A Dye-Assisted Paper-Based Assay to Rapidly Differentiate the Stress of Chlorophenols and Heavy Metals on Enterococcus faecalis and Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fabrication of PADs
2.3. Bacterial Culture
2.4. PAD Toxicity Detection Method and Data Analysis
2.5. Applicability for Heavy Metal Toxicity Detection
2.6. Traditional Toxicity Detection
3. Results and Discussion
3.1. The Kinetics of Color Change on PADs
3.2. For Bacterial Species
3.3. For Bacterial Density
3.4. Comparison with Traditional Toxicity Detection Methods
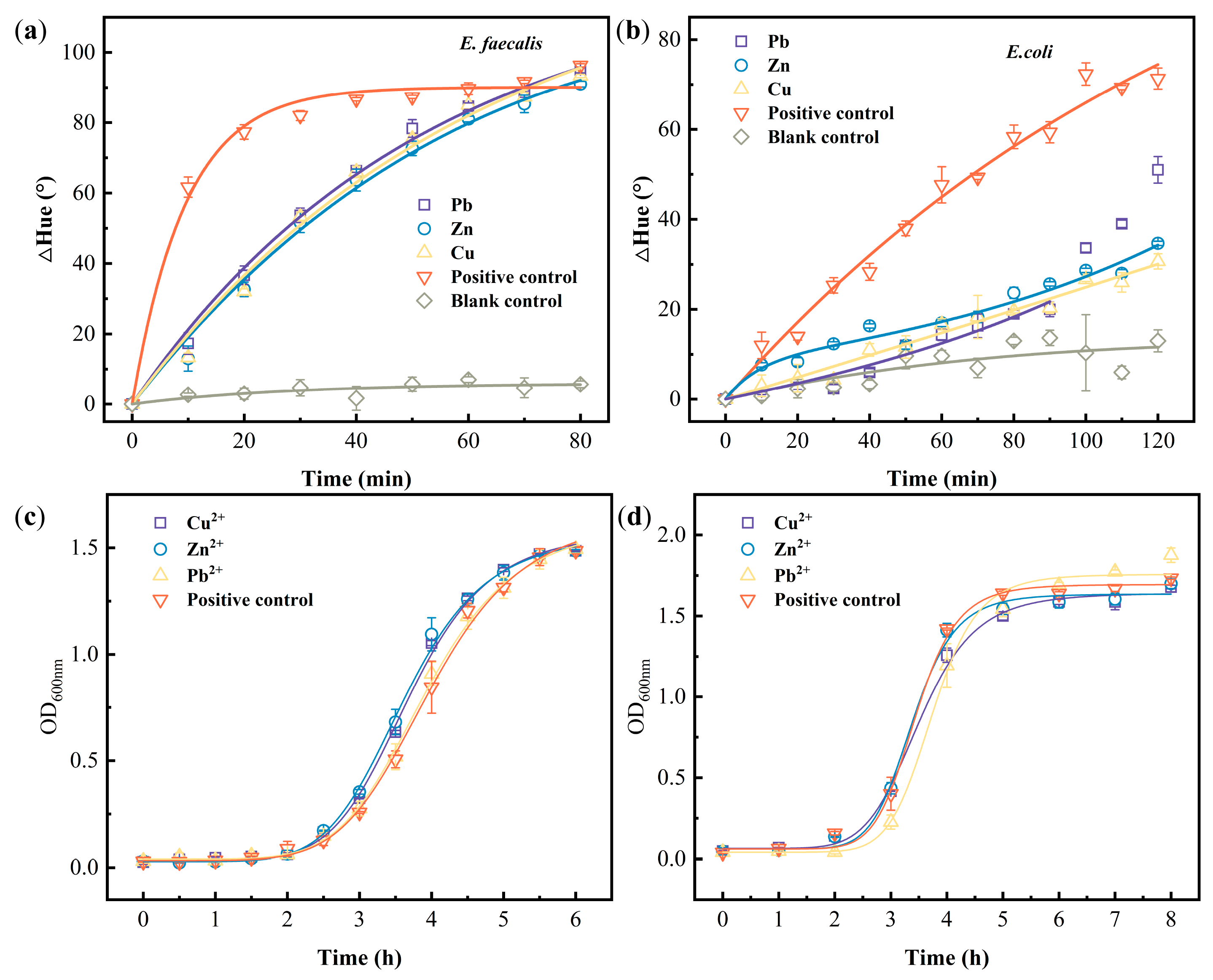
3.5. Toxicity Testing for Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tubic, A.; Loncarski, M.; Maletic, S.; Jazic, J.M.; Watson, M.; Trickovic, J.; Agbaba, J. Significance of Chlorinated Phenols Adsorption on Plastics and Bioplastics during Water Treatment. Water 2019, 11, 2358. [Google Scholar] [CrossRef]
- Ashraf, M.A. Persistent organic pollutants (POPs): A global issue, a global challenge. Environ. Sci. Pollut. Res. 2017, 24, 4223–4227. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Gallampois, C.M.J.; Haglund, P.; Timonen, S.; Rowe, O. Bacterial communities as indicators of environmental pollution by POPs in marine sediments. Environ. Pollut. 2021, 268, 11. [Google Scholar] [CrossRef]
