Abstract
MnFe2O4 magnetic nanoparticles have shown broad application prospects in the field of tumor diagnosis and treatment; however, precise particle size regulation within the 100–200 nm range, as well as the synergistic integration of physical and medical functionalities, remains challenging. As a commonly used method for synthesizing MnFe2O4 nanoparticles, the solvothermal method has been proven to enable the regulation of the particle size of products, particularly its ability to utilize the viscosity of solvents as a method for particle size regulation. Therefore, this work investigates the influence of the diethylene glycol (DEG) to ethylene glycol (EG) ratio on particle size regulation in solvothermal synthesis of MnFe2O4 nanoparticles, and constructs MnFe2O4@PB nanocomposite materials. The results demonstrate that with the DEG ratio increasing from 0 to 80% in a DEG:EG mixed solvent system, the average particle size of MnFe2O4 nanoparticles can be reduced from 266 nm to 105 nm. The MPB4.5 sample (MnFe2O4:PB molar ratio = 5:4.5 in the MnFe2O4@PB nanostructure) exhibits an optimal photothermal heating effect and good photothermal stability, demonstrating potential as a photothermal therapeutic agent. The resultant MnFe2O4@PB system provides a strategy for precise particle size regulation and functional integration for photothermal therapy of tumors with magnetic targeting potential.
1. Introduction
Magnetic nanoparticles have been widely used in biomedical fields due to their unique properties such as tunable magnetic performance and chemical stability [1,2]. Among numerous types of magnetic nanoparticles, MnFe2O4 has attracted extensive interest from researchers [3] due to its high magnetization [4] and excellent biocompatibility [5], showing great potential in applications such as targeted drug delivery [6] and magnetic resonance imaging [7].
Particle size control is crucial for facilitating the translational application of MnFe2O4 in clinical diagnosis and treatment [8]. Previous studies have made progress in controlled synthesis of MnFe2O4 magnetic nanoparticles, and the commonly adopted methods include co-precipitation [9], hydrothermal/solvothermal [10], thermal decomposition [11], and others. For instance, André C. Horta [12] fine-tuned the size of MnFe2O4 nanoparticles from 23 nm to 3 nm by adjusting the volume ratio of organic base MIPA to inorganic base NaOH in the co-precipitation method, although significant particle agglomeration was observed. Prashant Kumar [13] used PEG-200 surface-modified hydrothermal synthesis to prepare MnFe2O4 nanoparticles with reduced agglomeration and an average size of approximately 40 nm. In contrast, the solvothermal method is frequently employed to synthesize dispersed nanoparticles due to its ability to achieve a narrow size distribution and precise shape control [14]. Gaoqian Yuan [15] utilized the solvothermal method to obtain MnFe2O4 nanoparticles with an average size of 274 nm, featuring high sphericity, good dispersibility, and uniform particle size. Beyond the aforementioned approaches, the thermal decomposition method represents another technique for nanoparticle size regulation [16]; however, it is associated with inherent drawbacks, including the tendency for organic solvent residues [17], high energy consumption, and low yield [2]. In sharp contrast, the solvothermal method further exhibits the advantages of easily available raw materials and low cost [18]. However, despite the fact that recent syntheses of MnFe2O4 nanoparticles have predominantly focused on small sizes, the development of systems within the 100–200 nm range with both low aggregation and precise size control remains an area demanding further exploration.
Photothermal therapy (PTT), a non-invasive cancer treatment modality [19], capitalizes on the deep-tissue-penetration capability of near-infrared (NIR) light to trigger localized and controlled hyperthermia at the tumor site, thereby achieving selective eradication of cancer cells [20]. In specific therapeutic scenarios, PTT exhibits significant application potential for a variety of tumors. For hepatocellular carcinoma with poor efficacy of traditional single therapy, combining photothermal therapy with chemotherapy enables relatively effective treatment [21]. For superficial breast tissue, light penetration to deep tissues makes photothermal therapy a promising alternative or adjuvant option for breast cancer [22]. To enhance nanoparticle delivery to tumors, Patrick et al. [23] showed that ultrasound-guided slow-controlled injection improves nanoparticle delivery efficiency and precision, offering a clinically translatable real-time method for patient-specific drug administration.
Prussian blue (PB) exhibits strong metal-to-metal charge transfer absorption in the near-infrared region [24], and possesses advantages over other photothermal agents (e.g., transition metal chalcogenides) such as low cost, high photostability, and biocompatibility [25], positioning it as a promising photothermal agent. Bingquan Chen et al. [26] proposed a novel PB@Fe-EGCG polyphenol nanolayer nanosystem, which effectively inhibits in vitro cancer cell growth under 808 nm near-infrared laser irradiation. By combining photothermal therapy with chemotherapy, this system achieves a significant inhibition rate of up to 75%. Hwichan Hong [27] synthesized an injectable biocompatible composite of bacterial cellulose and Prussian blue nanoparticles (PB NPs), which exhibits good in vivo retention and enables tumor elimination through repeated photothermal therapy (PTT).
In selecting photothermal agent carriers, superparamagnetic nanoparticles outperform other photothermal carriers such as poly (lactic-co-glycolic acid) (PLGA) [28] as they efficiently deliver nanocomposites to tumors under an external magnetic field [29]. Among these magnetic nanoparticles, MnFe2O4 contains Mn2+ ions (with five unpaired d-electrons) that generate magnetic moments to accelerate longitudinal relaxation (T1), thereby endowing the material with a stronger T1 relaxation effect [30] and exhibiting bright signals in T1-weighted imaging [31]. Integrating the magnetic targeting capability of MnFe2O4 with the near-infrared responsive performance of photothermal materials to construct a synergistic therapeutic system has emerged as a cutting-edge research direction [32]. Notably, compared with smaller particles, photothermal agents loaded with 100–200 nm magnetic nanoparticles are more easily delivered to tumor regions via magnetic guidance [33,34], enabling more precise targeting and further exerting photothermal effects.
This study aims to synthesize MnFe2O4 magnetic nanoparticles with tunable sizes (100–200 nm), a narrow size distribution, and minimal agglomeration. By decorating MnFe2O4 nanoparticles with Prussian blue (PB), we constructed a magnetic–photothermal synergistic therapeutic composite material. This system, featuring both potential magnetic targeting capability and near-infrared photothermal conversion performance, offers a novel and high-potential material for magnetically targeted photothermal therapy of tumors and is expected to provide a new high-performance nanoplatform for precise tumor diagnosis and treatment.
2. Materials and Methods
2.1. Materials and Reagents
Manganese chloride tetrahydrate (MnCl2∙4H2O), iron (III) chloride hexahydrate (FeCl3∙6H2O), anhydrous sodium acetate (CH3COONa), polyethylene glycol 2000 (PEG2000), ethylene glycol (EG), diethylene glycol (DEG), sodium ferrocyanide decahydrate (Na4Fe(CN)6∙10H2O), hydrochloric acid (36–38%), and polyvinylpyrrolidone K30 (PVP K30) were obtained from Aladdin Biochemical Technology Co., Ltd., Shanghai, China. All chemical reagents were used directly without further purification.
