Abstract
Buildings consume 40% of global energy, over half of which is used for cooling and heating. Tungsten bronze (MxWO3) holds promise for smart windows due to its ability to block near-infrared (NIR) heat radiation while maintaining visible light transmittance. However, conventional high-temperature synthesis is energy intensive. Here, we develop a low-temperature hydrothermal method (170 °C) to prepare Li and Cs co-doped tungsten oxide using WCl6, LiF, and CsOH·H2O as precursors, with acetic acid as a crystallographic modulator. The material exhibits a hexagonal structure (P63/mcm) and Li+-induced lattice expansion (0.34 nm spacing). Combined XPS and ICP-OES analyses confirm the chemical composition as Cs0.31Li0.09WO3 and reveal a positive correlation between the W5+ content (15.76%) and oxygen vacancy concentration, which is identified as the key factor enhancing the NIR absorption. The material demonstrates excellent visible light transmission and NIR shielding properties. Our work provides a more energy-efficient and sustainable pathway for the production of smart window materials.
1. Introduction
The emission of greenhouse gases, such as carbon dioxide and methane, is contributing to global warming and causing various environmental problems. Energy-saving glass with NIR shielding performance can efficiently insulate the heat that primarily originates from the NIR region of sunlight, and reduce the indoor or car temperatures in summer. It can lower the electricity consumption of indoor space-cooling and lighting equipment, thus promoting energy-saving [1,2,3]. From a materials science perspective, one of the keys to achieving this functionality (i.e., high visible light transmission and selective near-infrared shielding) lies in transparent conductive oxides (TCOs). Traditional TCOs, such as indium-doped tin oxide (ITO), are widely used due to their excellent conductivity and transparency. However, their high cost and the scarcity of indium have prompted the exploration of alternative materials. In this context, a range of low-cost, elementally abundant alternative TCO systems have been developed, such as aluminum-doped zinc oxide (ZnO: Al, AZO) and niobium-doped titanium dioxide (TiO2: Nb). These materials introduce free charge carriers into wide-bandgap semiconductors through doping, thereby enabling the regulation of near-infrared light via plasmonic resonance effects, making them important members of the transparent thermal insulation material family [4,5]. Currently, various types of transparent insulating materials have been developed, including indium-doped tin oxide (ITO), antimony-doped tin oxide (ATO), non-stoichiometric tungsten oxide (WO3−X), gold nanoparticles (n-Au), silver nanoparticles (n-Ag), lanthanum hexaboride nanoparticles (LaB6), and hexagonal tungsten bronzes (MxWO3, where M = Cs+, Rb+, K+, Na+, NH4+, as well as H+, which is pivotal for the gasochromic effect) [6,7,8,9,10]. The non-stoichiometric tungsten oxides (WO3−x), in which the parameter x of the chemical formula normally ranges from 0 to 1 and represents the degree of oxygen deficiency, have proved superior to WO3 for various properties, due to the self-doping effect introduced by the oxygen vacancies in the materials [11,12]. Among these materials, tungsten bronzes have attracted increasing attention due to their relatively low cost and excellent near-infrared (NIR) shielding performance [13,14]. It is noteworthy that the foundational principle of modulating the optical properties of WO3 by ion insertion is shared with the well-established electrochromic phenomenon, which was introduced into smart window applications by C. G. Granqvist several decades ago [15,16]. In addition to electrochromism, WO3 also possesses an outstanding gasochromic effect. Prof. L. M. Thomas and his group pioneered the work on the gasochromic effect in WO3 thin films and also made comparative investigations on the gasochromic and electrochromic effect in the WO3 thin films [17,18,19]. To improve the optical property and boost the light modulation of WO3 thin films, cation doping was applied, such as Cs+ insertion in the WO3 lattice, forming a CsxWO3 structure. Prof. Li and his team found that the Cs0.3WO3 is formed through a dissolution–recombination route during the nucleation and growth process [20]. Cs+ doping into the WO3 crystal could effectively adjust the density of free carriers, thereby enhancing the small polariton absorption ability and the local surface plasmon resonance effect [21]. The continued increase in greenhouse gases is contributing to a warming global climate. According to global average surface temperature measurements, 2021 was one of the six hottest years on record, with lake surface temperatures reaching their highest levels on record and the number of warm days on land reaching an all-time high. Increased carbon emissions from human activities have become a major driver of global climate change, with growth in carbon emissions coming mainly from industry, transportation and energy supply, while residential and commercial buildings, among others, also contribute significant amounts of carbon dioxide, methane and other greenhouse gases [22]; air conditioning and heating systems account for more than 50% of total building energy consumption [23]. With the introduction of China’s “carbon peak and carbon neutral” strategy, controlling carbon emissions has become a key issue in China’s low-carbon transformation and development. Transparent insulation is destined to play an important role in slowing down carbon emissions. It plays a crucial role in reducing the use of indoor air conditioning and vehicle air conditioning.
Researchers have developed various materials that can be utilized as transparent thermal insulation coatings. These materials include conventional infrared shielding materials such as low-emissivity (low-E) coatings [24,25], vanadium dioxide (VO2) [26], ITO [27,28], and modified zinc oxide (ZnO) [29,30]. However, these materials possess inherent limitations that hinder their broader applications. The core component of low-E coatings is the low-emissivity Ag layer. To ensure the stability of the Ag layer during operation, a complex multilayer vacuum structure must be designed, resulting in high manufacturing costs [24,25]. VO2, one of the most widely studied thermochromic materials, holds significant promise for smart window applications due to its unique reversible metal-insulator transition (MIT). Nevertheless, its relatively high phase transition temperature (Tc = 68 °C), which exceeds room temperature, limited solar modulation ability, and poor oxidation resistance restrict its practical implementation [26]. Both ITO and modified ZnO exhibit excellent infrared shielding properties as transparent insulating materials [27,28]. Recently, hafnium-doped ZnO coatings have been investigated for constructing windows with self-cleaning effects and controllable solar transmittance [29,30]. However, the strong shielding range of ITO and modified ZnO is primarily concentrated in the 1500–2500 nm region, while their near-infrared (NIR) transmittance remains high in the 780–1500 nm range, leading to suboptimal NIR shielding efficiency [27]. Moreover, indium, a critical component of ITO, is a rare and expensive metal, further limiting its large-scale commercial viability.
Tungsten bronze materials (MxWO3, M = Li [31], Na [32], Cs [33], etc.) have garnered considerable research interest owing to their promising applications in energy-saving windows. The NIR shielding property of tungsten bronze powder is attributed to the localized surface plasmon resonance (LSPR) and small polaron transfer [34,35]. It is noteworthy that the fundamental understanding of the polaron absorption mechanism in reduced tungsten oxides dates back to seminal early studies. Saenger’s research group discovered that two polaron modes exist in the electron transitions between W4+ and W5+ and between W5+ and W6+ for tungsten ion sites [36]. Additionally, the direct spectroscopic evidence for these mixed valence states was determined through XPS investigations of the tungsten oxide films [37]. Among the many transparent insulation materials, alkali metal-doped tungsten bronze material is widely noticed for its excellent electrical and color rendering properties. Its main principle of action is the selective transmission of the visible wavelengths of sunlight and the selective absorption of the near-infrared wavelengths that can raise the ambient temperature, thus avoiding ambient heating and reducing the use of air-conditioning, thereby slowing down global warming and reducing carbon emissions. Bo xu et al. used ammonium meta-tungstate as the tungsten source and lithium chloride as the lithium source to obtain lithium–cesium co-doped tungsten-bronze by hydrothermal reaction at 240 °C for 24 h, and enhanced the spectral tunability of LSPR and small polaron transfer [38]. Hao yuan et al. mixed CsNO3, Li2CO3, and WO3 with deionized water, with M/W (M = Li + Cs) and Li/Cs molar ratios of 0.35 and 0.2:0.8, respectively. Then, the well-mixed raw materials were annealed in a high-temperature tube furnace at 650 °C under a H2 atmosphere for 30 min and naturally cooled to room temperature to obtain LimCsnWO3 nanoparticles. It has been confirmed that the LimCsnWO3 nanoparticles with Li/Cs = 0.2:0.8 prepared at 650 °C have the best near-infrared shielding performance, with the maximum visible light transmittance reaching 75.18%, and the NIR shielding rate attaining 97.65% [23].
