Abstract
Firefly luciferases catalyze the efficient production of yellow-green light under normal physiological conditions, having been extensively used for bioanalytical purposes for over 5 decades. Under acidic conditions, high temperatures and the presence of heavy metals, they produce red light, a property that is called pH-sensitivity or pH-dependency. Despite the demand for physiological intracellular biosensors for pH and heavy metals, firefly luciferase pH and metal sensitivities were considered drawbacks in analytical assays. We first demonstrated that firefly luciferases and their pH and metal sensitivities can be harnessed to estimate intracellular pH variations and toxic metal concentrations through ratiometric analysis. Using Macrolampis sp2 firefly luciferase, the intracellular pH could be ratiometrically estimated in bacteria and then in mammalian cells. The luciferases of Macrolampis sp2 and Cratomorphus distinctus fireflies were also harnessed to ratiometrically estimate zinc, mercury and other toxic metal concentrations in the micromolar range. The temperature was also ratiometrically estimated using firefly luciferases. The identification and engineering of metal-binding sites have allowed the development of novel luciferases that are more specific to certain metals. The luciferase of the Amydetes viviani firefly was selected for its special sensitivity to cadmium and mercury, and for its stability at higher temperatures. These color-tuning luciferases can potentially be used with smartphones for hands-on field analysis of water contamination and biochemistry teaching assays. Thus, firefly luciferases are novel color-tuning sensors for intracellular pH and toxic metals. Furthermore, a single luciferase gene is potentially useful as a dual bioluminescent reporter to simultaneously report intracellular ATP and/or luciferase concentrations luminometrically, and pH or metal concentrations ratiometrically, providing a useful tool for real-time imaging of intracellular dynamics and stress.
Keywords:
bioluminescence; pH indication; heavy metals; cadmium; mercury; ratiometric biosensors; bioimaging 1. Introduction
In the past decades, the firefly luciferin–luciferase system has been used in an enormous variety of bioanalytical applications. Firefly luciferase genes have been widely used as reporters in gene expression studies and for cell tracking in normal biological and pathological processes, including cell proliferation studies, cytotoxicity assays, and metastasis in model animals, emerging as a novel technology replacing animal testing by cell assays, and helping the pharmaceutical industry to find new therapeutic drugs [1,2,3]. Among these emerging technologies, firefly luciferases are being used as bioluminescent reporters to track pathogenic bacteria and viral infections, including SARS/COVID-19 [4], and in the development of high throughput drug screening assays (HTS) [5]. Firefly luciferases are also being used in luminescent biosensors [2,6].
Despite their many uses, luciferases have not been commonly used for intracellular pH indication and, with the exception of calcium, for metal biosensing. Currently, luminescent biosensors for pH and metals are mostly fluorescent. The use of bioluminescence in these types of bioluminescent sensors could potentially be useful for real-time bioimaging.
The estimation of intracellular pH is essential for cell homeostasis, cellular stress and intoxication. Intracellular and organelle pH variations are often associated with changes in the cellular cycle, such as cell division and apoptosis, and stress indicating pathologies such as inflammation, allergy and cancer [7,8]. On the other hand, metals may display either physiological functions, such as zinc and copper which are enzymatic cofactors, or toxic ones, especially the heavier metals such as mercury (Hg), cadmium (Cd) and lead (Pb). The estimated intracellular zinc concentration in human cells is around 200–300 µM [9]. Cadmium and mercury are among the most toxic metals causing serious damage to human health and the environment. The intracellular toxic concentrations of cadmium range from 1–300 µM [10], and its deleterious effects involve genotoxicity, generation of ROS, causing pathologies such as cancer, lung, bone and kidney injuries, and immunosuppression. Mercury’s toxic effects start from 1 to 100 µM, causing several cytotoxic effects. At 10 µM, HgCl2 causes three-fold more abnormalities/aberrations such as polycentrism of the chromosome and chromatid breakage [11]. In mammalian cells, 100 µM of HgCl2 affected cell division.
Several luminescent biosensors and indicators for pH were developed, most of them based on fluorescence intensity at a single wavelength [12,13,14,15], and some are based on fluorescence ratiometric analysis at distinct wavelengths [16]. Similarly, fluorescent biosensors for metals were also developed [17,18,19]. Bioluminescent sensors for metals relied first on bioluminescent bacterial light off sensors [20,21,22,23], and then on light on biosensors based on metal-inducible promoters coupled with luciferases [24].
A noteworthy but still overlooked property of firefly luciferases, from the bioanalytical point of view, is their pH and metal sensitivities, in which the bioluminescence color changes from green to red at an acidic pH, higher temperatures and in the presence of some heavy metals [25,26]. The mechanism of pH-sensitivity and bioluminescence color determination by firefly luciferases has been debated for over 5 decades, and recent studies by our group using firefly luciferases displaying distinct bioluminescence colors and spectral sensitivities [27,28,29,30] revealed the putative proton and metal binding sites responsible for pH and metal sensitivities [31].
Despite the demand for physiological intracellular biosensors for pH and heavy metals, firefly luciferase pH and metal sensitivities were originally considered drawbacks in analytical assays, and little attention was given to its potential utility for pH and metal indication. We recently demonstrated, for the first time, that firefly luciferases can be harnessed as ratiometric indicators of intracellular pH and heavy metals [32,33,34]. In this review, we briefly overview luminescent biosensors for pH and heavy metals and show the landmarks that lead to the exploration and engineering of firefly luciferases pH and metal sensitivities for ratiometric analysis of intracellular pH and toxic metals, providing their advantages, drawbacks and perspectives in real-time BL imaging.
2. An Overview of Current Luminescent Intracellular Sensors for pH and Heavy Metals
Luminescent biosensors for pH and metal detection can be divided into fluorescent and chemi- or bioluminescent. Most of the currently used luminescent biosensors are still fluorescent. Luminescent biosensors can be further divided into those based on the intensity at a single wavelength, in which the intensity increases or decreases in response to pH or metal concentration, and ratiometric ones, in which there are spectral changes that can be quantified by the ratio of intensities at different wavelengths. Each type of sensor has its own advantages and disadvantages. Herein, we briefly review the main types of fluorescent and bioluminescent sensors for pH and heavy metals.
Fluorescent sensors. The fluorescent sensors typically have the advantages of being simpler, convenient and inexpensive. Most of the currently used fluorescent biosensors are based on fluorescence intensity, which is linearly responsive to pH or increasing concentrations of metals, but may lack specificity [17]. On the other hand, the ratiometric fluorescent biosensors continue to increase, with advantages such as specificity and selectivity for metals. Whereas the fluorescence intensity at a single wavelength is sensitive to the actual concentration of the fluorophore and self-absorption variations, the ratio of intensities at different wavelengths is insensitive to fluorophore concentration, reflecting a more realistic concentration of the metal [17]. Despite their simplicity, fluorescent sensors have drawbacks such as the need for an external irradiation source, with auto-absorption by internal pigments, auto-fluorescence of the irradiated tissue, and phototoxicity effects caused by the irradiation with the external source.
Fluorescent pH sensors. For intracellular pH indication, specific fluorescent dyes and biosensors are usually used [7,8,12,13,14,15,17,35,36,37]. However, low molecular weight dyes may affect the physiology of the cell. Several pH-dependent fluorescent proteins (FPs) were also engineered and increasingly used to estimate intracellular pH [13,38,39,40], including ratiometric FPs based on either dual excitation or emission wavelengths [14,16,41,42,43]. However, as with any fluorescent dyes, FPs must be previously irradiated with blue light in order to emit fluorescence, which has potential drawbacks [12,44]. In order to reduce problems associated with autofluorescence and auto-absorption of light at shorter wavelengths in mammalian tissues, red-shifted emitting FPs have also been developed in the past 20 years [43]. Furthermore, because GFPs (Green Fluorescent Proteins) are quite stable proteins, they are usually well suited for ex vivo and microscopic fluorescence imaging, but not for real-time imaging.
Fluorescent Metal sensors. Fluorescent sensors were extensively used for distinct metals, either physiological ones such as calcium, zinc and copper, or toxic ones such as mercury, lead and cadmium [17]. Several fluorescent sensors to measure transition and heavy metals in biological systems use small fluorophores, whereas others use genetically encodable fluorescent proteins [40]. Here, we focus on luminescent sensors for physiological and toxic heavier metals such as zinc, copper, cadmium and mercury.
Ratiometric fluorescent sensors for the detection of Cu2+, in which increasing concentrations of Cu2+ decrease the ratio of fluorescence intensity at 470 nm and 355 nm (I470/I355), were developed [45]. Other ratiometric fluorescent sensors based on intramolecular charge transfer (ICT) using small fluorescent molecules with spectroscopic signatures of Zn2+, Hg2+and Pb2+ were also developed [46].
Intracellular Zn2+. Several fluorescent sensors that detect Zn2+ were developed [47,48,49,50], including ratiometric sensors [51] with detection limits lower than 50 µM. A highly selective fluorescent ratiometric sensor for Zn2+ based on bicarboxamidoquinoline, which uses the ratio of intensities at the wavelengths of 410 nm and 500 nm (I500/I410), was also developed [51]. Zinc fluorescent sensors based on fluorescent proteins are also popular [40]. Several zinc sensors are based on the principle of FRET, using zinc finger motifs in fluorescent proteins such as CFP (Cyan Fluorescent Protein) and YFP (Yellow Fluorescent Protein) [47,52].
