Enhanced Detection in Droplet Microfluidics by Acoustic Vortex Modulation of Particle Rings and Particle Clusters via Asymmetric Propagation of Surface Acoustic Waves
Abstract
:1. Introduction
2. Experimental Set-Up, Materials and Methods
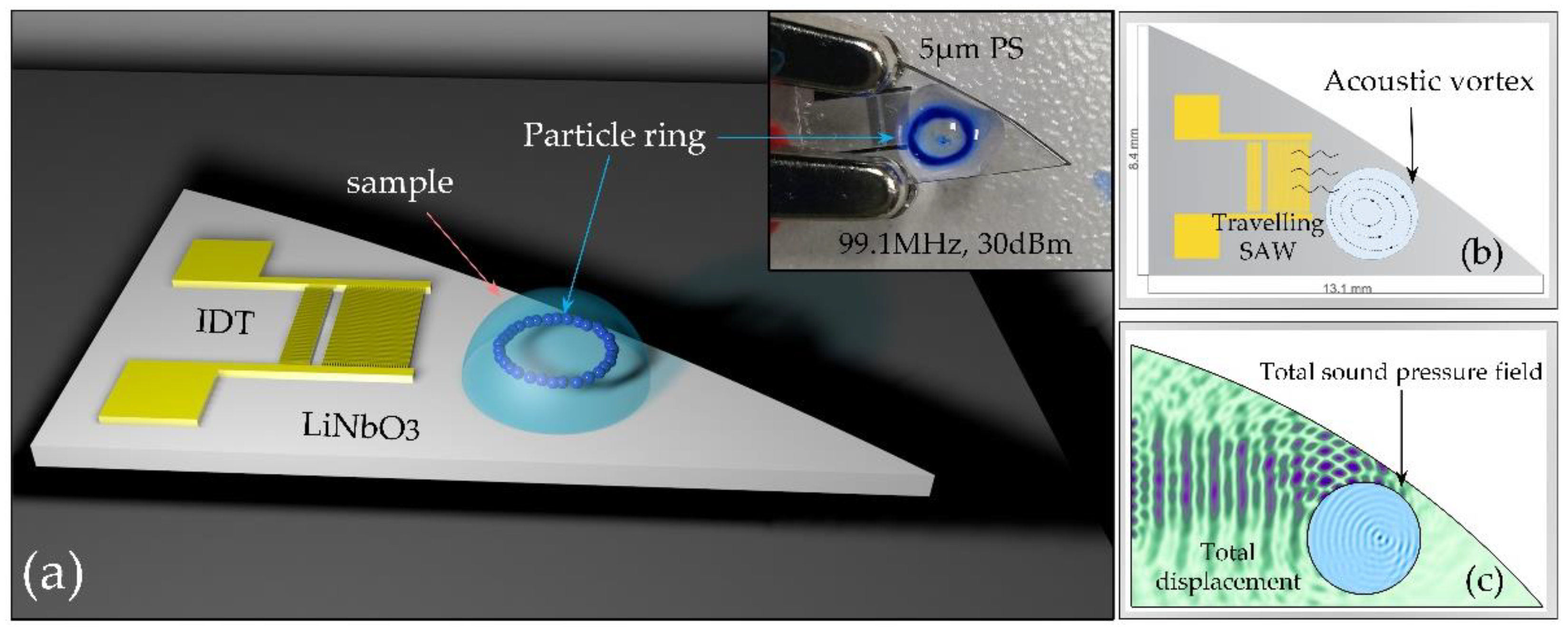
2.1. Experimental Set-Up, Materials and Methods
2.2. Methods
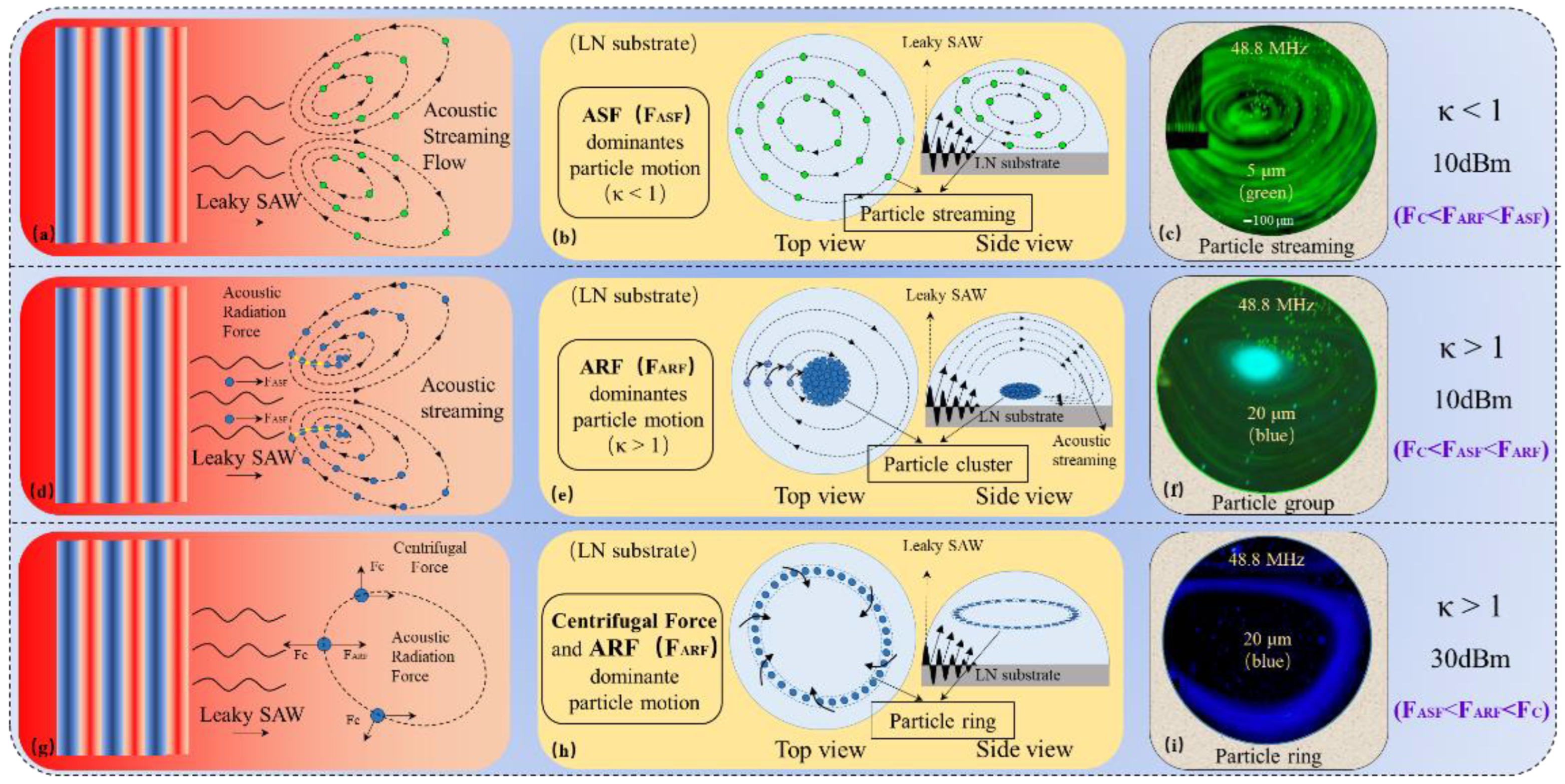
2.2.1. Particle Streaming and Particle Cluster
2.2.2. Particle Ring
3. Results
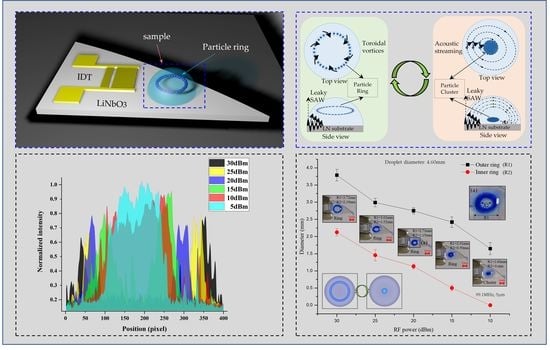
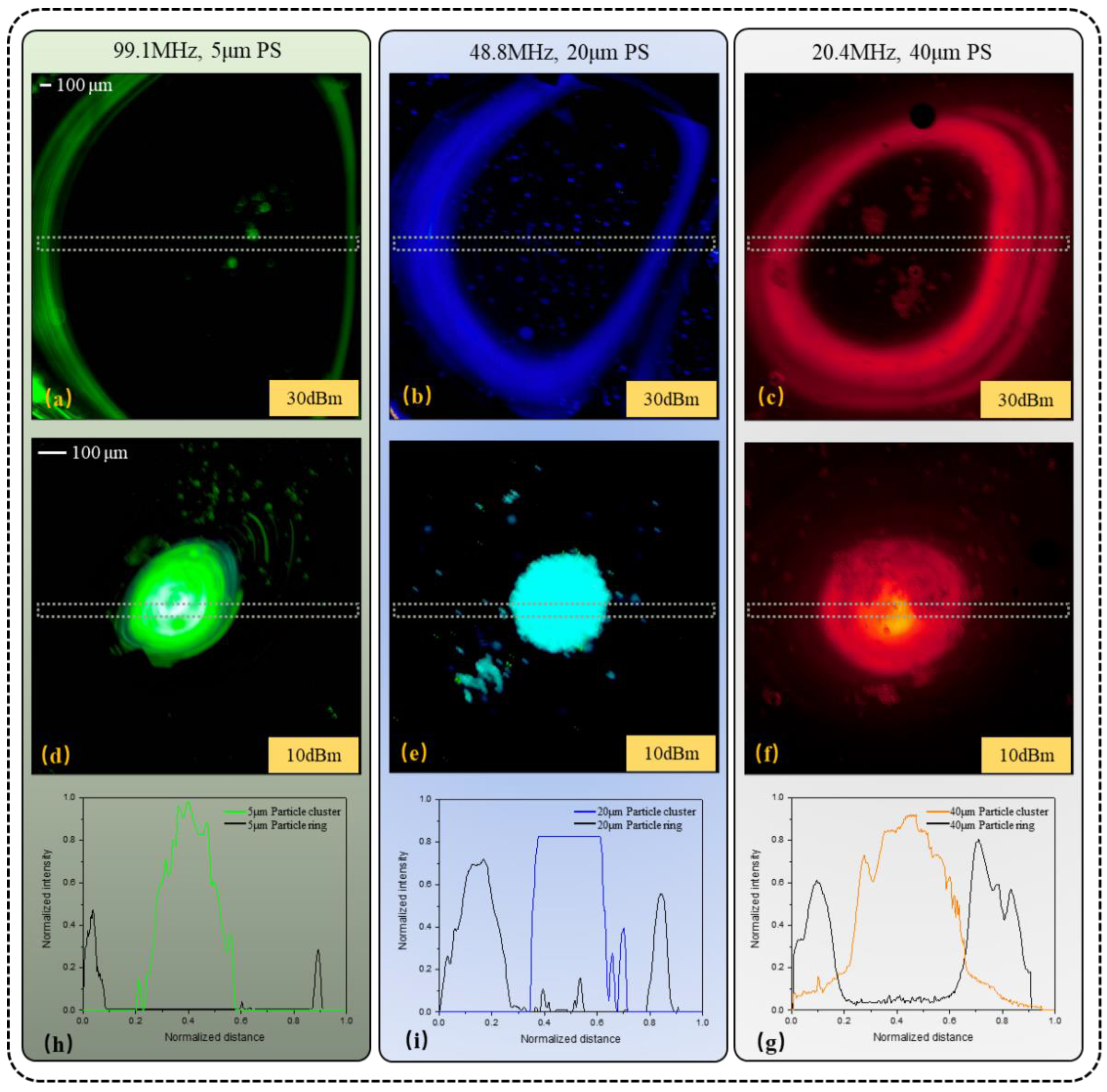
3.1. Testing of Particle Ring and Cluster with Different Particle Diameters
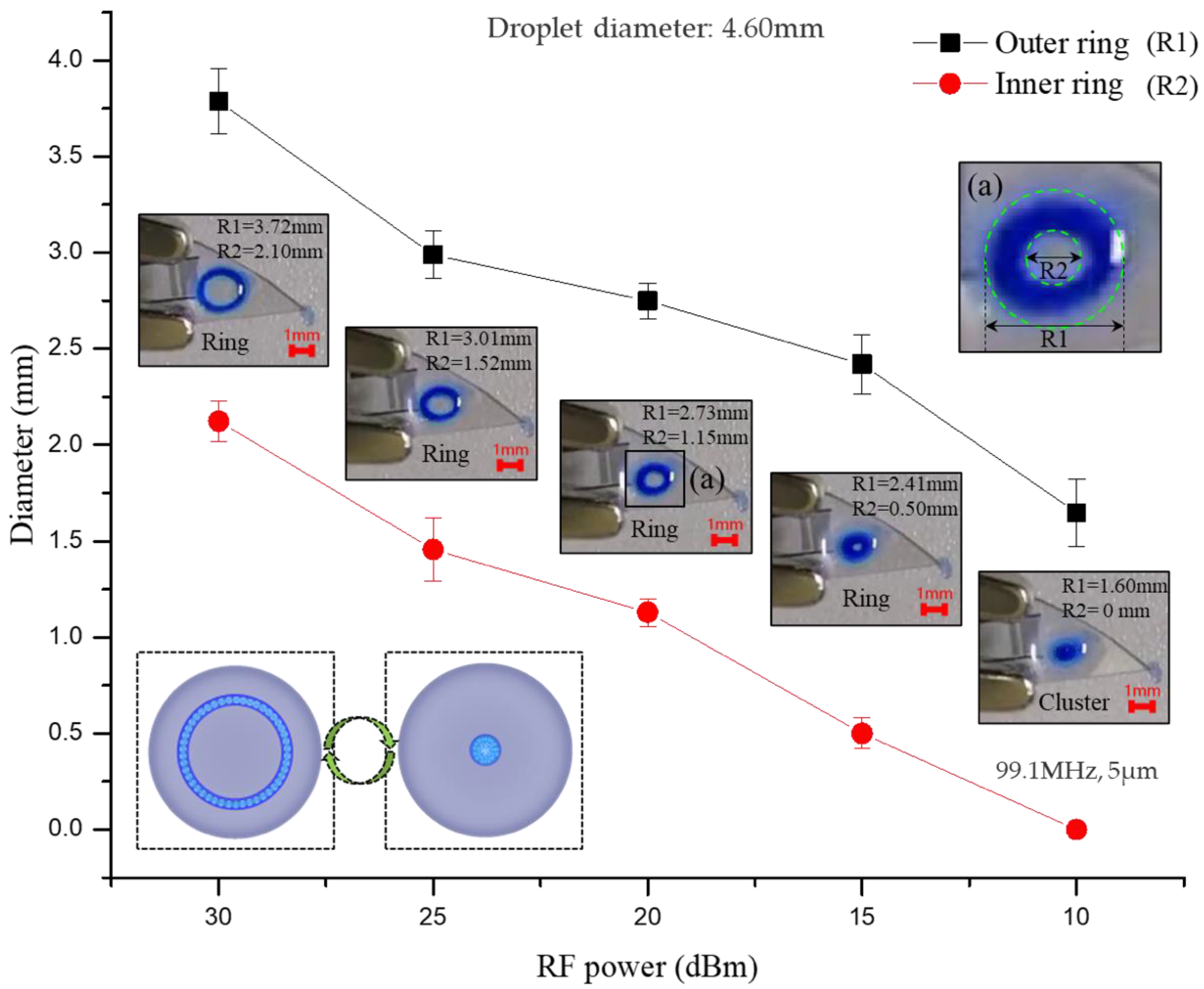
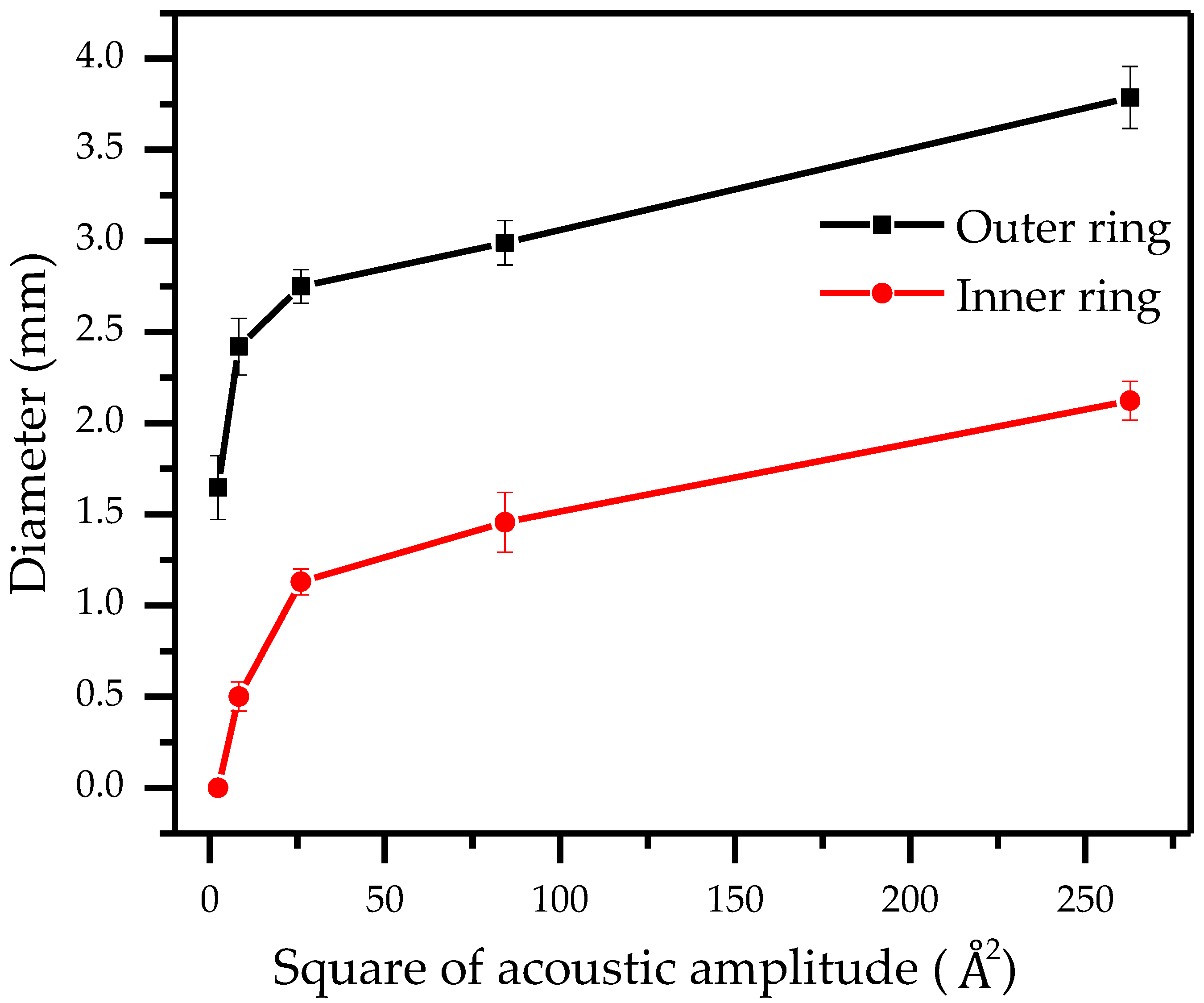
3.2. The Free Transition of Particle Ring and Particle Cluster
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Valpapuram, I.; Candeloro, P.; Coluccio, M.L.; Parrotta, E.I.; Giugni, A.; Das, G.; Cuda, G.; Di Fabrizio, E.; Perozziello, G. Waveguiding and SERS Simplified Raman Spectroscopy on Biological Samples. Biosensors 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandas, P.J.; Luo, J.; Quan, A.; Qiu, C.; Cao, W.; Fu, C.; Fu, Y.Q. Highly selective and label-free Love-mode surface acoustic wave biosensor for carcinoembryonic antigen detection using a self-assembled monolayer bioreceptor. Appl. Surf. Sci. 2020, 518, 146061. [Google Scholar] [CrossRef]