- Moon, H.B.; Lee, D.H.; Lee, Y.S.; Choi, M.; Choi, H.G.; Kannan, K. Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, and Organochlorine Pesticides in Adipose Tissues of Korean Women. Arch. Environ. Contam. Toxicol. 2012, 62, 176–184. [Google Scholar] [CrossRef]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control—A state-of-the-art report with special reference to literature published in Chinese journals. Environ. Sci. Pollut. Res. 2007, 14, 489. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 22. [Google Scholar] [CrossRef]
- Attaallah, R.; Amine, A. Highly selective and sensitive detection of cadmium ions by horseradish peroxidase enzyme inhibition using a colorimetric microplate reader and smartphone paper-based analytical device. Microchem. J. 2022, 172, 8. [Google Scholar] [CrossRef]
- Deiss, F.; Funes-Huacca, M.E.; Bal, J.; Tjhung, K.F.; Derda, R. Antimicrobial susceptibility assays in paper-based portable culture devices. Lab Chip 2014, 14, 167–171. [Google Scholar] [CrossRef]
- Rengaraj, S.; Cruz-Izquierdo, A.; Scott, J.L.; Di Lorenzo, M. Impedimetric paper-based biosensor for the detection of bacterial contamination in water. Sens. Actuators B Chem. 2018, 265, 50–58. [Google Scholar] [CrossRef]
- Zhang, X.X.; Song, Y.Z.; Fang, F.; Wu, Z.Y. Sensitive paper-based analytical device for fast colorimetric detection of nitrite with smartphone. Anal. Bioanal. Chem. 2018, 410, 2665–2669. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.C.; Adkins, J.A.; Bisha, B.; Mentele, M.M.; Goodridge, L.D.; Henry, C.S. Development of a Paper-Based Analytical Device for Colorimetric Detection of Select Foodborne Pathogens. Anal. Chem. 2012, 84, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, H.; Tashkhourian, J.; Hemmateenejad, B. A 