A Proof-of-Concept Electrochemical Cytosensor Based on Chlamydomonas reinhardtii Functionalized Carbon Black Screen-Printed Electrodes: Detection of Escherichia coli in Wastewater as a Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Algae Growth Conditions and Physiological Characterization of the Algal Liquid Cultures
- ✓
- F0 or fluorescence in the initial state: minimum fluorescence intensity in the state acclimated to the darkness, recorded when all PSII reaction centres are open (oxidized quinones);
- ✓
- FM or maximum fluorescence: maximum fluorescence intensity reached after 10 min of darkness and a subsequent saturating light pulse, recorded when all reaction centres of the PSII are closed (reduced);
- ✓
- FV/FM: maximum fluorescence yield of PSII photochemical reaction expressed as a ratio of variable fluorescence (FM − F0) and maximum fluorescence, calculated according to the Equation (1):
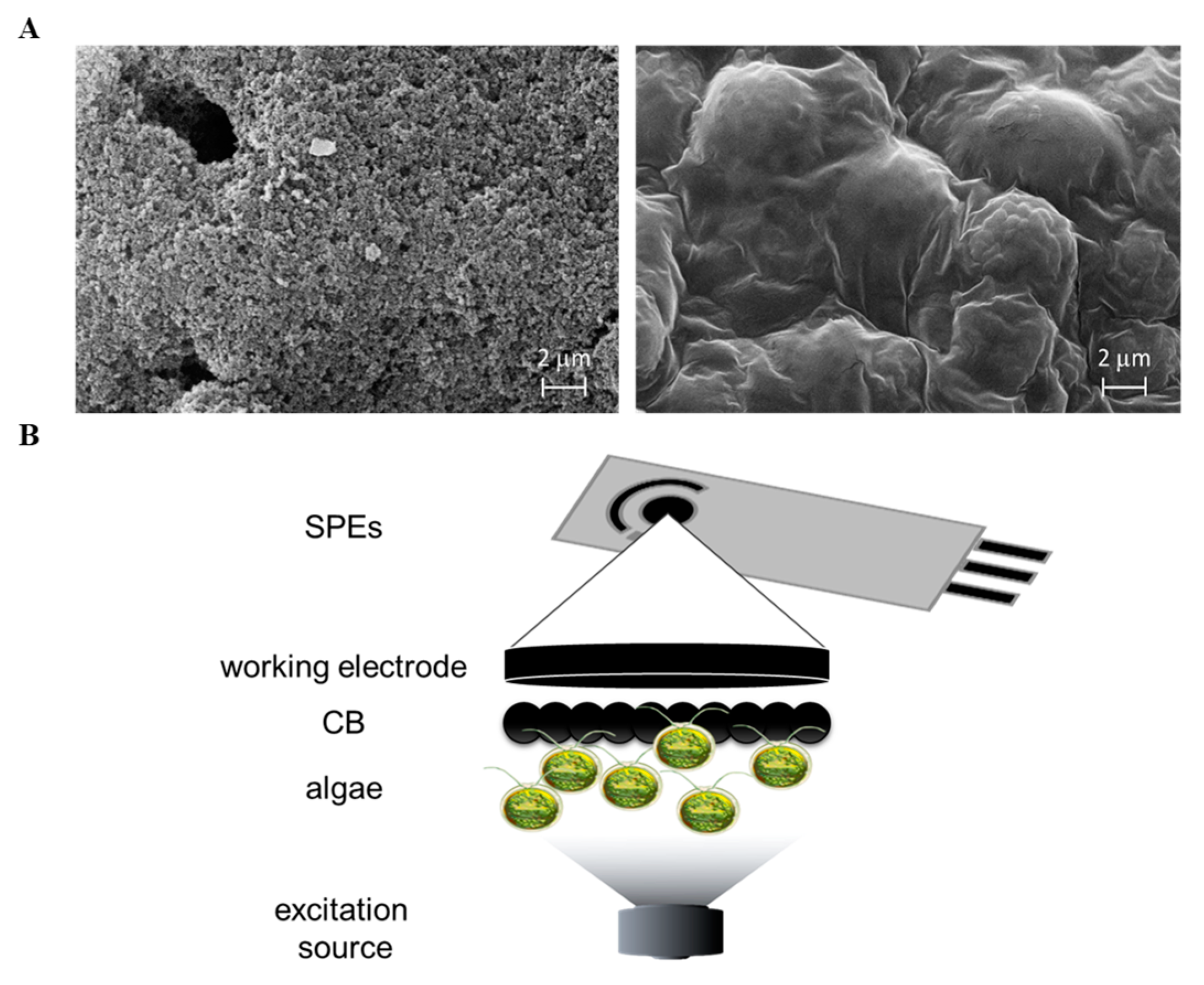
