Comparative Analysis of QCM and Electrochemical Aptasensors for SARS-CoV-2 Detection
Abstract
1. Introduction
2. Materials and Methods
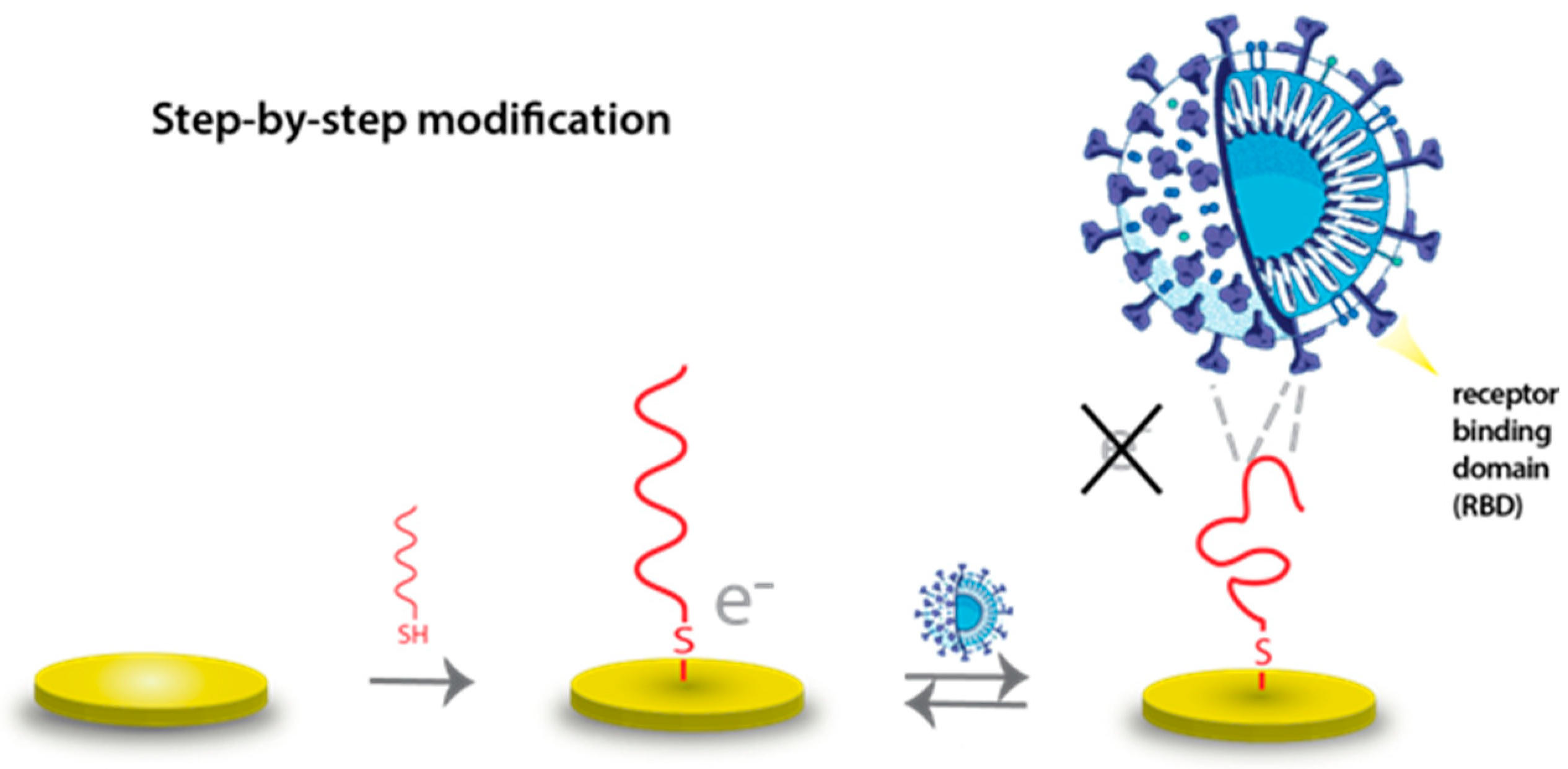
2.1. QCM Setup and Preparation of Aptasensors
2.2. Electrochemical Setup and Aptasensor Preparation
3. Results and Discussion
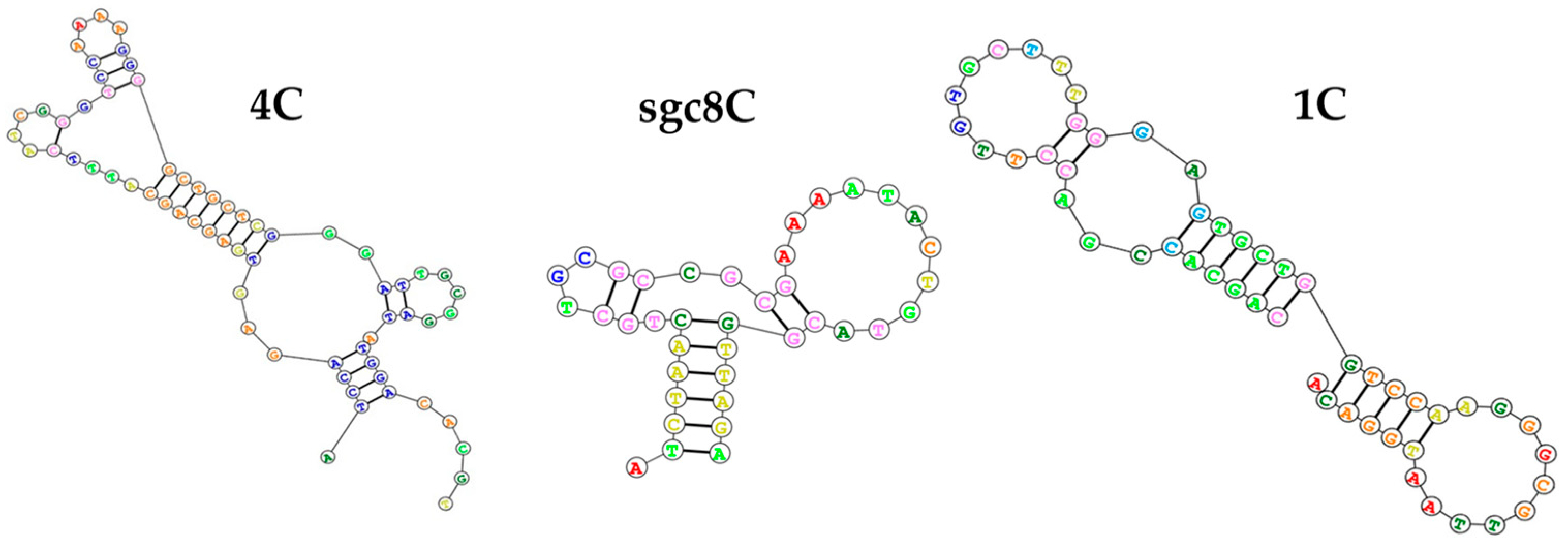
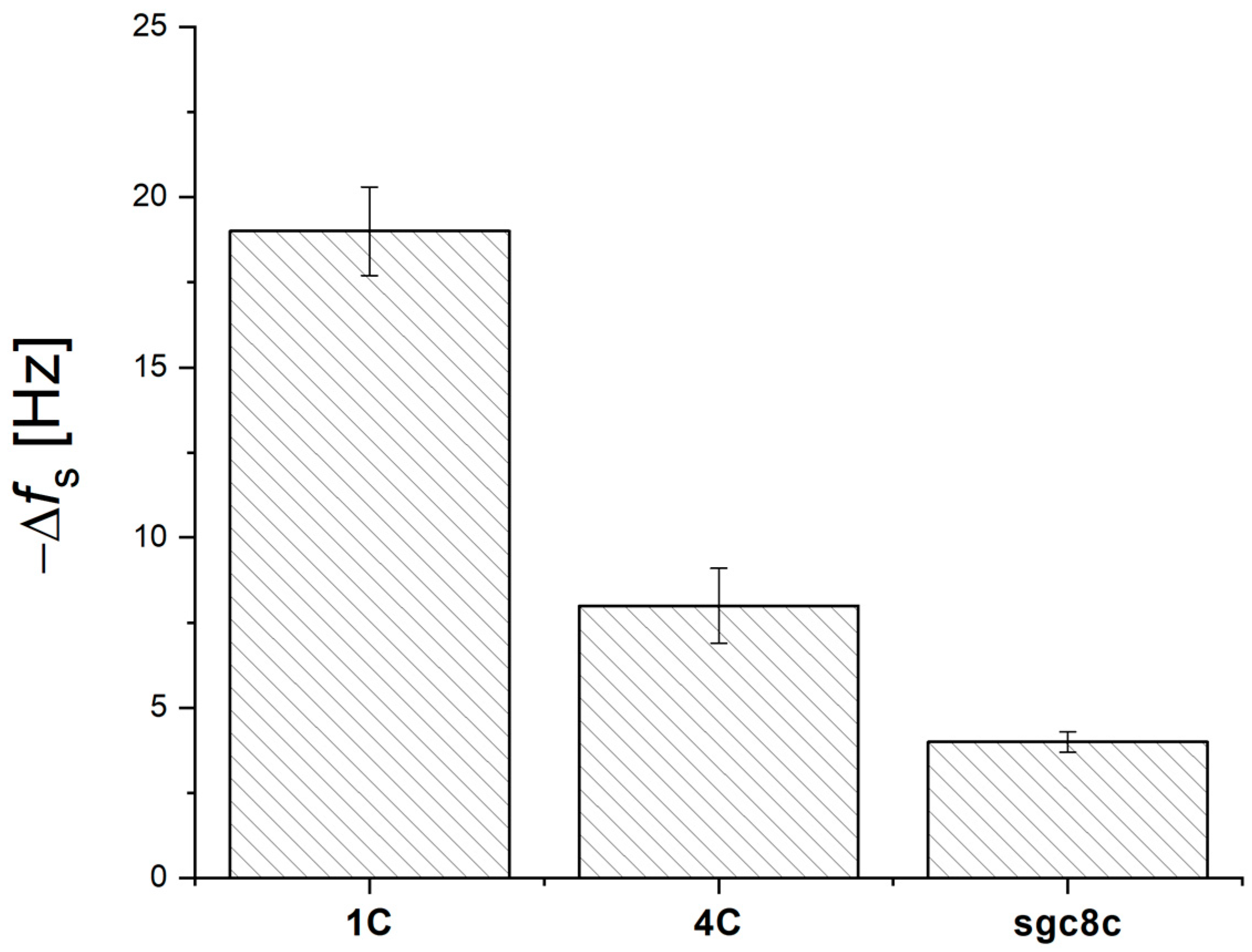
3.1. Optimization of QCM Aptasensor Preparation
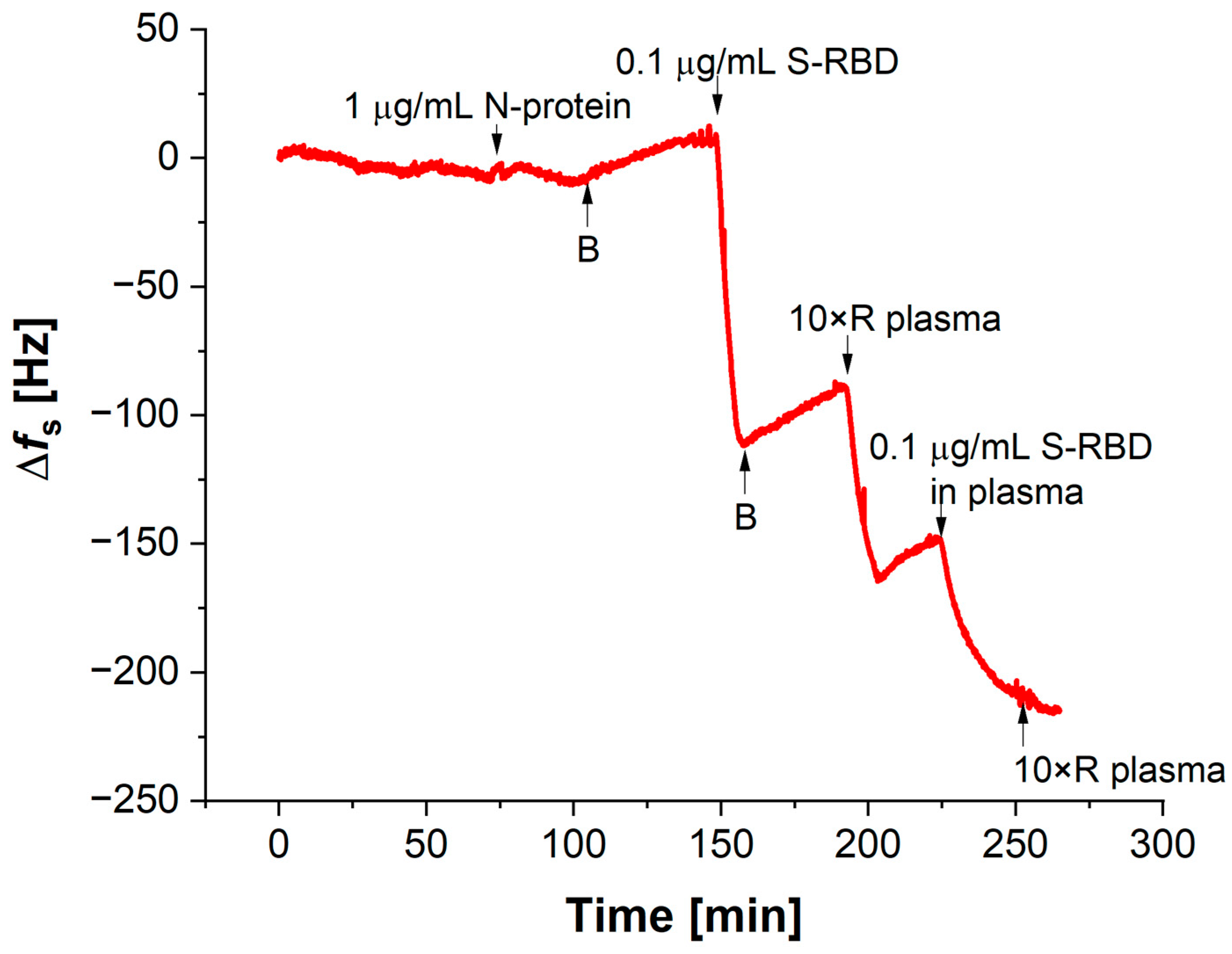
3.2. Verification of the Functionality and Stability of QCM Aptasensors in Biological Fluids
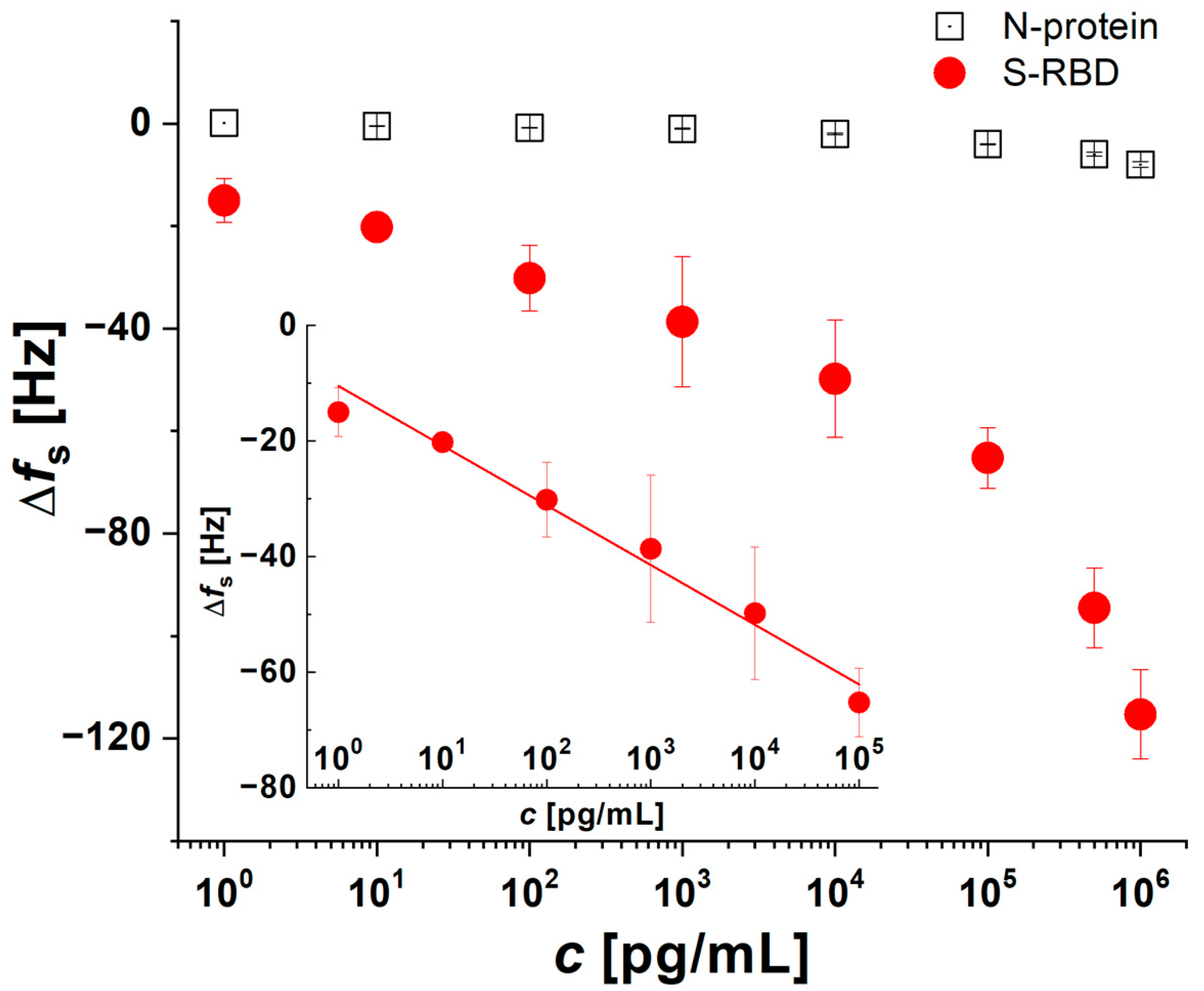
3.3. Electrochemical AuE Aptasensor Preparation and S-RBD Detection
3.4. Selectivity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Huang, Z.; Zhu, J.; Li, C.; Fang, Z.; Chen, K.; Zhang, Y. An Updated Review of SARS-CoV-2 Detection Methods in the Context of a Novel Coronavirus Pandemic. Bioeng. Transl. Med. 2023, 8, e10356. [Google Scholar] [CrossRef]
- Chavda, V.P.; Valu, D.D.; Parikh, P.K.; Tiwari, N.; Chhipa, A.S.; Shukla, S.; Patel, S.S.; Balar, P.C.; Paiva-Santos, A.C.; Patravale, V. Conventional and Novel Diagnostic Tools for the Diagnosis of Emerging SARS-CoV-2 Variants. Vaccines 2023, 11, 374. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid Supported Lipid Bilayers: From Biophysical Studies to Sensor Design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.; Barrias, S.; Chaves, R.; Adega, F.; Fernandes, J.R.; Martins-Lopes, P. Development of a QCM-Based Biosensor for the Detection of Non-Small Cell Lung Cancer Biomarkers in Liquid Biopsies. Talanta 2023, 260, 124624. [Google Scholar] [CrossRef]