Our study presents a breakthrough low-temperature (170 °C) hydrothermal method for synthesizing phase-pure CsxLiyWO3 nanocrystals. By employing acetic acid as a crystallographic modulator, we successfully achieved co-doping of Li+ and Cs+, and confirmed the chemical composition and W5+/W6+ mixed-valence states through characterization techniques such as XPS and ICP-OES. Notably, our O 1s XPS analysis established a direct correlation between the material’s performance and its oxygen vacancy concentration, providing experimental evidence for understanding its excellent near-infrared shielding properties. This synthesis strategy consumes significantly less energy compared to conventional high-temperature methods. Our work not only provides a scalable pathway for low-carbon material fabrication but also offers new perspectives for designing next-generation smart window materials by elucidating the relationship between oxygen vacancy concentration, valence state, and performance in co-doped tungsten bronzes.
2. Materials and Methods
2.1. Materials
The following reagents are of analytical grade and utilized as received without further treatment. Tungsten chloride (WCl6, 99%), Cesium hydroxide monohydrate (CsOH·H2O, AR, 95%), Lithium fluoride (LiF, AR, 99%), acetic acid (CH3COOH, AR, 99.5%), and anhydrous ethanol (C2H5OH) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
2.2. Synthesis of LimCs0.5WO3 Nanoparticles
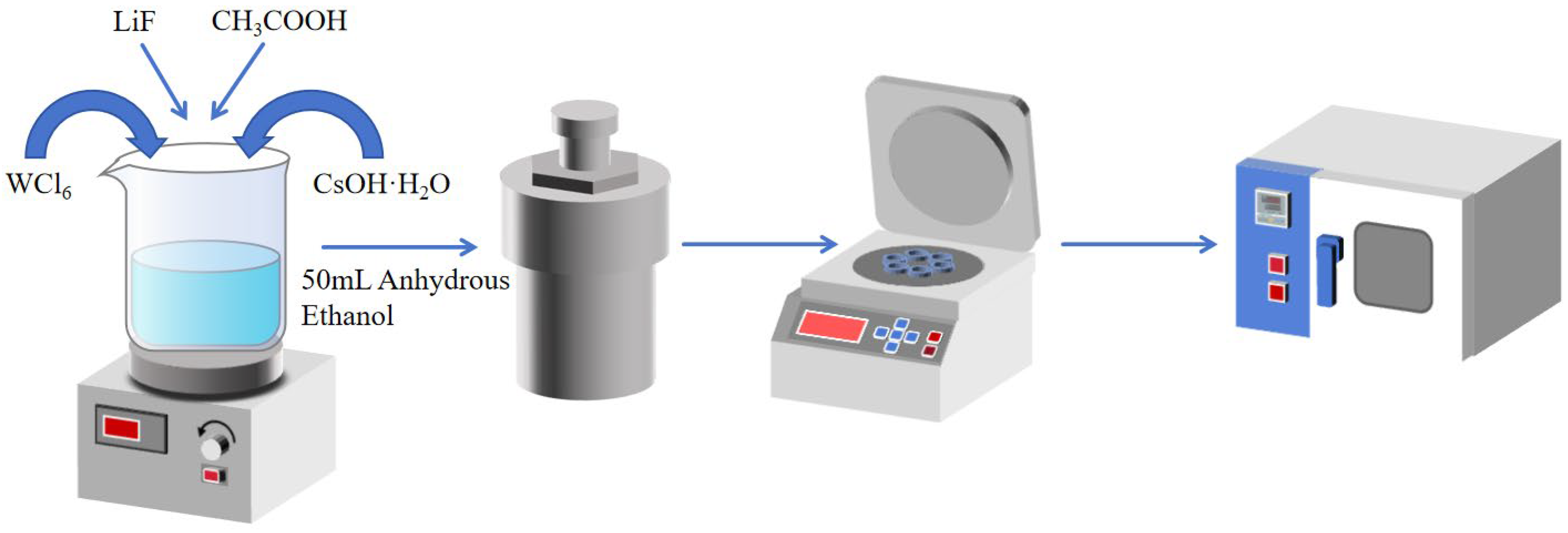
The synthesis of LimCs0.3WO3 was carried out according to the following procedure. 1 mmol of WCl6 was dissolved in 50 ml of ethyl alcohol, followed by the addition and complete dissolution of 0.5 mmol CsOH·H2O. The crystalline water in CsOH·H2O promotes its dissolution in ethanol, enabling molecular-level dispersion of the cesium precursor. This operation was repeated five times to produce five identical mixtures. After that, LiF with different molar masses (0.5 mmol, 1.0 mmol, 1.5 mmol, 2.0 mmol, and 2.5 mmol) was added to the five mixtures in turn. Despite its extremely low solubility in ethanol, LiF undergoes gradual etching under the following acidic hydrothermal conditions, thereby releasing Li+ ions. Then 12.5 mL of acetic acid was introduced to each mixture, followed by additional stirring. In this system, acetic acid functions dually as a crystallization moderator and reaction catalyst. Its role involves both proton-assisted acceleration of WCl6 hydrolysis/condensation and carboxyl-directed coordination that modulates crystallization kinetics, ultimately promoting the formation of a highly crystalline hexagonal nanostructure. Five mixtures were then transferred into five 100 mL Teflon-lined stainless-steel autoclaves (Shanghai Xiniu Leibo Instrument Co., Ltd., Shanghai, China) and heated at 220 °C for 24 h. After cooling to room temperature, the samples were washed with ethyl alcohol and dried in a vacuum oven (Shanghai Jinghong Experimental Equipment Co., Ltd., Shanghai, China) at 60 °C for 12 h (As shown in Figure 1). The obtained materials were labeled Li0.5Cs0.5WO3, Li1.0Cs0.5WO3, Li1.5Cs0.5WO3, Li2.0Cs0.5WO3, and Li2.5Cs0.5WO3 (LimCs0.5WO3). Li1.0Cs0.5WO3 was synthesized at different temperatures (160 °C, 170 °C, 180 °C, 190 °C, and 200 °C).

Figure 1.
Schematic workflow for the synthesis of Cs-Li co-doped tungsten bronze nanoparticles.
