Abstract
Choline is an important molecule in monitoring food safety and infant nutrition. Here, we report Ce nanogels synthesized by atom transfer radical polymerization (ATRP) employing Ce-coordinated acryloyl-lysine polymer brushes (Ce@SiO2 NGs) as highly efficient cascade nanozymes for colorimetric detection of choline. The synthesized Ce@SiO2 NGs demonstrated remarkable peroxidase-like activity with a porous exterior, which are essential to entrap choline oxidase (COx) to yield COx@Ce@SiO2 NGs and construct a cascade reaction system to detect choline. Immobilized COx catalyzed the oxidation of choline in food samples to produce H2O2, which subsequently induced the oxidation of chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (TMB) to produce blue color signals. This method enabled the selective and sensitive detection of target choline with a satisfactory linear range of 4–400 μM, which is sufficient to analyze foodborne choline. The practical utility of the COx@Ce@SiO2 NG-based assay was successfully validated to determine choline spiked in commercially available milk and infant formula with high accuracy and precision values. This approach provides a simple and affordable method of choline detection and has the potential to lead to more developments in ATRP-based nanozymes for diverse biosensing applications.
1. Introduction
Choline is an essential nutrient for developing the brain and maintaining overall health [1,2,3]. Several diseases, such as Alzheimer’s disease, liver cirrhosis, and growth disorder, were suggested to be induced by long-term choline deficiency [4,5]. Usually, the body can restore choline through food intake, and, thus, accurate determination of choline in foods is an important issue. The US Food and Drug Administration (FDA) as well as the food and nutrition board (FNB) of the institute of medicine thus set regulations that infant formula not made from cow’s milk must be supplemented with a high choline concentration (up to 200 mg/100 g sample), which is equivalent to that of breast milk (~1 mM) [6]. In addition, H2O2 is commonly used as a disinfectant in food preservation; however, its excessive levels can pose health risks, such as oxidative stress and tissue damage [7,8]. Therefore, ensuring accurate quantification of choline as well as H2O2 in foods is essential for maintaining health standards and meeting dietary needs [6,9]. The traditional detection methods for choline and H2O2, such as high-performance liquid chromatography (HPLC) and fluorescence-based techniques, are generally selective and sensitive. However, these approaches present several challenges, including time-consuming sample pre-/post-treatment, interference from background signals, and the inevitable use of complicated instrumentation [7,10,11]. Thus, the development of more convenient, reliable, and sensitive methods for detecting choline and residual H2O2 is vital to overcome these limitations and improve food safety and nutritional assessments.
Recent advancements in nanotechnology have highlighted the potential of nanomaterials with enzyme-like properties, known as nanozymes, to replace natural enzymes in versatile applications. Nanozymes possess several superiorities to natural enzymes, such as enhanced stability and durability, tunable activity, and more affordable synthesis [12,13]. Despite these advantages, natural enzymes often outperform nanozymes in terms of substrate specificity and catalytic efficiency. This is primarily due to the intricate and finely tuned structure of the active sites in natural enzymes, which nanozymes struggle to replicate. To bridge this gap, recent studies have focused on designing more sophisticated nanozyme architectures, with attention on engineering their active sites. Notably, materials with metal–nitrogen (M–N) active sites, such as Fe-N, Co-N, Zn-N, or metal–organic frameworks (MOFs), have emerged as highly promising candidates [14,15,16,17]. M–N active site materials have mimicked the active centers of natural metalloenzymes, while MOFs provide nanoscale cavities resembling the three-dimensional (3D) binding pockets of natural enzymes, creating a conducible environment for the catalytic process. These advances in nanozyme development provide new opportunities in versatile applications from biochemical sensing and environmental remediation to medical theranostics [18].
Building on these developments, nanogels synthesized via ATRP have emerged as a promising platform for improving the catalytic performance of nanozymes. ATRP is recognized as a controlled polymerization method that enables the precise synthesis of well-defined, biocompatible nanogels. These nanogels are characterized by exceptional biocompatibility, tunable porosity, and the ability to create dynamic microenvironments around catalytic centers that resemble the active sites of natural enzymes [19,20,21,22,23]. Recently, an enzyme-catalyzed variant of this method, called ATRPase, was introduced. This approach enables the synthesis of biocompatible polymer brushes through the interfacial polymerization of amino-acid-based monomers, such as N-acryloyl-l-lysine. By coordinating with certain metal ions including Fe, these polymeric nanogels exhibited unique enzyme-like activities [24,25]. Although the potential of ATRP-based nanozymes has been demonstrated, further investigations are required to study the effects of versatile metal ions regarding incorporation within the nanogels to induce affirmative enzyme-like activity for their practical applications.
Cerium (Ce) ions exhibit exceptional qualities as effective cross-linkers in the formation of various hydrogel-based materials. Their integration into the hydrogel matrix through cross-linking enhances the structural stability and mechanical properties of the hydrogels while enabling the creation of materials with tailored functionalities [26,27,28]. By leveraging the unique coordination chemistry of Ce ions, researchers have designed hydrogels with affirmative properties, such as improved stability, increased loading capacities, and enhanced catalytic activities, making them suitable for diverse applications in the biomedical and environmental fields [29,30]. Moreover, the ability of Ce ions to alternate between Ce3+ and Ce4+ oxidation states promotes strong peroxidase-like activity, making them highly effective for various biosensing applications [14]. Additionally, the monoatomic dispersion of Ce ions within the hydrophilic network of the nanogels possibly creates a high density of active sites, resulting in significantly improved reaction rates and overall catalytic efficiency [31,32]. These advantageous properties position Ce-based nanogels as promising materials for advanced catalytic and biosensing platforms.
Herein, we developed Ce nanogels synthesized by ATRP employing Ce-coordinated acryloyl-lysine polymer brushes (Ce@SiO2 NGs) as an efficient peroxidase mimic and scaffold for oxidative enzyme entrapment, which is designed for colorimetric detection of H2O2 and choline by incorporating COx within the nanogels. The Ce ions within the Ce@SiO2 NGs serve as both structural cross-linkers and active centers, leading to enhanced catalytic efficiency. Additionally, the ATRP-based nanogels provided a biocompatible and porous network that is capable of entrapping COx, resulting in COx@Ce@SiO2 NGs performing selective and sensitive choline detection via a cascade reaction. In the presence of choline, COx catalyzed its oxidation to produce H2O2, which subsequently activated the peroxidase-mimicking Ce@SiO2 NGs to oxidize a chromogenic substrate TMB to produce a blue color. This provides a dual-function platform capable of detecting choline as well as H2O2. By leveraging the catalytic properties of Ce@SiO2 NGs and COx@Ce@SiO2 NGs, a versatile, reliable, and scalable method has been developed to enhance food safety analysis, particularly in the context of dairy products and infant nutrition.
2. Materials and Methods
2.1. Reagents and Materials
2-Bromoisobutanoic acid N-hydroxysuccinimide ester (NHS-Bib), sodium hydroxide (NaOH), sodium carbonate (Na2CO3), sodium acetate (CH3COONa), sodium ascorbate, copper sulfate pentahydrate (CuSO4.5H2O), TMB, ammonia solution (28% in water), dimethyl sulfoxide (DMSO), tetraethyl orthosilicate (TEOS), cerium(III) nitrate hexahydrate (Ce(NO3)3.6H2O), absolute ethanol, acryloyl chloride, L-lysine hydrochloride, horseradish peroxidase (HRP), 3-aminopropyl triethoxysilane (APTES), phosphate buffered saline (PBS), and 8-hydroxyquinoline were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrogen peroxide was obtained from Samchun Chemical (Seoul, Republic of Korea). All solutions were prepared with deionized (DI) water purified by a Milli-Q Purification System (Millipore, Darmstadt, Germany).