- Bonyadi, F.; Kavruk, M.; Ucak, S.; Cetin, B.; Bayramoglu, G.; Dursun, A.D.; Arica, Y.; Ozalp, V.C. Real-Time Biosensing Bacteria and Virus with Quartz Crystal Microbalance: Recent Advances, Opportunities, and Challenges. Crit. Rev. Anal. Chem. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.S.; Thompson, M. Acoustic Coupling at Multiple Interfaces and the Liquid Phase Response of the Thickness Shear-Mode Acoustic Wave Sensor. Chem. Commun. 2004, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Antunes, B.; Ferreira, H.A. Nucleic Acid Aptamer-Based Biosensors: A Review. Biomedicines 2023, 11, 3201. [Google Scholar] [CrossRef]
- Dong, H.; Huang, J.; Guo, Z.; Jia, P.; Sun, Z.; Guo, Y.; Sun, X. Unleashing the Potential of QCM: A Comprehensive Review of Aptamer-Based QCM Sensing Analysis. Microchem. J. 2024, 200, 110344. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ku, T.-H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.-W.; Han, Y.; Lo, Y.-H. Nucleic Acid Aptamers: An Emerging Tool for Biotechnology and Biomedical Sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L. Aptamers: Problems, Solutions and Prospects. Acta Naturae 2013, 5, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Luo, F.; Liu, M.; Zhang, X.-L. Oligonucleotide Aptamers: Promising and Powerful Diagnostic and Therapeutic Tools for Infectious Diseases. J. Infect. 2018, 77, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Minunni, M.; Tombelli, S.; Gullotto, A.; Luzi, E.; Mascini, M. Development of Biosensors with Aptamers as Bio-Recognition Element: The Case of HIV-1 Tat Protein. Biosens. Bioelectron. 