2.3. Characterization
XRD patterns of the samples were recorded on a Bruker D8 ADVANCE X-ray diffractometer (Bruker AXS GmbH, Billerica, MA, USA) using filtered Cu-Kα radiation (λ = 1.5406 Å), generated at 40 kV and 40 mA. Scans for 2θ values were recorded at 6° min-1 between 10° and 90°. SEM images were obtained on a Hitachi (SU8020) scanning electron microscope (Hitachi Ltd., Tokyo, Japan). Energy dispersive spectroscopy (EDS, Hitachi Ltd., Tokyo, Japan) was employed for determining the elemental composition of the materials. TEM micrographs were taken on a JEOL (JEM-2100 F) transmission electron microscope (JEOL Ltd., Tokyo, Japan) operating at 200 kV. X-ray photoelectron spectra (XPS) were acquired with a Thermo ESCALAB 250 Xi spectrometer (Waltham, MA, USA). UV−visible−NIR absorption spectra were measured using a Shimadzu (UV-3600, 300–2500 nm) spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
3. Results
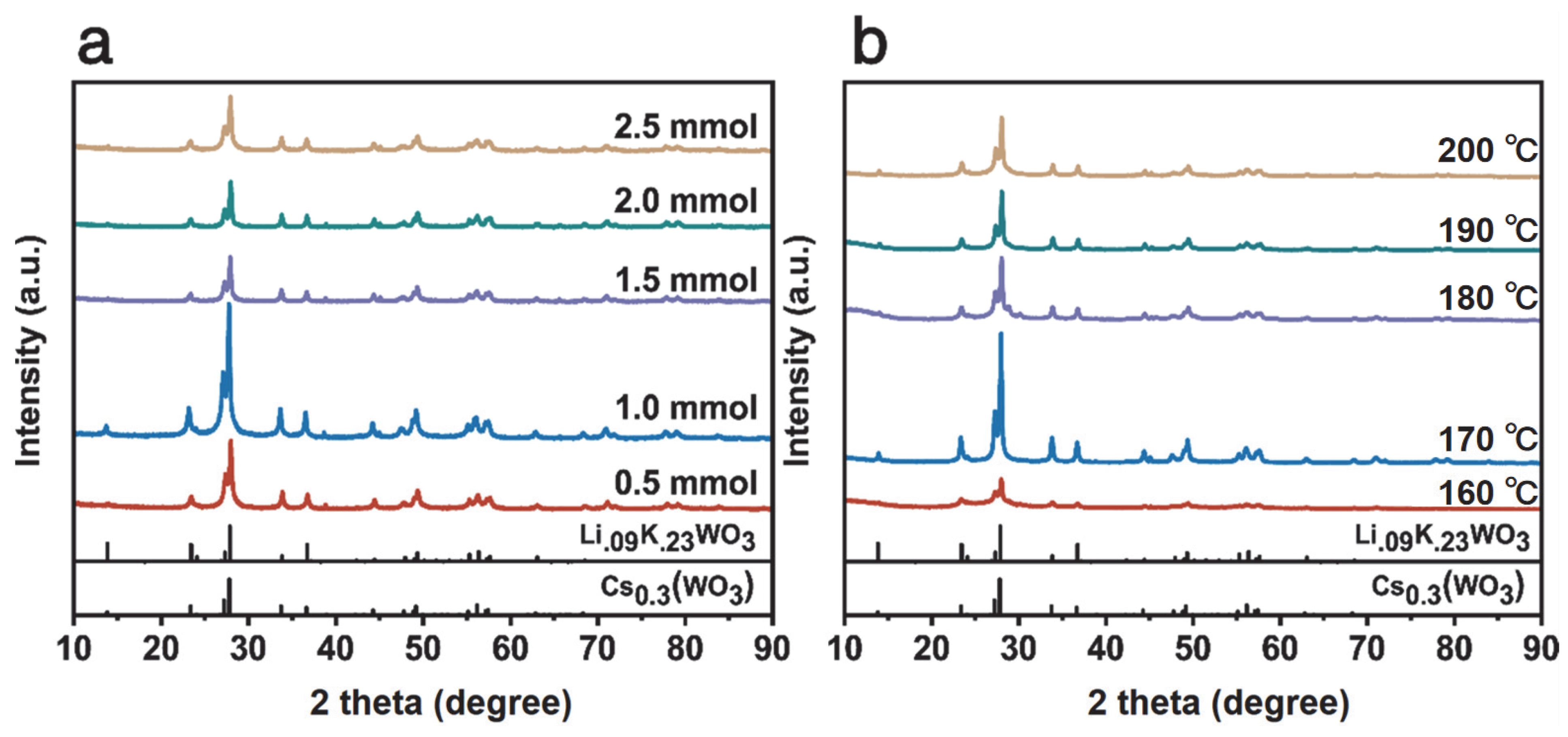
Figure 2a displays the X-ray diffraction (XRD) patterns of the Cs0.5LiyWO3 series. All samples exhibit sharp and intense diffraction peaks, indicating a highly crystalline hexagonal tungsten bronze (HTB) structure (space group: P63/mcm). The diffraction peaks observed at 23.35°, 27.27°, 27.97°, 33.84°, and 36.69° can be indexed to the (002), (102), (200), (112), and (202) planes of the HTB structure, respectively. This indexing was performed based on reference patterns for the isostructural hexagonal tungsten bronze compounds Cs0.3WO3 (PDF#97-007-2618) and Li0.09K0.23WO3 (PDF#97-006-5729) [39,40]. Notably, a systematic shift in diffraction peak positions is observed with variations in the Li precursor content. This phenomenon provides strong evidence for the introduction of microstrain within the crystal lattice by successful Li+ doping. Such peak shifts are an effective method for analyzing crystal strain [41]. Among the series, the sample with the nominal composition Cs0.5Li1.0WO3 demonstrates the highest diffraction peak intensity, signifying its optimal crystallinity. Figure 2b shows the XRD patterns of Cs0.31Li0.09WO3 samples synthesized at different temperatures (160–200 °C). The sample synthesized at 170 °C exhibits significantly higher intensity for the (200) peak at approximately 27.97° compared to other temperatures, indicating the most complete crystallization at this temperature. Furthermore, a discernible shift in this sample’s (200) peak to lower angles is observed when compared to the reference patterns. According to Bragg’s law, this directly corresponds to an increase in interplanar spacing, i.e., lattice expansion, thereby directly confirming the presence of tensile strain induced by Li+ doping [1,42]. This lattice strain, acting synergistically with the W5+/W6+ mixed valence states revealed by subsequent XPS analysis, is considered a key structural feature significantly enhancing the material’s near-infrared shielding performance [33,34].
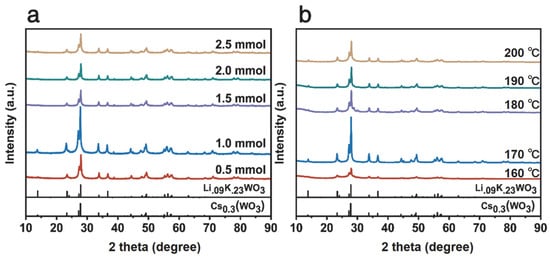
Figure 2.
XRD patterns of LimCs0.5WO3: (a) different Li contents; (b) Cs0.31Li0.09WO3 prepared at different temperatures.
ICP-OES was applied to accurately characterize the chemical composition of the prepared samples, as listed in Table 1, which presents the elemental weight percentages and the corresponding atomic ratios of Cs/W and Li/W. It is obvious that the detection of the ratio of Cs to W in the sample remains relatively stable, ranging between 0.29 and 0.34, which aligns with the capacity of the hexagonal tungsten bronze structure to accommodate large-radius alkali metal ions (the theoretical value of x = 0.33). By contrast, the Li content (y value) increases gradually from 0.30 to 1.07 with the increasing Li precursor dosage, demonstrating effective control over Li incorporation during the preparation process. The total alkali ion content (x + y) is a critical parameter for identifying the crystal phases of the synthesized samples. The (x + y) values for the samples with Li doping below 1 mmol range from 0.30 to 0.40, falling within the physical limit for the hexagonal tungsten bronzes (x ≈ 0.3–0.5). In contrast, the (x + y) values for samples of Li doping exceeding 1.5 mmol are as high as 0.83 and 1.07, significantly breaking the limit of 0.5. These results demonstrate a deviation from phase purity under high Li loading, where excessive Li incorporation probably leads to the formation of some secondary crystalline phases, such as lithium oxides or lithium tungstates.