Hg2+ and Cd2+. Fluorescent sensors for Hg2+ with sensitivities down to micromolar (µM) concentrations in water samples and living cells were also developed [50,53,54,55,56,57]. More recently, whole-cell fluorescent biosensors based on inducible CadC and CadR cadmium-binding proteins and GFP or Cherry were also developed, showing sensitivities to cadmium ranging from 0.1 to 400 µM [58]. Whole-cell E.coli biosensors based on CadC and GFP were also developed to detect cadmium in milk samples [59]. However, despite their sensitivity, a disadvantage of these biosensors is that the analysis requires longer times and considerable infrastructure because they require induction. Other fluorescent sensors include those based on FRET (Fluorescent Ressonance Energy Transfer) between ECFP (Cyan Fluorescent Protein) and cpVenus mediated by CadR cadmium-binding protein, with a Kd value around 250 nM [60]. Other biosensors based on ratiometric analysis of coumarin derivatives, with sensitivity between 40 and 660 pM [61], were also developed.
Bioluminescent sensors. Most bioluminescent biosensors are also based on luminescence intensity and are classified into light off and light on biosensors [6,62]. Non-specific light off biosensors based on natural and genetically transformed bioluminescent bacteria with the LUX operon [20,21,22,23,63,64] were the first whole-cell biosensors used to detect heavy metals and other toxic compounds. In the presence of these toxic metals, the respiratory chain of bacteria is inhibited and bioluminescence decreases. Bacterial light off biosensors using firefly luciferases that detect endogenous ATP were also proposed [21]. However, despite their practical use, the light off biosensors are rather non-specific regarding the toxic agents. On the other hand, light on bioluminescent biosensors based on the full lux-CDABE-operon were later developed to detect the bioavailability of Hg2+ based on the increase in the bioluminescence intensity [24,65,66]. A light on bacterial biosensor was also constructed using the firefly luciferase gene under the control of the ars promoter [65].
Bioluminescent reporters that use two or more emission colors are well known and were extensively used for dual and tricolor reporting in gene expression studies (www.promega.com.br/products/luciferase-assays/reporter-assays/dual_luciferase-reporter-assay-system/?catNum=E1910 (accessed 10 January 2022)) [67]. However, they usually rely on the use of two or more luciferase reporter genes [1] and are rather used to analyze gene expression instead of intracellular homeostasis indicators such as pH and metal concentrations.
The advantage of BL sensors over the fluorescent ones is usually the fact that they do not require external light irradiation in the UV and blue regions, eliminating problems associated with phototoxicity and autofluorescence. In general, they are more specific, emitting a specific light signal with a high signal-to-noise ratio that depends exclusively on the luciferin–luciferase reaction. Furthermore, luciferases, photoproteins and their luciferins usually do not display cytotoxicity. The main disadvantages, however, are the lower sensitivity of bioluminescence assays, due to much weaker signals than fluorescence, and their instability inside cells, which may reduce the potential signal. Despite that, their relative instability makes them advantageous for real-time imaging applications.
Bioluminescent pH sensors. Bioluminescence has not been commonly used to estimate pH. A photo-controllable bioluminescent protein based on firefly luciferase, which displays high sensitivity and low background, was constructed for real-time intracellular pH analysis [68]. Calcium sensors. The most widely known bioluminescent sensors for metals are aimed at detecting calcium, based on the well-known calcium-binding property of photoproteins and luciferases such as aequorin and obelin [69,70,71,72]. A bioluminescent sensor based on zinc finger protein was also developed [73]. BRET sensors. Bioluminescent ratiometric biosensors are much less common, and usually involve BRET (Bioluminescence Resonance Energy Transfer) systems, employing a luciferase or photoprotein and a fluorescent acceptor such as GFP which emit at different wavelengths [74]. Zhang et al. used Renilla RLuc8 as a donor for the permutated Venus FP acceptor to create the BRET-based tandem protein called pHlash, which shows an increase in emission ratio (525 nm/475 nm) as the pH increased [75,76]. Recently, Calflux, a BRET-based sensor for calcium that interposes a calcium-sensitive troponin-C sequence between the very bright Nanoluc and fluorescent proteins, was also constructed [77,78]. In neuronal cells, the release of calcium induces a conformational change of the troponin-C moiety, approximating NanoLuc and the fluorescent protein, allowing BRET to occur [79]. This ratiometric analysis provides a sensitive real-time estimation of released calcium with high a dynamic range, which is useful in conjunction with optogenetic probes such as melanopsin to measure calcium fluxes in neuronal cells. BRET sensors based on the fusion of NanoLuc and CFP (Cyan Fluorescent Protein) and eZincCh-2 were also developed to detect zinc, with sensitivities down to the pMolar range [80].
3. The Firefly Luciferases pH Sensitivity
Firefly luciferases display a bioluminescence color varying from green to yellow-orange under normal physiological conditions or slightly alkaline pH [25,26]. However, when dying or stressed at higher temperatures, firefly bioluminescence color can change from the usual yellow-green to orange (Figure 1). At acidic pH, the light intensity decreases and the spectrum becomes red-shifted. A more detailed analysis showed that their bioluminescence spectra are composed of at least two spectral components, green and red emissions, but a third orange component was also considered [81]. Quantum yield measurements showed that at an alkaline pH, the green component predominates with a reported value of 41% for P. pyralis firefly, whereas at an acidic pH the green component decreases and the red component becomes predominant [81]. Distinct firefly luciferases were shown to display different degrees of pH sensitivity and proportions of green and red light (Figure 2) [29,82]. Usually, the most blue-shifted ones are also less sensitive to such factors [83,84]. Among the firefly luciferases we studied, the luciferase of Amydetes viviani is the most blue-shifted (539 nm) and also the least pH-sensitive, whereas those of Cratomorphus distinctus and P. pyralis display intermediate values and pH sensitivities, and that of Macrolampis sp2 (569 nm) displays the broadest and more pH-sensitive spectrum [29].
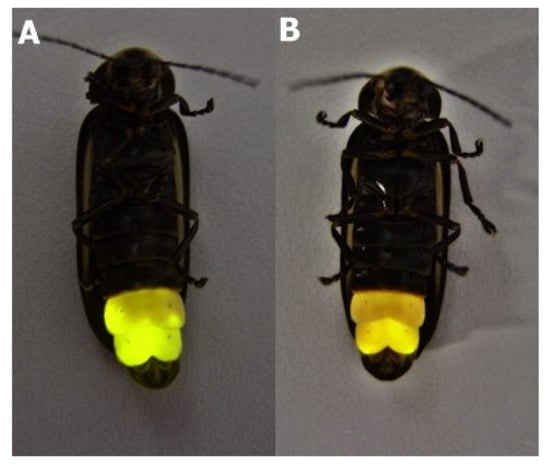
Figure 1.
The Macrolampis sp2 firefly-induced bioluminescence display time-dependent color change after stimulation by anesthesia/adrenalin: (A) in the beginning just after adrenalin injection the color is yellow-green; (B) after 5 min, a subtle color change to yellow-orange is observable. Continuous glow stimulation may induce lantern acidification, causing the color change.
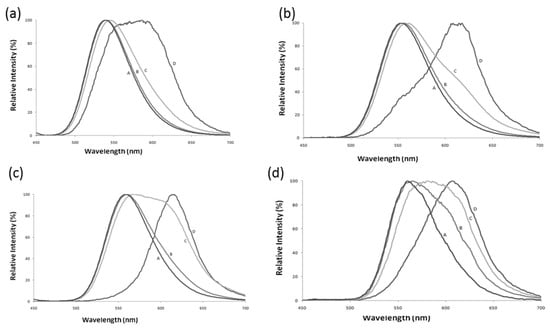
Figure 2.
Effect of pH on bioluminescence spectra of four firefly luciferases displaying distinct pH sensitivities. Reprinted with permission from ref. [29]. Copyright 2011 Royal Society of Chemistry. (a) Amydetes viviani; (b) Cratomorphus distinctus; (c) Photinus pyralis, and (d) Macrolampis sp2. (A) pH 6.5; (B) pH 7.0; (C) pH 7.5, and (D) pH 8.0.
Metal sensitivity. Almost all known adult lantern firefly luciferases also display bioluminescence spectra that are sensitive to certain heavier divalent metals such as zinc, nickel, mercury and lead. Similar to acidic pH, the presence of zinc was shown to decrease the emission quantum yield of the green component, leaving the red component predominant [77]. We found that firefly luciferases also varied in their degree of sensitivity to specific metals. For example, the luciferase of Cratomorphus distinctus displays a bioluminescence spectrum that is more sensitive to zinc, displaying a larger red shift in the presence of this metal (Figure 3) than Macrolampis sp2 firefly luciferase [33].
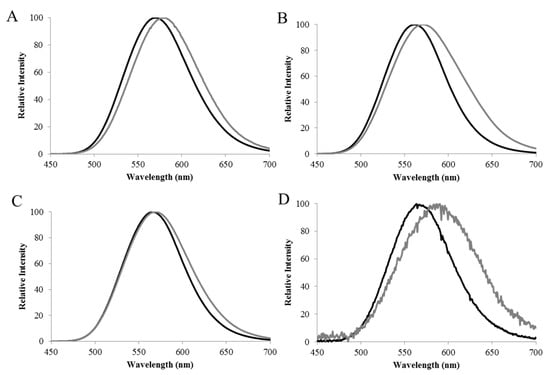
Figure 3.
