Abstract
The toxicity of commonly used drugs, such as acetaminophen (ACAP) and its degradation-derived metabolite of 4-aminophenol (4-AP), underscores the need to achieve an effective approach in their simultaneous electrochemical determination. Hence, the present study attempts to introduce an ultra-sensitive disposable electrochemical 4-AP and ACAP sensor based on surface modification of a screen-printed graphite electrode (SPGE) with a combination of MoS2 nanosheets and a nickel-based metal organic framework (MoS2/Ni-MOF/SPGE sensor). A simple hydrothermal protocol was implemented to fabricate MoS2/Ni-MOF hybrid nanosheets, which was subsequently tested for properties using valid techniques including X-ray diffraction (XRD), field emission-scanning electron microscopy (FE-SEM), energy dispersive X-ray spectroscopy (EDX), Fourier transformed infrared spectroscopy (FTIR), and N2 adsorption-desorption isotherm. The 4-AP detection behavior on MoS2/Ni-MOF/SPGE sensor was followed by cyclic voltammetry (CV), chronoamperometry and differential pulse voltammetry (DPV). Our experimental findings on the generated sensor confirmed a broad linear dynamic range (LDR) for 4-AP from 0.1 to 600 μM with a high sensitivity of 0.0666 μA/μM and a low limit of detection (LOD) of 0.04 μM. In addition, an analysis of real specimens such as tap water sample as well as a commercial sample (acetaminophen tablets) illuminated the successful applicability of as-developed sensor in determining ACAP and 4-AP, with an impressive recovery rate.
1. Introduction
HOC6H4NH2, p-Aminophenol or 4-aminophenol is known as a raw material with wide and varied applications in the manufacture of pharmaceutical products, thermal dyes, black and white photographic developer, antioxidants, polymer stabilizers, petroleum additives, fungicides, herbicides, and insecticides [1,2]. Such extensive applications have inevitably led to large concentrations of 4-AP being introduced into the environment and in particular water sources, which can leave toxic effects due to the presence of structural phenol and aniline. The penetration of 4-AP into the human body can be associated with serious health consequences such as dermatitis, eczema, nephrotoxicity, and teratogenic complications [3,4]. Therefore, some European countries and the United States have recommended that the maximum allowable dose of 4-AP in pharmacy should be up to 50 ppm [5]. Thus, both biochemically and environmentally hazardous 4-AP can contaminate and threaten both environmental resources and the health of life by easily penetrating the skin and membranes of plants.
N-acetyl-p-aminophenol, Paracetamol, Acetaminophen or ACAP, is known as one of the most common antipyretic and analgesic drugs that impact mainly the management of migraine pain, headache, arthritis, cancer pain, back pain, and postoperative pain [6,7,8]. ACAP can also be prescribed to aspirin-sensitive patients, such as those with hemophilia, varicella, and other bleeding conditions [9]. Despite these advantages, ACAP can be partially degraded to 4-AP in pharmaceutical formulations during synthesis or storage. In fact, toxic 4-AP can accumulate in the human body following over-administration of ACAP, leading to some severe diseases such as deformity, renal toxicity, liver toxicity, pancreatitis and skin rash [10,11]. In addition, the environmental accumulation of ACAP and 4-AP excreted is increasingly observed in a variety of water sources. The toxic nature of ACAP and 4-AP underscores the need to achieve an effective approach in their simultaneous determination.
Some of the techniques available in this field are spectrophotometry [12], chemiluminescence [13], high performance liquid chromatography [14], and capillary electrophoresis [15]. Despite the unique advantages of all these techniques, they suffer from some disadvantages such as being time-consuming, having high cost, and the need for precision tools, tedious operations, and boring sample preparation [16]. In the meantime, special attention has been paid to electrochemical measurement approaches due to advantages such as simplicity, simple preparation of specimens and sensors, high accuracy, and fast response [17,18,19,20,21,22,23,24]. Nevertheless, bare electrodes in electrochemical determinations have shown disadvantages such as low sensitivity, poor reproducibility and low electrochemical response [25,26,27,28,29,30,31]. In this context, the potentials of redox peak can be obscured by cross-interference when applying a bare screen printed graphite electrode for sensing ACAP and 4-AP [32]. Therefore, there is a need for a modifier for surface modification of the electrodes to achieve some advantages such as low LOD, broad working range, and high accuracy during analytical applications [33,34,35,36,37,38,39,40,41].
Ultrathin two-dimension (2D) nanosheets (NSs) have been introduced as promising electrode materials because of admirable attributes like huge specific surface area, impressive electronic behaviors, and high mechanical strength [42,43], particularly in the field of nanotechnology [44].
Molybdenum disulfide (MoS2) is a layered quasi-2D chalcogenide material, similar to graphite structure. Three atomic layers are found in MoS2, including a layer of Mo positioned between two layers of S with the aid of weak van der Waals forces [45,46]. It has a variety of applications in catalysts, lithium batteries, sensors and supercapacitors because of commendable catalytic ability, impressive electron mobility, huge surface area and high electronic density [47,48].
Metal-organic frameworks (MOFs) such as promising crystalline porous materials are formed by binding metal ions or clusters using organic ligands based on coordination chemistry [49,50,51]. Some of the fields of application of MOFs are drug, catalysis, gas separation, magnetism and fluorescence owing to their huge specific surface area, pore structure, functional nature, drug delivery and adjustable architecture [52]. Among these, 2D MOFs have shown special attributes when compared with bulk MOF materials, such as high porosity and nano-sized thickness that predispose excellent electron transfer and rapid mass transport. Unlike the bulk MOFs’ active sites, 2D MOFs show many available active sites on the layers’ surface, thereby facilitating the contact of active sites with substrates and consequently improving the catalytic behavior of MOFs [53,54,55,56]. It should be noted that many nano-sized metal sulfides are adsorbed into MOF NSs as in-situ to generate nano-hybrids. The activity can be improved due to the synergism of all components [57]. We used the synergistic properties of MoS2 and Ni-MOF NSs to achieve a facile protocol to fabricating the MoS2/Ni-MOF nanohybrid sheets, which was applied to modify the SPGE surface for 4-AP electrooxidation. The findings showed commendable electrocatalytic performance of MoS2/Ni-MOF/SPGE for the oxidation/reduction process of 4-AP. In addition, the proposed sensor was employed to voltammetrically detect 4-AP and ACAP in real specimens.
2. Experimental Section
2.1. Apparatus and Materials
(NH4)6Mo7O24•4H2O, thiourea, nickel (II) nitrate hexahydrate, terephthalic acid, KOH, N,N-Dimethylformamide (DMF), 4-aminophenol and acetaminophen were purchased from Sigma-Aldrich. All other reagents had analytical grade. Orthophosphoric acid was used to prepare phosphate buffer solution (0.1 M, PBS) as a supporting electrolyte.