Table 1.
Chemical composition of the as-synthesized samples determined by ICP-OES.
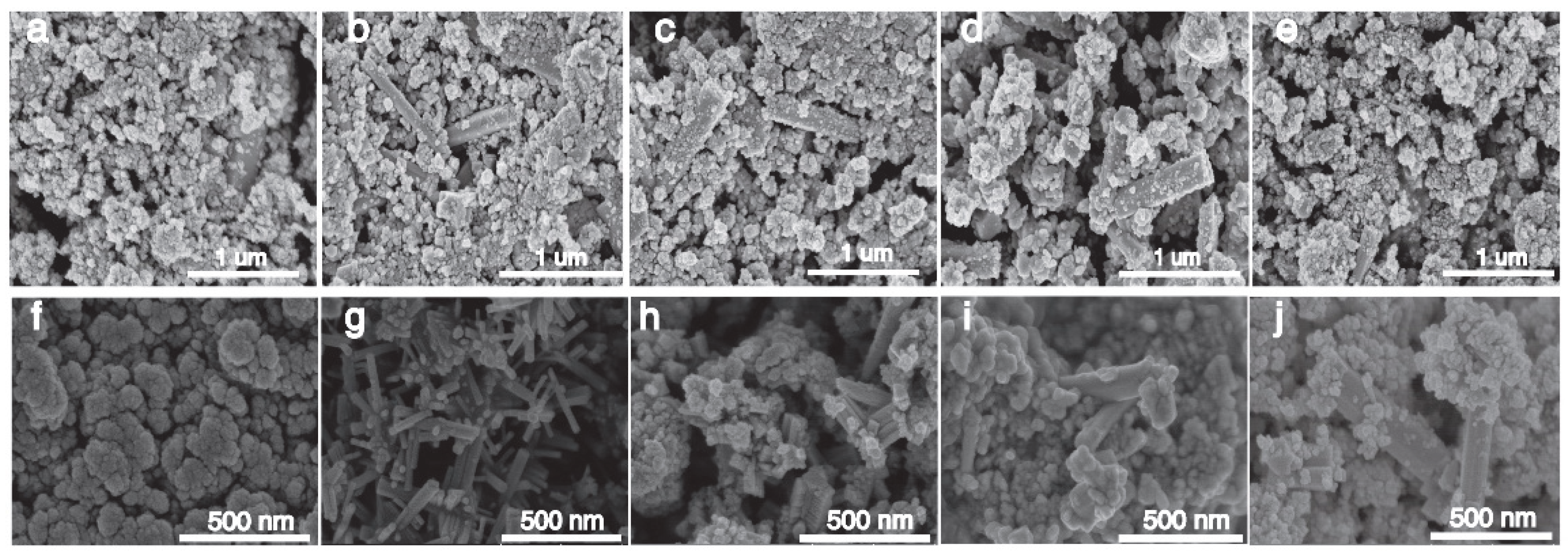
Figure 3a–e shows the SEM images of Cs0.5LiyWO3 doped with 0.5–2.5 mmol LiF, and the generation of strips can be clearly seen, especially the 1.0 mmol LiF doped sample, which generates strips with a smooth surface and no impurity particles attached. It shows that the reaction is more complete when the content of LiF is 1.0 mmol, which is more suitable for the generation of lithium–cesium co-doped tungsten oxide. Figure 3a–f shows the SEM images of the Cs0.31Li0.09WO3 samples at 160–200 °C, and it can be seen that a large number of strips are formed at 170 °C with interlaced growth, and the surface is smooth with no impurity particles attached, which indicates that the reaction of the Cs0.31Li0.09WO3 is more complete under the condition of 170 °C, and the product is more densely populated, which offers a possibility of a strong NIR shielding properties.
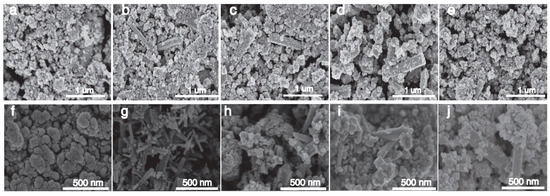
Figure 3.
SEM images of Cs0.5LiyWO3: (a–e) different Li precursor amounts (0.5–2.5 mmol); (f–j) Cs0.31Li0.09WO3 synthesized at different temperatures (160–200 °C) and elemental compositions.
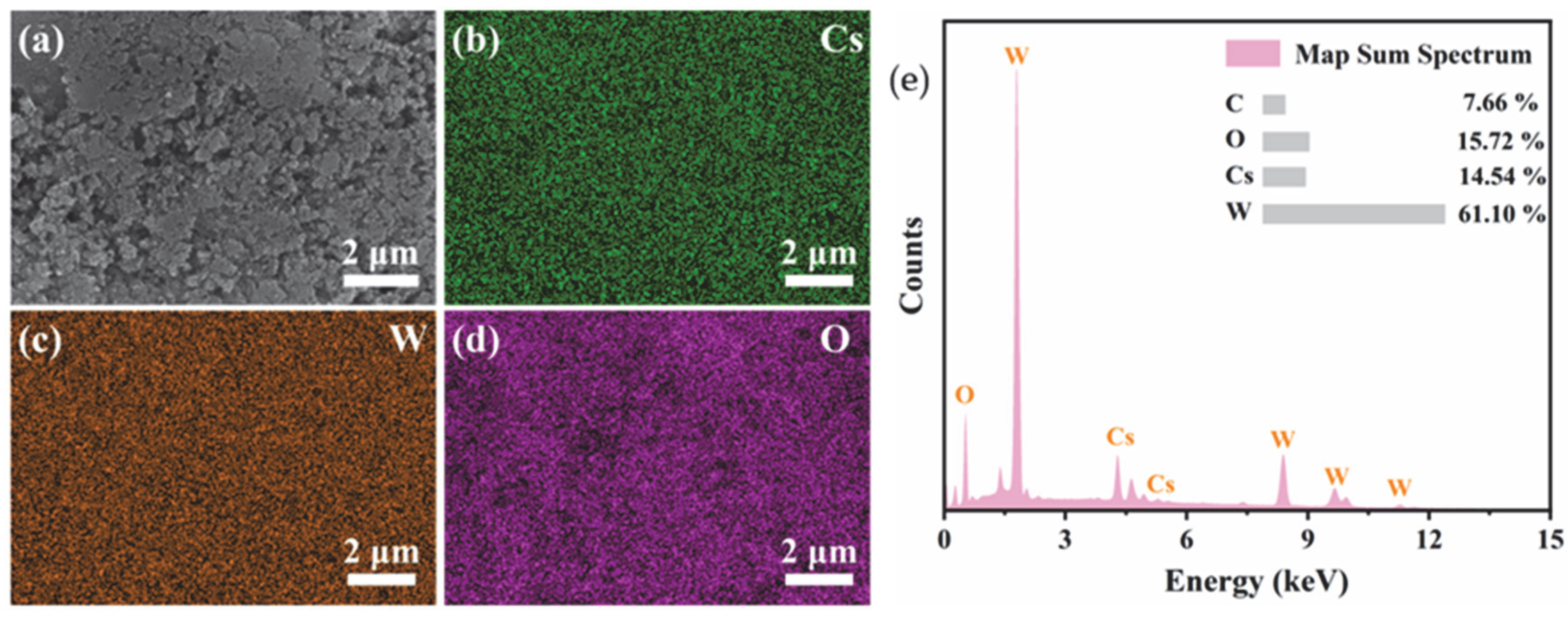
Figure 4 shows the SEM-EDS mapping and the corresponding elemental composition. It is seen that the Cs, W, and O elements are uniformly distributed on the feature in the selected mapping area. It is worth noting that the distribution of Cs perfectly coincides with that of W and O, indicating that Cs+ ions have been successfully and uniformly incorporated into the crystal tunnels of the hexagonal tungsten oxide. The characteristic X-ray peaks of Cs, W, O, and C are labeled in Figure 4e. The atomic percentages (at%) provided in the inset table yield a Cs/W atomic ratio of approximately 0.24.