2.2. Material Characterizations
Using a Field Emission Scanning Electron Microscope (JSM-7500F JEOL, Pleasanton, CA, USA) for scanning electron microscopy (SEM) and a Transmission Electron Microscope (FEI Tecnai, Hillsboro, OR, USA) for transmission electron microscopy (TEM) and high-resolution TEM (HR-TEM), the morphology of the Ce@SiO2 NGs was examined. Energy-dispersive spectroscopy (EDS) was used to analyze the elemental composition (Bruker, Billerica, MA, USA). The suspension of sonicated Ce@SiO2 NGs was allowed to dry overnight on a silicon wafer in preparation for the SEM examinations. Further, 5 μL of the sonicated nanogel suspension was deposited onto a carbon-coated copper TEM grid (Electron Microscopy Sciences, Hatfield, PA, USA) for the TEM studies, and it was then allowed to dry overnight at room temperature (RT). An FT-IR spectrophotometer (FT/IR-4600, JASCO, Easton, MD, USA) was used to acquire the Fourier transform infrared (FT-IR) spectra of Ce@SiO2 NGs. X-ray photoelectron spectroscopy (XPS) was conducted using an XPS reader (Sigma Probe, Thermo Scientific, Madison, WI, USA) to analyze the contribution of elements. Water contact angle was measured to investigate the hydrophilicity of Ce@SiO2 NGs using a Phoenix 300 contact angle analyzer (Surface Electro Optics, Suwon, Gyeonggi, Republic of Korea).
2.3. Synthesis of Ce@SiO2 NGs and Ce@SiO2 NGs Entrapping COx (COx@Ce@SiO2 NGs)
Ce@SiO2 NGs were synthesized from SiO2 nanoparticles (NPs), N-acryloyl-L-lysine brush, and Ce(NO3)2 solution following reported method with minor modifications [24]. First, N-acryloyl-L-lysine solution (50 mg/mL), SiO2–Br NP dispersion (5 mg/mL), and sodium ascorbate solution (1.5 mg/mL) were mixed thoroughly in PBS (10 mM, pH 6) under nitrogen atmosphere. Next, HRP (5 mg/mL) as an ATRP catalyst (ATRPase) was added and the solution was oscillated overnight at RT. In order to obtain the nanogel layer at the surface of nanoparticles, Ce(NO3)3·6H2O was added to the solution and stirred for 2 h at RT to induce the cross-linking process of lysine moieties at the surface of polymer brushes (N-acryloyl-L-lysine). The Ce-coordinated nanogel on SiO2 surfaces (Ce@SiO2 NGs) was obtained by centrifugation at 5000× g for 5 min and washing with H2O and absolute ethanol. SiO2-Pol(Lys) NPs were also synthesized via the same method except the incorporation of Ce.
COx@Ce@SiO2 NGs were prepared by mixing Ce@SiO2 NGs with several concentrations of COx (0.025, 0.05, 0.1, 0.2, and 0.4 mg/mL) for 30 min at RT. The samples were collected by centrifugation at 5000× g for 5 min, followed by washing with DI water to obtain COx@Ce@SiO2 NGs. The concentrations of COx before and after the immobilization remaining in the supernatant were measured using the bicinchoninic acid (BCA) assay to calculate the loading capacity.
2.4. Evaluation of Peroxidase-like Activity of Ce@SiO2 NGs
Peroxidase-like activity of Ce@SiO2 NGs was assessed by monitoring the oxidation of TMB in the presence of H2O2. In this assay, Ce@SiO2 NGs (0.1 mg/mL) were added into a reaction buffer (0.1 M sodium acetate (NaAc), pH 4.0) containing TMB (1 mM) and H2O2 (10 mM) and incubated for 5 min at RT. Following the incubation, the catalytic materials were separated by centrifugation (13,000× g, 2 min). The absorbance of the supernatant was measured in a scanning mode from 550 to 750 nm or at 652 nm using a microplate reader (Synergy H1, BioTek, Winooski, VT, USA).
Stability of Ce@SiO2 NGs was compared with that of HRP by evaluating their activity in NaAc buffer (0.1 M) across varying conditions of temperature (4 to 90 °C) and pH (3 to 9). After incubation for 2 h, the residual activities of Ce@SiO2 NGs and HRP were measured using aforementioned colorimetric assays.
Steady-state kinetic studies were conducted to evaluate the kinetic parameters of Ce@SiO₂ NGs. The experiments were performed in NaAc buffer (0.1 M, pH 4.0) containing 0.1 mg/mL Ce@SiO2 NGs. For TMB, the reaction buffer was supplemented with 10 mM H2O2 at varying concentrations of TMB, while, for H2O2, 1 mM TMB was added in reaction buffer at various concentrations of H2O2. After addition of TMB or H2O2, absorbance was monitored by measuring the color changes of reaction solution using a kinetic mode at 652 nm. The kinetic parameters were calculated using the equation v = Vmax × [S]/(Km + [S]), where v is the initial velocity, Vmax is maximal velocity, [S] is substrate concentration, and Km is Michaelis constant.
2.5. Quantitative Determination of H2O2 Using Ce@SiO2 NGs
H2O2 concentration was determined using TMB as a substrate in a transparent 96-well plate as follows. Various concentrations of H2O2 were added in NaAc buffer (0.1 M, pH 4.0) containing Ce@SiO2 NGs (0.1 mg/mL) and TMB (1 mM). After incubation for 5 min at RT, the Ce@SiO2 NGs were separated and the absorbance of supernatant was measured at 652 nm using a microplate reader.
2.6. Quantitative Determination of Choline Using COx@Ce@SiO2 NGs
The quantification of choline level was conducted by incubating COx@Ce@SiO2 NGs with varying concentrations of choline in HEPES buffer (0.05 M, pH 7.0) at RT for 15 min. Then, NaAc buffer (0.1 M, pH 4) containing 1 mM TMB was added and incubated for 5 min. Following the incubation, further procedures were the same as those described for H2O2 detection.
2.7. Detection of H2O2 and Choline in Milk and Infant Formula Samples
Fresh milk and infant formula samples were purchased from local market and pretreated with methanol to remove organic impurities before being diluted 100 times with HEPES buffer. The amounts of H2O2 and choline in diluted samples were measured by HRP-TMB-based assay and choline assay kit (Abcam, Cambridge, UK), respectively. Then the prescribed amounts of H2O2 (25, 50, and 100 µM) and choline (50, 100, and 200 µM) were added into diluted samples to create spiked samples. The levels of choline and H2O2 in these spiked samples were subsequently analyzed using the same detection protocols as described earlier for individual H2O2 and choline quantification. To evaluate the accuracy and reproducibility of the assay, recovery rate (%) and coefficient of variation (CV, %) were calculated based on three independent assay results for each spiked sample. Recovery rate (%) and CV (%) are defined by these equations: [recovery (%) = measured value/actual value × 100] and [CV (%) = SD/average × 100].