2004, 20, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hu, Y.; Li, D.; Chen, H.; Zhang, X.-L. CS-SELEX Generates High-Affinity SsDNA Aptamers as Molecular Probes for Hepatitis C Virus Envelope Glycoprotein E2. PLoS ONE 2009, 4, e8142. [Google Scholar] [CrossRef]
- Bai, H.; Wang, R.; Hargis, B.; Lu, H.; Li, Y. A SPR Aptasensor for Detection of Avian Influenza Virus H5N1. Sensors 2012, 12, 12506–12518. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Misono, T.S.; Kawasaki, K.; Mizuno, T.; Imai, M.; Odagiri, T.; Kumar, P.K.R. An RNA Aptamer That Distinguishes between Closely Related Human Influenza Viruses and Inhibits Haemagglutinin-Mediated Membrane Fusion. J. Gen. Virol. 2006, 87, 479–487. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Ren, J.; Qu, X. A Graphene Functionalized Electrochemical Aptasensor for Selective Label-Free Detection of Cancer Cells. Biomaterials 2011, 32, 2930–2937. [Google Scholar] [CrossRef]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-Based Rapid Diagnosis for Point-of-Care Application. Microfluid. Nanofluid. 2023, 27, 15. [Google Scholar] [CrossRef]
- Sochr, J.; Nemčeková, K.; Černicová, M.; Campbell, K.; Milata, V.; Farkašová, D.; Labuda, J. DNA Interaction with 17α-Ethinylestradiol Studied Using Electrochemical Biosensors and Biosensing in Solution. Electroanalysis 2019, 31, 1961–1968. [Google Scholar] [CrossRef]
- Svitková, V.; Vyskočil, V. Electrochemical Behavior of Methylene Blue at Bare and DNA-Modified Silver Solid Amalgam Electrodes. J. Solid State Electrochem. 2022, 26, 2491–2499. [Google Scholar] [CrossRef]