3D origami paper-based analytical device combined with PVC membrane for colorimetric assay of heavy metal ions: Application to determination of Cu(II) in water samples. Anal. Chim. Acta 2020, 1126, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cao, R.; Nilghaz, A.; Guan, L.; Zhang, X.; Shen, W. “Periodic-table-style” paper device for monitoring heavy metals in water. Anal. Chem. 2015, 87, 2555–2559. [Google Scholar] [CrossRef] [PubMed]
- Budlayan, M.L.; Lagare-Oracion, J.P.; Patricio, J.; De La Rosa, L.; Arco, S.; Alguno, A.; Manigo, J.; Capangpangan, R. Single-dip colorimetric detection of cyanide using paper-based analytic device based on immobilized silver nanoparticles. Int. Nano Lett. 2022, 12, 399–407. [Google Scholar] [CrossRef]
- Budlayan, M.L.; Lagare-Oracion, J.P.; Dela Rosa, L.; Rodriguez, M.J.; Manigo, J.; Alguno, A.; Austria, E.; Arco, S.; Patricio, J.; Deocaris, C.; et al. Gold nanoparticles-decorated paper-based sensor for rapid cyanide detection in water. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Travnickova, E.; Mikula, P.; Oprsal, J.; Bohacova, M.; Kubac, L.; Kimmer, D.; Soukupova, J.; Bittner, M. Resazurin assay for assessment of antimicrobial properties of electrospun nanofiber filtration membranes. AMB Express 2019, 9, 11. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Xiao, Y.; Steele, T.W.; Stuckey, D.C. Rapid fluorescence-based measurement of toxicity in anaerobic digestion. Water Res. 2015, 75, 123–130. [Google Scholar] [CrossRef]