3. Results and Discussion
3.1. Synthesis and Characterization of Ce@SiO2 NGs
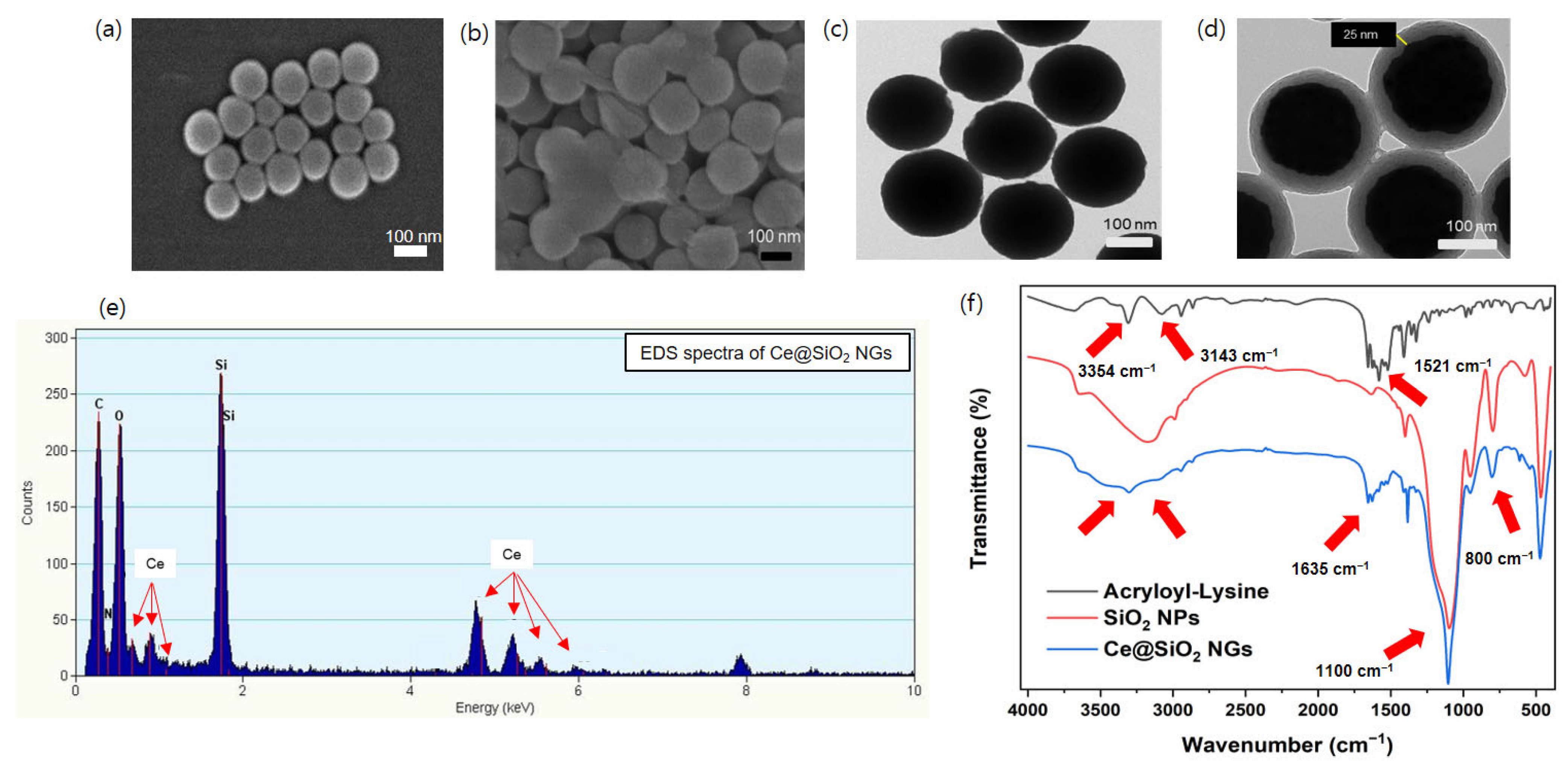
Peroxidase-like Ce@SiO2 NGs, synthesized via a biocatalytic ATRP reaction, were used to construct cascade nanozyme COx@Ce@SiO2 NGs by entrapping COx for the convenient colorimetric detection of choline (Figure 1). The morphological characteristics of the Ce@SiO2 NGs were analyzed using SEM, which revealed a notable change in the dispersion of the SiO2 NPs after the polymer brush incorporation (Figure 2a,b). The bare SiO2 NPs were highly dispersed, while the Ce@SiO2 NGs displayed marginal aggregation, suggesting successful coating with the polymer brushes. The TEM images also showed a uniform spherical morphology with a diameter of approximately 160 nm of the bare SiO2 NPs (Figure 2c). After the surface modification, the Ce@SiO2 NGs exhibited an enlarged particle size due to the formation of outer polymeric brushes, with ~25 nm thickness, on the SiO2 core (Figure 2d). The EDS analysis validated the presence of C, O, N, Si, and Ce elements within the Ce@SiO2 NGs (Figure 2e), confirming the successful incorporation of the Ce ions in the nanogel matrix. The FT-IR spectroscopy further confirmed the successful synthesis of the Ce@SiO2 NGs (Figure 2f). The presence of Si-O-Si linkages was evident from the strong absorption peaks observed between 800 and 1100 cm−1, one of the characteristics of silica networks. Additional peaks at 1521 cm−1, 1635 cm−1, 3143 cm−1, and 3354 cm−1 were attributed to the stretching vibrations of C=O, C-H, N-H, and O-H, respectively, indicating the presence of the functional groups associated with the polymeric network and Ce coordination [26,27,28]. In addition, the XPS full spectra for the Ce@SiO2 NGs revealed the presence of C, O, N, Si, and Ce elements, which is consistent with the EDS analysis (Figure S1). The high-resolution XPS spectra presented special peaks of Si 2p, N 1s (N–H, C–N), and Ce 3d, which were observed at 103.1, 399.2, and 401.2, respectively [33,34]. The Ce@SiO2 NGs showed an extremely hydrophilic property, demonstrated by the contact angle of ~16°, which was 3-fold smaller than that of the SiO2 NPs (Figure S2). The enhanced hydrophilicity of the Ce@SiO2 NGs is presumed by the carboxyl and hydroxyl groups on their surface, which was confirmed by the FT-IR analysis. These spectral features collectively confirm the successful formation of Ce-coordinated nanogels with the intended structural and functional characteristics.