2.2. Synthesis of MnFe2O4 Nanoparticles
A total of 4.0 mmol of MnCl2∙4H2O (0.8000 g) and 8.0 mmol of FeCl3∙6H2O (2.1624 g) were added to 80 mL of solvent (either ethylene glycol or diethylene glycol) and dispersed uniformly by mechanical stirring. Subsequently, 4.0 mmol of CH3COONa (5.2499 g) and 3.2586 g of PEG2000 were added to the above-mentioned solution. The mixture was mechanically stirred for 1 h, then transferred into a hydrothermal autoclave (Shanghai Xiniu Laibo Instrument Co., Ltd., Shanghai, China; 100 mL), which was then placed in a 200 °C forced-air drying oven (Tianjin Laibote Rui Instrument Equipment Co., Ltd., Tianjin, China; GFL-45) for a 12 h reaction, followed by cooling to room temperature. The product was washed several times with deionized water and anhydrous ethanol, and then dried in a vacuum oven (Shanghai Lichen Bangxi Instrument Technology Co., Ltd., Shanghai, China; DZF-6050AB) at 60 °C for 24 h for subsequent characterizations.
2.3. Synthesis of MnFe2O4@PB Nanocomposite
In total, 0.5470 g of Na4Fe(CN)6·10H2O and 0.1094 g of PVP were dissolved in 25 mL of hydrochloric acid solution (pH = 2) under magnetic stirring at 400 rpm to form solution A. An amount of 0.6487 g of FeCl3∙6H2O and 0.1297 g of PVP were dissolved in 25 mL of hydrochloric acid solution (pH = 2). After complete dissolution, 0.6919 g of MnFe2O4 nanoparticle powder was added to the solution, followed by stirring for an additional 20 min to form solution B.
Solution A was then added dropwise to Solution B under continuous magnetic stirring. After 30 min of reaction, the mixture was washed repeatedly with deionized water and anhydrous ethanol. The product was collected by centrifugation at 2000 rpm and dried under vacuum at 60 °C for 24 h.
Prussian-blue-coated MnFe2O4 magnetic nanoparticles (MnFe2O4@PB) were synthesized using the procedure described above. By varying the molar amounts of Na4Fe(CN)6∙10H2O (1.8 mmol, 3.6 mmol) and FeCl3∙6H2O (2.4 mmol, 4.8 mmol), nanoparticles with different MnFe2O4:PB molar ratios (5:1, 5:2, 5:3, 5:4, 5:4.5, 5:5.4) were achieved, labeled as MPB1, MPB2, MPB3, MPB4, MPB4.5, and MPB5.4, respectively (Table 1).

Table 1.
Sample designations for MnFe2O4@PB composites with varying modification molar ratios.
2.4. Characterization
The products were characterized by powder X-ray diffraction using a D8 FOCUS X-ray diffractometer from Bruker AXS GmbH, Germany. The microstructure of the prepared MnFe2O4 materials was observed via a Quanta 450 FEG scanning electron microscope (SEM) from FEI Hong Kong Limited. Infrared (IR) spectroscopy tests on the samples were conducted using an IRAffinity-1S infrared spectrometer from Shimadzu Corporation, Japan. The hysteresis loop measurements were performed using a LakeShore 7404 magnetometer from LakeShore Cryotronics, Inc., Westerville, OH, USA. The UV–Vis–NIR spectra were measured using a UV2700 UV–Vis spectrophotometer from Shimadzu Corporation, Kyoto, Japan.
For the photothermal performance experiments, a VCL-MO-2W laser from Beijing Honglan Optoelectronic Technology Co., Ltd., Beijing, China was used to irradiate the samples with a wavelength of 808 nm and an emission power density of 3.41 W/cm2. Thermal infrared images were captured, and temperature measurements were recorded using an S65 infrared thermal imager from FLIR Systems, Wilsonville, Oregon, USA.
3. Results and Discussion
3.1. Characterizations of MnFe2O4 Nanoparticles
In the synthesis of MnFe2O4 nanoparticles, ethylene glycol (EG) is commonly used as a solvent in solvothermal methods due to its strong coordination ability with metal ions—which forms stable chelates—and its high viscosity that can limit the reaction rate [35] These properties enable EG to form nanoparticles with uniform morphology and prevent agglomeration in the synthesis of magnetic nanoparticles [36]. In this work, EG was first employed as the primary solvent for synthesis. The morphology of the synthesized MnFe2O4 sample was observed by SEM, and the particle size distribution diagram was plotted based on the statistical data obtained from the SEM images. As shown in Figure 1, the average particle size of the MnFe2O4 sample was 266.37 ± 48.83 nm with a relative standard deviation (RSD) of 18.33%. The SEM elemental mapping shows that Mn and Fe elements exhibit a homogeneous distribution in the MnFe2O4 sample, with their distribution regions overlapping with one another. The morphology exhibited a dispersed spherical state, although the particle size exceeded the target range of 100–200 nm.
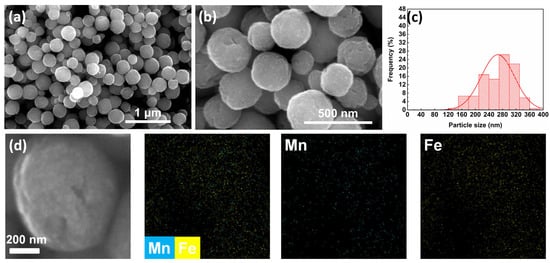
Figure 1.
(a) SEM image at 100,000× magnification, scale bar = 1 μm; (b) SEM image at 300,000× magnification, scale bar = 500 nm; (c) particle size distribution diagram; (d) SEM elemental mapping images of MnFe2O4 synthesized using ethylene glycol as solvent.
In the solvothermal process, when the energy barrier between nanoscale precursors is eliminated, a limited number of “agglomeration centers” are rapidly formed, and these precursors subsequently grow rapidly at the expense of consuming surrounding constituent subunits [37]. Based on this theory, the existing experimental reaction process may have the problem that the excessively fast growth rate leads to large particle size and broad particle size distribution of MnFe2O4. Combined with the literature research [38], it is found that at a pressure of 0.098 MPa, the viscosity of diethylene glycol (DEG) is always higher than that of ethylene glycol (EG)—at 293.15 K, the viscosity of DEG is 36.52 mPa∙s and that of EG is 21.10 mPa∙s; at 464.40 K, the viscosity of DEG is 0.789 mPa∙s and that of EG is 0.652 mPa∙s. In addition, this viscosity difference between solvents may have an impact on the solvothermal synthesis process of nanoparticles [39]. Therefore, DEG was selected as a secondary solvent here to investigate the possible influence of different solvent systems on size-controlled synthesis of MnFe2O4 nanoparticles. The experiment was designed with different ratios of EG to DEG, ranging the proportion of DEG in the solvent system from 50% to 80%. The particle sizes of different groups of experiments are shown in Table 2.

Table 2.
Particle size results of MnFe2O4 synthesized with different DEG ratios.
These data are further summarized as a trend in Figure 2, which clearly illustrates the variation in particle sizes of solvothermally synthesized MnFe2O4 nanoparticles with diethylene glycol ratios. With the increase in the proportion of diethylene glycol in the system, the average particle size of MnFe2O4 nanoparticles gradually decreased from 266 nm to 105 nm.
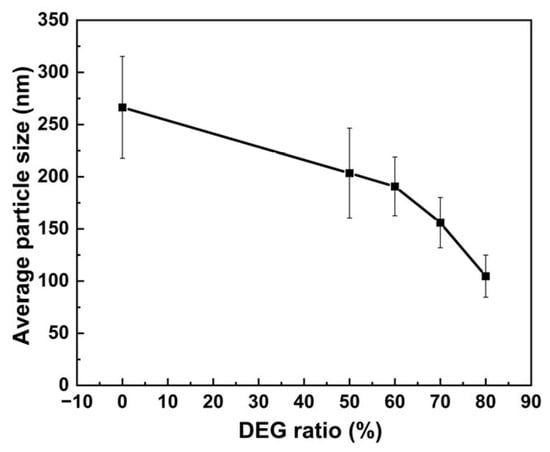
Figure 2.