The residue E354 is important for luciferase sensitivity to Zinc. Effect of 2 mM zinc on the bioluminescence spectra of: (A) Macrolampis sp2 wild-type; (B) C. distinctus wild-type; (C) C. distinctus E354N, and (D) Macrolampis sp2 N354E mutant (black line) without zinc, and (gray lines) in the presence of zinc. Reprinted with permission from ref. [33]. Copyright 2016 Springer.
Temperature sensitivity. Temperature also affects the spectrum of firefly luciferases [25,26]. We demonstrated that luciferases with the most blue-shifted spectra display lower sensitivity to pH [83]. These observations corroborate the hypothesis that the active sites of the most blue-shifted luciferases are more rigid, whereas those of the most red-shifted and pH-sensitive luciferases are more flexible [85]. Mochizuki et al. also investigated the effect of temperature on the quantum yield of firefly luciferase [86]. Similar to the effect of pH and heavy metals, they showed that only the green component is temperature-sensitive, whereas the red and orange components are insensitive [86].
4. Identification of the pH-Sensor and Metal Binding Site of Firefly Luciferases
A comparison of the above and other pH-sensitive firefly luciferase primary structures, modeling studies and site-directed mutagenesis showed important substitutions that affected the proportion of green and red light, as well as the pH sensitivity [28,82,87]. Among them, the natural substitution E354N in Macrolampis firefly luciferase was clearly shown to be responsible for the broader and more red-shifted spectrum of this luciferase in relation to the closer P.pyralis and Cratomorphus luciferases [28]. Indeed, the substitution of the negatively charged E354 in Cratomorphus luciferase, by the neutral asparagine, which is naturally found in Macrolampis luciferase, decreased the sensitivity to zinc, also providing the first evidence of the importance of this site for metal binding [28]. The identification of the electrostatic pair H310 and E354 by modeling studies in firefly luciferase [82,88] also led to the identification of the close salt bridge E311 and R337, which later was shown to stabilize the closed hydrophobic conformation of the luciferase luciferin-binding site [89]. These results finally led to the identification of the pH-sensor and metal-binding site which involve the carboxylates of E311 and E354, and the imidazole or other nucleophilic side-chains of H310 (S/T310) as the proton and metal-binding sites (Figure 4) responsible for pH and metal sensitivities in firefly luciferases [31]. The identity and geometry of these sites, which include histidines and glutamates, indeed resemble the metal-binding sites of several zinc and cadmium metal-binding proteins [90,91]. The E311 carboxylate is a critical base responsible for pH sensitivity and is very likely to be involved in the chemiexcitation step by accepting the oxyluciferin phenolate released proton (Figure 5; [31]), whereas protons and metals such as zinc, lead and mercury, apparently bind to the basic or negatively charged side-chains of H310, E311 and E354, disrupting the active site salt bridges, polarizing the excited oxyluciferin phenolate binding site and promoting red light emission (Figure 5; [31]). Whereas the phenol group of excited oxyluciferin was shown to be critical for pH sensitivity and for bioluminescence color modulation [92], the influence of the keto-enol tautomerization may also exert a critical influence on bioluminescence color modulation [93].
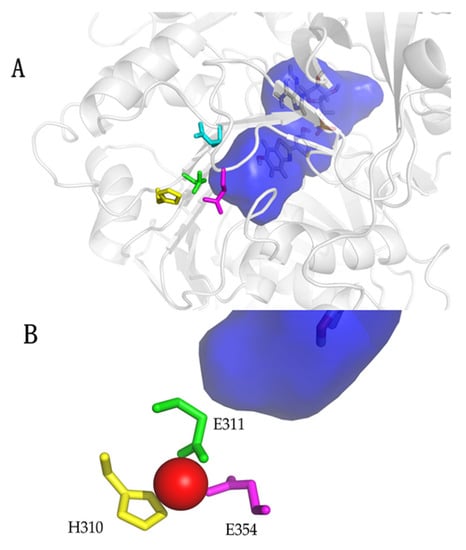
Figure 4.
Structure of the metal-binding site in Amydetes viviani firefly luciferase: (A) Zoom showing the metal-binding site residues (yellow: H310; green: E311; magenta: E354) and oxyluciferin phenolate binding site (deep blue); (B) Metal-binding site showing zinc in red, being coordinated by the side chains of H310, E311 and E354. Reprinted from ref. [31].
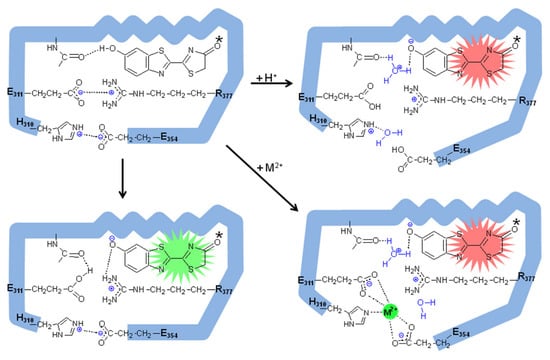
Figure 5.
The proposed mechanism of pH and metal sensitivity in firefly luciferases involves the oxyluciferin phenol/phenolate group excited state proton transfer and electrostatic interactions between residues E311 and R337, and H310 and E354, which keep the active site closed. Whereas the keto form of excited oxyluciferin was considered the most likely emitter in this figure for simplicity, the process of keto-enol tautomerization in bioluminescence color determination can not be ruled out. Reprinted from ref. [31].
5. Use of Firefly Luciferases as Color-Tuning Indicators of Intracellular pH
Considering that the firefly bioluminescence spectrum changes from green to red at an acidic pH, in 2005 we first proposed that pH sensitivity could be harnessed to estimate pH and metals [28]. We then analyzed the effect of the pH on the ratio of bioluminescence intensities in the green and red regions and found that there is a ratiometric relationship between pH 6.0 and 8.0 (Equation (1)) using Macrolampis sp2 and Cratomorphus distinctus firefly luciferases, allowing the estimation of intracellular pH changes in live E. coli bacteria [32]. We found that the bacterial intracellular pH was estimated to be approximately 7.1, in agreement with reported values using other methodologies. The results also showed that at an acidic pH, luciferin may act as a proton shuttle to the intracellular environment, initially acidifying the intracellular pH, producing reddish bioluminescence in bacteria (Figure 6), which then gradually changes to yellow-green, clearly showing that the intracellular buffering capacity of bacteria was recovered after some time.
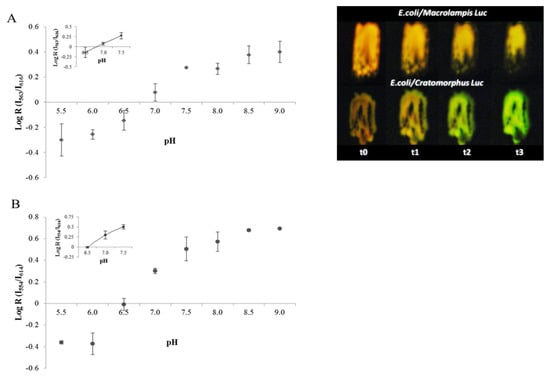
Figure 6.
(Left graph) ratiometric analysis of pH in bacteria using: (A) Macrolampis and (B) Cratomorphus firefly luciferases; (right image) effect of pH on in vivo bioluminescence of E. coli colonies expressing Macrolampis and Cratomorphus firefly luciferases; (t0–t3) represent the time (minutes) when the bioluminescence image was taken after addition of acidic D-luciferin. Reprinted with permission from ref. [32]. Copyright 2014 Royal Society of Chemistry.
Equation (1) Ratiometric estimation of pH. f is the reason between pH and the ratio of intensities in the green and red regions in the linear range from pH 6.0 to 8.0.
pH indication at the single-cell level in Mammalian cells. Using Macrolampis firefly luciferase, we also investigated whether this ratiometric methodology could be applied to estimate intracellular pH in mammalian cells [34]. We analyzed the intracellular pH in different cellular compartments, using filter-based luminometry, bioluminescence microscopy and spectroluminometry (Figure 7 and Figure 8), and found that the intracellular pH could be estimated, using Equation (1), at the single-cell level. The results also showed that the nucleus is more alkaline than the cytosol and that during cell division, the cytosol may also become more alkaline. However, the bioluminescence color of Macrolampis firefly luciferase is already quite red-shifted inside mammalian cells at 36 °C, which diminishes the visualization of color change.
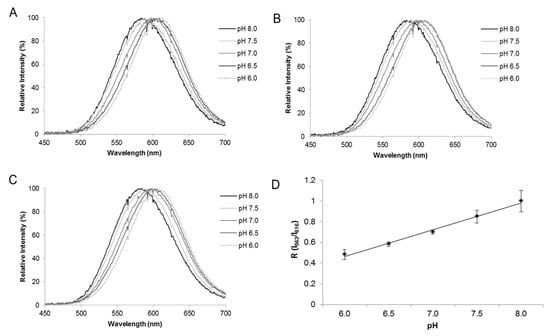
Figure 7.
Bioluminescence spectra in COS-1 cells transfected with Macrolampis firefly luciferase carrying vector, pCMV-Mac, and ratiometric curves. Effect of pH in cells transfected with: (A) pCMV-Mac;cytoplasm; (B) pCMV-Mac-Nucleus; (C) pCMV-Mac with calibration buffer containing nigericin at different pHs (pH 6.0, 6.5, 7.0, 7.5 and 8.0); (D) ratiometric analysis between R (Igreen/Ired) and pH. Reprinted with permission from ref. [34]. Copyright 2019 Royal Society of Chemistry.