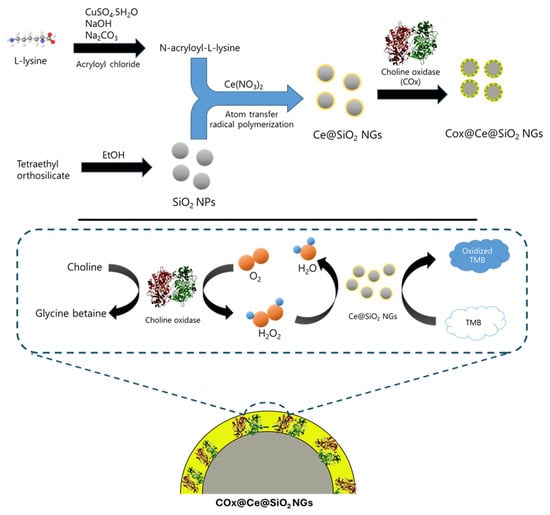
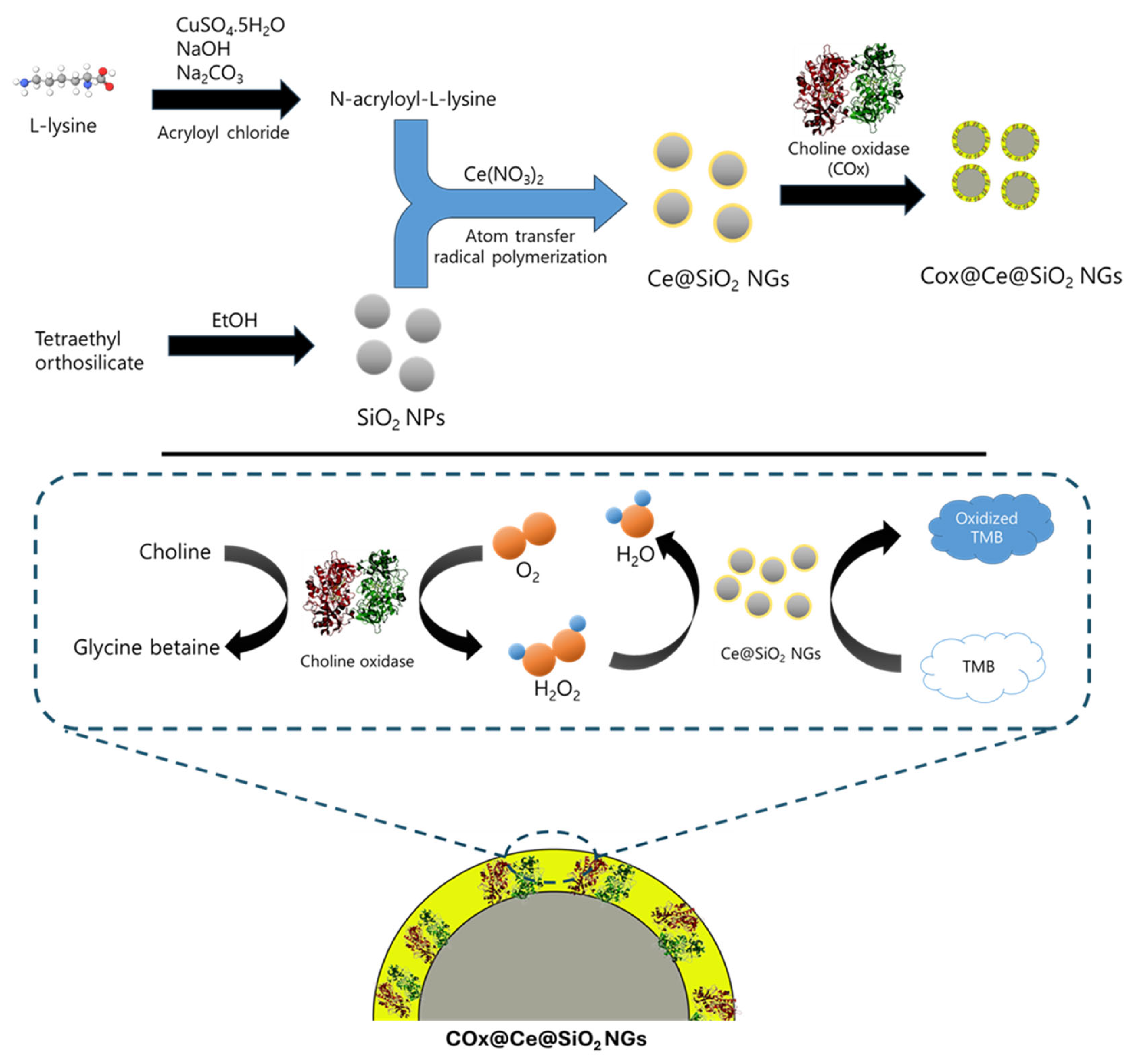
Figure 1.
Schematic illustration for the synthesis of Ce@SiO2 NGs and COx@Ce@SiO2 NGs, with their applications to colorimetrically detect H2O2 and choline.
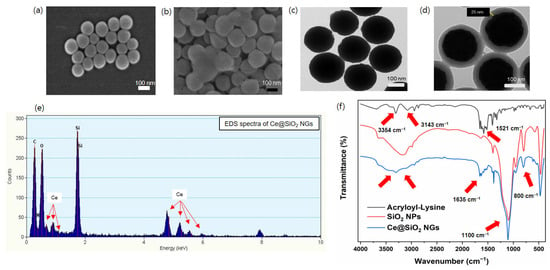
Figure 2.
SEM images of (a) SiO2 NPs and (b) Ce@SiO2 NGs. TEM images of (c) SiO2 NPs and (d) Ce@SiO2 NGs. (e) EDS and (f) FT-IR spectra of Ce@SiO2 NGs.
3.2. Investigation of Peroxidase-like Activity of Ce@SiO2 NGs
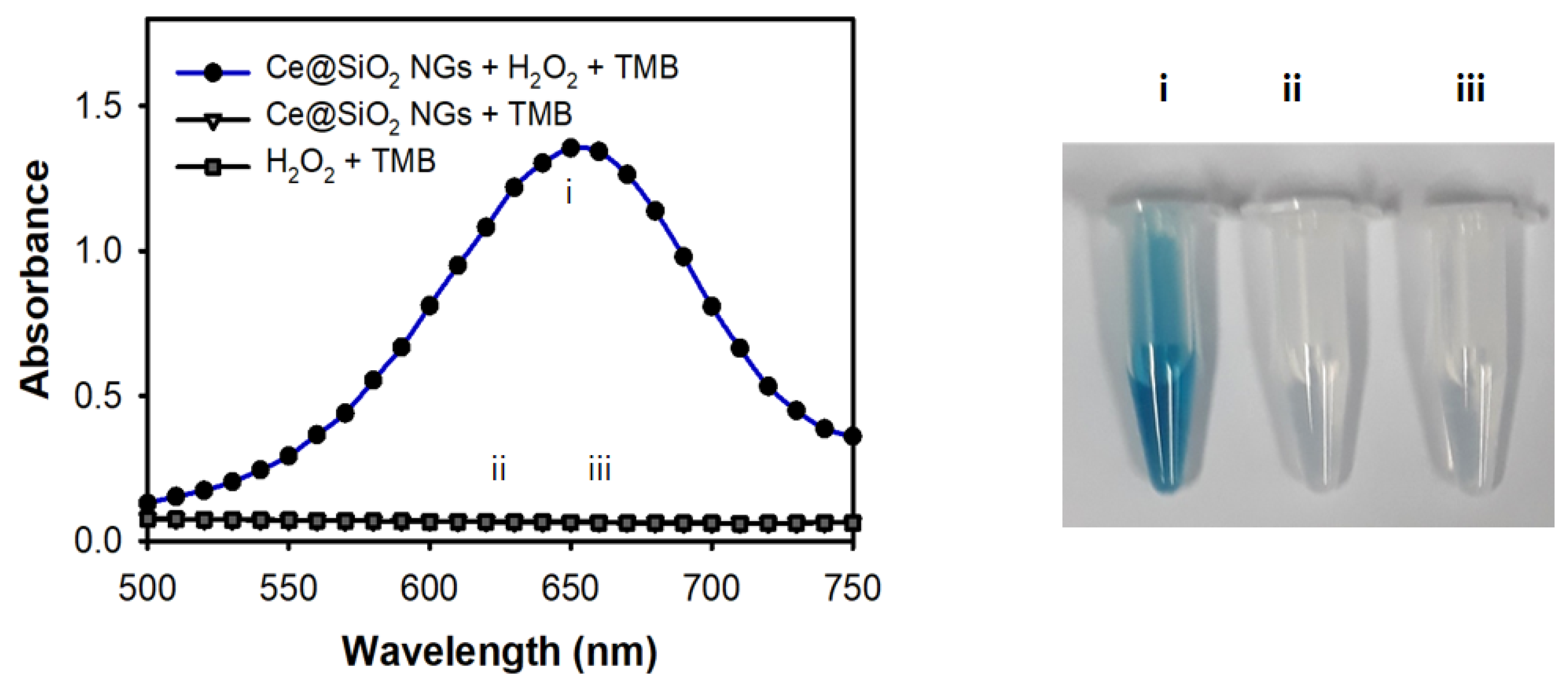
The peroxidase-like activity of the Ce@SiO2 NGs was evaluated by performing the peroxidase-mediated oxidation of TMB in the presence of H2O2. First, the influences of pH and temperature on the catalytic activity were explored. The results showed that the Ce@SiO₂ NGs exhibited their maximal activity at pH 4.0 and 37 °C (Figure S3a,b); however, to facilitate practical utilizations, RT was used in further assays where the activity was more than 80% of that observed at 37 °C. During only a 5 min reaction, the Ce@SiO2 NGs successfully catalyzed the oxidation of TMB, producing a strong blue color signal in the presence of H2O2, but showed no activity without H2O2 (Figure 3). In addition, the incorporation of Ce ions within the SiO2 NPs played a key role in mimicking the peroxidase activity when SiO2-Pol(Lys) NPs, as a control, did not oxidize TMB in the presence of H2O2 (Figure S4). Moreover, the Ce@SiO2 NGs exhibited remarkable stability across broad pH and temperature ranges, contrasting with HRP, which rapidly lost activity under acidic conditions and elevated temperatures (Figure S3c,d). During the catalytic action of the Ce@SiO2 NGs in the presence of H2O2, the production of hydroxyl radicals was confirmed by employing a terephthalic acid (TA) probe, indicating that TMB can be oxidized with the hydroxyl radicals, similar to HRP-mediated catalysis (Figure S5). These results proved that trapped Ce ions within SiO2 NPs using a biocatalytic ATRP reaction notably enhanced the peroxidase-like activity and stability, which may serve as a feasible alternative to HRP in versatile applications.