An Autolab potentiostat/galvanostat type PGSTAT302N made by Eco Chemie in Netherlands, which has been also provided with the General-Purpose Electrochemical System (GPES 4.9) was applied in each of the electrochemical experiments. The screen-printed graphite electrode (SPGE) was made by Dropsens (DRP-110) in Asturias, Spain. This electrode consists of a graphite working electrode, a silver pseudo reference electrode; and a graphite counter electrode. The solutions pH was adjusted by a pH-meter (Metrohm 710).
FE-SEM images were obtained on a MIRA3TESCAN microscope alongside energy-dispersive X–ray analysis. MoS2/Ni-MOF nanosheets structure was characterized using X-ray diffraction patterns (X’Pert Pro X-ray diffractometer, Panalytical, Netherlands) applying Cu/Kα radiation (λ = 1.5418 nm). Then, the Bruker spectrometer (KBr pellets, Tensor-27) made by Germany was utilized to record a FTIR spectrum on the wavelengths 4000 cm−1 to 400 cm−1. The MoS2/Ni-MOF hybrid nanosheets were also examined for their surface area by a BELSORP MINI II Brunauer–Emmett–Teller device (BET) with the Barrett–Joyner–Halenda (BJH) analysis for pore size determination.
2.2. Fabrication of MoS2
A typical protocol was followed to prepare MoS2 NSs [58,59]. Thus, (NH4)6Mo7O24·4H2O (3 mmol) and thiourea (2.3 g) were poured in deionized water (30 mL) and then heated for 24 h at 200 °C inside a 50-mL Teflon autoclave. The product was thoroughly rinsed with deionized water/ethanol (with a volume ratio of 1:1), and finally dried at 50 °C for six hours under vacuum condition.
2.3. Fabrication of MoS2/Ni-MOF Hybrid Nanosheets
In situ growth of Ni-MOF on MoS2 surface led to the formation of the hybrid NSs of MoS2/Ni-MOF. Thus, MoS2 (0.32 g) was poured in DMF (15 mL) under 30-min ultra-sonication, followed by adding nickel (II) nitrate hexahydrate (0.87 g, 3 mmol) dispersed in deionized water (10 mL) under another 30-min ultra-sonication. The obtained solution was then slowly appended with DMF solution (10 mL) including terephthalic acid (0.17 g, 1 mmol) and 0.4 M KOH (5 mL). Following another sonication for two hours, the obtained solution was subjected to solvothermal reaction inside the 50-mL Teflon stainless steel autoclave at 120 °C for 24 h. Next, the product was cooled down to room temperature, followed by centrifugation to obtain the precipitate that was subsequently rinsed with deionized water/DMF (with a volume ratio of 1:1) thoroughly, and finally dried at 65 °C for 24 h under vacuum condition.
Moreover, Ni MOF NSs were prepared as control, similar to the method used to produce MoS2/Ni MOF hybrid NSs, except for the addition of MoS2 prior to solvothermal treatment.
2.4. SPGE Modification with MoS2/Ni MOF Hybrid Nanosheets
In this step, SPGE was coated by the MoS2/Ni MOF hybrid NSs. Thus, 1 mg of MoS2/Ni MOF hybrid NSs was dispersed in 1 mL of aqueous solution through a 45-min ultrasonication to prepare a stock solution of the hybrid nanosheets of MoS2/Ni MOF. Then, the graphite working electrodes were used to cast 5 µL of the suspension solution of the MoS2/Ni MOF nanohybrid. Finally, we put the solvent at room temperature to evaporate to achieve the MoS2/Ni-MOF/SPGE.
2.5. Preparation of Real Specimens
In order to prepare the real sample of an ACAP tablet (labeled 325 mg), the first five tablets of the ACAP were powdered with a mortar and pestle. Then, 325 mg of this powder was dissolved in 25 mL deionized water under ultra-sonication. Then, in order to remove impurities and fillers in the tablet, the resulting sample was filtered by using filter paper. Then, a specific volume of this solution was transferred to a volumetric flask (25 mL) and diluted with 0.1 M PBS (pH = 7.0). Finally, the standard addition method was used to determine the ACAP and 4-AP content in tablet samples.
Tap water specimens filtrated with a membrane filter and added into 0.1 M PBS (pH = 7). At last, the ACAP and 4-AP contents were measured in the tap water samples using the developed protocol according to standard addition method.
3. Results and Discussion
3.1. Characterizations
The FE-SEM images captured from Ni-MOF (Figure 1a) shows approximately transparent 20-nanometer NSs and also well-defined two-dimensional layer of fabricated Ni-MOF. A flower-like MoS2 nanostructure constructed by the assembly of 2D NSs is shown in Figure 1b. The NSs cut with a smooth surface show an identical thickness and a distinct gap of the layers. Figure 1b illustrates the FE-SEM image captured from MoS2, highlighting a 13-nanometer nanosheet morphology. Moreover, the obtained MoS2/Ni-MOF hybrid NSs exhibit a hierarchical structure while retaining the 2D NS property (Figure 1c,d), so that the MoS2 NS surface is covered densely by Ni-MOF, leading to a relatively uneven surface.
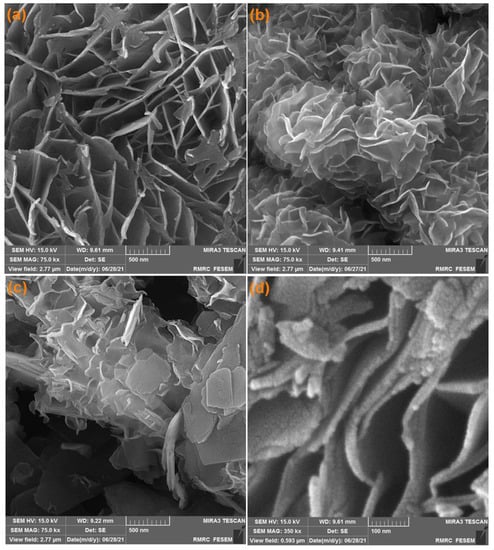
Figure 1.
FE-SEM images of Ni-MOF nanosheets (a), MoS2 flower-like nanosheets (b), MoS2/Ni-MOF hybrid NSs (c,d).
Energy dispersive X-ray findings (Figure 2) illustrate only the presence of Mo, S, C, O, and Ni in the structure of MoS2/NiMOF hybrid NSs.