- Nemčeková, K.; Labuda, J.; Milata, V.; Blaškovičová, J.; Sochr, J. Interaction of DNA and Mononucleotides with Theophylline Investigated Using Electrochemical Biosensors and Biosensing. Bioelectrochemistry 2018, 123, 182–189. [Google Scholar] [CrossRef]
- Nemčeková, K.; Svitková, V.; Gökçe, G. Electrochemical Aptasensing for the Detection of Mycotoxins in Food Commodities. Monatshefte Für Chem.-Chem. Mon. 2022, 153, 971–983. [Google Scholar] [CrossRef]
- Poturnayová, A.; Buríková, M.; Bízik, J.; Hianik, T. DNA Aptamers in the Detection of Leukemia Cells by the Thickness Shear Mode Acoustics Method. ChemPhysChem 2019, 20, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Poturnayová, A.; Dzubinová, Ľ.; Buríková, M.; Bízik, J.; Hianik, T. Detection of Breast Cancer Cells Using Acoustics Aptasensor Specific to HER2 Receptors. Biosensors 2019, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; et al. A Rapid Advice Guideline for the Diagnosis and Treatment of 2019 Novel Coronavirus (2019-NCoV) Infected Pneumonia (Standard Version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Song, J.; Wei, X.; Huang, M.; Sun, M.; Zhu, L.; Lin, B.; Shen, H.; Zhu, Z.; Yang, C. Discovery of Aptamers Targeting the Receptor-Binding Domain of the SARS-CoV-2 Spike Glycoprotein. Anal. Chem. 2020, 92, 9895–9900. [Google Scholar] [CrossRef]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers Evolved from Live Cells as Effective Molecular Probes for Cancer Study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef]