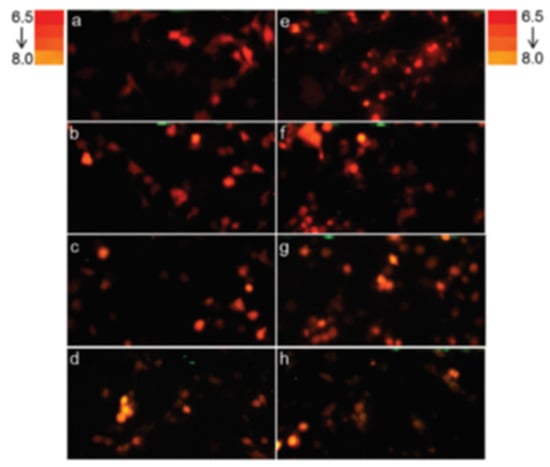
Figure 8.
Firefly luciferase color reporting of intracellular pH change in mammalian cells. In vivo bioluminescence color change of COS-1 cells transfected with pCMV-Mac expressing Macrolampis firefly luciferase at different pH in calibration buffer containing nigericin. Reprinted with permission from ref. [34]. Copyright 2019 Royal Society of Chemistry. (left panels: a–d) luciferase directed to cytoplasm; (right panel: e–h) luciferase directed to nucleus; (a,e) pH 6.5; (b,f) pH 7.0; (c,g) pH 7.5; and (d,h) pH 8.0. The reddish bioluminescence indicates acidic pH, whereas the orange-yellow indicates more alkaline pH.
6. Ratiometric Analysis of Temperature
We also showed a linear relationship between temperature and the ratio of green and red emissions (Figure 9; [85]), which could potentially be used to estimate intracellular temperature. However, it is questionable whether firefly luciferases can be practically used for temperature measurements.
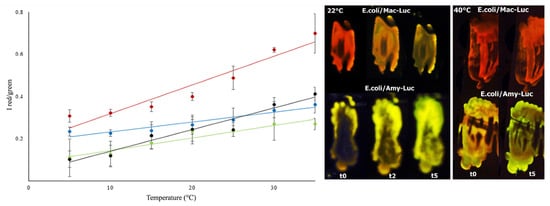
Figure 9.
Comparison of the effect of temperature on the ratio of bioluminescence color of beetle luciferases: (left graph) ratiometric estimation of temperature using distinct firefly luciferases. (red) Macrolampis sp2; (black) Photinus pyralis; (green) Cratomorphus distinctus, and (blue) Amydetes viviani; (right images) in vivo bioluminescence color of E.coli colonies expressing Macrolampis sp2 (Mac-Luc) and Amydetes viviani (Amy-Luc) luciferases, after spraying D-luciferin, at 22 and 40 °C. Reprinted with permission from ref. [85]. Copyright 2014 Royal Society of Chemistry. One can see that the luciferases with steeper curves such as Macrolampis luciferase (red) are the most sensitive to temperature, and those with less steep curves, such as Amydetes viviani luciferase (blue), are the least sensitive.
Compensation for temperature. As shown previously, high temperatures also induce a red shift in the spectrum of firefly luciferases. Thus, at higher temperatures (37 °C), the spectrum of most firefly luciferases, such as that of the Macrolampis firefly, is already red-shifted, decreasing the magnitude of the observable color and spectral change at different pH values (Figure 9) and the potential resolution in bioimaging analysis. Therefore, for bioanalytical assays performed at higher temperatures, it is important to compensate for the temperature effect. The ratiometric curves of temperature for a specific luciferase could in principle potentially be used to compensate for the spectral changes caused by pH at higher temperatures. Alternatively, luciferases such as the Amydetes firefly, which are less sensitive to temperature in the ratiometric analysis of pH, could be used.
7. Use of Firefly Luciferases as Color-Tuning Sensors for Heavy Metals
Based on a similar principle of pH sensitivity, we then analyzed the effect of different heavy metal concentrations on the ratio of green and red bioluminescence intensities using Macrolampis and Cratomorphus firefly luciferases and found that there is also a linear relationship (Figure 10; [33]) in the range of ~15–2000 μM, depending on the metal. The metal concentration can be estimated by the product of the ratio (R) of the bioluminescence intensity in red and green, according to Equation (2).
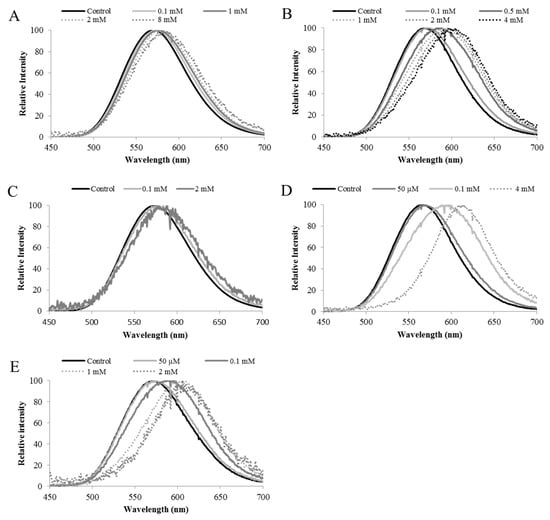
Figure 10.
Bioluminescence spectra of Macrolampis sp2 firefly luciferase and its mutants showing spectral change at different concentrations of ZnSO4: (A) Wild-type; (B) N354H; (C) H310C; (D) N354C; (E) H310C/N315C; Reprinted with permission from ref. [33]. Copyright 2016 Springer.
Equation (2) Ratiometric estimation of heavy metal concentration. f is the ratio between metal concentration and the ratio of intensities in the red and green regions.
Different firefly luciferases display distinct natural degrees of sensitivity to metals such as zinc, copper, cadmium and mercury. The luciferase of Cratomorphus and Photinus pyralis, for example, are more sensitive to zinc than Macrolampis firefly luciferase (Figure 3; Table 1).

Table 1.
pH-sensing and metal binding properties of firefly luciferasesand their engineered forms, and potential applicability.
8. Selection of Metal-Sensitive Luciferases and Engineering of the Metal-Binding Site
Based on the identified structure of the metal-binding site in firefly luciferases, and predictors of metal-binding sites in other proteins [91,94,95], we already started to engineer the metal-binding site of firefly luciferases by mutating the residues H310 and E/N354, in order to change the metal sensitivity. Furthermore, considering the natural differences in metal sensitivity displayed by some firefly luciferases, we started to select luciferases better suited for specific metals. For example, Cratomorphus distinctus larval firefly luciferase is more sensitive to zinc (Figure 3), whereas Macrolampis sp2 firefly luciferase is more sensitive to mercury. Table 1 compares the spectral sensitivity and other bioluminescence properties and applications of firefly luciferases and their mutants.
Indeed, by changing H310 and E354, we could change the metal sensitivity of the Macrolampis firefly luciferase (Figure 10), as can be seen by the detection limits that cause a minimal spectral change and the spectral amplitude caused by the metal (Table 1; [33]). As expected by the chelating property of the side-chains of the substituted residues, the substitution of N354 by His increased the sensitivity to nickel but also for mercury, whereas the substitution of H310 by cysteine increased the sensitivity for zinc (detection limit 20 µM) and mercury (detection limit 15 µM). The double mutant H310C/N354C showed a considerably increased sensitivity to zinc (detection limit 15 µM).
A cadmium- and mercury-selective luciferase. More recently, we found that Amydetes viviani firefly luciferase, which is the most blue-shifted and least pH-sensitive among the studied adult firefly luciferases [29], displays spectral sensitivity exclusively to Cd and Hg at concentrations below 2 mM (Figure 11) [84], in contrast to other studied firefly luciferases studied, which display similar spectral sensitivities to different metals such as zinc, cadmium and mercury at the same concentration. The minimum cadmium concentration that caused a measurable red shift in Amydetes luciferase was estimated to be 100 μM, whereas that for mercury was 62 μM. Furthermore, the activity of cadmium was less affected than that of mercury (Figure 11). Such spectral sensitivity and the improved catalytic properties make Amydetes firefly luciferase particularly suitable as an enzymatic sensor for cadmium detection.
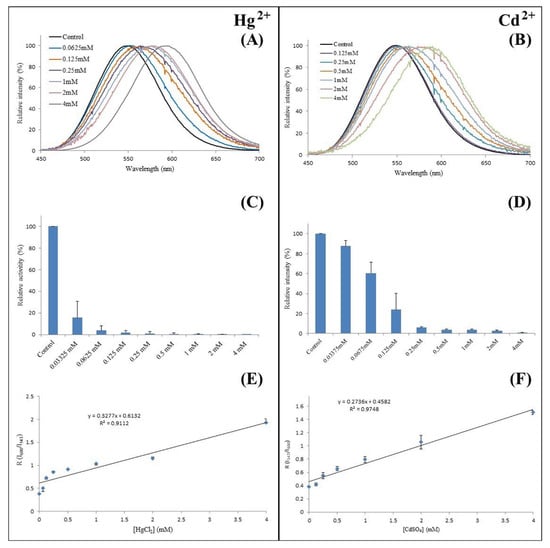
Figure 11.