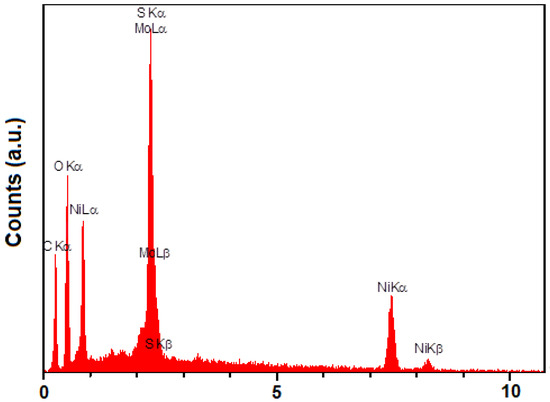
Figure 2.
EDX spectrum of MoS2/Ni-MOF hybrid NSs.
Figure 3 shows the XRD patterns captured for the pure MoS2 and MoS2/Ni-MOF hybrid NSs. Pure MoS2 curves exhibited high purity for the samples produced. The peaks appearing at 13.66°, 32.22°, 35.3° and 57.8° were indexed to planes of (002), (100), (103) and (110), sequentially [60]. Overall, the sharp diffraction peaks of MoS2 verifies a great crystallinity. MoS2/Ni-MOF hybrid NSs not only had the characteristic peaks related to MoS2, but also displayed clear related to Ni-MOF, corresponding to the planes of (100), (010), (10-1), (2-10), and (020) [61,62].
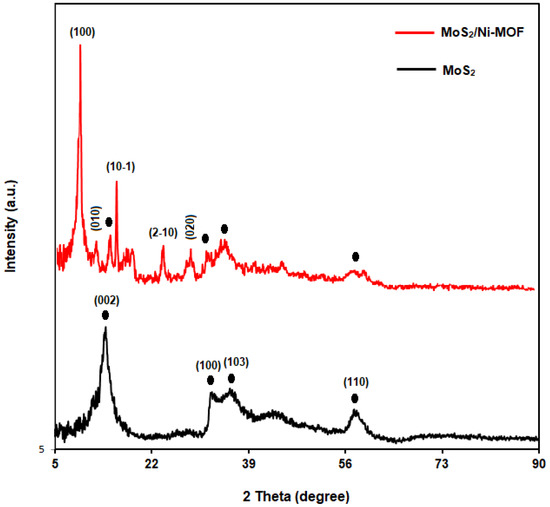
Figure 3.
XRD patterns of MoS2 and MoS2/Ni-MOF hybrid NSs.
The composition of as-produced hybrid NSs was explored using the Fourier transform infrared spectroscopy (Figure 4). The FT-IR spectrum revealed the peaks at 614.55 and 884 cm−1 for MoS2 (Figure 4; curve a) respectively corresponding to Mo-S and S-S vibrations. The peaks formed at 1100, 1642 and 3448 cm−1 corresponded to hydroxyl stretching vibration resulting from water molecules absorbed [63,64]. The FT-IR spectrum obtained for Ni-MOF (Figure 4; curve c) showed strong peaks at 3490 and 3320 cm−1 corresponding to hydroxyl (−OH) stretching vibration, and at 2960, 811 and 743 cm−1 corresponding to aromatic units’ C–H bonds, as well as at 1382 and 1581 cm−1 respectively corresponding to terephthalic anions’ Vs(COO) and Vas(COO). The peak at 545 cm−1 corresponding to ν(Ni–O) verifies a metal–oxo bond between the terephthalic acid’s carboxylic group and Ni atoms [62]. When comparing to Ni-MOF and MoS2, a new peak appeared strongly at 1650.6 cm−1 related to MoS2/Ni-MOF hybrid NSs (Figure 4; curve b) because of an interplay of MoS2 with Ni-MOF, boosting C=C stretching vibration. Consequently, the MoS2/Ni-MOF hybrid NSs possess all absorption bands of MoS2 and Ni-MOF [62].
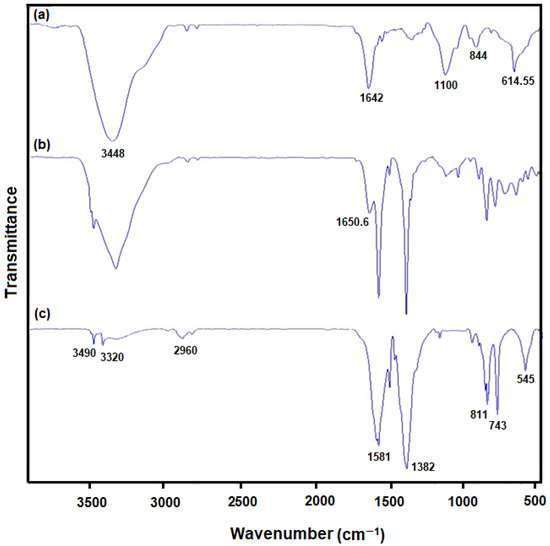
Figure 4.
FT-IR patterns of (a) MoS2 flower-like nanosheets, (b) MoS2/Ni-MOF hybrid NSs, and (c) Ni-MOF nanosheets.
Figure 5 shows the calculation of MoS2@Ni-MOF pore size and surface areas based on nitrogen adsorption−desorption isotherms adopted from Barrett-Joyner-Halenda and Brunauer-Emmett-Teller methods [62,65]. In accordance with the graphical isotherm of typical H3 hysteresis loop in Figure 5A, MoS2/Ni-MOF hybrid NSs follows the type-IV isotherms. The accumulation of pores is evident based on the hysteresis loop of MoS2/Ni-MOF at relative high pressure (p/p0). The surface area of 27.19 m2/g was computed for the MoS2/Ni-MOF. According to the distribution of pore size in Figure 5B, the MoS2/Ni-MOF had the pore diameter of 1.85 nm.
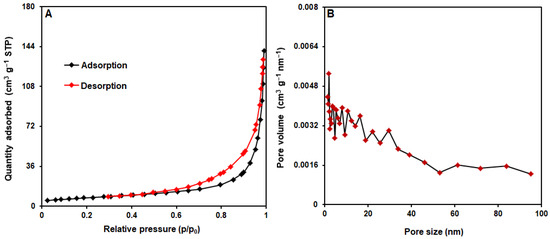
Figure 5.
(A) N2 adsorption−desorption isotherms and (B) pore size distribution of MoS2/Ni-MOF hybrid NSs.
3.2. Electrochemical Performance of 4-AP on the Surface of MoS2/Ni-MOF/SPGE
Research indicates that the pH of the electrolytes is one of the fundamental factors influencing the 4-AP response on the MoS2/Ni-MOF/SPGE. Hence, researchers cautiously used DPV in the pH ranges between 2.0 and 9.0 through 0.1 M PBS to determine the impact of the pH value on the electro-chemical behaviors of 4-AP. Analyses revealed that the peak current 4-AP of oxidation enhanced when pH value elevated to 7.0 but it was reduced with an increase in pH. For achieving higher sensitivity, we chose pH 7.0 as an optimal pH to electrochemically detect the 4-AP on the MoS2/Ni-MOF/SPGE.