- Domsicova, M.; Kurekova, S.; Babelova, A.; Jakic, K.; Oravcova, I.; Nemethova, V.; Razga, F.; Breier, A.; Gal, M.; Poturnayova, A. Advancements in Chronic Myeloid Leukemia detection: Development and evaluation of a novel QCM aptasensor for use in clinical practice. Biochem. Biophys. Rep. 2024, 39, 10816. [Google Scholar] [CrossRef]
- Xiao, Y.; Lai, R.Y.; Plaxco, K.W. Preparation of Electrode-Immobilized, Redox-Modified Oligonucleotides for Electrochemical DNA and Aptamer-Based Sensing. Nat. Protoc. 2007, 2, 2875–2880. [Google Scholar] [CrossRef]
- Oberhaus, F.V.; Frense, D.; Beckmann, D. Immobilization Techniques for Aptamers on Gold Electrodes for the Electrochemical Detection of Proteins: A Review. Biosensors 2020, 10, 45. [Google Scholar] [CrossRef]
- Hornychová, M. Application of Diamond Surfaces in Biosensing of Oncological Diseases. Master’s Thesis, Slovak University of Technology in Bratislava, Bratislava, Slovakia, 2024. [Google Scholar]
- Lin, Y.; Chen, C.-Y.; Ku, Y.-C.; Wang, L.-C.; Hung, C.-C.; Lin, Z.-Q.; Chen, B.-H.; Hung, J.-T.; Sun, Y.-C.; Hung, K.-F. A Modified SELEX Approach to Identify DNA Aptamers with Binding Specificity to the Major Histocompatibility Complex Presenting Ovalbumin Model Antigen. RSC Adv. 2023, 13, 32681–32693. [Google Scholar] [CrossRef] [PubMed]
- Kohlberger, M.; Wildner, S.; Regl, C.; Huber, C.G.; Gadermaier, G. Rituximab-Specific DNA Aptamers Are Able to Selectively Recognize Heat-Treated Antibodies. PLoS ONE 2020, 15, e0241560. [Google Scholar]