- Shin, J.H.; Seo, J.; Hong, J.; Chung, S.K. Hybrid optothermal and acoustic manipulations of microbubbles for precise and on-demand handling of micro-objects. Sensor Actuat. B-Chem. 2017, 246, 415–420. [Google Scholar] [CrossRef]
- Xu, M.; Lee, P.V.S.; Collins, D.J. Microfluidic acoustic sawtooth metasurfaces for patterning and separation using traveling surface acoustic waves. Lab Chip 2021, 22, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Liu, S.; Xiao, Y.; Zhang, G.; Cui, W.; Reed, M. A microfluidic chip with a serpentine channel enabling high-throughput cell separation using surface acoustic waves. Lab Chip 2021, 21, 4608–4617. [Google Scholar] [CrossRef]
- Habibi, R.; Neild, A. Sound wave activated nano-sieve (SWANS) for enrichment of nanoparticles. Lab Chip 2019, 19, 3032–3044. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, C.; Rufo, J.; Shen, C.; Huang, T.J. Acoustofluidic Holography for Micro- to Nanoscale Particle Manipulation. ACS Nano 2020, 14, 14635–14645. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Z.; Bachman, H.; Li, P.; Zhang, P.; Ren, L.; Wu, M.; Huang, T.J. Acoustic Cell Separation Based on Density and Mechanical Properties. J. Biomech. Eng. 2020, 142, 0310051–0310059. [Google Scholar] [CrossRef]