Variation in the average particle size of MnFe2O4 with the ratio of diethylene glycol.
The SEM images and particle size distribution diagrams of the samples are shown in Figure 3a1–a3, depicting the solvent system with 50% diethylene glycol (DEG), where the average particle size of MnFe2O4 nanoparticles is 203.43 ± 43.05 nm. Compared to the ethylene glycol system, the particle size significantly decreases, approaching the target size of 200 nm. However, the relative standard deviation (RSD) increases slightly to 21.16%. When the DEG proportion further increases to 60% [Figure 3b1–b3], the average particle size of MnFe2O4 is 190.69 ± 28.18 nm with an RSD of 14.78%, representing the smallest RSD among the designed proportional conditions. This sample shows the narrowest particle size distribution and the most uniform particle growth. In Figure 3c1–c3, with a DEG proportion of 70%, the average particle size is 155.99 ± 24.06 nm, showing a substantial decrease from the previous sample, while the RSD remains relatively stable at 15.42%. For Figure 3d1–d3 with 80% DEG, the average particle size of MnFe2O4 is 104.69 ± 20.19 nm, and the RSD increases to 19.29%, accompanied by a broadened particle size distribution and slight aggregation between particles.
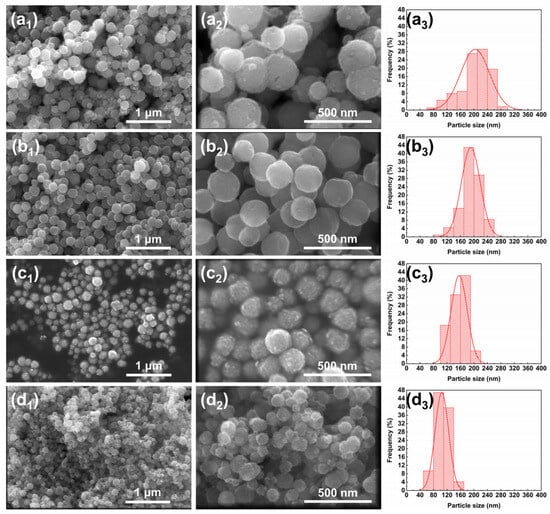
Figure 3.
SEM images and particle size distribution of MnFe2O4 nanoparticles synthesized with different DEG ratios: (a1–a3) 50%; (b1–b3) 60%; (c1–c3) 70%; (d1–d3) 80%. For each ratio, a1/b1/c1/d1 show low-magnification (100,000×) SEM images, scale bar = 1 μm; a2/b2/c2/d2 show high-magnification (300,000×) SEM images, scale bar = 500 nm; a3/b3/c3/d3 show the corresponding particle size distribution diagrams.
During the process of increasing the DEG proportion from 0% to 80%, the particle size of MnFe2O4 nanoparticles decreases from approximately 266 nm to 105 nm, basically achieving the target size control within 100–200 nm. Overall, the MnFe2O4 nanoparticles exhibit regular spherical morphologies with minimal aggregation. The realization of such particle size regulation effect can be attributed to the higher viscosity of diethylene glycol (DEG) and its stronger coordination ability with metal ions compared with ethylene glycol (EG) [40]. When pure EG is used, the crystal nuclei agglomerate rapidly due to their high surface energy and the existence of magnetic dipole–dipole interactions, eventually yielding nanoparticles with relatively large particle sizes [37]. After the introduction of diethylene glycol (DEG), compared with ethylene glycol (EG), the bulkier DEG can form more stable coordination complexes with metal ions. This not only slows down the growth rate of MnFe2O4 grains but also is more conducive to increasing the number of “agglomeration centers” [41]. Additionally, due to the higher viscosity of the system, MnFe2O4 crystal nuclei cannot agglomerate into larger spheres [42]; ultimately, MnFe2O4 with a smaller particle size is obtained.
From the perspective of particle size distribution, the relative standard deviation (RSD) of the size distribution for the samples in this study is approximately 15% or higher, which is slightly higher than that of MnFe2O4 synthesized via the solvothermal method (with a particle size of 274 ± 40 nm and a corresponding RSD of 14.6%) as previously reported by Gaoqian Yuan [15]. Overall, this result indicates that there are slight limitations in the monodispersity of the samples in this study. The particle size distribution can be further narrowed by optimizing synthetic conditions with such strategies as screening and using alternative surfactants [43].
The crystal structure of the MnFe2O4 nanoparticles was determined by XRD, and the corresponding XRD patterns are shown in Figure 4. Characteristic diffraction peaks were observed at 2θ = 18.18°, 29.90°, 35.20°, 42.71°, 53.05°, 57.08°, and 62.20°, corresponding to the (111), (220), (311), (400), (422), (511), and (440) crystal planes of the MnFe2O4 spinel structure (JCPDS 10-1039), indicating successful synthesis of MnFe2O4 nanoparticles via the solvothermal method. The XRD patterns of samples synthesized with varying diethylene glycol ratios in the solvent system were all in good agreement with the standard card, confirming that the nanoparticles synthesized with different solvent systems remained as MnFe2O4.
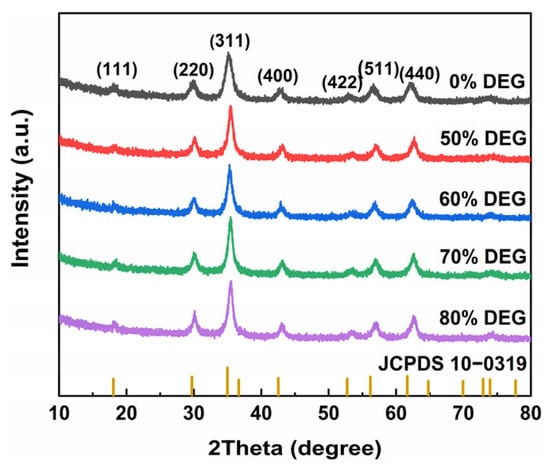
Figure 4.
XRD pattern of MnFe2O4 nanoparticles samples synthesized with different DEG:EG ratios.
3.2. Characterizations of MnFe2O4@PB Nanocomposite
In the synthesis of MnFe2O4@PB nanostructure, when the solution containing Na4[Fe(CN)6]∙10H2O is mixed with a solution of FeCl3∙6H2O, the mixed solution rapidly develops a bright blue color, indicating the formation of Prussian blue (PB). The powder diffraction pattern of the MnFe2O4@PB sample was first characterized, as shown in Figure 5a. For instance, sample MPB1 showed its peak positions at 30.22°, 35.53°, and 42.13°, matching the MnFe2O4 JCPDS standard (Card No.: 10-0319), which corresponds to the crystal planes (220), (311), and (400), respectively. Compared to pure MnFe2O4 nanoparticles, the additional diffraction peaks at 2θ = 17.35°, 24.80°, and 39.55° belong to the (200), (220), and (420) crystal planes of the Prussian blue standard card (73-0687) [44], confirming the combination of Prussian blue and MnFe2O4 materials.
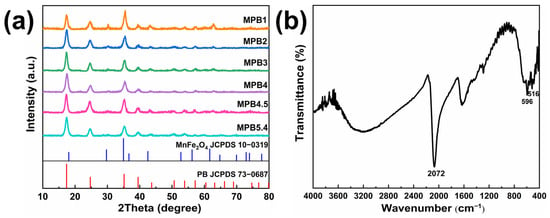
Figure 5.
(a) XRD pattern, MPB1, MPB2, MPB3, MPB4, MPB4.5, and MPB5.4 represent different MnFe2O4:PB molar ratios (5:1, 5:2, 5:3, 5:4, 5:4.5, 5:5.4), respectively; (b) infrared spectrum of MnFe2O4@PB nanocomposite.