Use of Amydetes viviani firefly luciferase as cadmium- and mercury-selective luciferase: (A,B) bioluminescence spectra in presence of mercury and cadmium; (C,D) bioluminescence activity in presence of mercury and cadmium; (E,F) ratio of luminescence intensities in green and red regions in presence of mercury and cadmium. Reprinted with permission from ref. [84]. Copyright 2019 National Library of Medicine.
9. Smartphone Detection of Cadmium Contamination in Water
We are harnessing Amydetes luciferase to detect cadmium and mercury contamination in water samples using smartphones (Figure 12). A contaminated water sample can be concentrated and then assayed with the Amydetes luciferase assay solution. If the water sample contained between 0.1–1 µM of cadmium or mercury salts, the bioluminescence color change could be visually observed and photographed using a standard CCD camera-based smartphone. This methodology provides a potentially easy, affordable and hands-on bioluminescent biosensor for fieldwork, and is very useful for teaching laboratory classes on the effects of heavy metals on protein function in biochemistry courses.
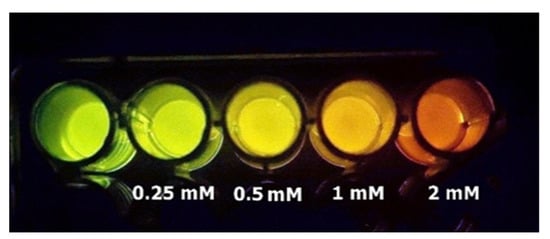
Figure 12.
Smartphone detection of bioluminescence color tuning by cadmium, using Amydetes viviani firefly luciferase.
10. Is it Possible to Report in Two Dimensions?
Dual reporting usually involves the use of two or more luciferase genes that emit distinct BL colors. Such a methodology is generally used to investigate gene expression processes in cells. We showed that firefly luciferases can be used as sensitive tools for the ratiometric analysis of intracellular pH or water contamination by toxic metals. The main advantage of this approach is the use of a single luciferase gene that does not require other fluorescent acceptors such as in BRET or other accessory proteins because the luciferase itself is the proteic sensor, making the analysis simpler, potentially cost-effective and free from interference.
Because luciferase bioluminescent activity has already been used to quantify intracellular ATP or gene expression, one may have the double advantage of using a single luciferase gene to report in two dimensions, the luminescence intensity to report intracellular ATP concentration or luciferase expression and the spectral ratiometric analysis to report a specific homeostatic event such as intracellular pH changes, variations in free intracellular concentrations of physiologic metals, or the presence of toxic metals. Such an approach could be a very powerful tool for the real-time assessment of cellular stress and intoxication in cell assays.
However, to be more effective and to gain more information, it is important to assess ATP concentrations or luciferase expression separately. Therefore, below, we briefly analyzed the gene expression and ATP levels independently.
Gene expression. Luciferases are widely used as reporter genes. Bioluminescence can be used to determine the cellular location, control and level of gene expression. At saturating concentrations of substrates, which usually occur inside the cells, the intensity of bioluminescence (I) depends exclusively on the concentration of expressed luciferase [Luc], its catalytic constant (kcat) and the quantum yield (QY) (Equation (3)). Once the quantum yield (QY) and kcat of some firefly luciferases are known [96], it is in principle possible to estimate the intracellular luciferase concentration at saturating substrate concentrations, according to Equation (3).
Equation (3). Equation showing the relationship between bioluminescence intensity (I) and luciferase concentration [Luc]. kcat is the catalytic constant (ratio of I/[luciferase]) and QY is the quantum yield of the bioluminescence reaction (number of emitted photons by number of luciferin molecules oxidized).
Recently, the bioluminescence intensity emitted by a single mammalian cell-expressing beetle luciferase was estimated to be between 1100–2500 photons s−1, and the number of luciferase molecules expressed inside the cell was estimated to be approximately 2000–3000 molecules [97]. However, one limiting factor for attaining high intensity could be oxygen availability, especially in deep tissues under anoxic conditions.
ATP assays. Reporting ATP inside cells using firefly luciferase is also possible in principle. However, the fluctuation of luciferase expression inside the cells may challenge the precise (ATP) estimation. Koop and Cobbold (1993) could first successfully measure intracellular ATP in cardiomyocytes and hepatocytes by microinjection of firefly luciferase. They showed that upon treating cells with the glycolysis and respiratory chain inhibitors, deoxyglucose and cyanide, a drop of bioluminescence signal usually took a long time (20–75 min), which could be attributed to the saturation of luciferase with the intracellular ATP and with the side-production of ATP by β-oxidation [98].
The KM for ATP is well known for most luciferases, usually ranging from 20–250 μM. The expected concentration of ATP in healthy cells usually ranges from 2–8 mM, which obviously saturates most luciferases. Considering that the usual concentrations of ATP in a healthy cell are well above the reported KM values for ATP for most beetle luciferases, it would be possible to estimate decreases in ATP concentrations only below 200 μM, which is of little practical use, given that most cells would die at such low concentrations. An alternative to estimating physiological intracellular ATP fluctuations is the use of luciferases with higher KM values for ATP, such as those of some railroad worms which are, however, pH-insensitive [99,100]. Furthermore, estimating ATP concentrations by luminescence intensity, simultaneously with pH changes by ratiometric analysis, poses additional difficulties because it is well known that the quantum yield and emission intensity also decrease at low pH or in the presence of heavy metals. However, considering that the KM value for ATP can be estimated at different pH values, it is in principle also possible to estimate the KM at different pH and wavelengths to compensate for the intensity changes.
In summary, intracellular luciferase concentrations could be estimated if the quantum yield of the luciferase and equipment intensity factor are provided. On the other hand, intracellular ATP concentrations could be estimated, providing a luciferase with a high enough KM value (>250 µM), and a curve of the effect of pH on KM values. If ATP or luciferase intracellular concentrations could be estimated, then the use of a single firefly luciferase gene could be very useful as a dual reporter, providing a holistic image of cell homeostasis during normal biological processes such as cell division, apoptosis and fermentation, or pathological processes such as inflammation, allergy, cancer and cell toxicity assays in the pharmaceutical and cosmetic industries.
11. Drawbacks and Perspectives: Comparison with Other Luminescent Biosensors
The color-tuning firefly luciferases described here provide a novel kind of ratiometric luminescent sensor for intracellular pH and toxic metals, with the main advantage of using a single firefly luciferase. The main drawbacks are the lower sensitivity of the assay in relation to fluorescent sensors, and the lower specificity, since these luciferases respond to either pH or some heavy metals. However, the signal-to-noise ratio is in general much better for bioluminescent sources, which have no competing intracellular chemiluminescent reactions. The fluorescent sensors, despite being more sensitive and simpler, have drawbacks such as the need for external irradiation by a light source to excite fluorescence, problems associated with phototoxicity due to irradiation in the ultraviolet and blue regions, self-absorption of the irradiated light and autofluorescence of endogenous compounds, which decrease the signal-to-noise ratio. Similar to the fluorescent ratiometric biosensors, the firefly luciferase bioluminescent ratiometric biosensors described here also eliminate problems associated with changes in luciferase expression and its inactivation, which are usually drawbacks in luminescent biosensors based on the light intensity at a single wavelength. Furthermore, because luciferases are unstable proteins, they do not accumulate inside cells as GFP does. The lower luminescent signal produced by the shorter half-life of luciferase could be overcome by the use of brighter luciferases, such as that of Amydetes viviani firefly. Altogether, these properties make pH-sensitive firefly luciferases promising bioindicators for real-time bioimaging and measurement of intracellular pH changes.
pH range. The pH sensitivity of firefly luciferases between pH 6 and 8.5 is well suited for physiological analysis but can not be used for analysis beyond such pH values. The distinct intensities produced at different pH values could also be a concern in the use of firefly luciferase in such analysis when using equipment based on photomultipliers that display lower sensitivities in the red region. However, such problems can be circumvented by using CCD-provided equipment which show more linear spectral photoresponse.
Tissue absorption. Another disadvantage of using firefly luciferases as pH indicators is their use for bioimaging purposes in deep live mammalian animals because firefly luciferases emit light in the range of green-orange/red, which is considerably absorbed by hemoglobin and other pigments. Therefore, currently, firefly luciferases can only be used to report pH in cell cultures and superficial tissues. This problem could be circumvented by engineering more far red-shifted luciferases, which are pH-sensitive with pH-sensitive luciferin analogs. It was shown that some luciferin analogs that preserve the phenol hydroxyl group also preserve pH sensitivity emitting in the FR and NIR [101].
Working at high temperatures. As shown, firefly luciferases display red-shifted spectra at higher temperatures, which may decrease the sensitivity of the pH estimation, especially in bioimaging. Luciferases can be engineered to improve the stability of bioluminescence spectra at higher temperatures. However, there is a trade-off between pH and temperature sensitivity: decreasing temperature sensitivity may also decrease pH sensitivity. The luciferase of Amydetes firefly is thermally more stable while preserving pH and metal sensitivity. We believe that further research is required to improve these properties.
