Synthesis, Characterization, and Toxicity Evaluation of Size-Dependent Iron-Based Metal–Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
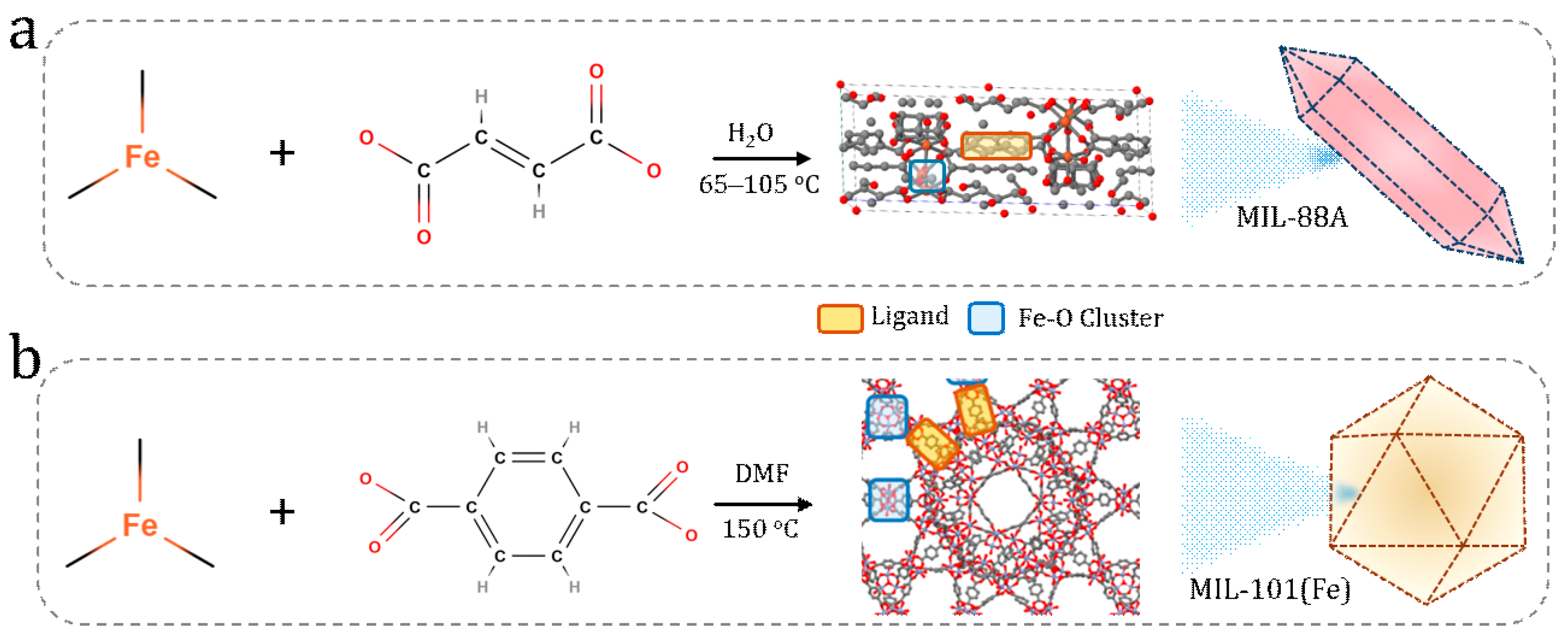
2.2. Synthesis of MOFs
2.2.1. Synthesis of MIL-101(Fe) Nanoparticles
2.2.2. Synthesis of Fe-MIL-88A Nanoparticles