- Breznan, D.; Das, D.; MacKinnon-Roy, C.; Simard, B.; Kumarathasan, P.; Vincent, R. Non-specific interaction of carbon nanotubes with the resazurin assay reagent: Impact on in vitro assessment of nanoparticle cytotoxicity. Toxicol. In Vitro 2015, 29, 142–147. [Google Scholar] [CrossRef]
- Rubin, A.E.; Zucker, I. Interactions of microplastics and organic compounds in aquatic environments: A case study of augmented joint toxicity. Chemosphere 2022, 289, 9. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.; Stuckey, D.C. Stimulation and inhibition of anaerobic digestion by nickel and cobalt: A rapid assessment using the resazurin reduction assay. Environ. Sci. Technol. 2016, 50, 11154–11163. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Chen, J.L.; Stuckey, D.C.; Steele, T.W.J. Poly(methyl methacrylate) Surface Modification for Surfactant-Free Real-Time Toxicity Assay on Droplet Microfluidic Platform. ACS Appl. Mater. Interfaces 2017, 9, 13801–13811. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Chen, J.L.; Stuckey, D.C.; Steele, T.W.J. Rapid serial diluting biomicrofluidic provides EC50 in minutes. Micro Nano Eng. 2019, 2, 92–103. [Google Scholar] [CrossRef]
- Ortiz, R.; Stuckey, D.C.; Steele, T.W.J. Rapid EC50 determination of hydrophobic toxicants in continuous droplet biomicrofluidics. Micro Nano Eng. 2019, 3, 82–91. [Google Scholar] [CrossRef]