Metal sensitivity and selectivity. The lower selectivity and sensitivity of ratiometric assays for metals using pH-sensitive firefly luciferases when compared to other reported fluorescent sensors are major drawbacks. As shown, the sensitivity and selectivity for specific metals can be improved by engineering the metal-binding site. Among the firefly luciferases we investigated (Table 1), the Macrolampis luciferase mutant N354C was the most sensitive to mercury, with a detection limit of 15 µM, however, it was not specific enough, displaying sensitivity to other metals, mainly zinc. The mutant H310C/N354C was the most sensitive to zinc with a detection limit of 15 µM, a value that is close to some fluorescent intracellular biosensors, and a spectral amplitude of 38 nm. However, similarly to the N354C mutant, the H310C/N354C also lacked selectivity, displaying sensitivity to other metals. Overall, currently, the Amydetes firefly luciferase was the one that displayed the best combination of properties, with special selectivity to cadmium and mercury, with detection limits of 100 µM for cadmium and 60 µM for mercury, and a spectral amplitude above 38 nm. For detecting heavy metal contamination in water samples, the lower sensitivity can be circumvented by concentrating the water samples to a volume in which the concentration of the metal reaches the micromolar (µM) range, as we have shown. However, for intracellular biosensing, the luciferase sensors are not as sensitive yet as the fluorescent sensors, which display sensitivity in the upper nanomolar to micromolar range. We anticipate that there may be some space for further improvement of this sensitivity. Nevertheless, although firefly luciferases are not specific metal-binding proteins, they could just be used as average intracellular sensing proteins to estimate toxic metal concentrations that cause average enzymatic and metabolic inhibition. Finally, considering that, often, the effect of these toxic metals is additive, luciferases could be used as fast average enzymatic indicators of total heavy metal concentration in a water sample.
12. Conclusions
Firefly luciferases can be harnessed to ratiometrically estimate intracellular pH in living bacteria and mammalian cells in the range from pH 6.0 to 8.5, and to estimate the concentration of physiologic metals such as Zn, and toxic heavy metals such as Hg, Cd and Pb down to the μM range. Whereas some firefly luciferases are not selective for distinct heavy metals, we showed that new luciferases can be selected or engineered in their metal-binding sites, providing a palette of selective metal color-tuning luciferases. Although firefly luciferases are not yet sensitive as intracellular sensors of these toxic metals, they could nevertheless be used in field enzymatic bioluminescent sensors of specific toxic metals and overall mixtures, and for educational purposes showing the average effect of these metals on protein function. The use of firefly luciferases as ratiometric biosensors for pH offers several advantages over other luminescent reporter genes: (1) the sensitivity of the ratiometric analysis, which is devoid of problems associated with fluorescence, such as self-absorption, autofluorescence and phototoxicity; (2) the ratiometric analysis is not affected by the level of luciferase expression or substrate concentrations, which could be a problem when using fluorescent and bioluminescent reporter genes based on intensity; (3) the relative instability of firefly luciferases, which is more appropriate for real-time in vivo imaging, and (4) and the possibility of using a single bioluminescent reporter gene for simultaneous dual reporting of gene expression or ATP concentration inside the cells using the light intensity, and intracellular pH changes using color tuning or the spectral ratiometric analysis. These color-tuning luciferases, together with the development of more sensitive detection devices such as CCD cameras, BL microscopes and filter luminometers, may provide a novel and promising technology to probe intracellular stress in cell assays. Furthermore, the development and miniaturization of photodetection systems, such as smartphones with sensitive CCD cameras, may enable the development of hands-on biosensors for in loco cell toxicity assays, and for field estimation of bioavailability and water contamination by toxic metals.
Author Contributions
V.R.V. idealized and conceptualized the work with pH- and metal-sensing luciferases, contributed with funding acquisition, administration, student supervision, wrote the manuscript and made some Figures. G.F.P. contributed with formal analysis, made Figures and revised metal-sensitive luciferases. V.R.B. contributed with the supervision of students, to the reviewing, writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP Grant number: 2010/05426-8 to VRV; 2021/11544-8 to GFP and 2020/07649-6 to VRB), National Research Council (CNPq 405060/2021-1 to VRV).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are deeply grateful to the collaborators that contributed with published manuscripts over the past years for the development of the research contained in this review, especially Gabriele Gabriel, Gabriela Oliveira (UFSCar) and Yoshiro Ohmiya (AIST). This work was funded by FAPESP 2010/05426-8; CNPq 401.867/2016-1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Viviani, V.R.; Ohmiya, Y. Beetle luciferases: Colorful lights on biological processes and diseases. In Photoproteins in Bioanalysis; Wiley-VCH: Weinheim, Germany, 2006; pp. 49–63. [Google Scholar] [CrossRef]
- Roda, A.; Mezzanotte, L.; Aldini, R.; Michelini, E.; Cevenini, L. A new gastric-emptying mouse model based on in vivo non-invasive bioluminescence imaging. Neurogastroenterol. Motil. 2010, 22, 1117–1288. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.J.; Anderson, J.C. Applications of bioluminescence in biotechnology and beyond. Chem. Soc. Rev. 2021, 50, 5668–5705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wei, L.; Niu, P. The novel coronavirus outbreak in Wuhan, China. Glob. Health Res. Policy 2020, 5, 6. [Google Scholar] [CrossRef]
- Shen, L.; Niu, J.; Wang, C.; Huang, B.; Wang, W.; Zhu, N.; Deng, Y.; Wang, H.; Ye, F.; Cen, S.; et al. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 2019, 93, e00023-19. [Google Scholar] [CrossRef] [PubMed]
- Mirasoli, M.; Michelini, E. Analytical bioluminescence and chemiluminescence. Anal. Bioanal. Chem. 2014, 406, 5529–5531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P. Apoptose: Programa molecular de morte celular. Einstein 2003, 1, 15–18. [Google Scholar]
- Maret, W. Analyzing free zinc (II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef]
- Howard, W.; Leonard, B.; Moody, W.; Kochhar, T.S. Induction of chromosome changes by metal compounds in cultured CHO cells. Toxicol. Lett. 1991, 56, 179–186. [Google Scholar] [CrossRef]
- Breeuwer, P.; Drocourt, J.L.; Rombouts, F.M.; Abee, T. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6)-carboxyfluoresceinsuccinimidyl ester. Appl. Environ. Microbiol. 1996, 62, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kneen, M.; Farinas, J.; Li, Y.; Verkman, A.S. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophys. J. 1998, 74, 1591–1599. [Google Scholar] [CrossRef]
- Bizzarri, R.; Arcangeli, C.; Arosio, D.; Ricci, F.; Faraci, P.; Cardarelli, F.; Beltram, F. Development of a novel GFP-based ratiometric excitation and emission pH indicator for intracellular studies. Biophys. J. 2006, 90, 3300–3314. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Qian, X.; Liu, F.; Zhang, R. Novel fluorescent pH sensors based on intramolecular hydrogen bonding ability of naphthalimide. Organicletters 2004, 6, 2757–2760. [Google Scholar] [CrossRef]
- Mahon, M.J. pHluorin2: An enhanced, ratiometric, pH-sensitive green florescent protein. Adv. Biosci. Biotechnol. 2011, 2, 132. [Google Scholar] [CrossRef]
- Valeur, B. Fluorescent Molecular Sensors of Ions and Molecules. In Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2001; pp. 273–349. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Eltzov, E.; Marks, R.S. Whole-cell aquatic biosensors. Anal. Bioanal. Chem. 2011, 400, 895–913. [Google Scholar] [CrossRef]
- Gabriel, G.V.; Lopes, P.S.; Viviani, V.R. Suitability of Macrolampis firefly and Pyrearinus click beetle luciferases for bacterial light off toxicity biosensor. Anal. Biochem. 2014, 445, 73–79. [Google Scholar] [CrossRef]
- Selifonova, O.; Burlage, R.; Barkay, T. Bioluminescent sensors for detection of bioavailable Hg (II) in the environment. Appl. Environ. Microbiol. 1993, 59, 3083–3090. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, S.M.; Virta MP, J.; Karp, M.T. Detecting bioavailable toxic metals and metalloids from natural water samples using luminescent sensor bacteria. Water Res. 2000, 34, 2661–2666. [Google Scholar] [CrossRef]