- Wildner, S.; Huber, S.; Regl, C.; Huber, C.G.; Lohrig, U.; Gadermaier, G. Aptamers as Quality Control Tool for Production, Storage and Biosimilarity of the Anti-CD20 Biopharmaceutical Rituximab. Sci. Rep. 2019, 9, 1111. [Google Scholar] [CrossRef]
- Hu, J.; Kim, J.; Easley, C.J. Quantifying Aptamer–Protein Binding via Thermofluorimetric Analysis. Anal. Methods 2015, 7, 7358–7362. [Google Scholar] [CrossRef] [PubMed]
- Abrego-Martinez, J.C.; Jafari, M.; Chergui, S.; Pavel, C.; Che, D.; Siaj, M. Aptamer-Based Electrochemical Biosensor for Rapid Detection of SARS-CoV-2: Nanoscale Electrode-Aptamer-SARS-CoV-2 Imaging by Photo-Induced Force Microscopy. Biosens. Bioelectron. 2022, 195, 113595. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, M.; Skouridou, V.; Jauset-Rubio, M.; Viéitez, I.; Fernández-Villar, A.; Cabrera Alvargonzalez, J.J.; Poveda, E.; Bofill, C.B.; Sans, T.; Bashammakh, A.; et al. Aptamer Sandwich Assay for the Detection of SARS-CoV-2 Spike Protein Antigen. ACS Omega 2021, 6, 35657–35666. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Ray, P.C. Aptamer Conjugated Gold Nanostar-Based Distance-Dependent Nanoparticle Surface Energy Transfer Spectroscopy for Ultrasensitive Detection and Inactivation of Corona Virus. J. Phys. Chem. Lett. 2021, 12, 2166–2171. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Li, Q.; Xing, W.; Luo, H.; Ji, H.; Fang, X.; Luo, Z.; Zhang, L. CRISPR/Cas12a-Derived Electrochemical Aptasensor for Ultrasensitive Detection of COVID-19 Nucleocapsid Protein. Biosens. Bioelectron. 2022, 200, 113922. [Google Scholar] [CrossRef]