- Sun, Q.D.; Tam, N.F.Y.; Han, J.; Peng, W.Y.K.; Zhu, Z.L.; Chen, J.L. A simple paper-based colorimetric analytical device for rapid detection of Enterococcus faecalis under the stress of chlorophenols. Talanta 2021, 225, 9. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Modeling and Application of a Rapid Fluorescence-Based Assay for Biotoxicity in Anaerobic Digestion. Environ. Sci. Technol. 2015, 49, 13463–13471. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.Z.; Tao, X.Y.; Zhang, X.; Si, Y.B. Acute biotoxicity assessment of heavy metals in sewage sludge based on the glucose consumption of Escherichia coli. R. Soc. Open Sci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Fang, D.Y.; Yu, Y.; Wu, L.Z.; Wang, Y.; Zhang, J.H.; Zhi, J.F. Bacillus subtilis-based colorimetric bioassay for acute biotoxicity assessment of heavy metal ions. RSC Adv. 2015, 5, 59472–59479. [Google Scholar] [CrossRef]
- Catterall, K.; Robertson, D.; Hudson, S.; Teasdale, P.R.; Welsh, D.T.; John, R. A sensitive, rapid ferricyanide-mediated toxicity bioassay developed using Escherichia coli. Talanta 2010, 82, 751–757. [Google Scholar] [CrossRef]
- Shil, A.; Chichger, H. Artificial Sweeteners Negatively Regulate Pathogenic Characteristics of Two Model Gut Bacteria, E. coli and E. faecalis. Int. J. Mol. Sci. 2021, 22, 5228. [Google Scholar] [CrossRef]
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Feria, J.M.; Hamad, M.; Valvano, M.A. Escherichia coli and Pseudomonas aeruginosa lipopolysaccharide O-antigen ligases share similar membrane topology and biochemical properties. Mol. Microbiol. 