2.3. Characterizations
2.4. Cytotoxicity Evaluation
2.4.1. Cell Culture
2.4.2. MTT Assay
2.4.3. Reactive Oxygen Species Assay
3. Results and Discussion
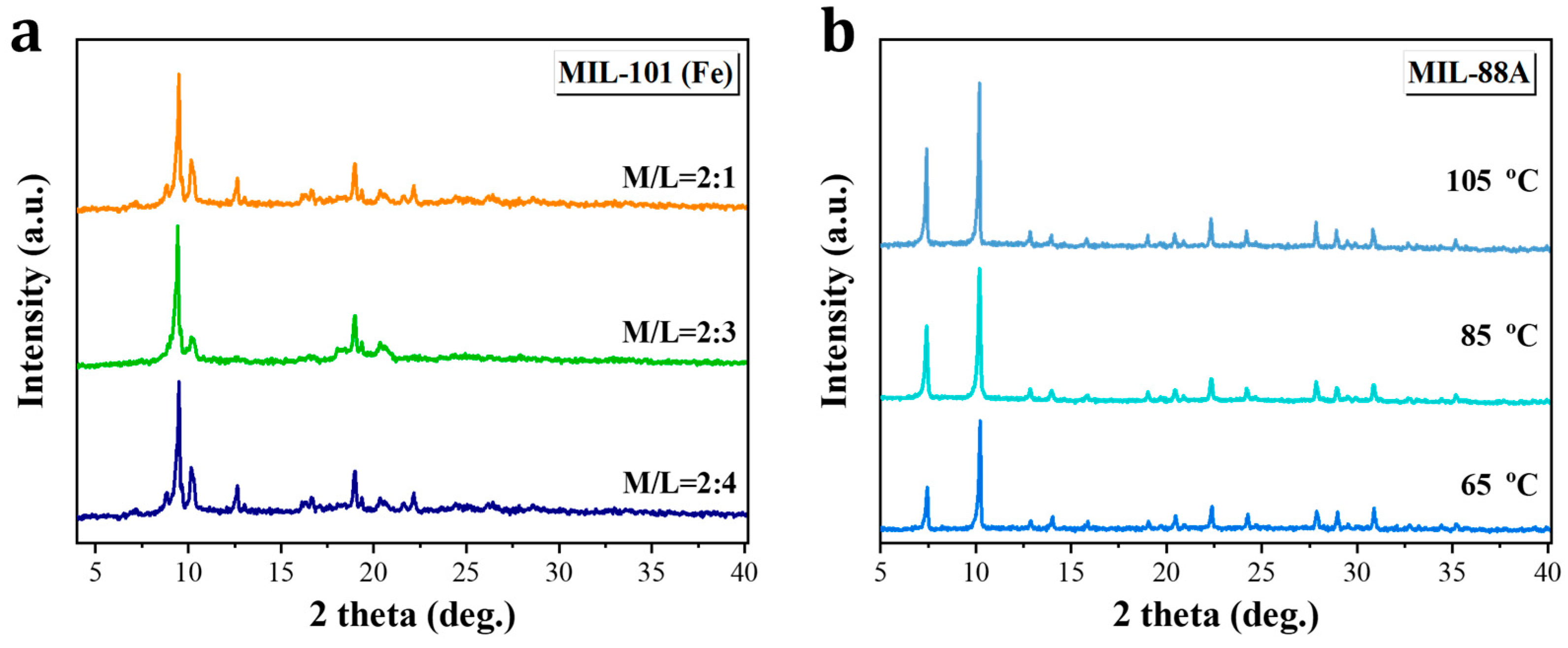
3.1. Synthesis of Size-Dependent Fe-MOFs
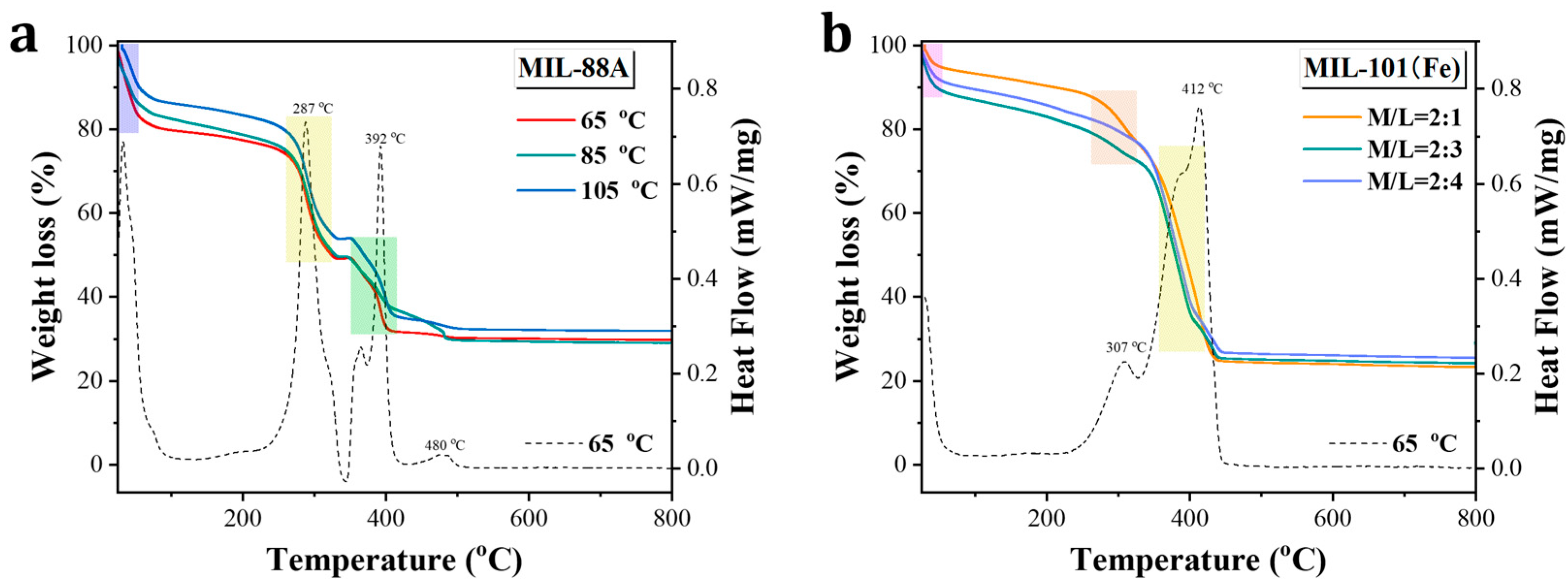
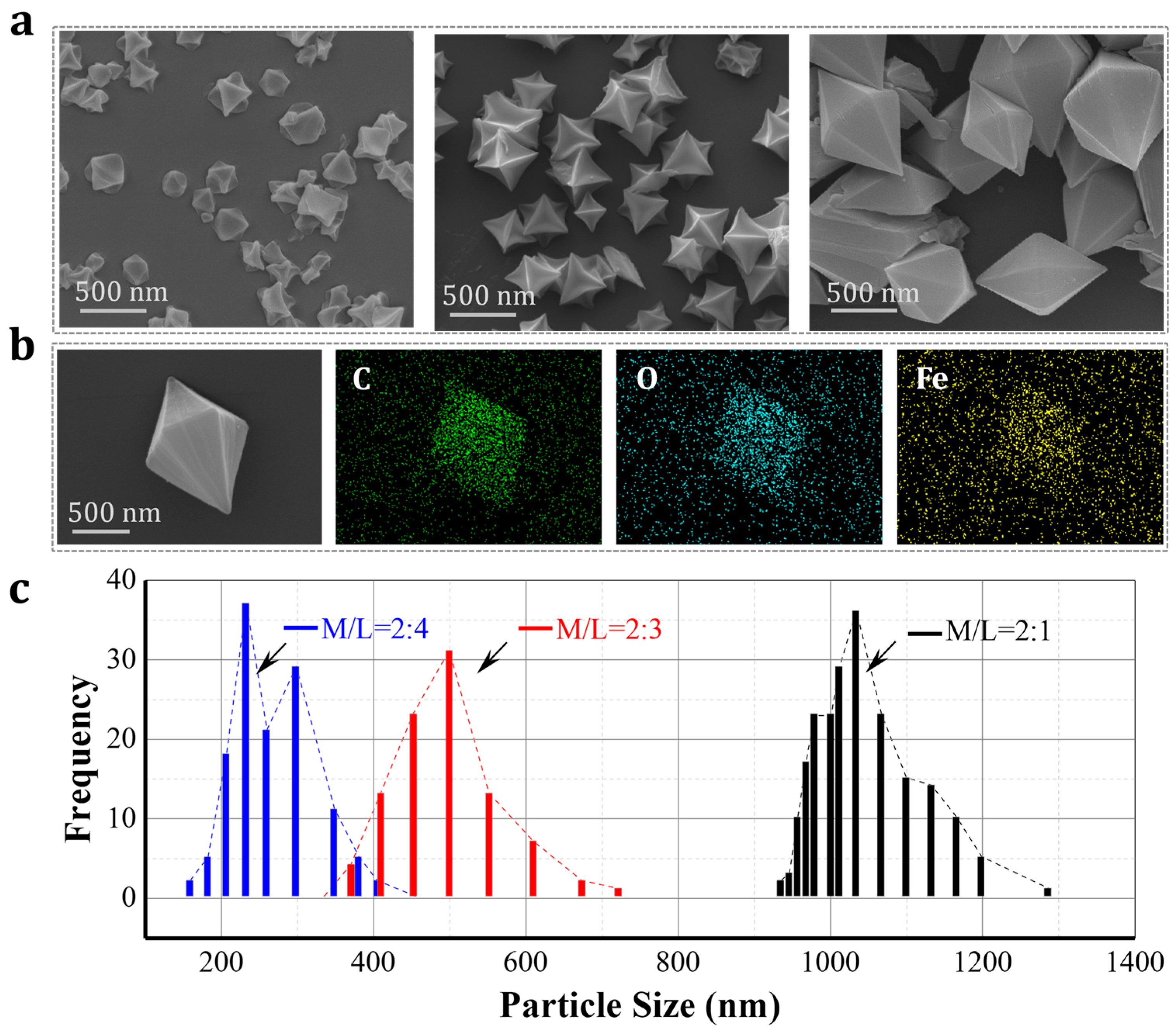