2.3. Algae Immobilization Protocol
2.4. Biosensor Prototype
2.5. Pathogen Detection
3. Results
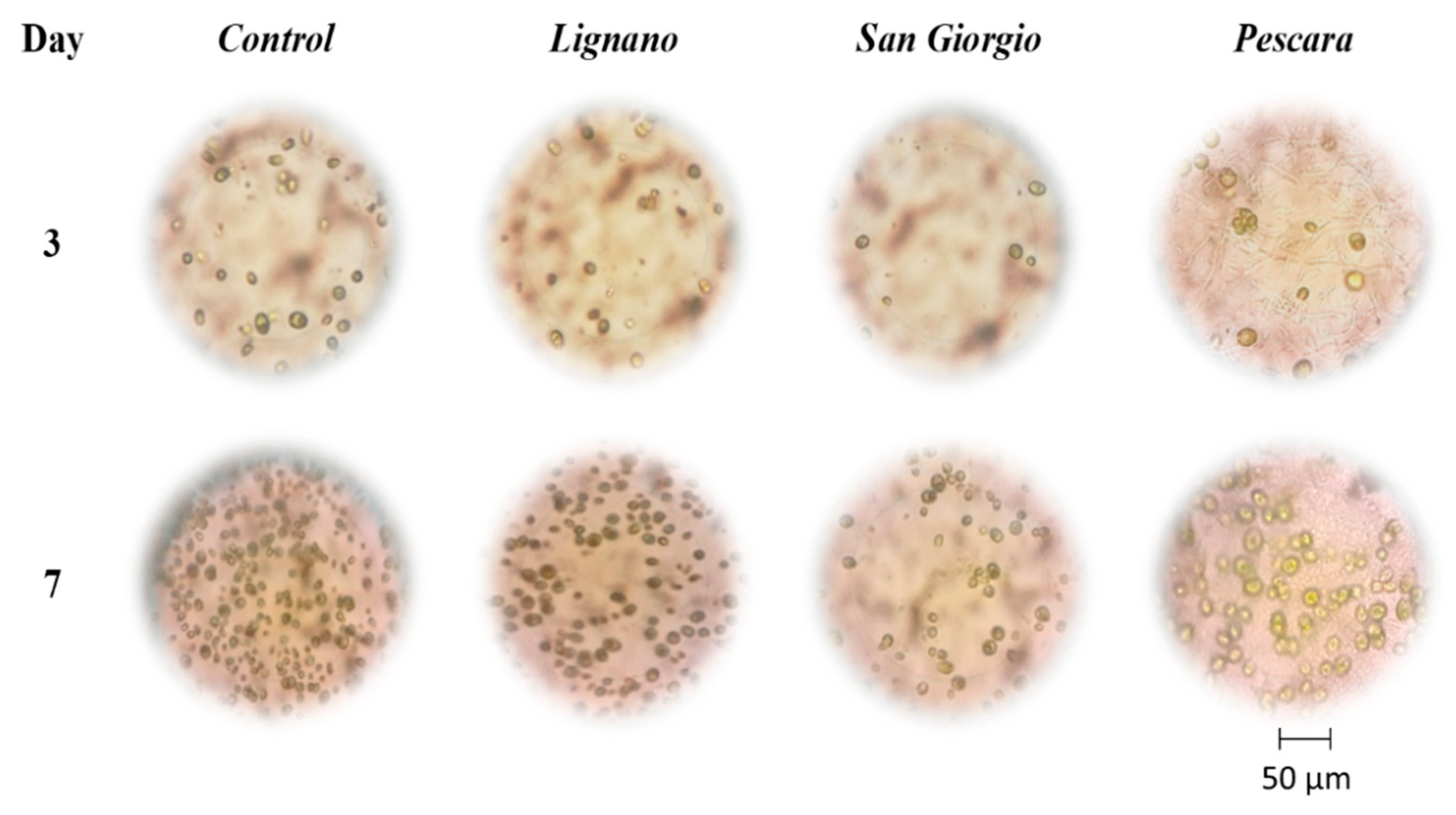
3.1. Effect of Wastewater Samples on the Alga C. Reinhardtii
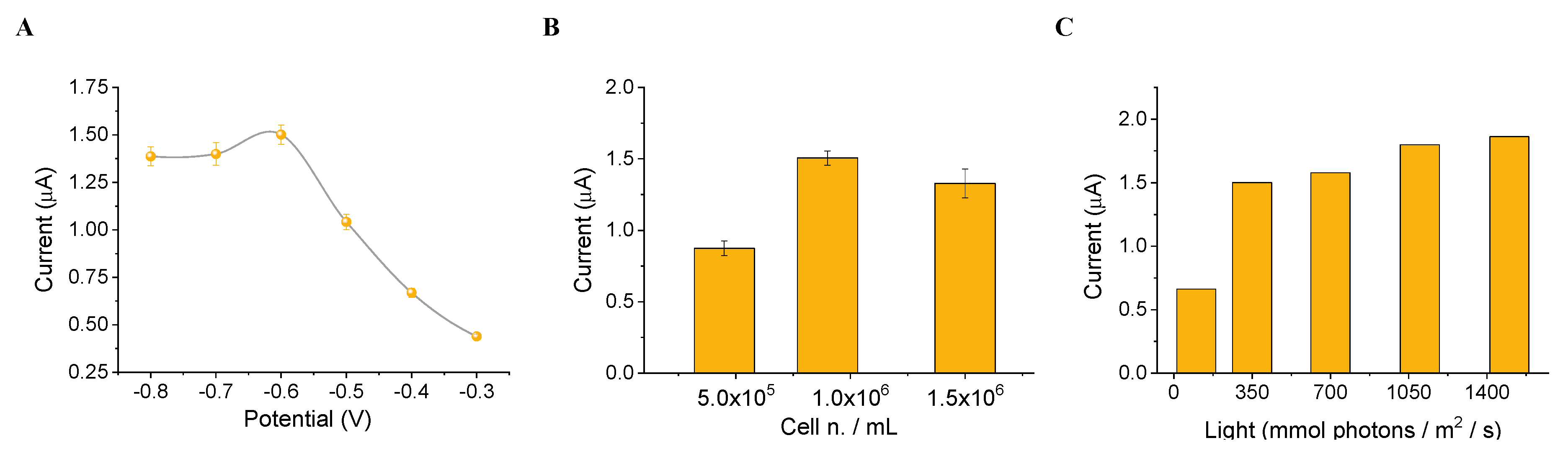
3.2. Set-Up of the Algal Cytosensor and Assessment of the Analytical Parameters
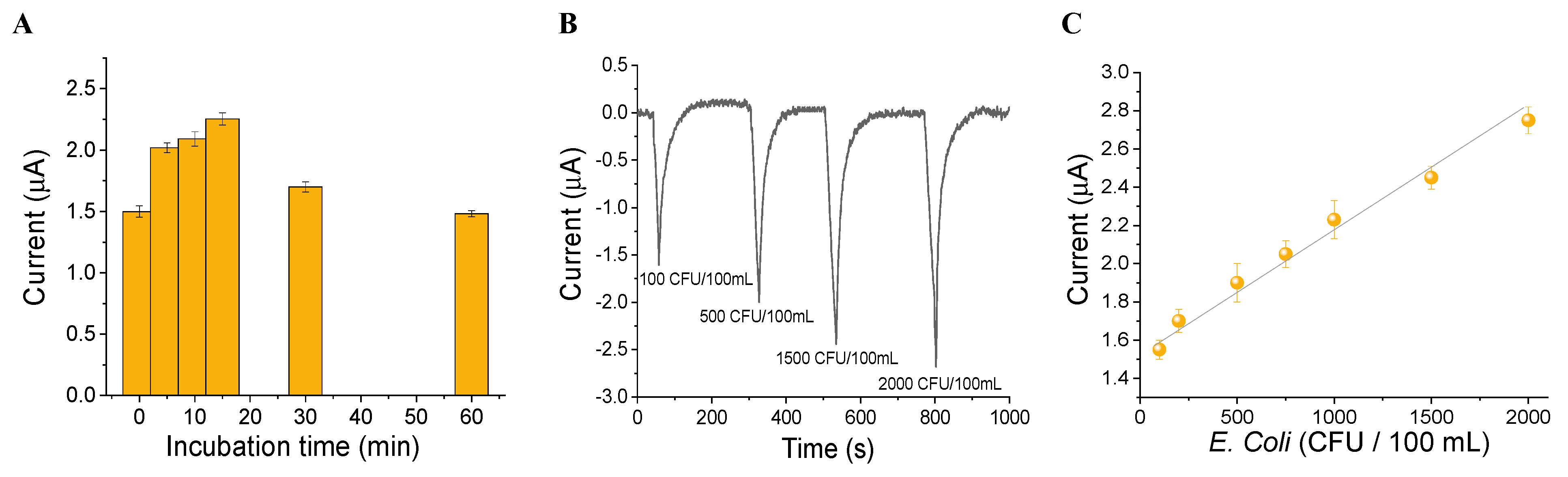
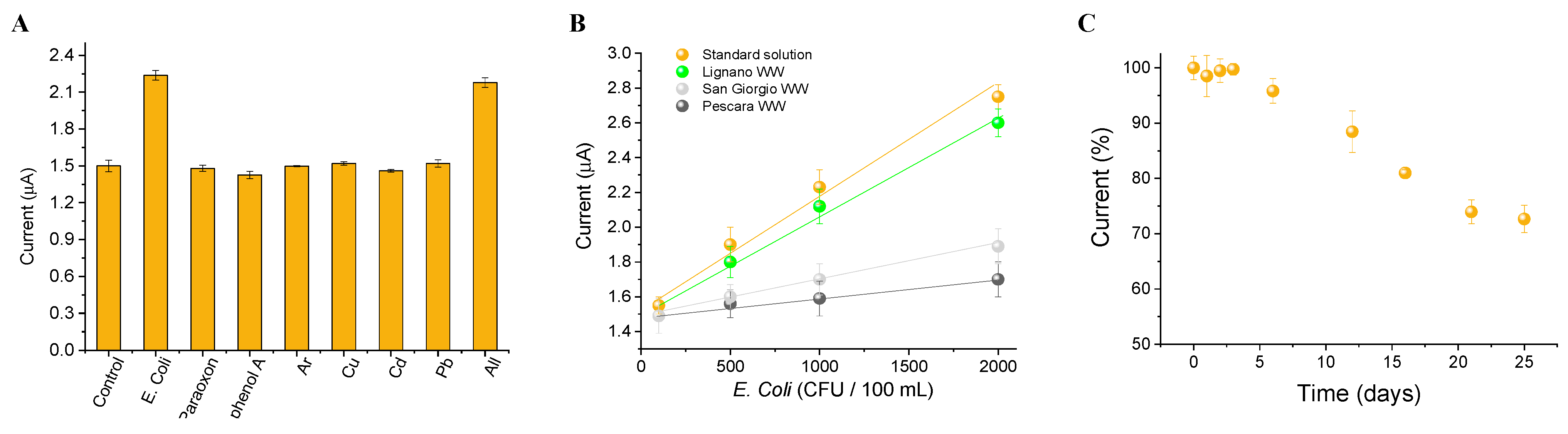
3.3. Algal Cytosensor Analytical Response to Pathogens
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inyinbor Adejumoke, A.; Adebesin Babatunde, O.; Oluyori Abimbola, P.; Adelani Akande Tabitha, A.; Dada Adewumi, O.; Oreofe Toyin, A. Water pollution: Effects, prevention, and climatic impact. Water Chall. Urban. World 2018, 33, 33–47. [Google Scholar]
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater Against Pollution and Deterioration. 2006. Available online: http://data.europa.eu/eli/dir/2006/118/oj/eng (accessed on 7 November 2021).