- Xu, L.; Ramadan, S.; Rosa, B.G.; Zhang, Y.; Yin, T.; Torres, E.; Shaforost, O.; Panagiotopoulos, A.; Li, B.; Kerherve, G.; et al. On-Chip Integrated Graphene Aptasensor with Portable Readout for Fast and Label-Free COVID-19 Detection in Virus Transport Medium. Sens. Diagn. 2022, 1, 719–730. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M. SARS-CoV-2 Virus Label-Free Electrochemical Nanohybrid MIP-Aptasensor Based on Ni3 (BTC) 2 MOF as a High-Performance Surface Substrate. Microchim. Acta 2022, 189, 287. [Google Scholar] [CrossRef]
- Shrikrishna, N.S.; Halder, S.; Kesarwani, V.; Nagamani, K.; Gandhi, S. Unveiling the Potential: High-Affinity Aptamers for Point of Care Detection of SARS-CoV-2 RBD Protein and It’s Validation in Clinical Samples. Chem. Eng. J. 2024, 493, 152841. [Google Scholar] [CrossRef]


| cS-RBD [pg/mL] | ΔfPBS [Hz] | Δfsaliva [Hz] | Δfplasma [Hz] | Recoverysaliva [%] | Recoveryplasma [%] |
|---|---|---|---|---|---|
| 102 | 30.2 ± 6.4 | 29.3 ± 2.5 | 29.3 ± 1.5 | 93.6 ± 3.6 | 97.4 ± 5.1 |
| 105 | 65.2 ± 5.9 | 71.3 ± 9.5 | 66.0 ± 3.6 | 109.5 ± 14.5 | 101.1 ± 5.3 |
| Type of Sensor | LOD | Linear Range | Reference |
|---|---|---|---|
| Electrochemical SPCE aptasensor | 66 pg/mL | 10 pM–25 nM | [36] |
| Optical sandwich aptamer assay | 21 ng/mL | ------------ | [37] |
| Nanoparticle surface energy transfer assay | 130 fg/mL | 10–500 virus mL−1 | [38] |
| CRISPR/Cas12a-derived electrochemical aptasensor | 16.5 pg/mL | 50 pg/mL–100 ng/mL | [39] |
| Al2O3 fabricated mercapto-silane AuNP aptasensor | 0.8 ng/mL | 2.5–40.0 ng/mL | [40] |