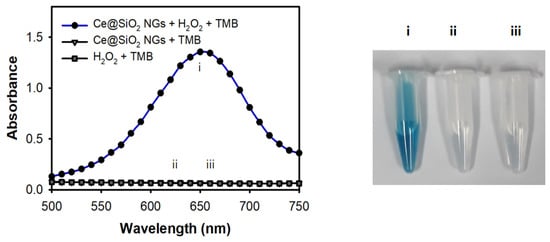
Figure 3.
Peroxidase-like activity of Ce@SiO2 NGs through the oxidation of TMB in the presence of H2O2. The assay was performed in NaAc buffer (0.1 M, pH 4) containing Ce@SiO2 NGs (0.1 mg/mL) and TMB (1 mM) for 5 min incubation at RT. Sample specifications: i: Ce@SiO2 NGs + H2O2 + TMB, ii: Ce@SiO2 NGs + TMB, and iii: H2O2 + TMB.
To fully elucidate the peroxidase activity of the Ce@SiO2 NGs, their steady-state kinetic parameters were determined. The results showed that the Ce@SiO2 NGs followed Michaelis–Menten kinetics for both TMB and H2O2 (Figure S6a,b), and the kinetic parameters, Km and Vmax, were determined via Lineweaver–Burk plots (Figure S6c,d). Importantly, the Km values of the Ce@SiO2 NGs for TMB and H2O2 were found to be 0.57 and 1.79 mM, respectively, demonstrating a strong affinity when compared with other conventional nanozymes and HRP (Table S1). The enhanced affinity may be due to the polymeric brushes located outside the nanogels, which provide dynamic microenvironments around coordinated Ce ions, providing improved hydrophilicity and facilitating substrate transfer to the active site of the Ce@SiO2 NGs. These kinetic parameters demonstrate the high catalytic efficiency of Ce@SiO2 NGs, highlighting their potential as a robust alternative to natural HRP for the colorimetric detection of H2O2 and biomarkers in various analytical applications.
3.3. Quantitative Detection of H2O2 Using Ce@SiO2 NGs
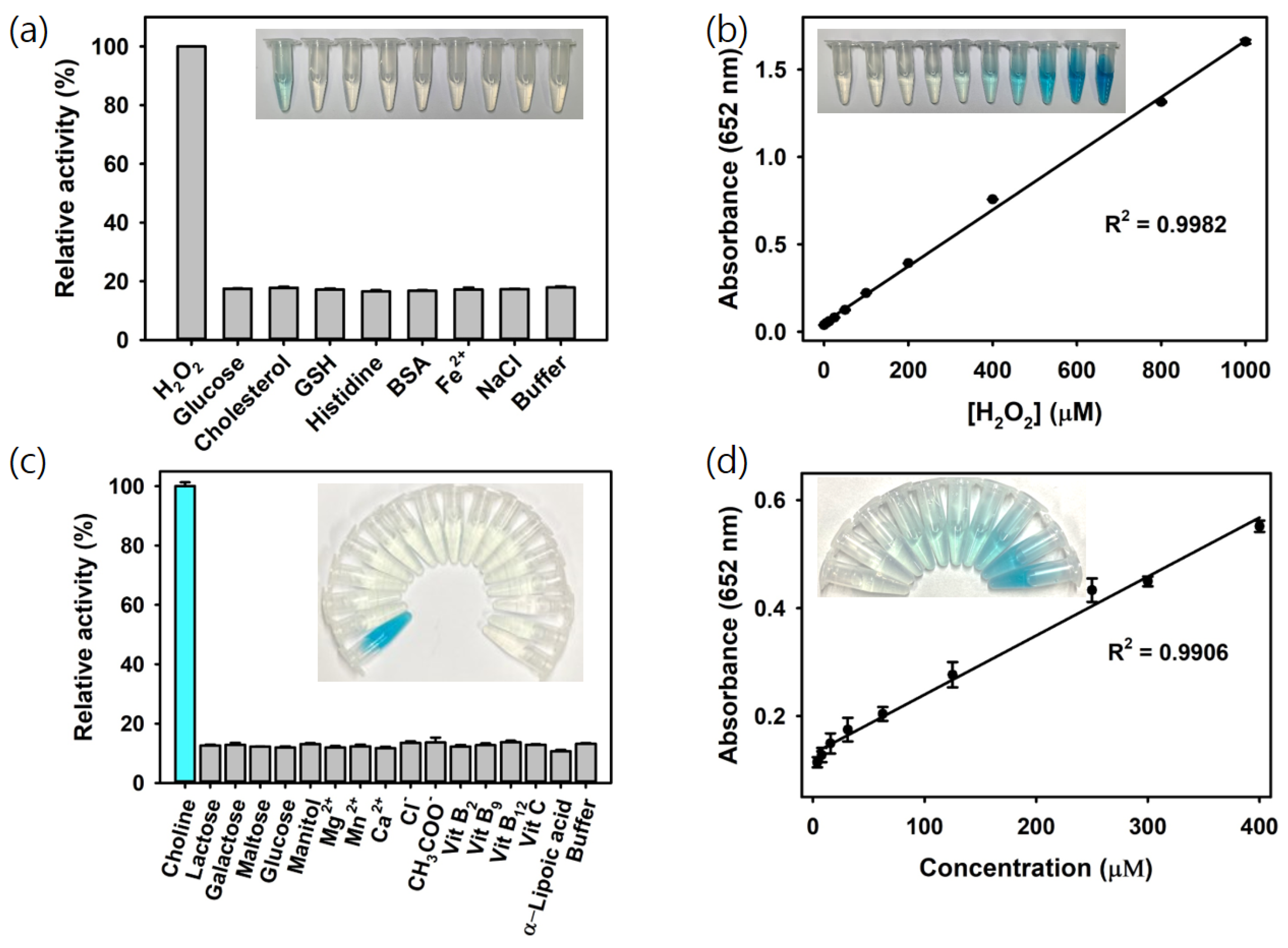
The oxidation of TMB via H2O2 catalyzed by Ce@SiO2 NGs produces a vivid blue color signal, directly proportional to the concentration of H2O2. Leveraging this reaction, a colorimetric strategy for H2O2 detection using Ce@SiO2 NGs was developed. As a result, H2O2 was specifically detected during a 5 min reaction, whereas no significant color change was observed for the negative control samples, such as glucose, cholesterol, glutathione (GSH), histidine, bovine serum albumin (BSA), Fe2+, and NaCl, at the same concentration with H2O2 (5 mM), confirming the excellent specificity of the assay system towards the target H2O2 (Figure 4a). The limit of detection (LOD) value was calculated using the formula LOD = 3 × δ/slope, where δ is the standard deviation of the blank and slope is the slope of the calibration curve. Through the analysis of the dose–response curve, the LOD for H2O2 was determined to be as low as 1.3 μM, with a linear range from 5 to 1000 μM (Figure 4b). The LOD and linear range values are among the most sensitive reported for colorimetric H2O2 detection, making this system highly advantageous for quantifying H2O2 and other biomarkers when combined with their respective oxidase enzymes (Table 1).