The sample was further analyzed by infrared (IR) spectroscopy, as shown in Figure 5b. The peaks at 516 cm−1 and 596 cm−1 are ascribed to the stretching vibrations of Mn-O and Fe-O bonds in MnFe2O4, respectively [45]. The broad peaks at 1630 cm−1 and 3121 cm−1 are attributed to the O-H bending vibration of adsorbed water molecules and the stretching vibration of -OH groups [46]. A strong characteristic peak at 2072 cm−1 originates from the C≡N stretching vibration in the Fe2+-CN-Fe3+ chemical unit of Prussian blue [47], further verifying the combination of Prussian blue and MnFe2O4.
The SEM images and particle size distribution in Figure 6 show that the PB-coated sample maintains a near-spherical particle morphology with an average particle size of 106.66 ± 19.45 nm and an RSD of 18.24%. The particle size and distribution exhibit no significant changes before and after coating, remaining within the 100–200 nm range.

Figure 6.
(a) SEM image at 100,000× magnification, scale bar = 1 μm; (b) SEM image at 300,000× magnification, scale bar = 500 nm; (c) particle size distribution diagram of MnFe2O4@PB nanocomposite.
To verify the magnetic properties of the synthesized samples and their potential for future magnetic targeting applications, their magnetic properties were measured using a vibrating sample magnetometer (VSM), and the resulting hysteresis loops are presented in Figure 7. Both MnFe2O4 and MnFe2O4@PB exhibit small coercivity and remanence, indicating that both possess excellent superparamagnetic properties [48]. The saturation magnetization (Ms) of MnFe2O4 is 66.53 emu/g, while that of MnFe2O4@PB is 18.74 emu/g. Specifically, the saturation magnetization (Ms) of MnFe2O4 in this study is essentially consistent with the upper limit of the 45–67 emu/g range for samples prepared by the chemical oxidation method [49], and it is also significantly higher than the 29.3 emu/g of the samples reported by Li et al. [50]. After modification with Prussian blue, MnFe2O4 still retains considerable magnetic ability, with its saturation magnetization (Ms) being higher than that of the multifunctional nanoparticles (MFNPs) containing Fe3O4 and PB components reported by Du et al. (approximately 8 emu/g) [51]. Notably, Du’s study has confirmed that their prepared MFNPs, under the guidance of an external magnetic field, can achieve a tumor accumulation level twice as high as that in the group without a magnetic field, and the photothermal therapeutic efficacy can be enhanced through magnetic targeting. Therefore, the MnFe2O4@PB nanocomposites in this study, which possess a higher saturation magnetization (18.74 emu/g), are expected to exhibit stronger magnetic response ability. This magnetic response characteristic enables it to achieve directional movement and enrichment under the guidance of an external magnetic field, demonstrating good magnetic targeting potential and providing an important basis for further exploration of its combined application with photothermal performance in future research.
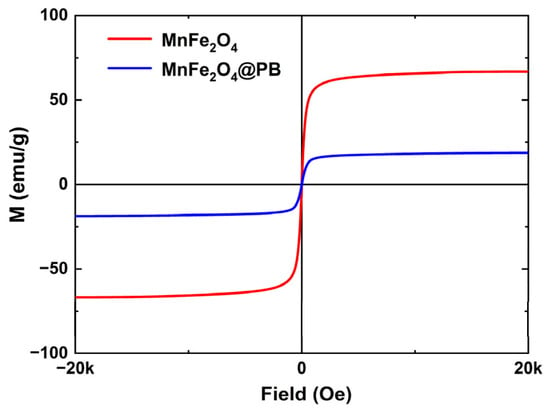
Figure 7.
Magnetization hysteresis loops of MnFe2O4 and MnFe2O4@PB.
3.3. The Photothermal Properties of MnFe2O4@PB Nanocomposite
The UV–Vis–NIR spectrum of the obtained MnFe2O4@PB, as shown in Figure 8, shows that it has a strong absorption capacity for near-infrared light (650–900 nm), which is basically consistent with the near-infrared absorption of Prussian blue alone. This indicates that the Prussian blue modification strategy endows MnFe2O4 with the characteristic of strong near-infrared absorption, providing crucial optical property support for expanding the near-infrared photothermal performance of MnFe2O4-based materials. Therefore, this study selected an 808 nm near-infrared laser, consistent with numerous previous studies [52,53], to test the photothermal performance of the samples.
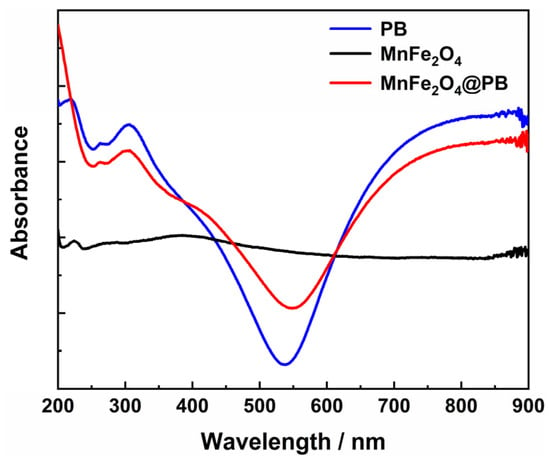
Figure 8.
UV–Vis–NIR spectra of PB, MnFe2O4, and MnFe2O4@PB.
Photothermal heating experiments were conducted on six groups of MnFe2O4@PB samples with different MnFe2O4:PB molar ratios and a control group of water, under the following experimental conditions: particle concentration of 2 g/L; number of repetitions, 3 times; laser wavelength of 808 nm; and laser power density of 3.41 W/cm2. An infrared thermal imager was used to record the temperature during the experiments. Taking sample MPB1 for instance, the infrared thermal images showing the temperature changes during the experiment—presented in Figure 9a–f sequentially—depict the temperature distribution of MPB1 under laser irradiation at 0, 1, 2, 3, 4, and 5 min. As the irradiation time increased, the color of the sample area in the images gradually shifted from red to brighter hues, indicating a continuous increase in temperature.
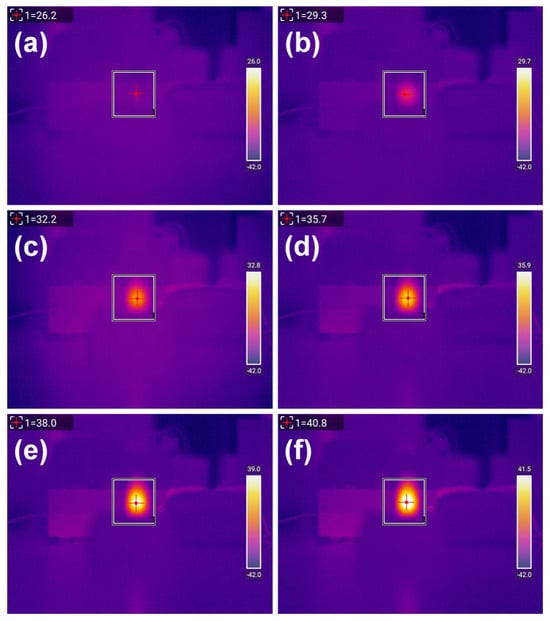
Figure 9.
Infrared thermal images of the MPB1 sample under laser irradiation at different times: (a) 0 min, (b) 1 min, (c) 2 min, (d) 3 min, (e) 4 min, (f) 5 min. Particle concentration: 2 g/L; number of repetitions: 3 times; laser wavelength: 808 nm; laser power density: 3.41 W/cm2.