- Seliger, H.H.; McElroy, W.D. The colors of firefly bioluminescence: Enzyme configuration and species specificity. Proc. Natl. Acad. Sci. USA 1964, 52, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Viviani, V.R.; Bechara, E.J. Bioluminescence of Brazilian fireflies (Coleoptera: Lampyridae): Spectral distribution and pH effect on luciferase-elicited colors. Comparison with elaterid and phengodid luciferases. Photochem. Photobiol. 1995, 62, 490–495. [Google Scholar] [CrossRef]
- Viviani, V.R.; Arnoldi, F.G.; Brochetto-Braga, M.; Ohmiya, Y. Cloning and characterization of the cDNA for the Brazilian Cratomorphus distinctus larval firefly luciferase: Similarities with European Lampyris noctiluca and Asiatic Pyrocoelia luciferases. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Viviani, V.R.; Oehlmeyer, T.L.; Arnoldi, F.G.C.; Brochetto-Braga, M.R. A New Firefly Luciferase with Bimodal Spectrum: Identification of Structural Determinants of Spectral pH-Sensitivity in Firefly Luciferases. Photochem. Photobiol. 2005, 81, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Viviani, V.R.; Amaral, D.; Prado, R.; Arnoldi, F.G. A new blue-shifted luciferase from the Brazilian Amydetes fanestratus (Coleoptera: Lampyridae) firefly: Molecular evolution and structural/functional properties. Photochem. Photobiol. Sci. 2011, 10, 1879–1886. [Google Scholar] [CrossRef]
- Carvalho, M.C.; Tomazini, A.; Amaral, D.T.; Murakami, M.T.; Viviani, V.R. Luciferase isozymes from the Brazilian Aspisoma lineatum (Lampyridae) firefly: Origin of efficient pH-sensitive lantern luciferases from fat body pH-insensitive ancestors. Photochem. Photobiol. Sci. 2020, 19, 1750–1764. [Google Scholar] [CrossRef]
- Viviani, V.R.; Gabriel, G.V.; Bevilaqua, V.R.; Simões, A.F.; Hirano, T.; Lopes-de-Oliveira, P.S. The proton and metal binding sites responsible for the pH-dependent green-red bioluminescence color tuning in firefly luciferases. Sci. Rep. 2018, 8, 17594. [Google Scholar] [CrossRef]
- Gabriel, G.V.; Viviani, V.R. Novel application of pH-sensitive firefly luciferases as dual reporter genes for simultaneous ratiometric analysis of intracellular pH and gene expression/location. Photochem. Photobiol. Sci. 2014, 13, 1661–1670. [Google Scholar] [CrossRef]
- Gabriel, G.V.; Viviani, V.R. Engineering the metal sensitive sites in Macrolampis sp2 firefly luciferase and use as a novel bioluminescent ratiometric biosensor for heavy metals. Anal. Bioanal. Chem. 2016, 408, 8881–8893. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.V.; Yasuno, R.; Mitani, Y.; Ohmiya, Y.; Viviani, V.R. Novel application of Macrolampis sp2 firefly luciferase for intracellular pH-biosensing in mammalian cells. Photochem. Photobiol. Sci. 2019, 18, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Arosio, D.; Ratto, G.M. Twenty years of fluorescence imaging of intracellular chloride. Front. Cell. Neurosci. 2014, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Barber, D.L.; Jacobson, M.P. Intracellular pH sensors: Design principles and functional significance. Physiology 2007, 22, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Van Beilen, J.W.; Brul, S. Compartment-specific pH monitoring in Bacillus subtilis using fluorescent sensor proteins: A tool to analyze the antibacterial effect of weak organic acids. Front. Microbiol. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Llopis, J.; McCaffery, J.M.; Miyawaki, A.; Farquhar, M.G.; Tsien, R.Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 6803–6808. [Google Scholar] [CrossRef]
- Miesenböck, G.; De Angelis, D.A.; Rothman, J.E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 1998, 394, 192–195. [Google Scholar] [CrossRef]
- Hao, Z.; Zhu, R.; Chen, P.R. Genetically encoded fluorescent sensors for measuring transition and heavy metals in biological systems. Curr. Opin. Chem. Biol. 2018, 43, 87–96. [Google Scholar] [CrossRef]
- Arosio, D.; Ricci, F.; Marchetti, L.; Gualdani, R.; Albertazzi, L.; Beltram, F. Simultaneous intracellular chloride and pH measurements using a GFP-based sensor. Nat. Methods 2010, 7, 516–518. [Google Scholar] [CrossRef]
- Chen, M.; Hombrebueno, J.R.; Luo, C.; Penalva, R.; Zhao, J.; Colhoun, L.; Pandi, S.P.S.; Forrester, J.V.; Xu, H. Age-and light-dependent development of localised retinal atrophy in CCL2−/− CX3CR1GFP/GFP mice. PLoS ONE 2013, 8, e61381. [Google Scholar] [CrossRef]
- Martynov, V.I.; Pakhomov, A.A.; Deyev, I.E.; Petrenko, A.G. Genetically encoded fluorescent indicators for live cell pH imaging. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2924–2939. [Google Scholar] [CrossRef] [PubMed]
- Benčina, M. Illumination of the spatial order of intracellular pH by genetically encoded pH-sensitive sensors. Sensors 2013, 13, 16736–16758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Tian, X.; Liu, A.; Jia, L. Carboxamidoquinoline–coumarin derivative: A ratiometric fluorescent sensor for Cu (II) in a dual fluorophore hybrid. Sens. Actuators B Chem. 2015, 218, 37–41. [Google Scholar] [CrossRef]
- Xu, W.; Ren, C.; Teoh, C.L.; Peng, J.; Gadre, S.H.; Rhee, H.W.; Lee, C.L.K.; Chang, Y.T. An artificial tongue fluorescent sensor array for identification and quantitation of various heavy metal ions. Anal. Chem. 2014, 86, 8763–8769. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Venkatesan, P.; Wu, S.P. A sensitive and selective fluorescent sensor for Zinc (II) and its application to living cell imaging. Sens. Actuators B Chem. 2014, 203, 719–725. [Google Scholar] [CrossRef]
- Hosseini, M.; Ghafarloo, A.; Ganjali, M.R.; Faridbod, F.; Norouzi, P.; Niasari, M.S. A turn-on fluorescent sensor for Zn2+ based on new Schiff’s base derivative in aqueous media. Sens. Actuators B Chem. 2014, 198, 411–415. [Google Scholar] [CrossRef]
- Tang, L.; Dai, X.; Zhong, K.; Wen, X.; Wu, D. A phenylbenzothiazole derived fluorescent sensor for Zn (II) recognition in aqueous solution through “Turn-On” excited-state intramolecular proton transfer emission. J. Fluoresc. 2014, 24, 1487–1493. [Google Scholar] [CrossRef]
- Song, E.J.; Park, G.J.; Lee, J.J.; Lee, S.; Noh, I.; Kim, Y.; Kim, S.J.; Kim, C.; Harrison, R.G. A fluorescence sensor for Zn2+ that also acts as a visible sensor for Co2+ and Cu2+. Sens. Actuators B Chem. 2015, 213, 268–275. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X.; Jia, L.; Yang, R.; Cao, G.; Liu, C. A fluorescent sensor based on bicarboxamidoquinoline for highly selective relay recognition of Zn2+ and citrate with ratiometric response. Sens. Actuators B Chem. 2015, 221, 923–929. [Google Scholar] [CrossRef]
- Hosseini, M.; Khabbaz, H.; Dezfoli, A.S.; Ganjali, M.R.; Dadmehr, M. Selective recognition of Glutamate based on fluorescence enhancement of graphene quantum dot. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1962–1966. [Google Scholar] [CrossRef]
- Aydin, Z.; Wei, Y.; Guo, M. An “off–on” optical sensor for mercury ion detection in aqueous solution and living cells. Inorg. Chem. Commun. 2014, 50, 84–87. [Google Scholar] [CrossRef]
- Han, A.; Liu, X.; Prestwich, G.D.; Zang, L. Fluorescent sensor for Hg2+ detection in aqueous solution. Sens. Actuators B Chem. 2014, 198, 274–277. [Google Scholar] [CrossRef]
- Erdemir, S.; Kocyigit, O.; Malkondu, S. Detection of Hg2+ ion in aqueous media by new fluorometric and colorimetric sensor based on triazole–rhodamine. J. Photochem. Photobiol. A Chem. 2015, 309, 15–21. [Google Scholar] [CrossRef]
- Maity, S.B.; Banerjee, S.; Sunwoo, K.; Kim, J.S.; Bharadwaj, P.K. A fluorescent chemosensor for Hg2+ and Cd2+ ions in aqueous medium under physiological pH and its applications in imaging living cells. Inorg. Chem. 2015, 54, 3929–3936. [Google Scholar] [CrossRef]
- Wu, B.; Xu, L.; Wang, S.; Wang, Y.; Zhang, W. A PEGylated colorimetric and turn-on fluorescent sensor based on BODIPY for Hg (II) detection in water. Polym. Chem. 2015, 6, 4279–4289. [Google Scholar] [CrossRef]
- Hui, C.Y.; Guo, Y.; Liu, L.; Yi, J. Recent advances in bacterial biosensing and bioremediation of cadmium pollution: A mini-review. World J. Microbiol. Biotechnol. 2022, 38, 9. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, N.; Singh, A.K. Development of cadmium specific recombinant biosensor and its application in milk samples. Sens. Actuators B Chem. 2017, 240, 248–254. [Google Scholar] [CrossRef]
- Chiu, T.Y.; Chen, P.H.; Chang, C.L.; Yang, D.M. Live-cell dynamic sensing of Cd2+ with a FRET-based indicator. PLoS ONE 2013, 8, e65853. [Google Scholar] [CrossRef]