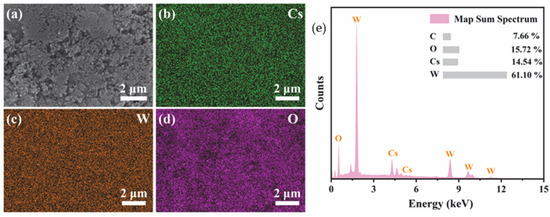
Figure 4.
SEM-EDS elemental mapping images of the CsxLiyWO3 sample. (a) Secondary electron image, (b) Cs map, (c) W map, (d) O map, and (e) elemental composition.
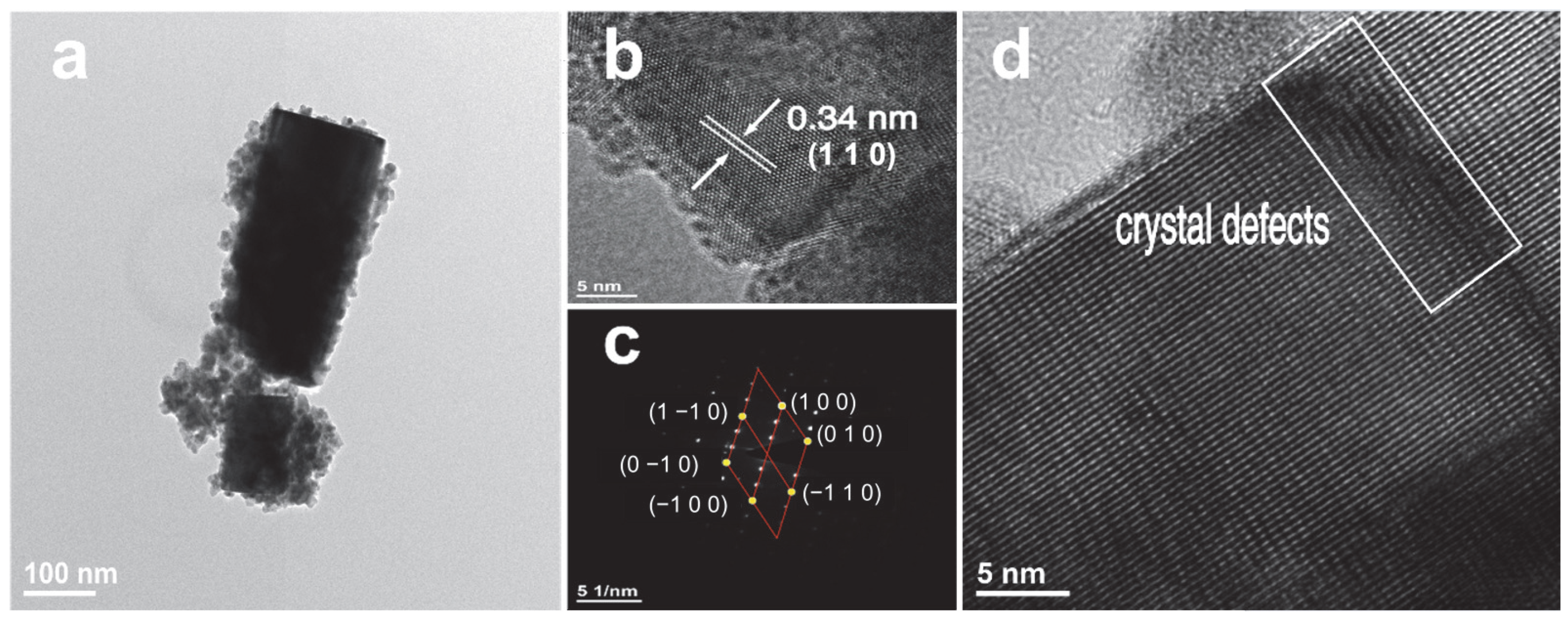
Figure 5 illustrates the TEM images of the prepared Cs0.31Li0.09WO3 sample. Figure 5a reveals the typical rod-like morphology with a diameter of approximately 150 nm. The HRTEM image in Figure 5b presents distinct lattice fringes with a measured spacing of 0.34 nm, which can be assigned to the (110) planes of the hexagonal CsxLiyWO3 structure. Normally, the interplanar spacing of (110) planes for Cs0.33WO3 is 0.37 nm. The slightly smaller value for the CsxLiyWO3 is probably ascribed to the lattice strain induced by the incorporation of Li ions. The corresponding selected-area electron diffraction (SAED) pattern is shown in Figure 5c. The sharp diffraction spots with clear sixfold symmetry confirm the hexagonal crystalline nature of the fabricated Cs0.31Li0.09WO3. Furthermore, the presence of crystal defects, as indicated by the rectangular box in Figure 5d, is consistent with the microscopic strain fields potentially introduced by Li+ incorporation into the host lattice.
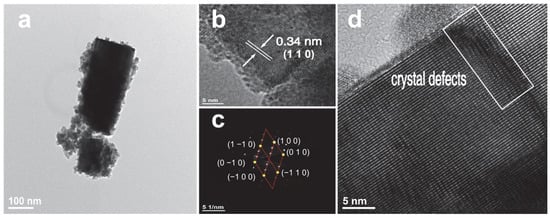
Figure 5.
(a–d) TEM images of Cs0.31Li0.09WO3 sample.