The photothermal heating experiments under laser irradiation were repeated three times for samples with different modification ratios in each group. The heating data are shown in Figure 9; this displays the infrared thermal images of the MPB1 sample under laser irradiation at different times: (a) 0 min, (b) 1 min, (c) 2 min, (d) 3 min, (e) 4 min, and (f) 5 min. The conditions applied were as follows: particle concentration: 2 g/L; number of repetitions: 3 times; laser wavelength: 808 nm; laser power density: 3.41 W/cm2.
Table 3, and the corresponding heating data plot is illustrated in Figure 10a. It can be observed that for the control group containing only water, the temperature curve is extremely smooth. After laser irradiation, the temperature increase is at most 1.6 °C. In contrast, the experimental groups with MnFe2O4@PB samples exhibit significant and stable temperature increases after laser irradiation.

Table 3.
Photothermal heating data of various MnFe2O4@PB samples.
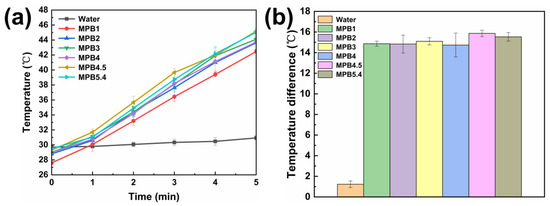
Figure 10.
(a) Temperature increase over time of various samples under laser irradiation; (b) temperature difference data of various samples between the 5th minute of laser irradiation and before illumination. The sample size (n value) = 3. The points in the figure represent the mean values, and the error bars indicate the standard deviation (SD).
During the 5 min photothermal heating test, sample MPB1 shows the smallest temperature increase, with an increment of only 14.6 °C. This is presumably because of a small amount of Prussian blue coated on the surface of MnFe2O4 nanoparticles, resulting in a limited charge transfer of Prussian blue upon laser irradiation and, thus, less heat generation. Sample MPB4.5 exhibited the highest temperature increase, reaching a maximum of 45.5 °C, which falls within the medical temperature range of 42–46 °C for triggering apoptotic programs to treat cancer cells [54]. However, for sample MPB5.4, with a further increased modification ratio, the heating effect is inferior to that of MPB4.5, with a maximum temperature increment of 16 °C. It is speculated that as the modification ratio of MnFe2O4 to PB reaches 5:4.5, Prussian blue has achieved the maximum coating on MnFe2O4 nanoparticles under the current experimental conditions. For MPB5.4, the excessive content of Prussian blue in the experiment may cause aggregation during the coating process, thereby affecting the photothermal heating performance.
Figure 10b presents a data plot of the temperature differences between the 5th minute of heating and the initial temperature before light irradiation for each group of samples. This plot clearly illustrates the comparison of the 5 min photothermal heating effects among six groups of samples with different molar modification ratios and the control group. Among these samples, MPB4.5 exhibits the greatest temperature increase, indicating that the sample under the condition of MPB4.5 has stronger heating capacity upon near-infrared laser irradiation and a better photothermal effect.
To verify the statistical significance of the differences between groups, a one-way analysis of variance (ANOVA) was performed on the photothermal heating data of six groups of samples with different molar modification ratios (n = 3 for each group). The results showed that the difference between groups was significant (F = 13.53, P = 1.4 × 10−4 < 0.05), indicating that the influence of sample conditions on the photothermal effect was not a random error. Based on the relatively higher heating trend of the MPB4.5 sample, in subsequent studies, investigation and analysis of the photothermal stability and photothermal conversion efficiency of samples will focus on the MPB4.5 sample under these conditions.
Sample MPB4.5 was prepared as a 2 g/L test solution for the examination of photothermal stability, as shown in Figure 11. After undergoing five cycles of stability testing, the solution was still able to reach the maximum temperature (43.9 °C) achieved during the first 5 min of light irradiation, demonstrating that this sample exhibits excellent photothermal stability.
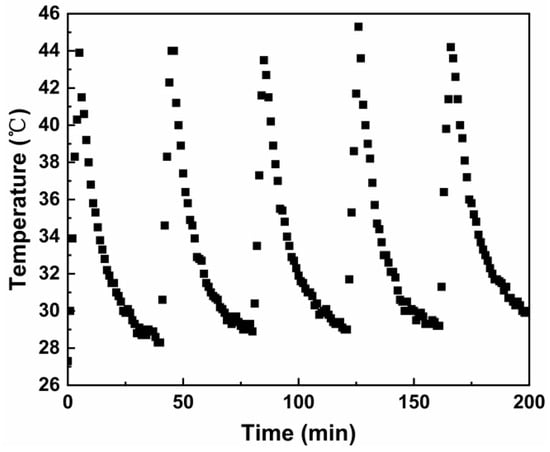
Figure 11.
Heating and natural cooling cycle of MPB4.5 under laser irradiation.
The photothermal conversion efficiency is a crucial parameter for evaluating the photothermal therapeutic efficacy of photothermal agents [55]. A high photothermal conversion efficiency enables more effective conversion of light energy into heat energy, enhancing the therapeutic effect while minimizing thermal damage to normal tissues. The photothermal conversion efficiency of sample MPB4.5 was investigated using the following method. The sample solution was irradiated with a laser until the temperature ceased to increase, after which the laser was removed, allowing the solution to cool naturally to room temperature. Figure 12a shows the photothermal conversion test curve of sample MPB4.5. In the initial stage of the test, the sample temperature increased rapidly. After approximately 10 min, the rate of temperature increment began to slow down. When the laser irradiation reached 18 min, the solution temperature essentially stopped increasing and remained around 57 °C. Subsequently, the laser was turned off, and the temperature decreased to room temperature.
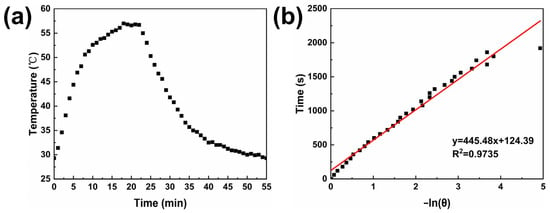
Figure 12.
(a) Time–temperature relationship of sample MPB4.5 cooling to room temperature after laser irradiation ceases to increase the temperature; (b) linear relationship between the negative natural logarithm of the cooling driving force −ln(θ) and time, y = 445.48 x + 124.39, R2 = 0.9735.
The photothermal conversion efficacy was calculated according to the following method [56]:
where η corresponds to the photothermal conversion efficiency; h denotes the heat transfer coefficient; A stands for the surface area of the container employed to hold the sample during the light irradiation experiment; ∆Tmax,mix represents the maximum temperature difference between the sample’s equilibrium temperature and its initial temperature; ∆Tmax,H2O signifies the maximum temperature variation in the aqueous solution from its initial temperature; the mass of water is denoted by md; Cd refers to the specific heat capacity of water; ΔT is the temperature difference from the initial temperature in the cooling phase; I denotes the laser power density; and Aλ corresponds to the sample’s absorbance at a wavelength of 808 nm.
Given that ∆Tmax,mix = 27.7 °C, ∆Tmax,H2O = 1.23 °C, md = 3 g, Cd = 4.2 J/(g·°C), I = 3.41 W/cm2, and Aλ= 2.6, by plotting the linear relationship between t and −ln(θ), as shown in Figure 12b, the slope was determined to be 445.48. Substituting this into Equation (2) yields hA = 0.0283. Overall, η was calculated to be 22.02%, with this photothermal conversion efficiency ranking at a moderate level in the field of Prussian blue photothermal applications. This value is close to the photothermal conversion efficiency of PB@Fe-EGCG-HA (23.5%) synthesized by Bingquan Chen [26], lower than the PEGylated PB NPs (36.7%) fabricated by Huajian Chen [57] and exceeds that of Fe3O4@GdPB NPs (16.1%) described earlier by Kale [58], thus indicating its promising potential as a photothermal therapeutic agent in the near-infrared region.