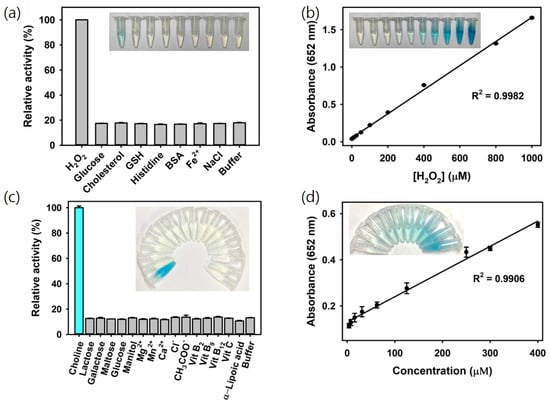
Figure 4.
(a) Selectivity and (b) linear calibration plots to show sensitivity for the colorimetric detection of H2O2 using Ce@SiO2 NGs. (c) Selectivity and (d) linear calibration plots to show sensitivity for the colorimetric detection of choline using COx@SiO2 NGs.

Table 1.
Comparison of analytical performances of various nanozymes for H2O2 detection.
3.4. Quantitative Detection of Choline Using COx@Ce@SiO2 NGs
H2O2 is a byproduct of the natural oxidase-mediated catalysis of biomarkers, and the COx@SiO2 NG-based system exhibited high selectivity and sensitivity in detecting H2O2. Based on these observations, we developed a detection system for choline, an important neurotransmitter and biomarker for monitoring various diseases and nutritional status, by incorporating COx into Ce@SiO2 NGs to construct COx@Ce@SiO2. In the presence of choline, the entrapped COx catalyzes the oxidation of choline to produce H2O2, which subsequently activates the peroxidase-mimicking Ce@SiO2 to produce a visible blue color signal from the oxidation of TMB. To optimize the preparation conditions of COx@Ce@SiO2, we investigated the effects of the COx concentration on its loading and choline detecting activity (Figure S7). The porous nature of the Ce@SiO2 NGs is expected to be advantageous to entrap COx, and, at 0.1 mg/mL COx, the maximum loading (~28 wt%) and activity were achieved, indicating that this concentration is optimal for the formation of COx@Ce@SiO2 NGs.
Using the optimized conditions with the COx@Ce@SiO2 NGs, choline was specifically detected by the intense blue color produced, while no noticeable color change occurred in the negative controls, including common interferes in milk such as lactose, galactose, maltose, glucose, manitol, Mg2+, Mn2+, Ca2+, Cl−, CH3COO−, vitamin B2, B9, B12, C, and α-lipoic acid, demonstrating the high specificity of the COx@Ce@SiO2 NG-based system towards choline (Figure 4c). The linear calibration plots showed the LOD as low as 2 μM, with a linear range from 4 to 400 μM (Figure 4d). These LOD and linear range values rank among the most sensitive reported for colorimetric choline detection (Table 2). These results proved that the developed biosensor could serve as a simple and highly sensitive approach for H2O2 and choline analysis without using expensive and specialized instruments or complex labeling procedures, making it a valuable tool for choline and food safety analysis in dairy products and infant nutrition.

Table 2.
Comparison of analytical performances of various nanozymes for choline detection.
The storage stabilities of the COx@Ce@SiO2 NGs and a free enzyme system comprising free HRP and free COx were evaluated under different temperature conditions (4 °C, RT, and 37 °C). The results showed the improved stability of the COx@Ce@SiO2 NGs under different temperature conditions (Figure S8), which may be attributed to the protective polymeric brushes of the nanogel. When nanogels were employed, the choline detecting activity was maintained over 80% during 6 days at RT, whereas the free system lost over 40% of its initial activity. When stored at 37 °C, there was a marginal decrease in the COx@Ce@SiO2 NGs, probably due to the temperature-dependent denaturation and release of the immobilized COx.
3.5. Choline and H2O2 Detection in Milk and Infant Formula Samples
Choline is predominantly found in infant formula and fresh milk, making its accurate detection crucial for ensuring nutritional quality. To assess the practical applicability of the COx@Ce@SiO2 NG-based assay, commercially available milk and infant formula were used to prepare the spiked sample for analysis. Initially, the original concentrations of choline in the fresh samples were determined using commercial assay kits, followed by the addition of specific amounts of choline to prepare the spiked samples. As a result, the choline levels in the spiked samples were measured with high precision and accuracy, with CVs in the range of 0.3 to 4.1% and recoveries of 97.5 to 105.3%. Additionally, the NGs designed for H2O2 detection demonstrated great analytical performance, with recoveries for the spiked H2O2 in the milk and infant formula samples ranging from 97.5 to 103.7% and CVs between 1.2 and 4.0%, underscoring the method’s reliability and reproducibility (Table 3). These results highlight the capability of using the COx@Ce@SiO2 NG-based assay to effectively analyze real food matrices, such as dairy products, ensuring the safety and nutritional adequacy of infant food sources.

Table 3.
Detection precision of COx@Ce@SiO2 NGs and Ce@SiO2 NGs for choline and H2O2 quantification, respectively, in spiked milk and infant formula samples.