- Umar, M.F.; Nasar, A. Reduced graphene oxide/polypyrrole/nitrate reductase deposited glassy carbon electrode (GCE/RGO/PPy/NR): Biosensor for the detection of nitrate in wastewater. Appl. Water Sci. 2018, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Campaña, A.L.; Florez, S.L.; Noguera, M.J.; Fuentes, O.P.; Puentes, P.R.; Cruz, J.C.; Osma, J.F. Enzyme-Based Electrochemical Biosensors for Microfluidic Platforms to Detect Pharmaceutical Residues in Wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Liu, H. Impact of heterotrophic denitrification on BOD detection of the nitrate-containing wastewater using microbial fuel cell-based biosensors. Chem. Eng. J. 2020, 394, 125042. [Google Scholar] [CrossRef]
- Schirmer, C.; Posseckardt, J.; Schröder, M.; Gläser, M.; Howitz, S.; Scharff, W.; Mertig, M. Portable and low-cost biosensor towards on-site detection of diclofenac in wastewater. Talanta 2019, 203, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Yashas, S.R.; Sandeep, S.; Shivakumar, B.P.; Swamy, N.K. A matrix of perovskite micro-seeds and polypyrrole nanotubes tethered laccase/graphite biosensor for sensitive quantification of 2,4-dichlorophenol in wastewater. Anal. Methods 2019, 11, 4511–4519. [Google Scholar] [CrossRef]
- Cui, L.; Li, H.-Z.; Yang, K.; Zhu, L.-J.; Xu, F.; Zhu, Y.-G. Raman biosensor and molecular tools for integrated monitoring of pathogens and antimicrobial resistance in wastewater. TrAC Trends Anal. Chem. 2021, 143, 116415. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Yang, Z. An integrated biosensor system with mobile health and wastewater-based epidemiology (iBMW) for COVID-19 pandemic. Biosens. Bioelectron. 2020, 169, 112617. [Google Scholar] [CrossRef]
- Gao, G.; Fang, D.; Yu, Y.; Wu, L.; Wang, Y.; Zhi, J. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Antonacci, A.; Scognamiglio, V. Biotechnological Advances in the Design of Algae-Based Biosensors. Trends Biotechnol. 2020, 38, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Antonacci, A.; Attaallah, R.; Arduini, F.; Amine, A.; Giardi, M.T.; Scognamiglio, V. A dual electro-optical biosensor based on Chlamydomonas reinhardtii immobilised on paper-based nanomodified screen-printed electrodes for herbicide monitoring. J. Nanobiotechnol. 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Antonacci, A.; Arduini, F.; Moscone, D.; Campos, E.V.; Fraceto, L.F.; Palleschi, G. An eco-designed paper-based algal biosensor for nanoformulated herbicide optical detection. J. Hazard. Mater. 2019, 373, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Attaallah, R.; Antonacci, A.; Mazzaracchio, V.; Moscone, D.; Palleschi, G.; Arduini, F.; Amine, A.; Scognamiglio, V. Carbon black nanoparticles to sense algae oxygen evolution for herbicides detection: Atrazine as a case study. Biosens. Bioelectron. 2020, 159, 112203. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Pezzotti, I.; Pezzotti, G.; Cano, J.; Manfredonia, I.; Buonasera, K.; Rodio, G.; Giardi, M.T. A new embedded biosensor platform based on micro-electrodes array (MEA) technology. Sensors Actuators B Chem. 2013, 176, 275–283. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Pezzotti, I.; Pezzotti, G.; Cano, J.; Manfredonia, I.; Buonasera, K.; Arduini, F.; Moscone, D.; Palleschi, G.; Giardi, M.T. Towards an integrated biosensor array for simultaneous and rapid multi-analysis of endocrine disrupting chemicals. Anal. Chim. Acta 2012, 751, 161–170. [Google Scholar] [CrossRef]
- Antonacci, A.; Lambreva, M.D.; Arduini, F.; Moscone, D.; Palleschi, G.; Scognamiglio, V. A whole cell optical bioassay for the detection of chemical warfare mustard agent simulants. Sens. Actuators B Chem. 2018, 257, 658–665. [Google Scholar] [CrossRef]
- Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 Laying Down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstuffs of Animal Origin, Repealing Council Regulation (EEC) No 2377/90 and Amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council (Text with EEA Relevance). 2009. Available online: http://data.europa.eu/eli/reg/2009/470/oj/eng (accessed on 7 November 2021).
- Commission Decision (EU) 2018/229 of 12 February 2018 Establishing, Pursuant to Directive 2000/60/EC of the European Parliament and of the Council, the Values of the Member State Monitoring System Classifications as a Result of the Intercalibration Exercise and Repealing Commission Decision 2013/480/EU (Notified under Document C(2018) 696)Text with EEA Relevance. 2018. Available online: http://data.europa.eu/eli/dec/2018/229/oj/eng (accessed on 7 November 2021).