In the further expansion of the material’s application potential, the development of its imaging function deserves focused attention. MnFe2O4 magnetic nanoparticles have been actively investigated as MRI contrast agents in diagnostic imaging by other researchers [59]. For example, Mauro Comes Franchini et al. reported MnFe2O4@SiO2@GNRs@PMs for magnetic–photoacoustic–optical trimodal imaging and verified their efficacy and applicability as diagnostic tools in medical research [60]. This points the MnFe2O4@PB in this paper toward MRI visibility examinations, and future work can further investigate its ability to identify tumor locations and evaluate therapeutic effects. Meanwhile, it should be noted that MRI may perform poorly in quantifying high concentrations of magnetic particles due to signal loss saturation. To overcome this limitation, a radiolabeling strategy compatible with magnetic nanoparticles can be adopted to achieve a more comprehensive evaluation of delivery status [61]. Building on this, imaging-based photothermal therapy enables more precise targeting of lesions. In subsequent preclinical applications, although Prussian-blue-based materials may have potential side effects [62], these effects can be regulated through corresponding strategies, thereby reducing their impact to a great extent [25].
In addition, the solvothermal method and co-precipitation method involved in this study are widely recognized and easily scalable for the synthesis of nanoparticles [63]. As shown in Wang’s research [64], with sodium citrate used as a modifier in the EG-DEG binary solvent, after scaling up the solvothermal reaction system for Fe3O4@SiO2 nanoparticles from 50 mL to 600 mL, only slight changes were observed in the size (approximately 120 nm) and size distribution of the product. This not only provides a robust and scalable approach to increase the yield from the milligram-to-gram scale and confirms the great potential of the solvothermal method for high-yield industrial production but also offers a reference basis for the feasibility of large-scale production of the MnFe2O4@PB nanocomposite in this study.
4. Conclusions
In summary, following the design concept of “precise particle size regulation–functional material integration–photothermal synergistic application”, this study innovatively adjusted the ratio of EG to DEG in the solvothermal method, successfully regulating the average particle size of MnFe2O4 nanoparticles from 266 nm to 105 nm and providing an effective control strategy for the precise regulation of MnFe2O4 particle size. By integrating the magnetic targeting property of MnFe2O4 and the photothermal property of PB, MnFe2O4@PB nanocomposite photothermal material with magnetic targeting potential was prepared. Among them, the MPB4.5 sample exhibits the strongest photothermal heating effect, good photothermal stability, and a moderate level of photothermal conversion efficiency, which not only confirms the application potential of the MnFe2O4@PB-based nanoplatform in photothermal therapy but also lays a materials science foundation for its further extension to the field of theranostics.
Author Contributions
Conceptualization, M.W., M.Z. and M.S.; methodology, M.W. and M.Z.; software, M.W.; validation, M.W., M.Z., Z.L. and M.S.; formal analysis, M.W. and Z.L.; investigation, M.W.; resources, M.S.; data curation, M.W.; writing—original draft preparation, M.W.; writing—review and editing, M.Z. and M.S.; visualization, M.W. and Z.L.; supervision, M.Z. and M.S.; project administration, M.Z. and M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21878067.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Ming Zhang, affiliated with Xiucun Pharmaceutical Development Co., Ltd., provided only experimental-related guidance during the research process. There was no conflict of interest in this study.
References
- Yang, F.; Li, J.; Chen, T.; Ren, W.; Gao, C.; Lin, J.; Xu, C.; Ma, X.; Xing, J.; Bao, H.; et al. Applications of magnetic nanoparticles for boundarics in biomedicine. Fundam. Res. 2025, 5, 1401–1422. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, T.; Philip, J. A review on synthesis, capping and applications of superparamagnetic magnetic nanoparticles. Adv. Colloid Interface Sci. 2024, 334, 103314. [Google Scholar] [CrossRef]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-w.; Seo, J.-w.; Jang, J.-t.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, N.; Najafpour-Darzi, G. Manganese ferrite (MnFe2O4) Nanoparticles: From synthesis to application—A review. J. Ind. Eng. Chem. 2021, 103, 292–304. [Google Scholar] [CrossRef]
- Akila, B.; Kogularasu, S.; Vasu, D.; Sakthinathan, S.; Chen, Y.-L.; Chiu, T.-W.; Chang-Chien, G.-P. Bio-waste derived hierarchical hydroxyapatite core–Shell manganese ferrite ceramic biomaterial: Structural, morphological characterization, and controlled drug release properties. Ceram. Int. 2025, 51, 22698–22706. [Google Scholar] [CrossRef]
- Peters, J.A. Relaxivity of manganese ferrite nanoparticles. Prog. Nucl. Magn. Reson. Spectrosc. 2020, 120–121, 72–94. [Google Scholar] [CrossRef]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Raland, R.D.; Saikia, D.; Borgohain, C.; Borah, J.P. Heating efficiency and correlation between the structural and magnetic properties of oleic acid coated MnFe2O4 nanoparticles for magnetic hyperthermia application. J. Phys. D Appl. Phys. 2017, 50, 325004. [Google Scholar] [CrossRef]
- Rafique, M.Y.; Pan, L.-Q.; Iqbal, M.Z.; Qiu, H.-M.; Farooq, M.H.; Guo, Z.-G.; Tanveer, M. Growth of monodisperse nanospheres of MnFe2O4 with enhanced magnetic and optical properties. Chin. Phys. B 2013, 22, 107101. [Google Scholar] [CrossRef]
- Chandunika, R.K.; Vijayaraghavan, R.; Sahu, N.K. Magnetic hyperthermia application of MnFe2O4 nanostructures processed through solvents with the varying boiling point. Mater. Res. Express 2020, 7, 064002. [Google Scholar] [CrossRef]
- Horta, A.C.; Poças, A.; Amaral, J.S.; Amorim, C.O. Fine-Tuning of Mn-Ferrite nanoparticle size using a Dual-Base coprecipitation method. Mater. Sci. Eng. B 2025, 314, 118058. [Google Scholar] [CrossRef]