4. Conclusions
We demonstrated that Ce@SiO2 NGs and COx@Ce@SiO2 NGs present a significant advancement in the colorimetric detection of H2O2 and choline, respectively. The ATRP-based synthesis of Ce@SiO2 NGs resulted in well-defined polymer-coated nanoparticles with excellent peroxidase-like activity. High selectivity and sensitivity towards target H2O2 and choline were achieved, underscoring their applicability in real-world scenarios. Validation through assays using commercial milk and infant formula confirmed the reliability and accuracy in practical applications. This research highlights the potential of ATRP-based nanozymes not only for enhancing food safety analysis in dairy products but also for broader biosensing applications, paving the way for future nanozymatic innovations in this field.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/bios14120563/s1, Figure S1: XPS spectra of (a) Ce@SiO2 NGs and their high-resolution XPS spectra of (b) Si 2p, (c) N 1s, and (d) Ce 3d; Figure S2: Contact angle of (a) SiO2 NPs and (b) Ce@SiO2 NGs; Figure S3: Effects of (a) pH and (b) temperature on the peroxidase-like activity of Ce@SiO2 NGs. Catalytic stabilities of Ce@SiO2 NGs and HRP in ranges of (c) pH and (d) temperature; Figure S4: Peroxidase-like activity of (i) Ce@SiO2 NGs, (ii) SiO2-Pol(Lys) NPs, and (iii) 10 mM H2O2; Figure S5: Demonstration of hydroxyl radicals produced during the catalytic action of Ce@SiO2 NGs and HRP in the presence of H2O2. In the assays, 10 mM H2O2 and 0.625 mM TA were employed; Figure S6: Steady-state kinetic assays of Ce@SiO2 NGs for (a) TMB and (b) H2O2 and their corresponding double reciprocal (Lineweaver–Burk) plots of activity; Figure S7: (a) COx loading percentage within Ce@SiO2 NGs (1mg/mL) and (b) relative activity to detect choline prepared at various initial COx concentrations; Figure S8: Storage stability of (a) free enzyme system comprising free HRP and free COx and (b) COx@Ce@SiO2 NGs under different temperature conditions; Table S1: Comparison of peroxidase-like kinetic parameters of Ce@SiO2 NGs and other catalysts. References [45,46] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, T.H.V.; investigation, validation, writing—review and editing, B.J.Y.; and conceptualization, supervision, writing—review and editing, M.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT [NRF-2023R1A2C2007833]) and the Basic Science Research Program through the NRF funded by the Ministry of Education (Grant No. 2021R1A6A1A03038996). This study has also been conducted with the support of the Korea Institute of Industrial Technology as “Development of nano material and process for smart living sensor (KITECH EO-24–0003)”.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeisel, S.H. Nutritional importance of choline for brain development. J. Am. Coll. Nutr. 2004, 23 (Suppl. S6), 621S–626S. [Google Scholar] [CrossRef]
- Moretti, A.; Paoletta, M.; Liguori, S.; Bertone, M.; Toro, G.; Iolascon, G. Choline: An essential nutrient for skeletal muscle. Nutrients 2020, 12, 2144. [Google Scholar] [CrossRef]
- Cater, R.J.; Mukherjee, D.; Gil-Iturbe, E.; Erramilli, S.K.; Chen, T.; Koo, K.; Santander, N.; Reckers, A.; Kloss, B.; Gawda, T.; et al. Structural and molecular basis of choline uptake into the brain by FLVCR2. Nature 2024, 629, 704–709. [Google Scholar] [CrossRef]
- Arai, T.; Tanaka, M.; Goda, N. HIF-1-dependent lipin1 induction prevents excessive lipid accumulation in choline-deficient diet-induced fatty liver. Sci. Rep. 2018, 8, 14230. [Google Scholar] [CrossRef]
- Velazquez, R.; Ferreira, E.; Winslow, W.; Dave, N.; Piras, I.S.; Naymik, M.; Huentelman, M.J.; Tran, A.; Caccamo, A.; Oddo, S. Maternal choline supplementation ameliorates Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations. Mol. Psychiatry 2020, 25, 2620–2629. [Google Scholar] [CrossRef]
- Abd El-Rahman, M.K.; Mazzone, G.; Mahmoud, A.M.; Sicilia, E.; Shoeib, T. Novel choline selective electrochemical membrane sensor with application in milk powders and infant formulas. Talanta 2021, 221, 121409. [Google Scholar] [CrossRef]
- Mathivanan, D.; Devi, K.S.; Sathiyan, G.; Tyagi, A.; da Silva, V.; Janegitz, B.; Prakash, J.; Gupta, R.K. Novel polypyrrole-graphene oxide-gold nanocomposite for high performance hydrogen peroxide sensing application. Sens. Actuator A-Phys. 2021, 328, 112769. [Google Scholar] [CrossRef]
- Giaretta, J.E.; Duan, H.; Farajikhah, S.; Oveissi, F.; Dehghani, F.; Naficy, S. A highly flexible, physically stable, and selective hydrogel-based hydrogen peroxide sensor. Sens. Actuator B-Chem. 2022, 371, 132483. [Google Scholar] [CrossRef]
- Tian, X.; Qin, Y.; Jiang, Y.; Guo, X.; Wen, Y.; Yang, H. Chemically renewable SERS sensor for the inspection of H2O2 residue in food stuff. Food Chem. 2024, 438, 137777. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, M.H.; Vu, M.T.; Duong, H.A.; Pham, H.V.; Mai, T.D. Dual-channeled capillary electrophoresis coupled with contactless conductivity detection for rapid determination of choline and taurine in energy drinks and dietary supplements. Talanta 2019, 193, 168–175. [Google Scholar] [CrossRef]
- Giaretta, J.E.; Oveissi, F.; Dehghani, F.; Naficy, S. Paper-Based, Chemiresistive Sensor for Hydrogen Peroxide Detection. Adv. Mater. Technol. 2021, 6, 2001148. [Google Scholar] [CrossRef]
- Wu, W.; Huang, L.; Wang, E.; Dong, S. Atomic engineering of single-atom nanozymes for enzyme-like catalysis. Chem. Sci. 2020, 11, 9741–9756. [Google Scholar] [CrossRef]
- Shamsabadi, A.; Haghighi, T.; Carvalho, S.; Frenette, L.C.; Stevens, M.M. The nanozyme revolution: Enhancing the performance of medical biosensing platforms. Adv. Mater. 2024, 36, 2300184. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Lee, J.; Cho, A.; Kim, M.S.; Choi, D.; Han, J.W.; Kim, M.I.; Lee, J. Rational development of co-doped mesoporous ceria with high peroxidase-mimicking activity at neutral ph for paper-based colorimetric detection of multiple biomarkers. Adv. Funct. Mater. 2022, 32, 2112428. [Google Scholar] [CrossRef]
- Vu, T.H.; Nguyen, P.T.; Kim, M.I. Polydopamine-coated Co3O4 nanoparticles as an efficient catalase mimic for fluorescent detection of sulfide ion. Biosensors 2022, 12, 1047. [Google Scholar] [CrossRef]
- Lee, J.; Le, X.A.; Chun, H.; Vu, T.H.; Choi, D.; Han, B.; Kim, M.I.; Lee, J. Active site engineering of Zn-doped mesoporous ceria toward highly efficient organophosphorus hydrolase-mimicking nanozyme. Biosens. Bioelectron. 2024, 246, 115882. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Vu, T.H.; Kim, M.I. Histidine–cysteine–copper hybrid nanoflowers as active site-inspired laccase mimics for the colorimetric detection of phenolic compounds in PDMS microfluidic devices. Sens. Actuator B-Chem. 2024, 413, 135845. [Google Scholar] [CrossRef]