The CVs of 4-AP at the scanning rate of 50 mV s−1 in the 0.1 M PBS (pH = 7.0) on the bare SPGE (curve a) as well as the MoS2/Ni-MOF/SPGE (curve b) are depicted in Figure 6. As seen in Figure 6 (curve a), the cathodic and anodic peaks of 4-AP on the bare SPGE were observed at −70 and 145 mV, respectively, while the separation between peak potentials (ΔEp) was observed at 213 mV. Figure 6 (curve b) demonstrates the further enhancement of the 4-AP oxidation peak current on the MoS2/Ni-MOF/SPGE compared to that of the bare SPGE, negative shift of the anodic peak potential to 105 mV as well as positive shift of the cathodic peak potential to −35 mV. Furthermore, ΔEp of 4-AP on the MoS2/Ni-MOF/SPGE equaled 140 mV. Consequently, we found a considerable increase of the 4-AP redox peak currents on the MoS2/Ni-MOF/SPGE caused by acceptable conductivity and electrocatalytic feature of MoS2/Ni-MOF.
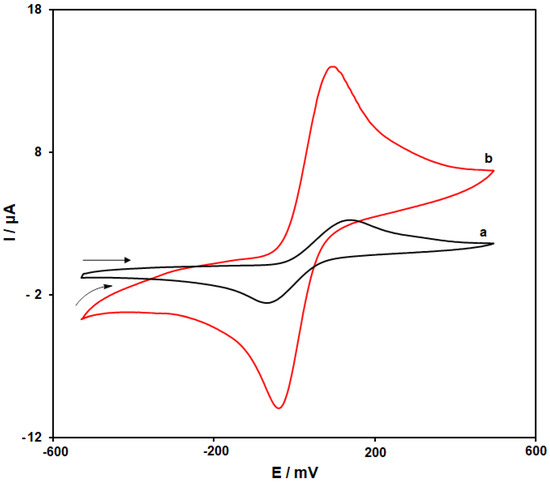
Figure 6.
CVs of the bare SPGE (a) and MoS2/Ni-MOF/SPGE (b) in the 0.1 M PBS (pH = 7.0) with 200.0 µM 4-AP at the scanning rate of 50 mVs−1.
3.3. The Effect of the Scanning Rate
We used CV in this step to determine the impact of the scanning rate on the redox reaction of 4-AP on the surface of MoS2/Ni-MOF/SPGE. Figure 7 represents the CV curves of 400.0 µM 4-AP on the MoS2/Ni-MOF/SPGE with diverse scanning rates from 5–900 mVs−1. Considering the figure, the cathodic and anodic peak currents (Ipc, Ipa) of 4-AP were elevated by enhancing the scanning rates. Therefore, values of Ipc and Ipa exhibit an acceptable linear correlation to the square root of the scanning rate (υ1/2) (see Inset in Figure 7). Thus, it could be concluded that the electrode reaction of 4-AP on the MoS2/Ni-MOF/SPGE would be a process controlled by diffusion.
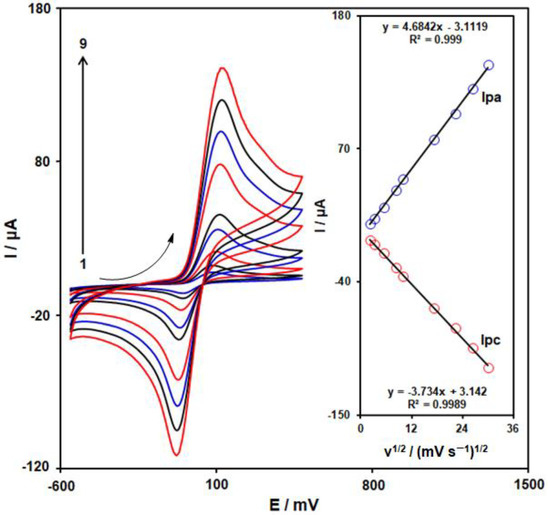
Figure 7.
CVs observed on the MoS2/Ni-MOF/SPGE with 400 µM 4-AP in the 0.1 M PBS (pH = 7.0) at different scanning rates of 1–9: 5, 10, 30, 70, 100, 300, 500, 700, and 900 mV/s. Inset: Plot of the variation of Ipc and Ipa vs. υ1/2 for 4-AP electrooxidation.
3.4. Chronoamperometric Measurements
Chronoamperometry was employed to study the 4-AP electro-oxidation using a MoS2/Ni-MOF/SPGE (see Figure 8). Therefore, the potential of the working electrode was set at 0.14 V as the first-step potential to measure the chronoamperometry of several concentrations of 4-AP on the MoS2/Ni-MOF/SPGE sensor. In this way, the diffusion coefficient, D, of 4-AP in 0.1 M PBS was identified by chronoamperometric tests. Then, we applied the experimental plots of Ip vs. t−1/2 with the best fits for several concentrations of 4-AP (see Figure 8A). Moreover, the slopes shown for the final straight lines were drawn vs. 4-AP concentrations (refer to Figure 8B) and thus D = 3.2 × 10−5 cm2/s by the Cottrell equation and the obtained slopes.
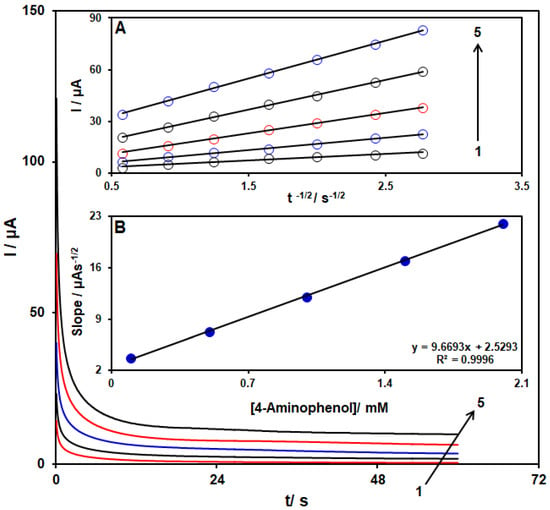
Figure 8.
4-AP chronoamperograms with several concentrations (from 1 to 5: 0.1, 0.5, 1.0, 1.5, and 2.0 mM) on the MoS2/Ni-MOF/SPGE in the 0.1 M PBS (pH = 7). Inset (A). I vs. t−1/2 plot for 4-AP electro-oxidation achieved by chronoamperoms 1–5. Inset (B). The slope plot from the straight lines vs. level of 4-AP.