| Molecular imprinting polymer electrochemical SPCE aptasensor | 3.3 PFU mL−1 | 101–108 PFU mL−1 | [41] |
| Electrochemical SPCE aptasensor | 1.1 pM | 1 pM–100 nM | [42] |
| Aptamer-based QCM sensor | 70 fg/mL | 1 pg/mL–0.1 μg/mL | This work |
| Aptamer-based electrochemical AuE | 132 ng/mL | 175 ng/mL to 5 μg/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemčeková, K.; Korčeková, J.; Svitková, V.; Baraniak, D.; Domšicová, M.; Melníková, E.; Hornychová, M.; Szebellaiová, V.; Gál, M.; Poturnayová, A. Comparative Analysis of QCM and Electrochemical Aptasensors for SARS-CoV-2 Detection. Biosensors 2024, 14, 431. https://doi.org/10.3390/bios14090431
Nemčeková K, Korčeková J, Svitková V, Baraniak D, Domšicová M, Melníková E, Hornychová M, Szebellaiová V, Gál M, Poturnayová A. Comparative Analysis of QCM and Electrochemical Aptasensors for SARS-CoV-2 Detection. Biosensors. 2024; 14(9):431. https://doi.org/10.3390/bios14090431
Chicago/Turabian StyleNemčeková, Katarína, Jana Korčeková, Veronika Svitková, Denis Baraniak, Michaela Domšicová, Eva Melníková, Michaela Hornychová, Viktória Szebellaiová, Miroslav Gál, and Alexandra Poturnayová. 2024. "Comparative Analysis of QCM and Electrochemical Aptasensors for SARS-CoV-2 Detection" Biosensors 14, no. 9: 431. https://doi.org/10.3390/bios14090431
APA StyleNemčeková, K., Korčeková, J., Svitková, V., Baraniak, D., Domšicová, M., Melníková, E., Hornychová, M., Szebellaiová, V., Gál, M., & Poturnayová, A. (2024). Comparative Analysis of QCM and Electrochemical Aptasensors for SARS-CoV-2 Detection. Biosensors, 14(9), 431. https://doi.org/10.3390/bios14090431