- Hengoju, S.; Shvydkiv, O.; Tovar, M.; Roth, M.; Rosenbaum, M.A. Advantages of optical fibers for facile and enhanced detection in droplet microfluidics. Biosens. Bioelectron. 2022, 200, 113910. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.L.; Fan, Z.H. Sessile droplets for chemical and biological assays. Lab Chip 2017, 17, 2150–2166. [Google Scholar] [CrossRef]
- Han, J.L.; Hu, H.; Huang, Q.Y.; Lei, Y.L. Particle Separation by Standing Surface Acoustic Waves inside a Sessile Droplet. Sensor Actuat. A-Phys. 2021, 326, 112731. [Google Scholar] [CrossRef]
- Richard, C.; Fakhfouri, A.; Colditz, M.; Striggow, F.; Kronstein-Wiedemann, R.; Tonn, T.; Medina-Sanchez, M.; Schmidt, O.G.; Gemming, T.; Winkler, A. Blood platelet enrichment in mass-producible surface acoustic wave (SAW) driven microfluidic chips. Lab Chip 2019, 19, 4043–4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tengfei, Z.; Chaohui, W.; Dong, N.; Weitao, J.; Yongsheng, S.; Lei, Y.; Bangdao, C.; Hongzhong, L.; Yucheng, D. Exploitation of surface acoustic waves to drive nanoparticle concentration within an electrification-dependent droplet. RSC Adv. 2014, 4, 46502–46507. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.C.