3.5. Calibration Plot and Detection Limit
According to the research design, we employed DPV at optimum conditions to measure several concentrations of 4-AP in the 0.1 M PBS (pH = 7.0) for evaluating the analytical functions of the MoS2/Ni-MOF/SPGE sensor. With regard to Figure 9 the increased peak current was observed as the concentration of 4-AP elevated from curve 1 to 14, reflecting a satisfactory linear correlation to the 4-AP concentration (as depicted in the inset of Figure 9) in ranges 0.1–600.0 μM, which followed the correlation equation of Ipa = 0.0666 C4-AP + 0.9904 (R2 = 0.9995). It should be noted that LOD equaled 0.04 µM. Two-dimentional Ni-MOFs have shown special attributes such as high porosity and nano-sized thickness that predispose excellent electron transfer and rapid mass transport. Also, the synergistic effect between Ni-MOF and Mos2 increases the conductivity and electro-catalytic activity of the proposed MoS2/Ni-MOF/SPG sensor. The electro-analytical performance for 4-AP at MoS2/Ni-MOF/SPGE was compared with the other chemically modified electrodes (Table 1). These findings revealed the acceptable analytical function of MoS2/Ni-MOF/SPGE sensor for 4-AP detection.
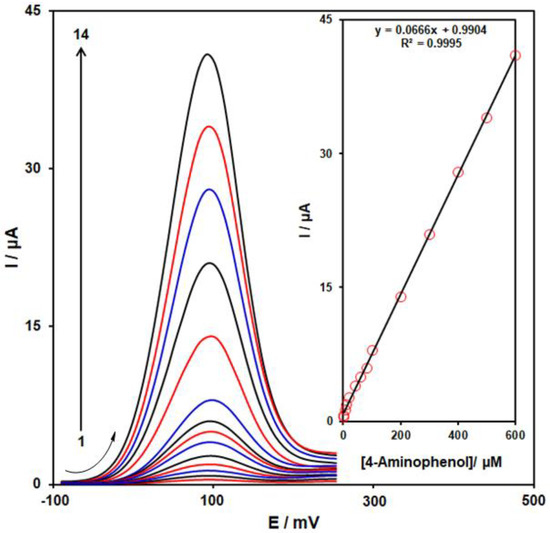
Figure 9.
DPVs of 4-AP with diverse concentrations (from 1 to 14: 0.1, 1.0, 5.0, 10.0, 20.0, 40.0, 60.0, 80.0, 100.0, 200.0, 300.0, 400.0, 500.0, & 600.0 µM) on the MoS2/Ni-MOF/SPGE in 0.1 M PBS (pH = 7). Inset: linear relationship of the peak current with 4-AP concentration (from 0.1 μM to 600.0 μM).

Table 1.
Comparison of various modified electrodes for the detection of 4- AP with MoS2/Ni-MOF/SPGE.
Table 1.
Comparison of various modified electrodes for the detection of 4- AP with MoS2/Ni-MOF/SPGE.
| Electrochemical Sensor | Method | Linear Range | LOD | Real Samples | Ref. |
|---|---|---|---|---|---|
| Chitosan-Au nanoparticles-Pd-reduced graphene oxide nanohybrid/glassy carbon electrode | DPV | 1.0–300.0 μM | 0.12 μM | Water | [66] |
| Hemin-molecularly imprinted polymer/glassy carbon electrode | Amperometric | 10.0–90.0 μM | 3.0 μM | Tap and river water | [67] |
| Graphene/hydroxyapatite nanocomposite/glassy carbon electrode | Square wave voltammetry | 0.1–425.0 μM | 0.29 μM | Tap water | [68] |
| Graphene–chitosan composite/glassy carbon electrode | DPV | 0.2–550.0 μM | 0.057 μM | River water, Lake water, Waste water, and Tap water | [69] |
| Graphene–polyaniline nanocomposite/glassy carbon electrode | DPV | 0.2–100.0 μM | 0.065 μM | - | [70] |
| MoS2/Ni-MOF/SPGE | DPV | 0.1–600.0 μM | 0.04 μM | Acetaminophen tablet and tap water | This Work |
3.6. Determination of 4-AP in Presence ACAP
Upon the creation of the optimized conditions, as a result of its more acceptable resolution and greater current sensitivity, DPV was employed for the quantitative simultaneous detection of 4-AP and ACAP. Figure 10 presents DPVs of various concentrations of 4-AP and ACAP on the MoS2/Ni-MOF/SPGE surface. Considering Figure 10, we observe 2 complete oxidation peaks at 100 mV and 440 mV for 4-AP and ACAP. Moreover, 4-AP and ACAP concentrations exhibit an acceptable linear relationship to the respective oxidation peak currents in ranges between 1.0 µM and 400.0 µM (see Insets A and B). In addition, sensitivity to 4-AP in the case of the presence and absence of ACAP equaled 0.0674 µA/µM (see Figure 10 Inset A) and 0.0666 µA/µM (see inset of Figure 9), respectively. Hence, we inferred that it is possible to use MoS2/Ni-MOF/SPGE successfully for the simultaneous detection of 4-AP and ACAP with higher sensitivity and better selectivity.
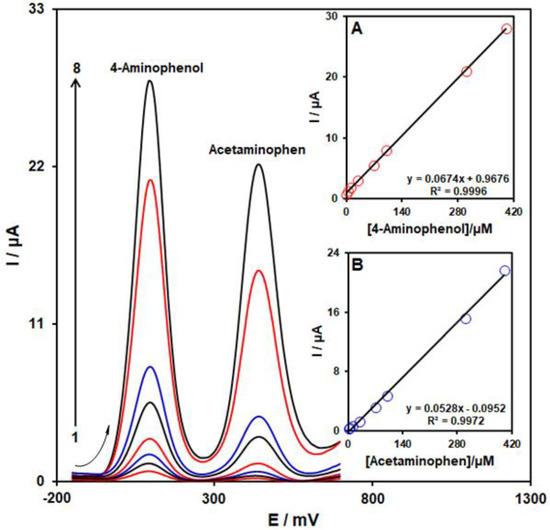
Figure 10.
DPVs of 4-AP and ACAP with several concentrations (from 1 to 8: 1.0 + 1.0, 5.0 + 5.0, 10.0 + 10.0, 30.0 + 30.0, 70.0 + 70.0, 100.0 + 100.0, 300.0 + 300.0, and 400.0 + 400.0 µM) on the MoS2/Ni-MOF/SPGE in 0.1 M PBS (pH = 7). Inset: (A) the peak current plot as the function of concentration of 4-AP and (B) the peak current plot as the function of ACAP concentration.
