoassay for Ultra-Sensitive and On-Site Detection of Porcine Epidemic Diarrhea Virus. Biosensors. 2025; 15(11):736. https://doi.org/10.3390/bios15110736
Chicago/Turabian StyleWang, Bin, Xufei Feng, Qian He, Hongwei Shi, Wei Hou, Jianjun Geng, and Haidong Wang. 2025. "Aggregation-Induced Emission-Based Lateral Flow Immunoassay for Ultra-Sensitive and On-Site Detection of Porcine Epidemic Diarrhea Virus" Biosensors 15, no. 11: 736. https://doi.org/10.3390/bios15110736
APA StyleWang, B., Feng, X., He, Q., Shi, H., Hou, W., Geng, J., & Wang, H. (2025). Aggregation-Induced Emission-Based Lateral Flow Immunoassay for Ultra-Sensitive and On-Site Detection of Porcine Epidemic Diarrhea Virus. Biosensors, 15(11), 736. https://doi.org/10.3390/bios15110736