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Tan, M.K.; Friend, J.R.; Yeo, L.Y. Interfacial Jetting Phenomena Induced by Focused Surface Vibrations. Phys. Rev. Lett. 2009, 103, 024501. [Google Scholar] [CrossRef] [Green Version]
- Rezk, A.R.; Friend, J.R.; Yeo, L.Y. Simple, low cost MHz-order acoustomicrofluidics using aluminium foil electrodes. Lab Chip 2014, 14, 1802–1805. [Google Scholar] [CrossRef]
- Li, H.; Friend, J.R.; Yeo, L.Y. Surface acoustic wave concentration of particle and bioparticle suspensions. Biomed. Microdevices 2007, 9, 647–656. [Google Scholar] [CrossRef]
- Bussonniere, A.; Miron, Y.; Baudoin, M.; Matar, O.B.; Grandbois, M.; Charette, P.; Renaudin, A. Cell detachment and label-free cell sorting using modulated surface acoustic waves (SAWs) in droplet-based microfluidics. Lab Chip 2014, 14, 3556–3563. [Google Scholar] [CrossRef]
- Salehi-Reyhani, A.; Gesellchen, F.; Mampallil, D.; Wilson, R.; Reboud, J.; Ces, O.; Willison, K.R.; Cooper, J.M.; Klug, D.R. Chemical-free lysis and fractionation of cells by use of surface acoustic waves for sensitive protein assays. Anal. Chem. 2015, 87, 2161–2169. [Google Scholar] [CrossRef] [Green Version]
- Akther, A.; Marqus, S.; Rezk, A.R.; Yeo, L.Y. Submicron Particle and Cell Concentration in a Closed Chamber Surface Acoustic Wave Microcentrifuge. Anal. Chem. 2020, 92, 10024–10032. [Google Scholar] [CrossRef]