- Taki, M.; Desaki, M.; Ojida, A.; Iyoshi, S.; Hirayama, T.; Hamachi, I.; Yamamoto, Y. Fluorescence imaging of intracellular cadmium using a dual-excitation ratiometricchemosensor. J. Am. Chem. Soc. 2008, 130, 12564–12565. [Google Scholar] [CrossRef]
- Michelini, E.; Cevenini, L.; Mezzanotte, L.; Roda, B.; Dolci, L.S.; Roda, A. Bioluminescent reporter proteins for multicolor assays. Minerva Biotecnol. 2009, 21, 87–96. [Google Scholar]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Tauriainen, S.; Karp, M.; Chang, W.; Virta, M. Recombinant luminescent bacteria for measuring bioavailable arsenite and antimonite. Appl. Environ. Microbiol. 1997, 63, 4456–4461. [Google Scholar] [CrossRef]
- Tauriainen, S.; Karp, M.; Chang, W.; Virta, M. Luminescent bacterial sensor for cadmium and lead. Biosens. Bioelectron. 1998, 13, 931–938. [Google Scholar] [CrossRef]
- Nakajima, Y.; Ikeda, M.; Kimura, T.; Honma, S.; Ohmiya, Y.; Honma, K.I. Bidirectional role of orphan nuclear receptor RORα in clock gene transcriptions demonstrated by a novel reporter assay system. FEBS Lett. 2004, 565, 122–126. [Google Scholar] [CrossRef]
- Hattori, M.; Ozawa, T. Split luciferase complementation for analysis of intracellular signaling. Anal. Sci. 2014, 30, 539–544. [Google Scholar] [CrossRef]
- Agulhon, C.; Platel, J.C.; Kolomiets, B.; Forster, V.; Picaud, S.; Brocard, J.; Faure, P.; Brulet, P. Bioluminescent imaging of Ca2+ activity reveals spatiotemporal dynamics in glial networks of dark-adapted mouse retina. J. Physiol. 2007, 583, 945–958. [Google Scholar] [CrossRef]
- Rogers, K.L.; Stinnakre, J.; Agulhon, C.; Jublot, D.; Shorte, S.L.; Kremer, E.J.; Brûlet, P. Visualization of local Ca2+ dynamics with genetically encoded bioluminescent reporters. Eur. J. Neurosci. 2005, 21, 597–610. [Google Scholar] [CrossRef]
- Saito, K.; Chang, Y.F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 2012, 3, 1262. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef]
- Ghose, A.; Maltsev, O.V.; Humbert, N.; Hintermann, L.; Arntz, Y.; Naumov, P.; Mély, Y.; Didier, P. Oxyluciferin Derivatives: A Toolbox of Environment-Sensitive Fluorescence Probes for Molecular and Cellular Applications. J. Phys. Chem. B 2017, 121, 1566–1575. [Google Scholar] [CrossRef]
- Ohmiya, Y. Basic and Applied aspects of color tuning of BL systems. Jpn. J. Appl. Phys. 2005, 44, 6368–6379. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Q.; Robertson, J.B.; Johnson, C.H. pHlash: A New Genetically Encoded and Ratiometric Luminescence Sensor of Intracellular pH. PLoS ONE 2012, 7, e43072. [Google Scholar] [CrossRef]
- Zhang, Y.; Robertson, J.B.; Xie, Q.; Johnson, C.H. Monitoring Intracellular pH Change with a Genetically Encoded and Ratiometric Luminescence Sensor in Yeast and Mammalian Cells. Methods Mol. Biol. (Clifton N.J.) 2016, 1461, 117–130. [Google Scholar] [CrossRef]
- Wang, Y.; Kubota, H.; Yamada, N.; Irie, T.; Akiyama, H. Quantum yields and quantitative spectra of firefly bioluminescence with various bivalent metal ions. Photochem. Photobiol. 2011, 87, 846–852. [Google Scholar] [CrossRef]
- Yang, J.; Johnson, C.H. Bioluminescent Sensors for Ca++ Flux Imaging and the Introduction of a New Intensity-Based Ca++ Sensor. Front. Bioeng. Biotechnol. 2021, 1036, 773353. [Google Scholar] [CrossRef]
- Yang, J.; Cumberbatch, D.; Centanni, S.; Shi, S.Q.; Winder, D.; Webb, D.; Johnson, C.H. Coupling optogenetic stimulation with NanoLuc-based luminescence (BRET) Ca++ sensing. Nat. Commun. 2016, 7, 13268. [Google Scholar] [CrossRef]
- Merkx, M.; Golynskiy, M.V.; Lindenburg, L.H.; Vinkenborg, J.L. Rational design of FRET sensor proteins based on mutually exclusive domain interactions. Biochem. Soc. Trans. 2013, 41, 1201–1205. [Google Scholar] [CrossRef]
- Ando, Y.; Niwa, K.; Yamada, N.; Enomoto, T.; Irie, T.; Kubota, H.; Ohmiya, Y.; Akiyama, H. Firefly bioluminescence quantum yield and colour change by pH-sensitive green emission. Nat. Photonics 2008, 2, 44–47. [Google Scholar] [CrossRef]
- Viviani, V.R.; Arnoldi, F.G.C.; Neto, A.S.; Oehlmeyer, T.L.; Bechara, E.J.H.; Ohmiya, Y. The structural origin and biological function of pH-sensitivity in firefly luciferases. Photochem. Photobiol. Sci. 2008, 7, 159–169. [Google Scholar] [CrossRef]
- Oliveira, G.; Viviani, V.R. Comparison of the thermostability of recombinant luciferases from Brazilian bioluminescent beetles: Relationship with kinetics and bioluminescence colours. Luminescence 2018, 33, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Pelentir, G.F.; Bevilaqua, V.R.; Viviani, V.R. A highly efficient, thermostable and cadmium selective firefly luciferase suitable for ratiometric metal and pH biosensing and for sensitive ATP assays. Photochem. Photobiol. Sci. 2019, 18, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Viviani, V.R. Temperature effect on the bioluminescence spectra of firefly luciferases: Potential applicability for ratiometric biosensing of temperature and pH. Photochem. Photobiol. Sci. 2019, 18, 2682–2687. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Wang, Y.; Hiyama, M.; Akiyama, H. Robust Red-Emission Spectra and Yields in Firefly Bioluminescence against Temperature Changes. Appl. Phys. Lett. 2014, 104, 213704. [Google Scholar] [CrossRef]
- Branchini, B.R.; Southworth, T.L.; Murtiashaw, M.H.; Magyar, R.A.; Gonzalez, S.A.; Ruggiero, M.C.; Stroh, J.G. An alternative mechanism of bioluminescence color determination in firefly luciferase. Biochemistry 2004, 43, 7255–7262. [Google Scholar] [CrossRef]
- Ugarova, N.N.; Brovko, L.Y. Protein structure and bioluminescent spectra for firefly bioluminescence. Lumin. J. Biol. Chem. Lumin. 2002, 17, 321–330. [Google Scholar] [CrossRef]
- Viviani, V.R.; Simões, A.; Bevilaqua, V.R.; Gabriel GV, M.; Arnoldi FG, C.; Hirano, T. Glu311 and Arg337 stabilize a closed active-site conformation and provide a critical catalytic base and countercation for green bioluminescence in beetle luciferases. Biochemistry 2016, 55, 4764–4776. [Google Scholar] [CrossRef]
- Granier, T.; Comberton, G.; Gallois, B.; d’Estaintot, B.L.; Dautant, A.; Crichton, R.R.; Précigoux, G. Evidence of new cadmium binding sites in recombinant horse L-chain ferritin by anomalous Fourier difference map calculation. Proteins 1998, 31, 477–485. [Google Scholar] [CrossRef]
- Friedman, R. Structural and computational insights into the versatility of cadmium binding to proteins. Dalton Trans. 2014, 43, 2878–2887. [Google Scholar] [CrossRef]
- Hirano, T.; Hasumi, Y.; Ohtsuka, K.; Maki, S.; Niwa, H.; Yamaji, M.; Hashizume, D. Spectroscopic studies of the light-color modulation mechanism of firefly (beetle) bioluminescence. J. Am. Chem. Soc. 2009, 131, 2385–2396. [Google Scholar] [CrossRef]
- Carrasco-Lopez, C.; Lui, N.M.; Schramm, S.; Naumov, P. The elusive relationship between structure and color emission in beetle luciferases. Nat. Rev. Chem. 2021, 5, 4–20. [Google Scholar] [CrossRef]
- Regan, L. Protein Design: Novel metal-binding sites. Trends Biochem. Sci. 1995, 20, 280–285. [Google Scholar] [CrossRef]
- Sciortino, G.; Garribba, E.; Rodríguez-Guerra Pedregal, J.; Maréchal, J.D. Simple Coordination Geometry Descriptors Allow to Accurately Predict Metal-Binding Sites in Proteins. ACS Omega 2019, 4, 3726–3731. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Ichino, Y.; Kumata, S.; Nakajima, Y.; Hiraishi, Y.; Kato, D.I.; Viviani, V.R.; Ohmiya, Y. Quantum yields and kinetics of the firefly bioluminescence reaction of beetle luciferases. Photochem. Photobiol. 2010, 86, 1046–1049. [Google Scholar] [CrossRef]
- Enomoto, T.; Kubota, H.; Mori, K.; Shimogawara, M.; Yoshita, M.; Ohmiya, Y.; Akiyama, H. Absolute bioluminescence imaging at the single-cell level with a light signal at the Attowatt level. Biotechniques 2018, 64, 270–274. [Google Scholar] [CrossRef]
- Koop, A.; Cobbold, P.H. Continuous bioluminescent monitoring of cytoplasmic ATP in single isolated rat hepatocytes during metabolic poisoning. Biochem. J. 1993, 295, 165–170. [Google Scholar] [CrossRef]
- Viviani, V.R.; Bechara, E.J.; Ohmiya, Y. Cloning, sequence analysis, and expression of active Phrixothrix railroad-worms luciferases: Relationship between bioluminescence spectra and primary structures. Biochemistry 1999, 38, 8271–8279. [Google Scholar] [CrossRef]
- Ohmiya, Y.; Sumiya, M.; Viviani, V.R.; Ohba, N. Comparative aspects of a luciferase molecule from the Japanese luminous beetle, Rhagophthalmus ohbai. Sci. Rep. Yokosuka City Mus 2000, 47, 31–38. [Google Scholar]
- Jathoul, A.P.; Grounds, H.; Anderson, J.C.; Pule, M.A. A dual-color far-red to near-infrared firefly luciferin analogue designed for multiparametric bioluminescence imaging. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 13059–13063. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).