- Kumar, P.; Pathak, S.; Jain, K.; Singh, A.; Kuldeep; Basheed, G.A.; Pant, R.P. Low-temperature large-scale hydrothermal synthesis of optically active PEG-200 capped single domain MnFe2O4 nanoparticles. J. Alloys Compd. 2022, 904, 163992. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.; Cao, H.; Wu, Y. Design of Functional Magnetic Nanocomposites for Bioseparation. Colloids Surf. B Biointerfaces 2020, 191, 111014. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Li, K.; Zhang, J.; Wang, H.; Huang, Z.; Li, F.; Zhang, H.; Jia, Q.; Zhang, S. Myrica rubra-like MnFe2O4 microsphere: A high efficiency microwave reduction catalyst for Cr (VI) removal from water. Sep. Purif. Technol. 2022, 286, 120434. [Google Scholar] [CrossRef]
- Uddin, M.K.; Deb, N.; Rashid, R.; Das, H.; Syed, I.M.; Hoque, S.M. Physical properties with high specific loss power of magnetite (Fe3O4) synthesized via thermal decomposition technique. AIP Adv. 2023, 13, 105105. [Google Scholar] [CrossRef]
- Mahin, J.; Torrente-Murciano, L. Continuous synthesis of monodisperse iron@iron oxide core@shell nanoparticles. Chem. Eng. J. 2020, 396, 125299. [Google Scholar] [CrossRef]
- Gavilán, H.; Rizzo, G.M.R.; Silvestri, N.; Mai, B.T.; Pellegrino, T. Scale-up approach for the preparation of magnetic ferrite nanocubes and other shapes with benchmark performance for magnetic hyperthermia applications. Nat. Protoc. 2023, 18, 783–809. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, Y.; Wu, R.; Lei, J.; Li, T.; Zheng, Y. Aggregable gold nanoparticles for cancer photothermal therapy. J. Mater. Chem. B 2024, 12, 8048–8061. [Google Scholar] [CrossRef]
- Liang, P.; Mao, L.; Dong, Y.; Zhao, Z.; Sun, Q.; Mazhar, M.; Ma, Y.; Yang, S.; Ren, W. Design and Application of Near-Infrared Nanomaterial-Liposome Hybrid Nanocarriers for Cancer Photothermal Therapy. Pharmaceutics 2021, 13, 2070. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, W.; Liu, X.; Song, X.; Gao, X.; Xu, K.; Tang, B. Homotypic cell membrane-cloaked biomimetic nanocarrier for the accurate photothermal-chemotherapy treatment of recurrent hepatocellular carcinoma. J. Nanobiotechnol. 2020, 18, 60. [Google Scholar] [CrossRef]
- Xiong, Y.; Rao, Y.; Hu, J.; Luo, Z.; Chen, C. Nanoparticle-Based Photothermal Therapy for Breast Cancer Noninvasive Treatment. Adv. Mater. 2025, 37, 2305140. [Google Scholar] [CrossRef] [PubMed]
- Patrick, P.S.; Stuckey, D.J.; Zhu, H.; Kalber, T.L.; Iftikhar, H.; Southern, P.; Bear, J.C.; Lythgoe, M.F.; Hattersley, S.R.; Pankhurst, Q.A. Improved tumour delivery of iron oxide nanoparticles for magnetic hyperthermia therapy of melanoma via ultrasound guidance and 111In SPECT quantification. Nanoscale 2024, 16, 19715–19729. [Google Scholar] [CrossRef]
- Blasa, S.; Borzenkov, M.; Pastori, V.; Doveri, L.; Pallavicini, P.; Chirico, G.; Lecchi, M.; Collini, M. Prussian Blue Nanoparticle-Mediated Scalable Thermal Stimulation for In Vitro Neuronal Differentiation. Nanomaterials 2022, 12, 2304. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.; Zheng, Y.; Cai, X. Prussian Blue Nanoparticle: From a Photothermal Conversion Agent and a Drug Delivery System, to a Bioactive Drug. Acc. Mater. Res. 2024, 5, 687–698. [Google Scholar] [CrossRef]
- Chen, B.; Wu, M.; Qin, J.; Zhang, L.; Cao, X.; Jiang, K. Prussian blue-EGCG nanoparticles for synergistic photothermal and chemo anticancer therapy in vitro. Dye. Pigment. 2025, 232, 112487. [Google Scholar] [CrossRef]
- Hong, H.; Kim, M.; Lee, W.; Jeon, M.; Lee, C.; Kim, H.; Im, H.-J.; Piao, Y. Injectable biocompatible nanocomposites of Prussian blue nanoparticles and bacterial cellulose as a safe and effective photothermal cancer therapy. J. Nanobiotechnol. 2023, 21, 365. [Google Scholar] [CrossRef]
- Zhang, Y.; Ang, C.Y.; Zhao, Y. Polymeric nanocarriers incorporating near-infrared absorbing agents for potent photothermal therapy of cancer. Polym. J. 2016, 48, 589–603. [Google Scholar] [CrossRef]
- Xue, P.; Sun, L.; Li, Q.; Zhang, L.; Guo, J.; Xu, Z.; Kang, Y. PEGylated polydopamine-coated magnetic nanoparticles for combined targeted chemotherapy and photothermal ablation of tumour cells. Colloids Surf. B Biointerfaces 2017, 160, 11–21. [Google Scholar] [PubMed]
- Zhu, D.; Liu, F.; Ma, L.; Liu, D.; Wang, Z. Nanoparticle-Based Systems for T1-Weighted Magnetic Resonance Imaging Contrast Agents. Int. J. Mol. Sci. 2013, 14, 10591–10607. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yang, H.; Zhang, S.; Yang, Y.; Zhang, D.; Li, Z.; Zou, L. Targeting T1 and T2 dual modality enhanced magnetic resonance imaging of tumor vascular endothelial cells based on peptides-conjugated manganese ferrite nanomicelles. Int. J. Nanomed. 2016, 11, 4051–4063. [Google Scholar] [CrossRef] [PubMed]
- Gorgizadeh, M.; Behzadpour, N.; Salehi, F.; Daneshvar, F.; Vais, R.D.; Nazari-Vanani, R.; Azarpira, N.; Lotfi, M.; Sattarahmady, N. A MnFe2O4/C nanocomposite as a novel theranostic agent in MRI, sonodynamic therapy and photothermal therapy of a melanoma cancer model. J. Alloys Compd. 2020, 816, 152597. [Google Scholar] [CrossRef]
- Patrick, P.S.; Pankhurst, Q.A.; Payne, C.; Kalber, T.L.; Lythgoe, M.F. Magnet-Targeted Delivery and Imaging. In Design and Applications of Nanoparticles in Biomedical Imaging; Bulte, J.W.M., Modo, M.M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 123–152. [Google Scholar]
- Mohseni, M.; Connell, J.J.; Payne, C.; Patrick, P.S.; Baker, R.; Yu, Y.; Siow, B.; Zaw-Thin, M.; Kalber, T.L.; Pankhurst, Q.A.; et al. Scalable magnet geometries enhance tumour targeting of magnetic nano-carriers. Mater. Des. 2020, 191, 108610. [Google Scholar] [CrossRef]
- Kozakova, Z.; Kuritka, I.; Kazantseva, N.E.; Babayan, V.; Pastorek, M.; Machovsky, M.; Bazant, P.; Saha, P. The formation mechanism of iron oxide nanoparticles within the microwave-assisted solvothermal synthesis and its correlation with the structural and magnetic properties. Dalton Trans. 2015, 44, 21099–21108. [Google Scholar] [CrossRef] [PubMed]
- Long, X.-Y.; Li, J.-Y.; Sheng, D.; Lian, H.-Z. Spinel-type manganese ferrite (MnFe2O4) microspheres: A novel affinity probe for selective and fast enrichment of phosphopeptides. Talanta 2017, 166, 36–45. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, F.; Wang, Y.-X.J.; Yu, J.C.; Leung, K.C.-F. Facile synthesis of size-controllable monodispersed ferrite nanospheres. J. Mater. Chem. 2010, 20, 5086–5094. [Google Scholar] [CrossRef]
- Sagdeev, D.I.; Fomina, M.G.; Mukhamedzyanov, G.K.; Abdulagatov, I.M. Experimental study of the density and viscosity of polyethylene glycols and their mixtures at temperatures from 293K to 465K and at high pressures up to 245MPa. Fluid Phase Equilibria 2012, 315, 64–76. [Google Scholar] [CrossRef]