- Akther, A.; Walsh, E.P.; Reineck, P.; Gibson, B.C.; Ohshima, T.; Abe, H.; McColl, G.; Jenkins, N.L.; Hall, L.T.; Simpson, D.A.; et al. Acoustomicrofluidic Concentration and Signal Enhancement of Fluorescent Nanodiamond Sensors. Anal. Chem. 2021, 93, 16133–16141. [Google Scholar] [CrossRef] [PubMed]
- Connacher, W.; Zhang, N.; Huang, A.; Mei, J.; Zhang, S.; Gopesh, T.; Friend, J. Micro/nano acoustofluidics: Materials, phenomena, design, devices, and applications. Lab Chip 2018, 18, 1952–1996. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.V.; Friend, J.R.; Yeo, L.Y. Particle concentration via acoustically driven microcentrifugation: MicroPIV flow visualization and numerical modelling studies. Microfluid. Nanofluid. 2010, 8, 73–84. [Google Scholar] [CrossRef]
- Bourquin, Y.; Reboud, J.; Wilson, R.; Cooper, J.M. Tuneable surface acoustic waves for fluid and particle manipulations on disposable chips. Lab Chip 2010, 10, 1898–1901. [Google Scholar] [CrossRef]
- Eckart, C. Vortices and Streams Caused by Sound Waves. Phys. Rev. 1948, 73, 68–76. [Google Scholar] [CrossRef]