3.7. ACAP and 4-AP Detection in the Real Samples
Since our research aimed for assessing the new approach practically, we employed MoS2/Ni-MOF/SPGE for ACAP and 4-AP detection in the acetaminophen tablet and tap water samples with the standard addition method. Hence, each measurement was iterated give times in similar conditions. See Table 2 for more results. As seen in the table, recovery of ACAP and 4-AP equaled 97.0–103.3% and 96.2–104.3%, indicating the possible use of our electrochemical sensor to detect ACAP and 4-AP in the real samples.

Table 2.
Findings for 4-AP and ACAP detection in real samples using MoS2/Ni-MOF/SPGE. All concentrations are in µM (n = 5).
4. Conclusions
We produced new MoS2/Ni-MOF hybrid nanosheets as the modifier for screen-printed graphite electrode surface modification to fabricate an ultra-sensitive sensor for the electrochemical detection of 4-AP. Findings presented an admirable electro-catalytic behavior for as-developed hybrid nanosheets towards the oxidation of 4-AP owing to commendable mass transfer, abundant active sites, impressive conductivity and huge surface area. As-developed MoS2/Ni-MOF/SPGE had a broad LDR (0.1–600.0 μM) and a narrow LOD towards the oxidation of 4-AP. Moreover, 4-AP and ACAP were electrochemically detected concurrently on the modified electrode surface, with a peak potential separation of 340 mV. In addition, the analysis of real specimens such as tap water sample as well as a commercial sample (acetaminophen tablets) illuminated the successful applicability of the as-developed sensor in determining ACAP and 4-AP, with an impressive recovery rate.
Author Contributions
Formal analysis, Z.D.; Writing—original draft, Z.D., I.S. and S.M.; Supervision, I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.; Xing, Z.; Pan, M.; Wang, H.B.; Liu, Y.M. Highly sensitive and selective electrochemical determination of 4-aminophenol based on flower-like Ag-Au nanocomposites modified glassy carbon electrode. J. Electrochem. Soc. 2020, 167, 126504. [Google Scholar] [CrossRef]
- Sun, L.; Yang, M.; Guo, H.; Zhang, T.; Wu, N.; Wang, M.; Yang, W. COOH-MWCNT connected COF and chemical activated CTF as a novel electrochemical sensing platform for simultaneous detection of acetaminophen and p-aminophenol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129092. [Google Scholar] [CrossRef]
- Rahman, M.M. Selective and sensitive 4-Aminophenol chemical sensor development based on low-dimensional Ge-doped ZnO nanocomposites by electrochemical method. Microchem. J. 2020, 157, 104945. [Google Scholar] [CrossRef]
- Chen, S.; Huang, R.; Zou, J.; Liao, D.; Yu, J.; Jiang, X. A sensitive sensor based on MOFs derived nanoporous carbons for electrochemical detection of 4-aminophenol. Ecotoxicol. Environ. Saf. 2020, 191, 110194. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Reddy, T.M.; Palakollu, V.N.; Karpoormath, R.; Rao, Y.S.; Venkataprasad, G.; Gopal, P. Multi walled carbon nanotubes supported CuO-Au hybrid nanocomposite for the effective application towards the electrochemical determination of acetaminophen and 4-aminophenol. Synt. Metals 2019, 252, 29–39. [Google Scholar] [CrossRef]
- Guan, Q.; Guo, H.; Wu, N.; Cao, Y.; Wang, M.; Zhang, L.; Yang, W. Highly sensitive determination of acetaminophen and 4-aminophenol based on COF/3D NCNF-T/Au NPs composite electrochemical sensing platform. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127624. [Google Scholar] [CrossRef]
- Ariavand, S.; Ebrahimi, M.; Fooladi, E. The Simultaneous Spectrophotometric Determination of Acetaminophen, Celecoxib, Diazepam, and Famotidine in Environmental Samples by Partial Least Squares. Chem. Methodol. 2021, 5, 82–89. [Google Scholar]
- Dou, N.; Zhang, S.; Qu, J. Simultaneous detection of acetaminophen and 4-aminophenol with an electrochemical sensor based on silver–palladium bimetal nanoparticles and reduced graphene oxide. RSC Adv. 2019, 9, 31440–31446. [Google Scholar] [CrossRef]
- Calam, T.T. A modified pencil graphite electrode with 2-thiobarbituric acid for the efficient and cheap voltammetric sensing of 4-aminophenol in water samples and child syrup sample. J. Food Compos. Anal. 2021, 98, 103809. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Yu, S.; Yang, Q.; Tong, Y.; Ye, B.C. Electrochemical sensor based on N-doped carbon dots decorated with manganese oxide nanospheres for simultaneous detection of p-aminophenol and paracetamol. Analyst 2021, 146, 5135–5142. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, M.; Zhang, L. 3D multiporous Co, N co-doped MoO2/MoC nanorods hybrids as improved electrode materials for highly sensitive simultaneous determination of acetaminophen and 4-aminophenol. Electrochim. Acta 2019, 302, 56–64. [Google Scholar] [CrossRef]
- Bloomfield, M.S. A sensitive and rapid assay for 4-aminophenol in paracetamol drug and tablet formulation, by flow injection analysis with spectrophotometric detection. Talanta 2002, 58, 1301–1310. [Google Scholar] [CrossRef]
- Easwaramoorthy, D.; Yu, Y.C.; Huang, H.J. Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Anal. Chim. Acta 2001, 439, 95–100. [Google Scholar] [CrossRef]
- Merrikhi Khosroshahi, A.; Aflaki, F.; Saemiyan, N.; Abdollahpour, A.; Asgharian, R. Simultaneous determination of paracetamol, 4-Aminophenol, 4-Chloroacetanilid, Benzyl alcohol, Benzaldehyde and EDTA by HPLC methodin paracetamol injection ampoule. J. Pharma. Health Sci. 2016, 4, 61–69. [Google Scholar]
- Chu, Q.; Jiang, L.; Tian, X.; Ye, J. Rapid determination of acetaminophen and p-aminophenol in pharmaceutical formulations using miniaturized capillary electrophoresis with amperometric detection. Anal. Chim. Acta 2008, 606, 246–251. [Google Scholar] [CrossRef]
- Palanna, M.; Mohammed, I.; Aralekallu, S.; Nemakal, M.; Sannegowda, L.K. Simultaneous detection of paracetamol and 4-aminophenol at nanomolar levels using biocompatible cysteine-substituted phthalocyanine. New J. Chem. 2020, 44, 1294–1306. [Google Scholar] [CrossRef]