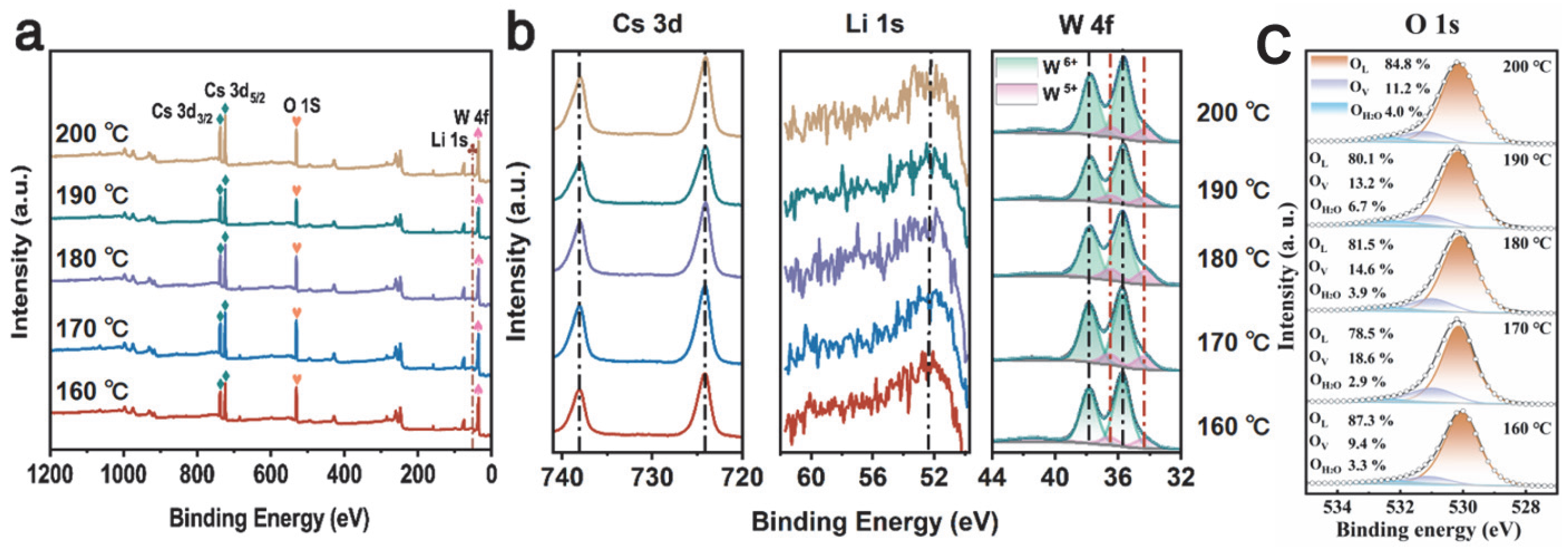
The surface chemical composition of the 160–200 °C Cs0.31Li0.09WO3 samples was determined by XPS characterization in Figure 6a. The survey spectra and high-resolution Cs 3d, Li 1s, W 4f XPS of 160–200 °C Cs0.31Li0.09WO3 samples are shown in Figure 6. The peaks at 724.21 eV and 738.07 eV correspond with Cs+ 3d5/2 and Cs+ 3d3/2 orbitals, respectively. The peaks at 52.5 eV can be attributed to the Li 1s orbital, which is in good agreement with the reported XPS results of Li [42]. The high-resolution XPS of W 4f can be fitted to two spin–orbit dual states of W 4f7/2 and W 4f5/2. W6+ and W5+ are responsible for the spin–orbit peaks at 35.66 eV/37.85 eV and 34.33 eV/36.54 eV, respectively. The content of W5+ in 160 °C Li1.0Cs0.5WO3 sample is the least, accounting for 12.84% of the total W atoms. The content of W5+ increases and then decreases with the rise in temperature, and the highest relative content of 15.76% is found at the temperature of 170 °C. It is due to increased lithium doping, and the afforded electron plays the role of the reducing agent for the W atoms [43]. This is also responsible for the enhanced NIR shielding performance [32]. The relative content of W5+ decreases from 15.55% at 180 °C to 14.31% and finally to 13.91% at 200 °C. It is consistent with the TEM results. Figure 6c presents the high-resolution XPS spectrum of O 1s, in which the different components originating from lattice oxygen (OL, at 530.4 eV), oxygen vacancies (OV, at 531.5 eV), and surface-adsorbed water/hydroxyl groups (OH2O, at 532.8 eV) are deconvoluted to investigate the role of oxygen vacancies in-depth. Quantitative analysis of OV reveals that its concentration increases from 9.4% at 160 °C to a peak of 18.6% at 170 °C, and then decreases gradually to 13.2% with the increasing preparation temperature. This trend shows a very strong positive correlation with the W5+ fraction from W 4f XPS, which suggests that the W5+ states originate directly from the oxygen vacancies, not from the Li doping. Each oxygen vacancy produces two electrons, locally reducing two W6+ to W5+ (or one to W4+) for charge balance. Thus, the W5+ maximum at 170 °C is intrinsically governed by the peak in oxygen vacancy concentration at this optimal temperature, confirming an oxygen vacancy-dominated charge compensation mechanism.
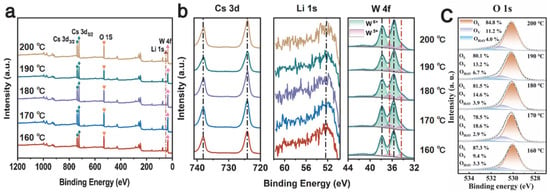
Figure 6.
XPS of Cs0.5Li1.0WO3 powders synthesized at different temperatures: (a) survey, (b) Cs 3d, Li 1s, W 4f and (c) O 1s spectra.
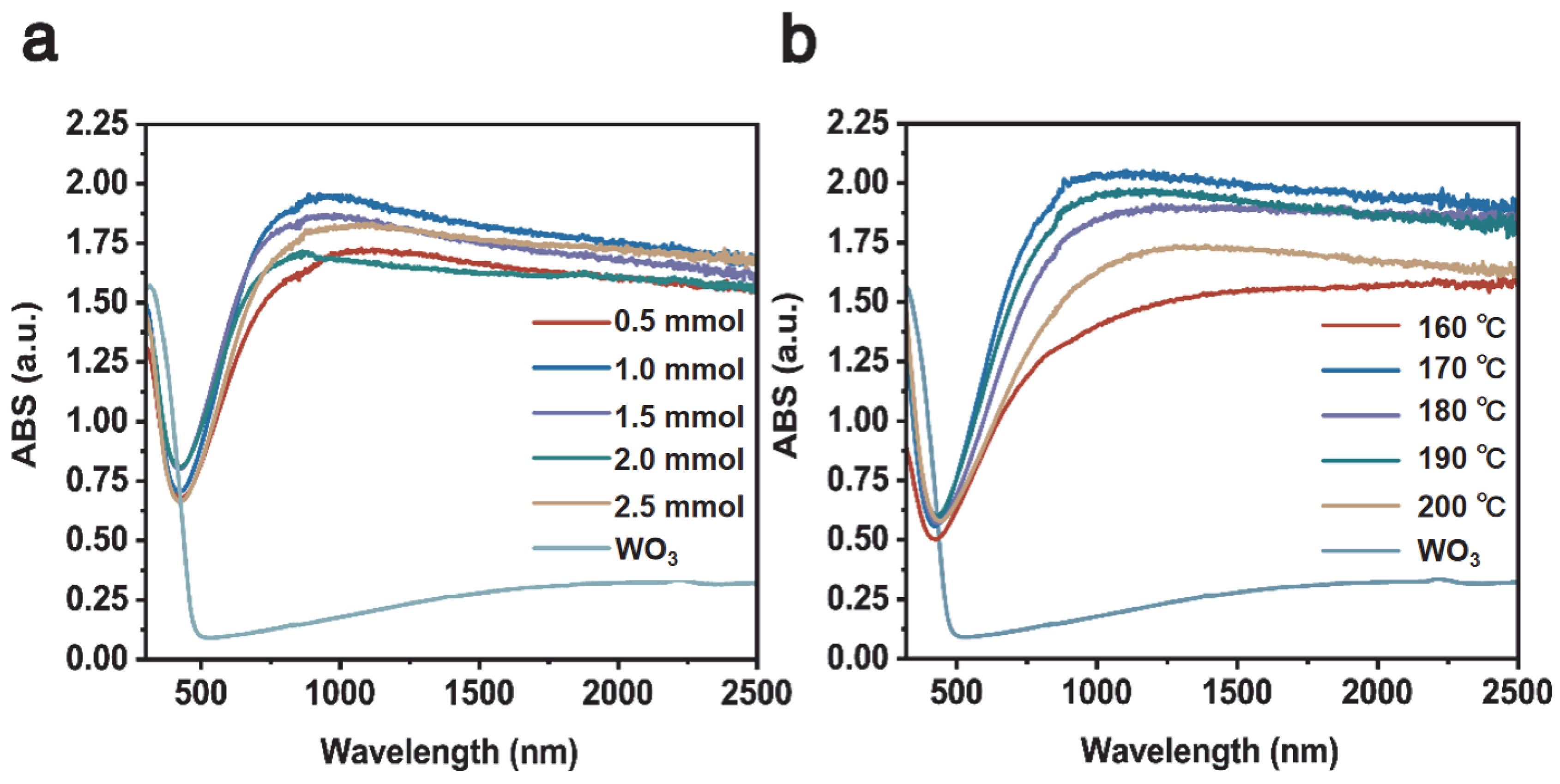
Figure 7a shows the UV–Visible–NIR absorption of Cs0.31LiyWO3 samples with varying Li doping. It is obvious that the sample of Cs0.31Li0.09WO3 possesses a better absorption ability in the NIR wavelength. Figure 7b shows the UV–Visible–NIR absorption of the Cs0.31Li0.09WO3 sample prepared at varying temperatures, in which the sample synthesized at 170 °C exhibits the best absorption property in the wavelength range between 800 nm and 2500 nm while maintaining almost the same level of absorption in the visible range for the doped WO3. The results are consistent with the findings of the lithium intercalated tungsten-bronze system [42]. These optical measurements demonstrate that the optimum Cs:Li for synthesizing high-quality CsxLiyWO3 for the transparent heat insulation applications is 0.5:1.0 and the optimum temperature is 170 °C.