- Destgeer, G.; Cho, H.; Ha, B.H.; Jung, J.H.; Park, J.; Sung, H.J. Acoustofluidic particle manipulation inside a sessile droplet: Four distinct regimes of particle concentration. Lab Chip 2016, 16, 660–667. [Google Scholar] [CrossRef]
- Destgeer, G.; Ha, B.; Park, J.; Sung, H.J. Lamb Wave-Based Acoustic Radiation Force-Driven Particle Ring Formation inside a Sessile Droplet. Anal. Chem. 2016, 88, 3976–3981. [Google Scholar] [CrossRef]
- Dentry, M.B.; Yeo, L.Y.; Friend, J.R. Frequency effects on the scale and behavior of acoustic streaming. Phys. Rev. E 2014, 89, 013203. [Google Scholar] [CrossRef] [Green Version]
- Destgeer, G.; Ha, B.H.; Jung, J.H.; Sung, H.J. Submicron separation of microspheres via travelling surface acoustic waves. Lab Chip 2014, 14, 4665–4672. [Google Scholar] [CrossRef]
- Fakhfouri, A.; Devendran, C.; Ahmed, A.; Soria, J.; Neild, A. The size dependant behaviour of particles driven by a travelling surface acoustic wave (TSAW). Lab Chip 2018, 18, 3926–3938. [Google Scholar] [CrossRef]
- Xie, Y.; Rufo, J.; Zhong, R.; Rich, J.; Li, P.; Leong, K.W.; Huang, T.J. Microfluidic Isolation and Enrichment of Nanoparticles. ACS Nano 2020, 14, 16220–16240. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.H.; Lee, K.S.; Destgeer, G.; Park, J.; Choung, J.S.; Jung, J.H.; Shin, J.H.; Sung, H.J. Acoustothermal heating of polydimethylsiloxane microfluidic system. Sci. Rep.