- Peyman, H. Design and Fabrication of Modified DNA-Gp Nano-Biocomposite Electrode for Industrial Dye Measurement and Optical Confirmation. Prog. Chem. Biochem. Res. 2022, 5, 391–405. [Google Scholar]
- Beitollahi, H.; Tajik, S.; Aflatoonian, M.R.; Makarem, A. Glutathione detection at carbon paste electrode modified with ethyl 2-(4-ferrocenyl-[1,2,3] triazol-1-yl) acetate, ZnFe2O4nano-particles and ionic liquid. J. Electrochem. Sci. Eng. 2022, 12, 209–217. [Google Scholar] [CrossRef]
- Almandil, N.B.; Ibrahim, M.; Ibrahim, H.; Kawde, A.N.; Shehatta, I.; Akhtar, S. A hybrid nanocomposite of CeO 2–ZnO–chitosan as an enhanced sensing platform for highly sensitive voltammetric determination of paracetamol and its degradation product p-aminophenol. RSC adv. 2019, 9, 15986–15996. [Google Scholar] [CrossRef]
- Beitollahi, H.; Raoof, J.B.; Hosseinzadeh, R. Electroanalysis and simultaneous determination of 6-thioguanine in the presence of uric acid and folic acid using a modified carbon nanotube paste electrode. Anal. Sci. 2011, 27, 991. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S.; Rajendran, S.; Karimi-Maleh, H. Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 2020, 566, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Maleki, N.; Moradlou, O. A selective and sensitive method for simultaneous determination of traces of paracetamol and p-aminophenol in pharmaceuticals using carbon ionic liquid electrode. Electroanalysis 2008, 20, 2158–2162. [Google Scholar] [CrossRef]
- Taleat, Z.; Ardakani, M.M.; Naeimi, H.; Beitollahi, H.; Nejati, M.; Zare, H.R. Electrochemical behavior of ascorbic acid at a 2, 2’-[3, 6-dioxa-1, 8-octanediylbis (nitriloethylidyne)]-bis-hydroquinone carbon paste electrode. Anal. Sci. 2008, 24, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Harismah, K.; Mirzaei, M.; Da’i, M.; Roshandel, Z.; Salarrezaei, E. In silico investigation of nanocarbon biosensors for diagnosis of COVID-19. Eurasian Chem. Commun. 2021, 3, 95–102. [Google Scholar]
- Scandurra, G.; Antonella, A.; Ciofi, C.; Saitta, G.; Lanza, M. Electrochemical detection of p-aminophenol by flexible devices based on multi-wall carbon nanotubes dispersed in electrochemically modified nafion. Sensors 2014, 14, 8926–8939. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. Simultaneous determination of droxidopa and carbidopa using a carbon nanotubes paste electrode. Sens. Actuators B Chem. 2013, 188, 923–930. [Google Scholar] [CrossRef]
- Peyman, H.; Roshanfekr, H.; Babakhanian, A.; Jafari, H. PVC Membrane Electrode Modified by Lawson as Synthetic Derivative Ionophore for Determination of Cadmium in Alloy and Wastewater. Chem. Methodol. 2021, 5, 446–453. [Google Scholar]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–436. [Google Scholar] [CrossRef]
- Mohabis, R.M.; Fazeli, F.; Amini, I.; Azizkhani, V. An overview of recent advances in the detection of ascorbic acid by electrochemical techniques. J. Electrochem. Sci. Eng. 2022, 12, 1081–1098. [Google Scholar]
- Beitollahi, H.; Sheikhshoaie, I. Novel nanostructure-based electrochemical sensor for simultaneous determination of dopamine and acetaminophen. Mater. Sci. Eng. C 2012, 32, 375–380. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, Y.; Liu, S. A novel enhanced electrochemical sensor based on the peroxidase-like activity of Fe3O4@ Au/MOF for the detection of p-aminophenol. J. Appl. Electrochem. 2022, 52, 989–1002. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Zhao, J.; Zhang, R.; Zhao, N.; Ren, H.; Li, Y. Simultaneous determination of paracetamol and p-aminophenol using glassy carbon electrode modified with nitrogen-and sulfur-co-doped carbon dots. Microchim. Acta 2019, 186, 733. [Google Scholar] [CrossRef] [PubMed]
- Roshanfekr, H. A Simple Specific Dopamine Aptasensor Based on Partially Reduced Graphene Oxide–AuNPs composite. Prog. Chem. Biochem. Res. 2023, 6, 79–88. [Google Scholar]
- Shi, P.; Xue, R.; Wei, Y.; Lei, X.; Ai, J.; Wang, T.; Yang, W. Gold nanoparticles/tetraaminophenyl porphyrin functionalized multiwalled carbon nanotubes nanocomposites modified glassy carbon electrode for the simultaneous determination of p-acetaminophen and p-aminophenol. Arab. J. Chem. 2020, 13, 1040–1051. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Aflatoonian, M.R.; Makarem, A. A sensitive Cu (salophen) modified screen-printed electrode for simultaneous determination of dopamine and uric acid. J. Electrochem. Sci. Eng. 2022, 12, 199–208. [Google Scholar] [CrossRef]
- Miraki, M.; Karimi-Maleh, H.; Taher, M.A.; Cheraghi, S.; Karimi, F.; Agarwal, S.; Gupta, V.K. Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa. J. Mol. Liq. 2019, 278, 672–676. [Google Scholar] [CrossRef]
- Mustafa, Y.F.; Chehardoli, G.; Habibzadeh, S.; Arzehgar, Z. Electrochemical detection of sulfite in food samples. J. Electrochem. Sci. Eng. 2022, 12, 1061–1079. [Google Scholar] [CrossRef]
- Calam, T.T.; Uzun, D. Rapid and Selective Determination of Vanillin in the Presence of Caffeine, its Electrochemical Behavior on an Au Electrode Electropolymerized with 3-amino-1, 2, 4-triazole-5-thiol. Electroanalysis 2019, 31, 2347–2358. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Hosseini Fakhrabad, A.; Sanavi Khoshnood, R.; Abedi, M.R.; Ebrahimi, M. Fabrication a composite carbon paste electrodes (CPEs) modified with Multi-Wall Carbon Nano-Tubes (MWCNTs/N, N-Bis (salicyliden)-1, 3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 2021, 3, 627–634. [Google Scholar]
- Cerda, V.; Rennan, G.O.A.; Ferreira, S.L. Revising Flow-through Cells for Amperometric and Voltammetric Detections Using Stationary Mercury and Bismuth Screen Printed Electrodes. Prog. Chem. Biochem. Res. 2022, 5, 351–366. [Google Scholar]