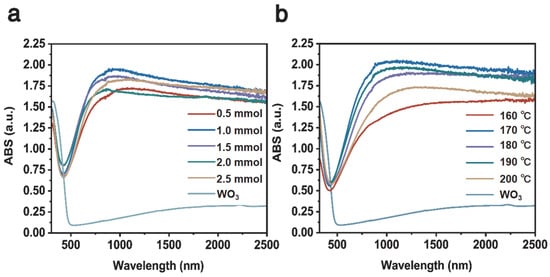
Figure 7.
UV–Visible–NIR absorption spectrum of (a) the Cs0.5LiyWO3 with varying Li doping; (b) the Cs0.5Li1.0WO3 prepared at varying temperatures of 160–200 °C.
4. Conclusions
In this study, Cs0.31Li0.09WO3 material with high NIR shielding performance was successfully synthesized via a simple low-temperature hydrothermal method. Compared to conventional high-temperature synthesis, this approach significantly reduces energy consumption. The effects of LiF doping content and reaction temperature on the product’s morphology, crystal structure, elemental composition, valence state, and optical properties were systematically investigated. The results indicate that the sample synthesized at 170 °C with a Cs/Li molar ratio of 0.5:1.0 possesses the highest crystallinity. O 1s XPS analysis confirmed that this optimal sample has the highest oxygen vacancy concentration, which correlates directly with the peak in W5+ content (15.76%) observed in W 4f XPS. This identifies the oxygen vacancy-dominated charge compensation mechanism as the intrinsic reason for the enhanced NIR shielding performance, rather than the effect of Li doping content alone. This optimal sample exhibits excellent overall optical properties. This work not only provides a feasible strategy for the green preparation of high-performance tungsten bronze materials but also deepens the understanding of the relationship between oxygen vacancies and optical properties in co-doped systems. Future work will focus on evaluating the long-term stability of this material under simulated practical conditions and fabricating it into smart window coatings for actual application testing to assess its real-world energy-saving performance, thereby accelerating its potential for industrial adoption.
Author Contributions
Conceptualization, Y.L. (Yue Liu), X.S. and S.W.; methodology, Y.L. (Yue Liu); software, S.W.; validation, X.S., S.W. and Y.L. (Yue Liu); formal analysis, Y.L. (Yan Luo); investigation, X.S.; resources, S.W.; data curation, Z.S.; writing—original draft preparation, Y.L. (Yue Liu) and X.S.; writing—review and editing, S.W.; visualization, L.W.; supervision, S.W.; project administration, S.W.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Planning Project of Lhasa (Grant No. LSKJ202307), the Disciplinary Construction Project of Xizang University (Grant No. 00061353/002), and the High-Level Talent Cultivation Program for Postgraduates of Xizang University (No. 2025-GSP-S071).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xu, X.; Zhang, W.; Hu, Y.; Wang, Y.; Lu, L.; Wang, S. Preparation and overall energy performance assessment of wide waveband two-component transparent NIR shielding coatings. Sol. Energy Mater. Sol. Cells 2017, 168, 119–129. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Y.; Lu, L.; Yang, H. Enhanced spectral modulation of CsxWO3 nanocrystals through anionic doping for energy-efficient glazing. Sol. Energy Mater. Sol. Cells 2022, 236. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Z.; Zhang, M.; Zhang, Z.; Li, T.; Chen, M.; Dong, W. Flexible core–shell CsxWO3-based films with high UV/NIR filtration efficiency and stability. Nanoscale Adv. 2021, 3, 3177–3183. [Google Scholar] [CrossRef]
- Minami, T. Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 2005, 20, S35–S44. [Google Scholar] [CrossRef]
- Hitosugi, T.; Ueda, A.; Nakao, S.; Yamada, N.; Furubayashi, Y.; Hirose, Y.; Shimada, T.; Hasegawa, T. Fabrication of highly conductive Ti1−xNbxO2 polycrystalline films on glass substrates via crystallization of amorphous phase grown by pulsed laser deposition. Appl. Phys. Lett. 2007, 90, 212106. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Y.; Lu, L.; Yang, H. Synthesis and characterization of Sb-doped SnO2 with high near-infrared shielding property for energy-efficient windows by a facile dual-titration co-precipitation method. Ceram. Int. 2020, 46, 18518–18525. [Google Scholar] [CrossRef]
- Chao, L.; Bao, L.; Wei, W.; Tegus, O. A review of recent advances in synthesis, characterization and NIR shielding property of nanocrystalline rare-earth hexaborides and tungsten bronzes. Sol. Energy 2019, 190, 10–27. [Google Scholar] [CrossRef]
- Thummavichai, K.; Xia, Y.; Zhu, Y. Recent progress in chromogenic research of tungsten oxides towards energy-related applications. Prog. Mater. Sci. 2017, 88, 281–324. [Google Scholar] [CrossRef]
- Li, G.; Wu, G.; Guo, C.; Wang, B. Fabrication of one-dimensional W18O49 nanomaterial for the near infrared shielding. Mater. Lett. 2016, 169, 227–230. [Google Scholar] [CrossRef]
- Li, S.-Y.; Niklasson, G.A.; Granqvist, C.G. Nanothermochromics: Calculations for VO2 nanoparticles in dielectric hosts show much improved luminous transmittance and solar energy transmittance modulation. J. Appl. Phys. 2010, 108, 063525. [Google Scholar] [CrossRef]
- Bandi, S.; Srivastav, A.K. Review: Oxygen-deficient tungsten oxides. J. Mater. Sci. 2021, 56, 6615–6644. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Li, D.; Xu, J. Multifunctional non-stoichiometric tungsten oxides: Synthesis, properties and application. J. Power Sources 2025, 631, 236222. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Dong, Q.; Kimura, T.; Tanaka, M.; Hang, L.T.; Wu, X.; Sato, T. Solvothermal fabrication of rubidium tungsten bronze for the absorption of near infrared light. J. Nanosci. Nanotechnol. 2013, 13, 3236–3239. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Dong, Q.; Sato, T. Simple route to (NH4)xWO3 nanorods for near infrared absorption. Nanoscale 2012, 4, 3394–3398. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Granqvist, C. Electrochromic coatings for “smart windows”. Sol. Energy Mater. 1985, 12, 391–402. [Google Scholar] [CrossRef]
- Granqvist, C. Progress in electrochromics: Tungsten oxide revisited. Electrochim. Acta 1999, 44, 3005–3015. [Google Scholar] [CrossRef]
- Gogova, D.; Thomas, L.-K.; Camin, B. Comparative study of gasochromic and electrochromic effect in thermally evaporated tungsten oxide thin films. Thin Solid Films 2009, 517, 3326–3331. [Google Scholar] [CrossRef]