-UK 2015, 5, 11851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronek, V.; Rambach, R.W.; Schmid, L.; Haase, K.; Franke, T. Particle deflection in a poly(dimethylsiloxane) microchannel using a propagating surface acoustic wave: Size and frequency dependence. Anal. Chem. 2013, 85, 9955–9959. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab Chip 2012, 12, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Sachs, S.; Baloochi, M.; Cierpka, C.; Konig, J. On the acoustically induced fluid flow in particle separation systems employing standing surface acoustic waves—Part I. Lab Chip 2022, 22, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Destgeer, G.; Ha, B.H.; Park, J.; Jung, J.H.; Alazzam, A.; Sung, H.J. Microchannel anechoic corner for size-selective separation and medium exchange via traveling surface acoustic waves. Anal. Chem. 2015, 87, 4627–4632. [Google Scholar] [CrossRef] [PubMed]
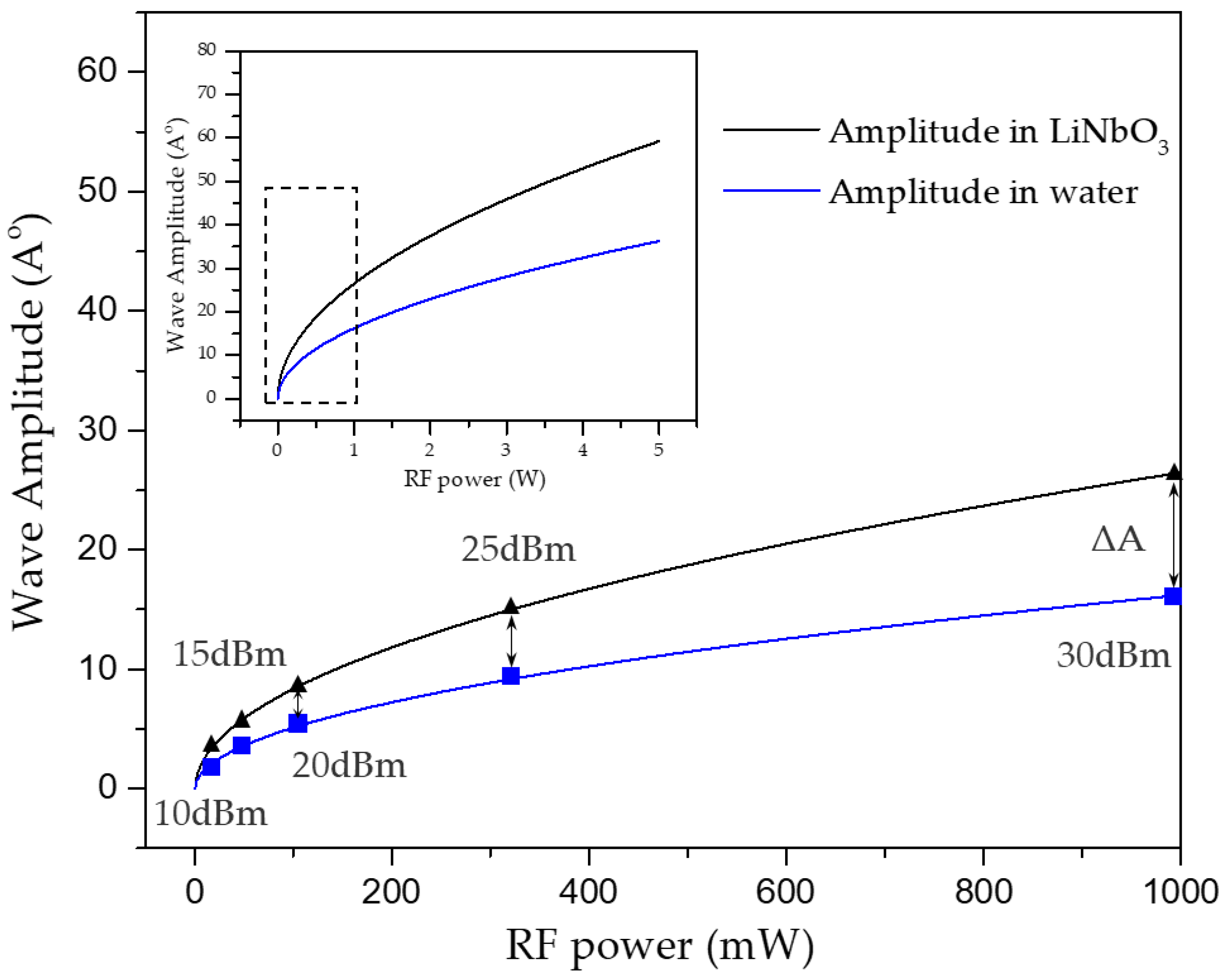



| RF Power (dBm) | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|
| Amplitude in LiNbO3 () | 2.51 | 4.71 | 8.34 | 14.90 | 26.49 |