- Zaid Almarbd, Z.; Mutter Abbass, N. Synthesis and characterization of TiO2, Ag2O, and graphene oxide nanoparticles with polystyrene as a nonocomposites and some of their applications. Eurasian Chem. Commun. 2022, 4, 1033–1043. [Google Scholar]
- Zheng, S.; Li, B.; Tang, Y.; Li, Q.; Xue, H.; Pang, H. Ultrathin nanosheet-assembled [Ni3(OH)2(PTA)2(H2O)4]·2H2O hierarchical flowers for high-performance electrocatalysis of glucose oxidation reactions. Nanoscale 2018, 10, 13270–13276. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Li, J.; Liu, Y.M. Electrochemical sensing based on layered MoS2–graphene composites. Sens. Actuators B Chem. 2013, 178, 671–677. [Google Scholar] [CrossRef]
- Su, S.; Sun, H.; Xu, F.; Yuwen, L.; Wang, L. Highly sensitive and selective determination of dopamine in the presence of ascorbic acid using gold nanoparticles-decorated MoS2 nanosheets modified electrode. Electroanalysis 2013, 25, 2523–2529. [Google Scholar] [CrossRef]
- Kukkar, M.; Sharma, A.; Kumar, P.; Kim, K.H.; Deep, A. Application of MoS2 modified screen-printed electrodes for highly sensitive detection of bovine serum albumin. Anal. Chim. Acta 2016, 939, 101–107. [Google Scholar] [CrossRef]
- Wu, Q.; Ji, C.; Zhang, L.; Shi, Q.; Wu, Y.; Tao, H. A simple sensing platform based on a 1T@ 2H-MoS 2/cMWCNTs composite modified electrode for ultrasensitive detection of illegal Sudan I dye in food samples. Anal. Methods 2022, 14, 549–559. [Google Scholar] [CrossRef]
- Shayegan, H.; Safarifard, V.; Taherkhani, H.; Rezvani, M.A. Efficient removal of cobalt(II) ion from aqueous solution using amide-functionalized metal-organic framework. J. Appl. Organomet. Chem. 2022, 2, 109–118. [Google Scholar]
- Taghavi, R.; Rostamnia, S. Four-Component Synthesis of Polyhydroquinolines via Unsymmetrical Hantzsch Reaction Employing Cu-IRMOF-3 as a Robust Heterogeneous Catalyst. Chem. Methodol. 2022, 6, 639–648. [Google Scholar]
- Akeremale, O.K. Metal-Organic Frameworks (MOFs) as Adsorbents for Purification of Dye-Contaminated Wastewater: A Review. J. Chem. Rev. 2022, 4, 1–14. [Google Scholar]
- Ko, M.; Mendecki, L.; Mirica, K.A. Conductive two-dimensional metal–organic frameworks as multifunctional materials. Chem. Commun. 2018, 54, 7873–7891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.T.; Yin, H.; An, P.; Tang, Z. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar]
- Zhu, D.; Liu, J.; Zhao, Y.; Zheng, Y.; Qiao, S.Z. Engineering 2D metal–organic framework/MoS2 interface for enhanced alkaline hydrogen evolution. Small 2019, 15, 1805511. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. 2D layered metal organic framework nanosheets as an emerging platform for electrochemical sensing. J. Electrochem. Soc. 2020, 167, 136502. [Google Scholar]
- Zhao, H.; Du, X.; Dong, H.; Jin, D.; Tang, F.; Liu, Q.; Li, Y. Electrochemical immunosensor based on Au/Co-BDC/MoS2 and DPCN/MoS2 for the detection of cardiac troponin I. Biosens. Bioelectron. 2021, 175, 112883. [Google Scholar] [CrossRef]
- Ge, Y.; Chu, H.; Chen, J.; Zhuang, P.; Feng, Q.; Smith, W.R.; Shen, J. Ultrathin MoS2 nanosheets decorated hollow CoP heterostructures for enhanced hydrogen evolution reaction. ACS Sust. Chem. Eng. 2019, 7, 10105–10111. [Google Scholar] [CrossRef]
- Liu, Y.; Han, M.; Xiong, Q.; Zhang, S.; Zhao, C.; Gong, W.; Zhao, H. Dramatically enhanced ambient ammonia electrosynthesis performance by in-operando created Li–S interactions on MoS2 electrocatalyst. Adv. Energy Mater. 2019, 9, 1803935. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Q.; Guo, Z.; Zhang, M.; Zhou, M.; Zhai, Z.; Xu, Y. Self-assembly of MoS2 nanosheet on functionalized pomelo peel derived carbon and its electrochemical sensor behavior toward taxifolin. Inorg. Chem. Commun. 2021, 129, 108631. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–organic frameworks: A new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Yang, W.; Guo, H.; Fan, T.; Zhao, X.; Zhang, L.; Guan, Q.; Yang, W. MoS2/Ni(OH)2 composites derived from in situ grown Ni-MOF coating MoS2 as electrode materials for supercapacitor and electrochemical sensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126178. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Shanmugapriya, K.; Sriram, S. Photocatalytic activity of MoS2 nanoparticles: An experimental and DFT analysis. Appl. Phys. A 2019, 125, 817. [Google Scholar] [CrossRef]
- Feng, W.; Chen, L.; Qin, M.; Zhou, X.; Zhang, Q.; Miao, Y.; He, C. Flower-like PEGylated MoS2 nanoflakes for near-infrared photothermal cancer therapy. Sci. Rep. 2015, 5, 17422. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, S.; Qu, J. Electrochemical sensor based on palladium-reduced graphene oxide modified with gold nanoparticles for simultaneous determination of acetaminophen and 4-aminophenol. Talanta 2018, 178, 188–194. [Google Scholar] [CrossRef]
- Neto, J.D.R.M.; Santos, W.D.J.R.; Lima, P.R.; Tanaka, S.M.C.N.; Tanaka, A.A.; Kubota, L.T. A hemin-based molecularly imprinted polymer (MIP) grafted onto a glassy carbon electrode as a selective sensor for 4-aminophenol amperometric. Sens. Actuators B Chem. 2011, 152, 220–225. [Google Scholar] [CrossRef]
- Lavanya, N.; Sudhan, N.; Kanchana, P.; Radhakrishnan, S.; Sekar, C. A new strategy for simultaneous determination of 4-aminophenol, uric acid and nitrite based on a graphene/hydroxyapatite composite modified glassy carbon electrode. RSC Adv. 2015, 5, 52703–52709. [Google Scholar] [CrossRef]
- Yin, H.; Ma, Q.; Zhou, Y.; Ai, S.; Zhu, L. Electrochemical behavior and voltammetric determination of 4-aminophenol based on graphene–chitosan composite film modified glassy carbon electrode. Electrochim. Acta 2010, 55, 7102–7108. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.H.; Yang, C.P.; Yu, M.; Liu, P. Graphene–polyaniline composite film modified electrode for voltammetric determination of 4-aminophenol. Sens. Actuators B Chem. 2011, 157, 669–674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).