2018, 110, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, J.A.; Thomas, J.A.; Marolda, C.L.; Valvano, M.A. Surface expression of O-specific lipopolysaccharide in Escherichia coli requires the function of the TolA protein. Mol. Microbiol. 2002, 38, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Metabolic reduction of resazurin; location within the cell for cytotoxicity assays. Biotechnol. Bioeng. 2018, 115, 351–358. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, J.-H. Toxicity of chlorophenols to Pseudokirchneriella subcapitata under air-tight test environment. Chemosphere 2006, 62, 503–509. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
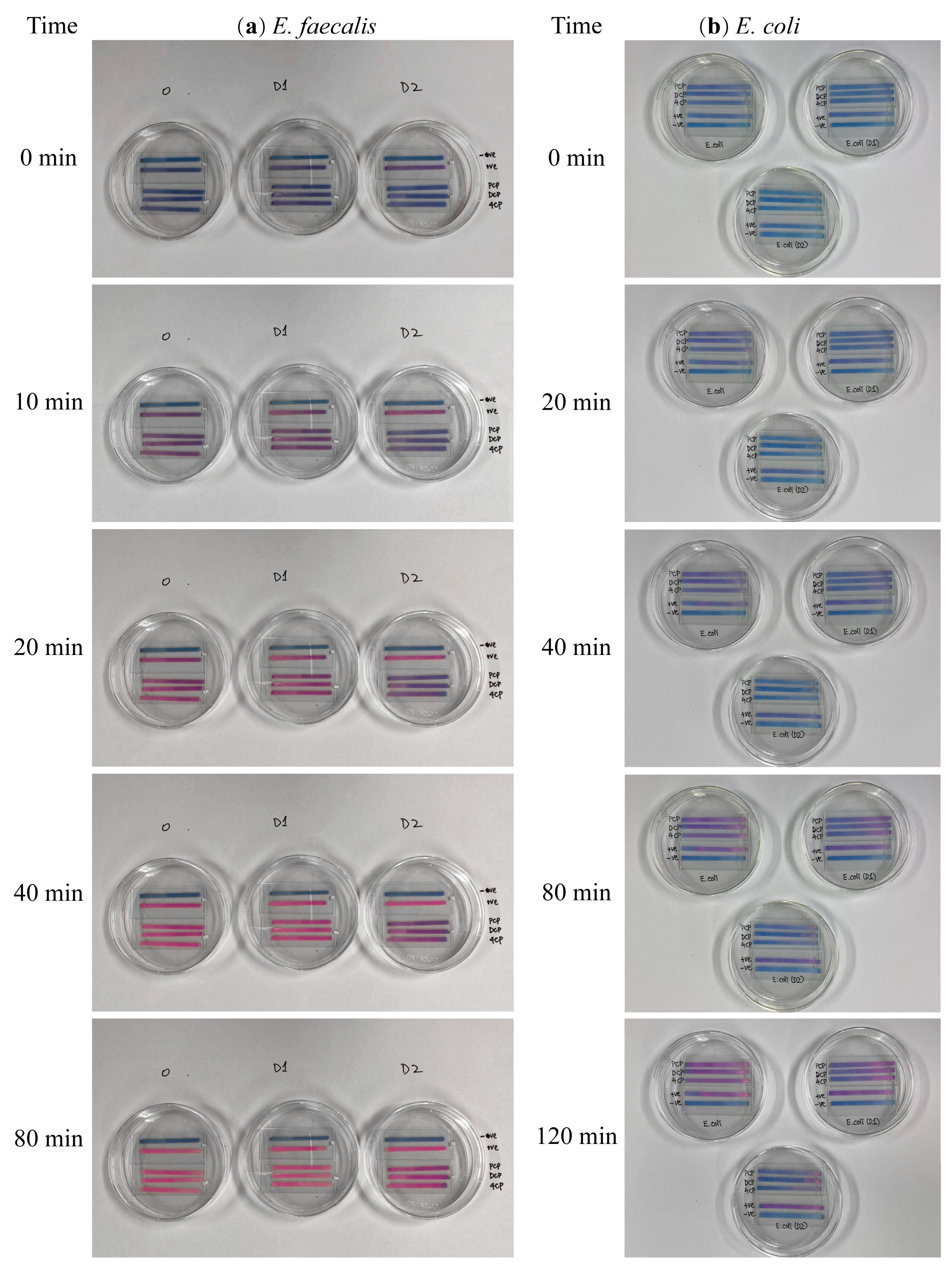

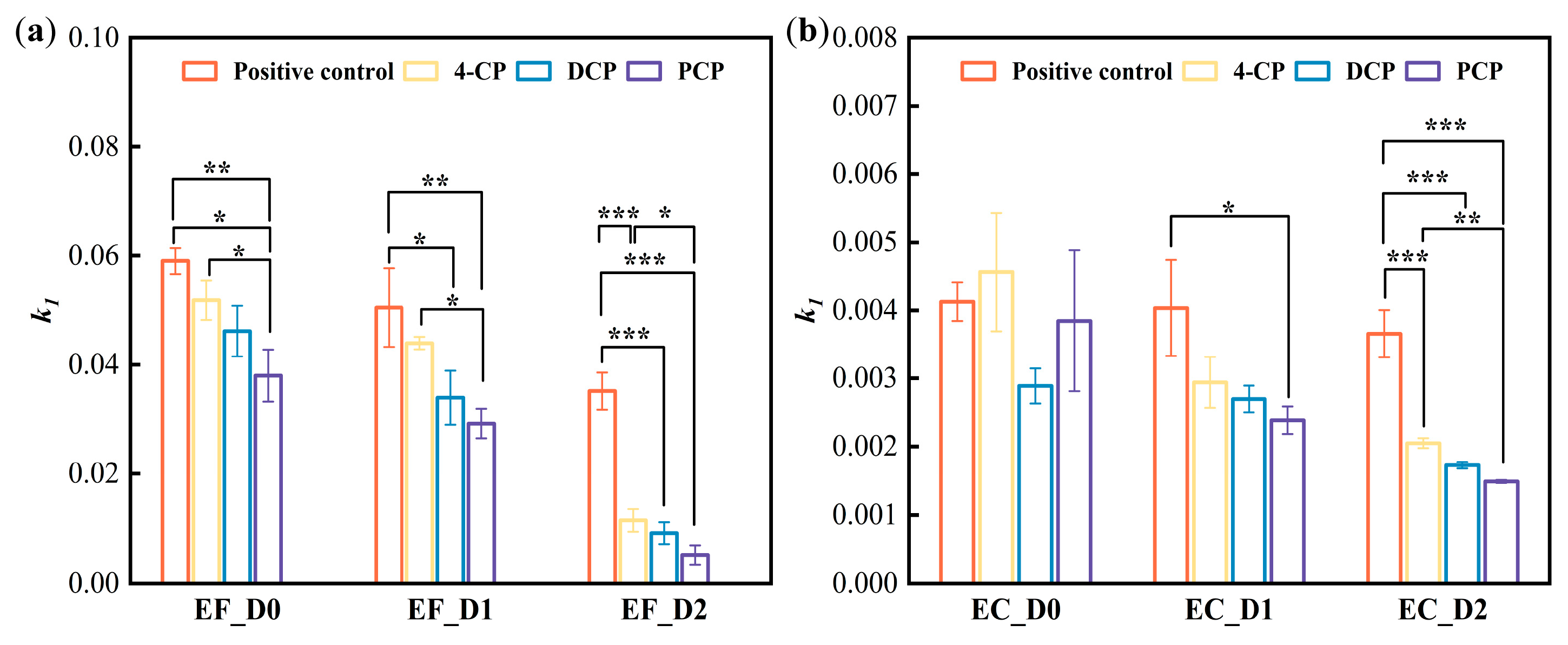
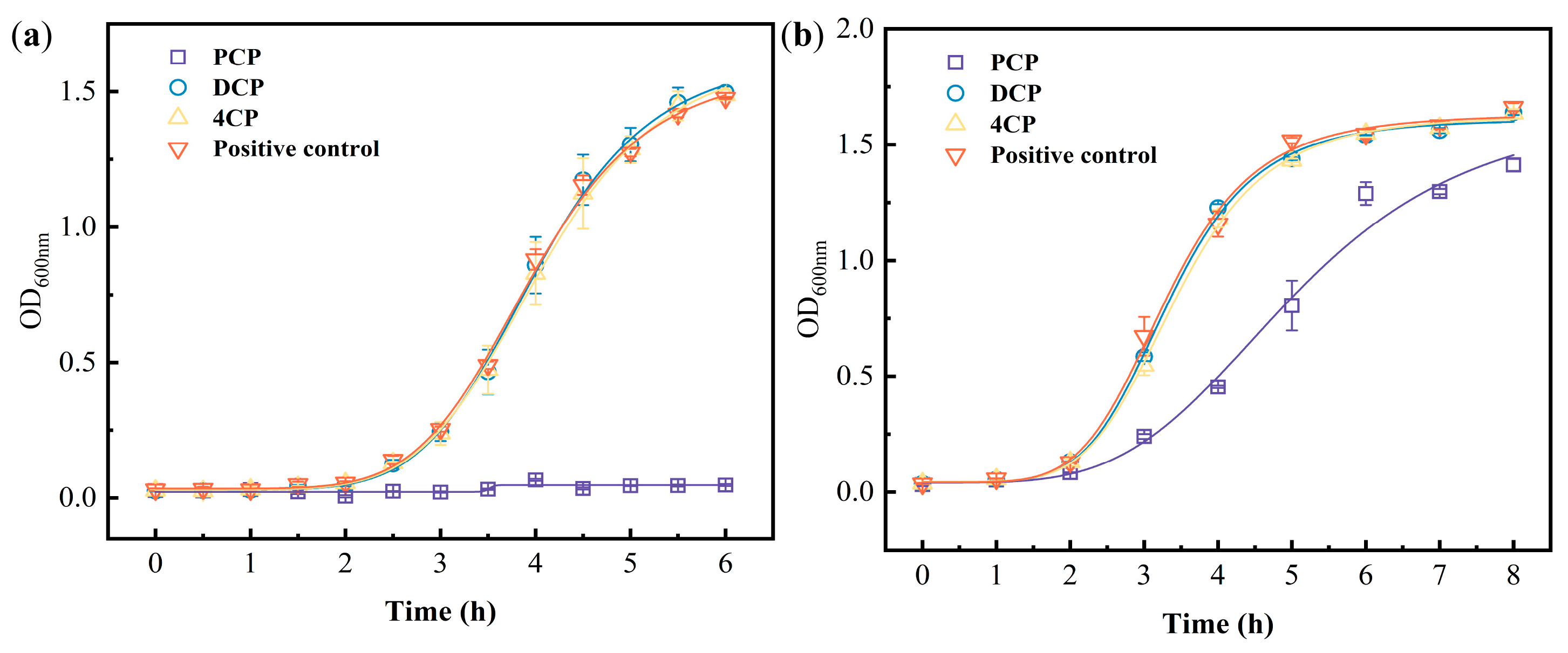
| Bacteria | Bacterial Density (OD600nm) | Group | k1 # | k2 # | R2 * |
|---|---|---|---|---|---|
| E. faecalis (n = 9) | 1.4 | Positive control | 0.059 ± 0.002 | 1.04 × 10−18 ± 6.20 × 10−19 | 0.998 |
| PCP | 0.038 ± 0.005 | 1.67 × 10−18 ± 1.80 × 10−18 | 0.994 | ||
| 2,4-DCP | 0.046 ± 0.005 | 6.62 × 10−21 ± 5.32 × 10−18 | 0.995 | ||
| 4-CP | 0.052 ± 0.004 | 2.41 × 10−21 ± 3.40 × 10−21 | 0.994 | ||
| 0.85 | Positive control | 0.050 ± 0.007 | 6.18 × 10−25 ± 4.12 × 10−25 | 0.989 | |
| PCP | 0.029 ± 0.003 | −1.41 × 10−5 ± 1.96 × 10−4 | 0.991 | ||
| 2,4-DCP | 0.034 ± 0.005 | 8.10 × 10−5 ± 1.05 × 10−4 | 0.987 | ||
| 4-CP | 0.044 ± 0.001 | 6.76 × 10−23 ± 3.15 × 10−23 | 0.991 | ||
| 0.45 | Positive control | 0.035 ± 0.003 | 6.61 × 10−22 ± 9.07 × 10−22 | 0.982 | |
| PCP | 0.005 ± 0.001 | 5.21 × 10−3 ± 1.46 × 10−3 | 0.962 | ||