| Amplitude in water () | 1.54 | 2.88 | 5.10 | 9.18 | 16.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ji, M.; Yu, N.; Zhao, C.; Xue, G.; Fu, W.; Qiao, X.; Zhang, Y.; Chou, X.; Geng, W. Enhanced Detection in Droplet Microfluidics by Acoustic Vortex Modulation of Particle Rings and Particle Clusters via Asymmetric Propagation of Surface Acoustic Waves. Biosensors 2022, 12, 399. https://doi.org/10.3390/bios12060399
Liu Y, Ji M, Yu N, Zhao C, Xue G, Fu W, Qiao X, Zhang Y, Chou X, Geng W. Enhanced Detection in Droplet Microfluidics by Acoustic Vortex Modulation of Particle Rings and Particle Clusters via Asymmetric Propagation of Surface Acoustic Waves. Biosensors. 2022; 12(6):399. https://doi.org/10.3390/bios12060399
Chicago/Turabian StyleLiu, Yukai, Miaomiao Ji, Nanxin Yu, Caiqin Zhao, Gang Xue, Wenxiao Fu, Xiaojun Qiao, Yichi Zhang, Xiujian Chou, and Wenping Geng. 2022. "Enhanced Detection in Droplet Microfluidics by Acoustic Vortex Modulation of Particle Rings and Particle Clusters via Asymmetric Propagation of Surface Acoustic Waves" Biosensors 12, no. 6: 399. https://doi.org/10.3390/bios12060399
APA StyleLiu, Y., Ji, M., Yu, N., Zhao, C., Xue, G., Fu, W., Qiao, X., Zhang, Y., Chou, X., & Geng, W. (2022). Enhanced Detection in Droplet Microfluidics by Acoustic Vortex Modulation of Particle Rings and Particle Clusters via Asymmetric Propagation of Surface Acoustic Waves. Biosensors, 12(6), 399. https://doi.org/10.3390/bios12060399