- Stolze, M.; Gogova, D.; Thomas, L.-K. Analogy for the maximum obtainable colouration between electrochromic, gasochromic, and electrocolouration in DC-sputtered thin WO3−y films. Thin Solid Films 2005, 476, 185–189. [Google Scholar] [CrossRef]
- Stolze, M.; Camin, B.; Galbert, F.; Reinholz, U.; Thomas, L. Nature of substoichiometry in reactively DC-sputtered tungsten oxide thin films and its effect on the maximum obtainable colouration by gases. Thin Solid Films 2002, 409, 254–264. [Google Scholar] [CrossRef]
- Deb, S.K. Optical and photoelectric properties and colour centres in thin films of tungsten oxide. Philos. Mag. 1973, 27, 801–822. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.Q.; Wang, D.Y.; An, F.H.; Xu, L. Boosted near-infrared shielding properties of cesium-tungsten bronze based on solid state reaction with optimized precursor. J. Phys. D Appl. Phys. 2024, 57, 145501. [Google Scholar] [CrossRef]
- Long, Z.; Zhang, Z.; Liang, S.; Chen, X.; Ding, B.; Wang, B.; Chen, Y.; Sun, Y.; Li, S.; Yang, T. Spatially explicit carbon emissions at the county scale. Resour. Conserv. Recycl. 2021, 173, 105706. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Shi, F.; Jiang, S.; Song, X.; Wasim, M.; Song, X.; Ma, J. Controlling the growth of hexagonal CsxWO3 nanorods by Li+-doping to further improve its near infrared shielding performance. Sol. Energy Mater. Sol. Cells 2022, 238, 111612. [Google Scholar] [CrossRef]
- Abundiz-Cisneros, N.; Sanginés, R.; Rodríguez-López, R.; Peralta-Arriola, M.; Cruz, J.; Machorro, R. Novel Low-E filter for architectural glass pane. Energy Build. 2020, 206, 109558. [Google Scholar] [CrossRef]
- Pu, J.; Shen, C.; Wang, J.; Zhang, Y.; Zhang, C.; Kalogirou, S.A. Near-infrared absorbing glazing for energy-efficient windows: A critical review and performance assessments from the building requirements. Nano Energy 2023, 110, 108334. [Google Scholar] [CrossRef]
- Yang, J.; Li, D.; Wang, X.; Jin, H.; Li, J. Optimizing phase transition temperature and visible transmittance of VO2 films driven by synergistic effect of La-Mo co-doping. Appl. Surf. Sci. 2022, 600, 154074. [Google Scholar] [CrossRef]
- Sibin, K.; Selvakumar, N.; Kumar, A.; Dey, A.; Sridhara, N.; Shashikala, H.; Sharma, A.K.; Barshilia, H.C. Design and development of ITO/Ag/ITO spectral beam splitter coating for photovoltaic-thermoelectric hybrid systems. Sol. Energy 2017, 141, 118–126. [Google Scholar] [CrossRef]
- Chu, X.; Tao, H.; Liu, Y.; Ni, J.; Bao, J.; Zhao, X. VO2/AZO double-layer films with thermochromism and low-emissivity for smart window applications. J. Non-Crystalline Solids 2014, 383, 121–125. [Google Scholar] [CrossRef]
- Nundy, S.; Ghosh, A.; Mesloub, A.; Noaime, E.; Touahmia, M. Comfort Analysis of Hafnium (Hf) Doped ZnO Coated Self-Cleaning Glazing for Energy-Efficient Fenestration Application. Materials 2022, 15, 4934. [Google Scholar] [CrossRef]
- Nundy, S.; Ghosh, A.; Tahir, A.; Mallick, T.K. Role of Hafnium Doping on Wetting Transition Tuning the Wettability Properties of ZnO and Doped Thin Films: Self-Cleaning Coating for Solar Application. ACS Appl. Mater. Interfaces 2021, 13, 25540–25552. [Google Scholar] [CrossRef]
- Takayanagi, M.; Tsuchiya, T.; Ueda, S.; Higuchi, T.; Terabe, K. In situ hard X-ray photoelectron spectroscopy on the origin of irreversibility in electrochromic LixWO3 thin films. Appl. Surf. Sci. 2021, 568, 150898. [Google Scholar] [CrossRef]
- Yang, G.; Hu, D.; Yang, C.; Qi, Y.; Liu, B.; Chen, H.; Zhang, L.; Cui, Y.; Yao, X.; Takats, V. Alkali metal tungsten bronze-doped energy-saving glasses for near-infrared shielding applications. Ceram. Int. 2021, 47, 31122–31129. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, L.; Chen, Z.; Cao, C.; Gao, Y.; Luo, H. Synthesis of CsxWO3 nanoparticles and their NIR shielding properties. Ceram. Int. 2018, 44, 13469–13475. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Y.; Lu, L.; Yang, H. Oxygen defect-induced small polaron transfer for controlling the near-infrared absorption coefficient of hexagonal cesium tungsten bronze nanocrystals. Ceram. Int. 2022, 48, 6942–6947. [Google Scholar] [CrossRef]
- Tegg, L.; Keast, V.J. A Review of Alkali Tungsten Bronze Nanoparticles for Applications in Plasmonics. Plasmonics 2022, 18, 49–71. [Google Scholar] [CrossRef]
- Saenger, M.F.; Höing, T.; Hofmann, T.; Schubert, M. Polaron transitions in charge intercalated amorphous tungsten oxide thin films. Phys. Status Solidi (a) 2008, 205, 914–917. [Google Scholar] [CrossRef]
- Deepa, M.; Singh, D.; Shivaprasad, S.; Agnihotry, S. A comparison of electrochromic properties of sol–gel derived amorphous and nanocrystalline tungsten oxide films. Curr. Appl. Phys. 2007, 7, 220–229. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Y.; Lu, L.; Yang, H. Enhancing the spectral tunability of localized surface plasmon resonance and small polaron transfer in Li-doped CsxWO3 nanocrystals for energy-efficient windows. Sol. Energy 2022, 231, 228–235. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.-F.; Zeng, X.; Cheng, D.; Huang, H.; Cao, D. Enhanced near-infrared shielding ability of (Li,K)-codoped WO3for smart windows: DFT prediction validated by experiment. Nanotechnology 2016, 27, 075203. [Google Scholar] [CrossRef]
- Heymann, G.; Selb, E.; Kogler, M.; Götsch, T.; Köck, E.-M.; Penner, S.; Tribus, M.; Janka, O. Li3Co1.06(1)TeO6: Synthesis, single-crystal structure and physical properties of a new tellurate compound with CoII/CoIIImixed valence and orthogonally oriented Li-ion channels. Dalton Trans. 2017, 46, 12663–12674. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Yang, G.; Gao, Y. Electronic properties, optical properties and diffusion behavior of WO3 with H+, Li+ and Na+ intercalated ions: A first-principles study. J. Solid State Chem. 2021, 297, 122082. [Google Scholar] [CrossRef]
- Porqueras, I.; Bertran, E. Efficiency of Li doping on electrochromic WO3 thin films. Thin Solid Films 2000, 377–378, 129–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).