- Qi, X.; Liu, M.; Zhu, W.; Wei, Z.; Liang, Y.; Sun, C.; Bao, C.; Zhao, W. Spherical Fe3O4 morphology modulation for enhancing infrared emissivity and radiant heat dissipation. Opt. Mater. 2024, 152, 115541. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, W.; Zhao, Y.; Shen, H.; Lin, H.; Liang, J. Preparation and Characterization of Fe3O4 Particles with Novel Nanosheets Morphology and Magnetochromatic Property by a Modified Solvothermal Method. Sci. Rep. 2015, 5, 9320. [Google Scholar] [CrossRef]
- Wang, W.; Tang, B.; Wu, S.; Gao, Z.; Ju, B.; Teng, X.; Zhang, S. Controllable 5-sulfosalicylic acid assisted solvothermal synthesis of monodispersed superparamagnetic Fe3O4 nanoclusters with tunable size. J. Magn. Magn. Mater. 2017, 423, 111–117. [Google Scholar] [CrossRef]
- Ji, J.; Huang, Y.; Yin, J.; Zhao, X.; Cheng, X.; He, J.; Wang, J.; Li, X.; Liu, J. Electromagnetic Wave Absorption Performance on Fe3O4 Polycrystalline Synthesized by the Synergy Reduction of Ethylene Glycol and Diethylene Glycol. J. Phys. Chem. C 2018, 122, 3628–3637. [Google Scholar] [CrossRef]
- AboGabal, R.; Shokeir, D.; Oraby, A.H. Design and synthesis of biologically inspired biocompatible various polymeric magnetic nanoparticles for imaging and biomedical applications. Nano-Struct. Nano-Objects 2023, 36, 101048. [Google Scholar] [CrossRef]
- Chang, L.; Chang, S.; Chen, W.; Han, W.; Li, Z.; Zhang, Z.; Dai, Y.; Chen, D. Facile one-pot synthesis of magnetic Prussian blue core/shell nanoparticles for radioactive cesium removal. RSC Adv. 2016, 6, 96223–96228. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/chitosan modified biochar composite for enhanced methyl orange removal based on adsorption and photo-Fenton process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126104. [Google Scholar] [CrossRef]
- Ibrahim, I.; Ali, I.O.; Salama, T.M.; Bahgat, A.A.; Mohamed, M.M. Synthesis of magnetically recyclable spinel ferrite (MFe2O4, M=Zn, Co, Mn) nanocrystals engineered by sol gel-hydrothermal technology: High catalytic performances for nitroarenes reduction. Appl. Catal. B Environ. 2016, 181, 389–402. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, N.; Tian, K.; Qing, T.; Hao, Y.; Liang, P.; Li, M. Nitrilotriacetic acid modified magnetic Prussian blue for efficient removal of cadmium from wastewater. Appl. Surf. Sci. 2022, 600, 154102. [Google Scholar] [CrossRef]
- Patade, S.R.; Andhare, D.D.; Somvanshi, S.B.; Jadhav, S.A.; Khedkar, M.V.; Jadhav, K. Self-heating evaluation of superparamagnetic MnFe2O4 nanoparticles for magnetic fluid hyperthermia application towards cancer treatment. Ceram. Int. 2020, 46, 25576–25583. [Google Scholar] [CrossRef]
- Arun, T.; Kumar, T.K.; Udayabhaskar, R.; Morel, M.J.; Rajesh, G.; Mangalaraja, R.; Akbari-Fakhrabadi, A. Size dependent magnetic and capacitive performance of MnFe2O4 magnetic nanoparticles. Mater. Lett. 2020, 276, 128240. [Google Scholar] [CrossRef]
- Li, Z.; Si, F.; Ma, Y.; Geng, J.; Wang, G.; Wang, Z.; Yu, R.; Mu, J.; Hou, J.; Wang, Y.; et al. Self-assembled bio-inspired cauliflower-like MnFe2O4 nanospheres as dispersed materials for high-stability magnetorheological fluid. J. Magn. Magn. Mater. 2023, 571, 170589. [Google Scholar] [CrossRef]
- Du, B.; Cao, X.; Zhao, F.; Su, X.; Wang, Y.; Yan, X.; Jia, S.; Zhou, J.; Yao, H. Multimodal imaging-guided, dual-targeted photothermal therapy for cancer. J. Mater. Chem. B 2016, 4, 2038–2050. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, C.; Zou, B.; Wang, Y. Hollow prussian blue nanospheres for photothermal/chemo-synergistic therapy. Int. J. Nanomed. 2020, 15, 5165–5177. [Google Scholar] [CrossRef]
- Zou, H.; Wang, H.; Zhong, Y.; Zhang, Z.; Wang, Z.; Shang, T. Prussian blue nanoparticles coated with tumor cell membranes for precise photothermal therapy and subsequent inflammation reduction. Biochem. Biophys. Res. Commun. 2024, 723, 150173. [Google Scholar] [CrossRef]
- Valente-Rodrigues, C.L.; Caraballo-Vivas, R.J.; Santos, E.C.S.; Sharma, S.K.; Garcia, F. Investigation of Cr3+ doped Zn-Co nanoferrites as potential candidate for self-regulated magnetic hyperthermia applications. Phys. Scr. 2023, 98, 095913. [Google Scholar] [CrossRef]
- Cai, Y.; Chai, T.; Nguyen, W.; Liu, J.; Xiao, E.; Ran, X.; Ran, Y.; Du, D.; Chen, W.; Chen, X. Phototherapy in cancer treatment: Strategies and challenges. Signal Transduct. Target. Ther. 2025, 10, 115. [Google Scholar] [CrossRef]
- Ren, W.; Yan, Y.; Zeng, L.; Shi, Z.; Gong, A.; Schaaf, P.; Wang, D.; Zhao, J.; Zou, B.; Yu, H.; et al. A Near Infrared Light Triggered Hydrogenated Black TiO2 for Cancer Photothermal Therapy. Adv. Healthc. Mater. 2015, 4, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, Y.; Wang, X.; Wu, X.; Zha, Z. Facile synthesis of Prussian blue nanoparticles as pH-responsive drug carriers for combined photothermal-chemo treatment of cancer. RSC Adv. 2017, 7, 248–255. [Google Scholar] [CrossRef]
- Kale, S.S.; Burga, R.A.; Sweeney, E.E.; Zun, Z.; Sze, R.W.; Tuesca, A.; Subramony, J.A.; Fernandes, R. Composite iron oxide–Prussian blue nanoparticles for magnetically guided T1-weighted magnetic resonance imaging and photothermal therapy of tumors. Int. J. Nanomed. 2017, 12, 6413–6424. [Google Scholar] [CrossRef]
- Augustine, R.; Lee, H.R.; Kim, H.; Zhang, Y.; Kim, I. Hyperbranched lipopolymer-folate-stabilized manganese ferrite nanoparticles for the water-soluble targeted MRI contrast agent. React. Funct. Polym. 2019, 144, 104352. [Google Scholar] [CrossRef]
- Monaco, I.; Armanetti, P.; Locatelli, E.; Flori, A.; Maturi, M.; Del Turco, S.; Menichetti, L.; Comes Franchini, M. Smart assembly of Mn-ferrites/silica core–shell with fluorescein and gold nanorods: Robust and stable nanomicelles for in vivo triple modality imaging. J. Mater. Chem. B 2018, 6, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Patrick, P.S.; Bogart, L.K.; Macdonald, T.J.; Southern, P.; Powell, M.J.; Zaw-Thin, M.; Voelcker, N.H.; Parkin, I.P.; Pankhurst, Q.A.; Lythgoe, M.F.; et al. Surface radio-mineralisation mediates chelate-free radiolabelling of iron oxide nanoparticles. Chem. Sci. 2019, 10, 2592–2597. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Gong, S.-W.; Zhang, Y.; Yang, T.; Wang, C.-Y.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, A.; Jiang, Y.; Lan, D.; Lu, F.; Zheng, L.; Zhuang, L.; Hong, R. Large-scale preparation of size-controlled Fe3O4@SiO2 particles for electrophoretic display with non-iridescent structural colors. RSC Adv. 2019, 9, 498–506. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).