| 2,4-DCP | 0.009 ± 0.002 | 5.04 × 10−3 ± 3.91 × 10−3 | 0.981 | ||
| 4-CP | 0.013 ± 0.001 | 5.52 × 10−3 ± 6.10 × 10−3 | 0.984 | ||
| E. coli (n = 13) | 1.4 | Positive control | 0.0041 ± 0.000 | −6.15 × 10−3 ± 1.43 × 10−3 | 0.979 |
| PCP | 0.0038 ± 0.001 | −1.85 × 10−3 ± 1.33 × 10−3 | 0.958 | ||
| 2,4-DCP | 0.0029 ± 0.000 | −1.15 × 10−3 ± 5.77 × 10−4 | 0.918 | ||
| 4-CP | 0.0046 ± 0.001 | −1.50 × 10−3 ± 3.95 × 10−4 | 0.982 | ||
| 0.85 | Positive control | 0.0040 ± 0.0007 | −5.73× 10−3 ± 7.04 × 10−4 | 0.974 | |
| PCP | 0.0024 ± 0.0002 | −1.74 × 10−2 ± 1.77 × 10−2 | 0.957 | ||
| 2,4-DCP | 0.0027 ± 0.0002 | −2.90 × 10−2 ± 2.13 × 10−2 | 0.930 | ||
| 4-CP | 0.0029 ± 0.0001 | −1.59 × 10−3 ± 1.02 × 10−4 | 0.951 | ||
| 0.45 | Positive control | 0.0037 ± 0.0003 | −6.54 × 10−3 ± 1.10 × 10−3 | 0.980 | |
| PCP | 0.0015 ± 0.0000 | −0.0245 ± 0.0022 | 0.949 | ||
| 2,4-DCP | 0.0017 ± 0.0000 | 0.00732 ± 0.0055 | 0.857 | ||
| 4-CP | 0.0020 ± 0.0001 | 1.29 × 10−3 ± 2.68 × 10−3 | 0.909 |
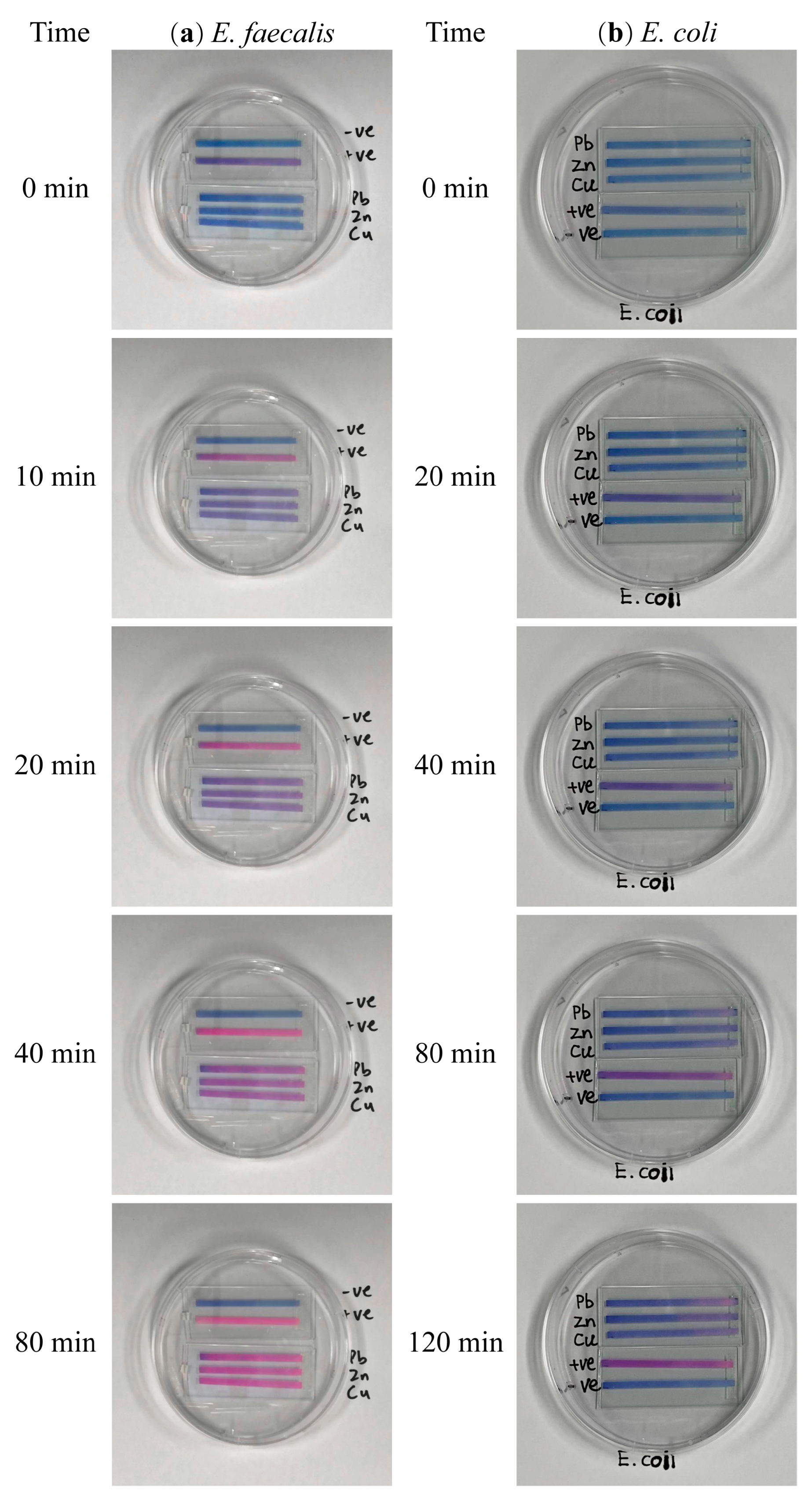
| Bacteria | Group | k1 # | k2 # | R2 * |
|---|---|---|---|---|
| E. faecalis (n = 9) | Positive control | 0.0366 ± 0.0023 a | 7.10 × 10−21 ± 2.34 × 10−3 | 0.977 |
| Pb | 0.0084 ± 0.0004 b | 8.45 × 10−3 ± 3.97 × 10−3 | 0.991 | |
| Zn | 0.0076 ± 0.0001 c | 7.62 × 10−3 ± 1.48 × 10−4 | 0.983 | |
| Cu | 0.0074 ± 0.0001 c | 7.44 × 10−3 ± 1.02 × 10−4 | 0.982 | |
| E. coli (n = 13) | Positive control | 0.00397 ± 0.0000 a | 4.07 × 10−3 ± 2.59 × 10−5 | 0.976 |