- Commission Directive 2009/90/EC of 31 July 2009 Laying Down, Pursuant to Directive 2000/60/EC of the European Parliament and of the Council, Technical Specifications for Chemical Analysis and Monitoring of Water Status (Text with EEA Relevance). 2009. Available online: http://data.europa.eu/eli/dir/2009/90/oj/eng (accessed on 7 November 2021).
- Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31991L0271 (accessed on 3 December 2019).
- Chirac, C.; Casadevall, E.; Largeau, C.; Metzger, P. Bacterial Influence upon Growth and Hydrocarbon Production of the Green Alga Botryococcus Braunii 1. J. Phycol. 1985, 21, 380–387. [Google Scholar] [CrossRef]
- Jones, J.G. Studies on Freshwater Bacteria: Association with Algae and Alkaline Phosphatase Activity. J. Ecol. 1972, 60, 59. [Google Scholar] [CrossRef]
- Mouget, J.-L.; Dakhama, A.; Lavoie, M.C.; De La Noüe, J. Algal growth enhancement by bacteria: Is consumption of photosynthetic oxygen involved? FEMS Microbiol. Ecol. 1995, 18, 35–43. [Google Scholar] [CrossRef]
- DECRETO LEGISLATIVO 3 Aprile 2006, n. 152. 2006. Available online: https://www.gazzettaufficiale.it/dettaglio/codici/materiaAmbientale (accessed on 9 November 2021).
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. 2013. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 9 June 2022).
- Hassan, A.-R.H.A.-A.; de la Escosura-Muñiz, A.; Merkoçi, A. Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags. Biosens. Bioelectron. 2015, 67, 511–515. [Google Scholar] [CrossRef]
- Radke, S.M.; Alocilja, E.C. A high density microelectrode array biosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 2005, 20, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124–125, 143–149. [Google Scholar] [CrossRef]
- Lamanna, L.; Rizzi, F.; Bhethanabotla, V.R.; De Vittorio, M. Conformable surface acoustic wave biosensor for E-coli fabricated on PEN plastic film. Biosens. Bioelectron. 2020, 163, 112164. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Pillai, R.G.; Lee, W.E.; Jemere, A.B. An impedimetric biosensor for E. coli O157:H7 based on the use of self-assembled gold nanoparticles and protein G. Mikrochim. Acta 2019, 186, 169. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Amaya, S.; Lin, L.-K.; Deering, A.J.; Stanciu, L.A. Aptamer-based SERS biosensor for whole cell analytical detection of E. coli O157:H7. Anal. Chim. Acta 2019, 1081, 146–156. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonacci, A.; Arduini, F.; Attaallah, R.; Amine, A.; Giardi, M.T.; Scognamiglio, V. A Proof-of-Concept Electrochemical Cytosensor Based on Chlamydomonas reinhardtii Functionalized Carbon Black Screen-Printed Electrodes: Detection of Escherichia coli in Wastewater as a Case Study. Biosensors 2022, 12, 401. https://doi.org/10.3390/bios12060401
Antonacci A, Arduini F, Attaallah R, Amine A, Giardi MT, Scognamiglio V. A Proof-of-Concept Electrochemical Cytosensor Based on Chlamydomonas reinhardtii Functionalized Carbon Black Screen-Printed Electrodes: Detection of Escherichia coli in Wastewater as a Case Study. Biosensors. 2022; 12(6):401. https://doi.org/10.3390/bios12060401
Chicago/Turabian StyleAntonacci, Amina, Fabiana Arduini, Raouia Attaallah, Aziz Amine, Maria Teresa Giardi, and Viviana Scognamiglio. 2022. "A Proof-of-Concept Electrochemical Cytosensor Based on Chlamydomonas reinhardtii Functionalized Carbon Black Screen-Printed Electrodes: Detection of Escherichia coli in Wastewater as a Case Study" Biosensors 12, no. 6: 401. https://doi.org/10.3390/bios12060401
APA StyleAntonacci, A., Arduini, F., Attaallah, R., Amine, A., Giardi, M. T., & Scognamiglio, V. (2022). A Proof-of-Concept Electrochemical Cytosensor Based on Chlamydomonas reinhardtii Functionalized Carbon Black Screen-Printed Electrodes: Detection of Escherichia coli in Wastewater as a Case Study. Biosensors, 12(6), 401. https://doi.org/10.3390/bios12060401