- Tian, Q.; Li, S.; Tang, Z.; Zhang, Z.; Du, D.; Zhang, X.; Niu, X.; Lin, Y. Nanozyme-enabled biomedical diagnosis: Advances, trends, and challenges. Adv. Healthc. Mater. 2024, 2401630. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Q.; Shang, Y.; Zhang, Q.; Wang, Q. D-Serine enzymatic metabolism induced formation of a powder-remoldable PAAM–CS hydrogel. Chem. Commun. 2017, 53, 12270–12273. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Ahn, H.T.; Kim, M.I. Reagent-free colorimetric assay for galactose using agarose gel entrapping nanoceria and galactose oxidase. Nanomaterials 2020, 10, 895. [Google Scholar] [CrossRef]
- Kim, M.I.; Park, C.Y.; Seo, J.M.; Kang, K.S.; Park, K.S.; Kang, J.; Hong, K.S.; Choi, Y.; Lee, S.Y.; Park, J.P.; et al. In situ biosynthesis of a metal nanoparticle encapsulated in alginate gel for imageable drug-delivery system. ACS Appl. Mater. Interfaces 2021, 13, 36697–36708. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Lee, D.H.; Nguyen, P.T.; Le, P.G.; Kim, M.I. Foldable paper microfluidic device based on single iron site-containing hydrogel nanozyme for efficient glucose biosensing. Chem. Eng. J. 2023, 454, 140541. [Google Scholar] [CrossRef]
- Qi, M.; Pan, H.; Shen, H.; Xia, X.; Wu, C.; Han, X.; He, X.; Tong, W.; Wang, X.; Wang, Q. Nanogel multienzyme mimics synthesized by biocatalytic ATRP and metal coordination for bioresponsive fluorescence imaging. Angew. Chem.-Int. Ed. 2020, 132, 11846–11851. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yan, X.; Du, Y.; Pu, F.; Ren, J.; Qu, X. An ATPase-Mimicking MXene nanozyme pharmacologically breaks the ironclad defense system for ferroptosis cancer therapy. Biomaterials 2024, 307, 122523. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Von Klitzing, R.; Erim, F.B. Antimicrobial cerium ion-chitosan crosslinked alginate biopolymer films: A novel and potential wound dressing. Int. J. Biol. Macromol. 2017, 105, 1161–1165. [Google Scholar] [CrossRef]
- Ma, T.; Zhai, X.; Huang, Y.; Zhang, M.; Li, P.; Du, Y.J. Cerium ions crosslinked sodium alginate-carboxymethyl chitosan spheres with antibacterial activity for wound healing. J. Rare Earths 2022, 40, 1407–1416. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.; Wang, J. Cerium alginate cross-linking with biochar beads for fast fluoride removal over a wide pH range. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 636, 128161. [Google Scholar] [CrossRef]
- Li, X.-J.; Yan, C.-J.; Luo, W.-J.; Gao, Q.; Zhou, Q.; Liu, C.; Zhou, S. Exceptional cerium (III) adsorption performance of poly (acrylic acid) brushes-decorated attapulgite with abundant and highly accessible binding sites. Chem. Eng. J. 2016, 284, 333–342. [Google Scholar] [CrossRef]
- Chen, Z.; Song, S.; Zeng, H.; Ge, Z.; Liu, B.; Fan, Z. 3D printing MOF nanozyme hydrogel with dual enzymatic activities and visualized glucose monitoring for diabetic wound healing. Chem. Eng. J. 2023, 471, 144649. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M.; Khodaei, M. Cerium oxide nanoparticles: Synthesis methods and applications in wound healing. Mater. Today Bio 2023, 23, 100823. [Google Scholar] [CrossRef]
- Othman, A.; Gowda, A.; Andreescu, D.; Hassan, M.H.; Babu, S.; Seo, J.; Andreescu, S. Two decades of ceria nanoparticles research: Structure, properties and emerging applications. Mater. Horiz. 2024, 11, 3213–3266. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Doong, R.A. Nitrogen doped graphene quantum dot-decorated earth-abundant nanotubes for enhanced capacitive deionization. Environ. Sci.-Nano 2020, 7, 228–237. [Google Scholar] [CrossRef]
- Anandan, C.; Bera, P. XPS studies on the interaction of CeO2 with silicon in magnetron sputtered CeO2 thin films on Si and Si3N4 substrates. Appl. Surf. Sci. 2013, 283, 297–303. [Google Scholar] [CrossRef]
- Hosu, O.; Lettieri, M.; Papara, N.; Ravalli, A.; Sandulescu, R.; Cristea, C.; Marrazza, G. Colorimetric multienzymatic smart sensors for hydrogen peroxide, glucose and catechol screening analysis. Talanta 2019, 204, 525–532. [Google Scholar] [CrossRef]
- Li, P.; Zhang, S.; Xu, C.; Zhang, L.; Liu, Q.; Chu, S.; Li, S.; Mao, G.; Wang, H. Coating Fe3O4 quantum dots with sodium alginate showing enhanced catalysis for capillary array-based rapid analysis of H2O2 in milk. Food Chem. 2022, 380, 132188. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Huang, Z.; Jia, Q. Investigation of efficient synergistic and protective effects of chitosan on copper nanoclusters: Construction of highly active and stable nanozyme for colorimetric and fluorometric dual-signal biosensing. Sens. Actuator B-Chem. 2021, 332, 129522. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Yang, B.; Liu, Z.; Liu, Q.; Zhang, X. Colorimetric and ultrasensitive detection of H2O2 based on Au/Co3O4-CeOx nanocomposites with enhanced peroxidase-like performance. Sens. Actuator B-Chem. 2018, 271, 336–345. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, H.; Gao, L.-N.; Fu, M.; Luo, X.; Zhang, X.; Liu, Q.; Zeng, R.-C. Compounds. Fe-doped Ag2S with excellent peroxidase-like activity for colorimetric determination of H2O2. J. Alloys Compd. 2019, 785, 1189–1197. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, H.; Huang, Y.; Jiang, H.; Yang, N.; Shahzad, S.A.; Meng, L.; Yu, C. Silver nanoparticles decorated and tetraphenylethene probe doped silica nanoparticles: A colorimetric and fluorometric sensor for sensitive and selective detection and intracellular imaging of hydrogen peroxide. Biosens. Bioelectron. 2018, 121, 236–242. [Google Scholar] [CrossRef]
- Remani, K.; Binitha, N.N. Cobalt doped ceria catalysts for the oxidative abatement of gaseous pollutants and colorimetric detection of H2O2. Mater. Res. Bull. 2021, 139, 111253. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Qin, X.; Shen, Y.; Wei, X.; Liu, G. Metalloporphyrin and gold nanoparticles modified hollow zeolite imidazole Framework-8 with excellent peroxidase like activity for quick colorimetric determination of choline in infant formula milk powder. Food Chem. 2022, 384, 132552. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, D.; Zhang, W.; Fang, S.; Zhou, Q.; Zhang, H.; Liang, Z.; Li, Y.J. Choline oxidase-integrated copper metal–organic frameworks as cascade nanozymes for one-step colorimetric choline detection. J. Agric. Food Chem. 2022, 70, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Chen, G.-Y.; Chai, T.-Q.; Chen, L.-X.; Chen, H.; Yang, F.-Q. Construction of Mn-decorated zeolitic imidazolate framework-90 nanostructure as superior oxidase-like mimic for colorimetric detection of glucose and choline. Talanta 2024, 271, 125708. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Hallaj, R. Mimicking peroxidase-like activity of Co3O4-CeO2 nanosheets integrated paper-based analytical devices for detection of glucose with smartphone. Sens. Actuator B-Chem. 2019, 288, 44–52. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).