| Pb | 0.00181 ± 0.0003 b | −3.00 × 10−2 ± 1.17 × 10−2 | 0.914 | |
| Zn | 0.00199 ± 0.0001 b | −4.52 × 10−3 ± 8.10 × 10−4 | 0.813 | |
| Cu | 0.00250 ± 0.0003 b | 7.35 × 10−3 ± 9.23 × 10−3 | 0.944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Inumbra, B.; Wong, P.Y.; Sarmiento, A.; Yau, Y.; Han, J.; Mao, G.; Peng, Y.-K.; Chen, J.L. A Dye-Assisted Paper-Based Assay to Rapidly Differentiate the Stress of Chlorophenols and Heavy Metals on Enterococcus faecalis and Escherichia coli. Biosensors 2023, 13, 523. https://doi.org/10.3390/bios13050523
Dai W, Inumbra B, Wong PY, Sarmiento A, Yau Y, Han J, Mao G, Peng Y-K, Chen JL. A Dye-Assisted Paper-Based Assay to Rapidly Differentiate the Stress of Chlorophenols and Heavy Metals on Enterococcus faecalis and Escherichia coli. Biosensors. 2023; 13(5):523. https://doi.org/10.3390/bios13050523
Chicago/Turabian StyleDai, Wanqing, Bibi Inumbra, Po Yu Wong, Alma Sarmiento, Ying Yau, Jie Han, Guozhu Mao, Yung-Kang Peng, and Jian Lin Chen. 2023. "A Dye-Assisted Paper-Based Assay to Rapidly Differentiate the Stress of Chlorophenols and Heavy Metals on Enterococcus faecalis and Escherichia coli" Biosensors 13, no. 5: 523. https://doi.org/10.3390/bios13050523
APA StyleDai, W., Inumbra, B., Wong, P. Y., Sarmiento, A., Yau, Y., Han, J., Mao, G., Peng, Y.-K., & Chen, J. L. (2023). A Dye-Assisted Paper-Based Assay to Rapidly Differentiate the Stress of Chlorophenols and Heavy Metals on Enterococcus faecalis and Escherichia coli. Biosensors, 13(5), 523. https://doi.org/10.3390/bios13050523