3.2. Characterizations of Fe-MOF Nanoparticles
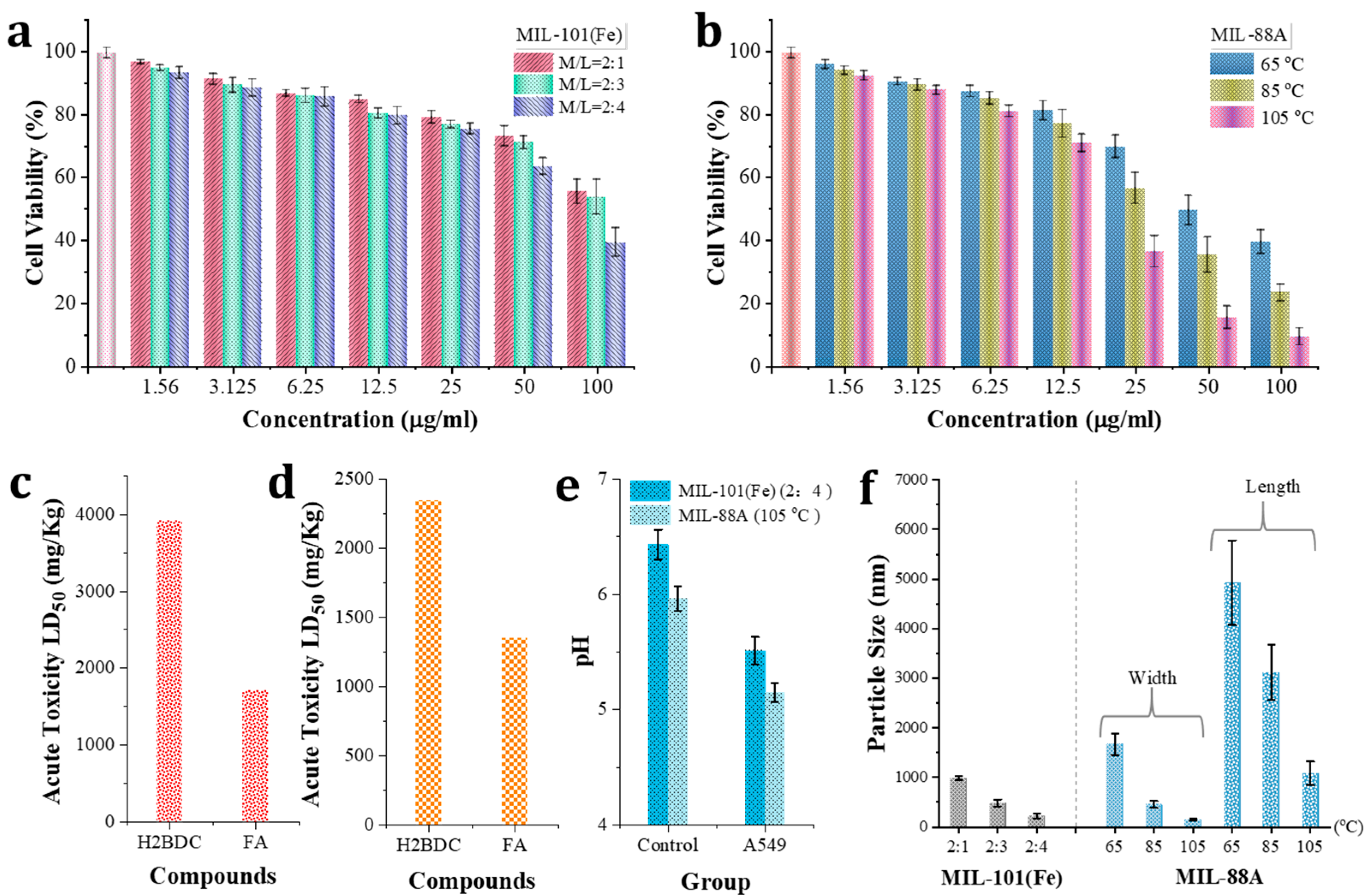
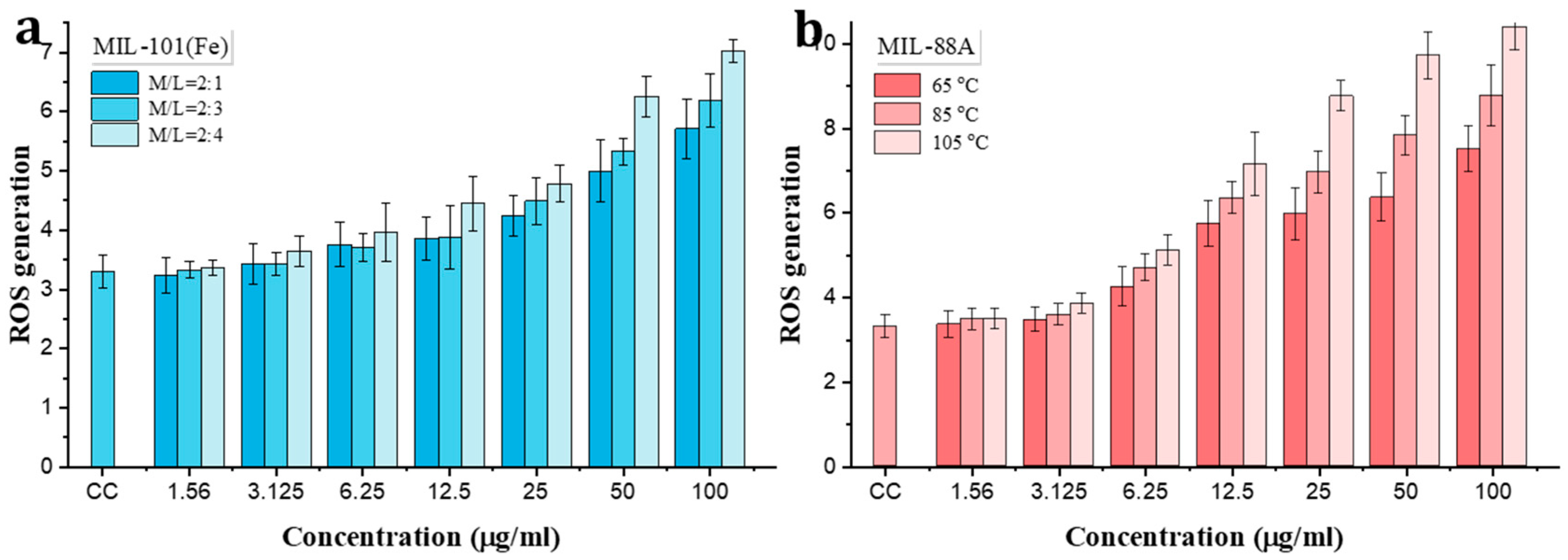
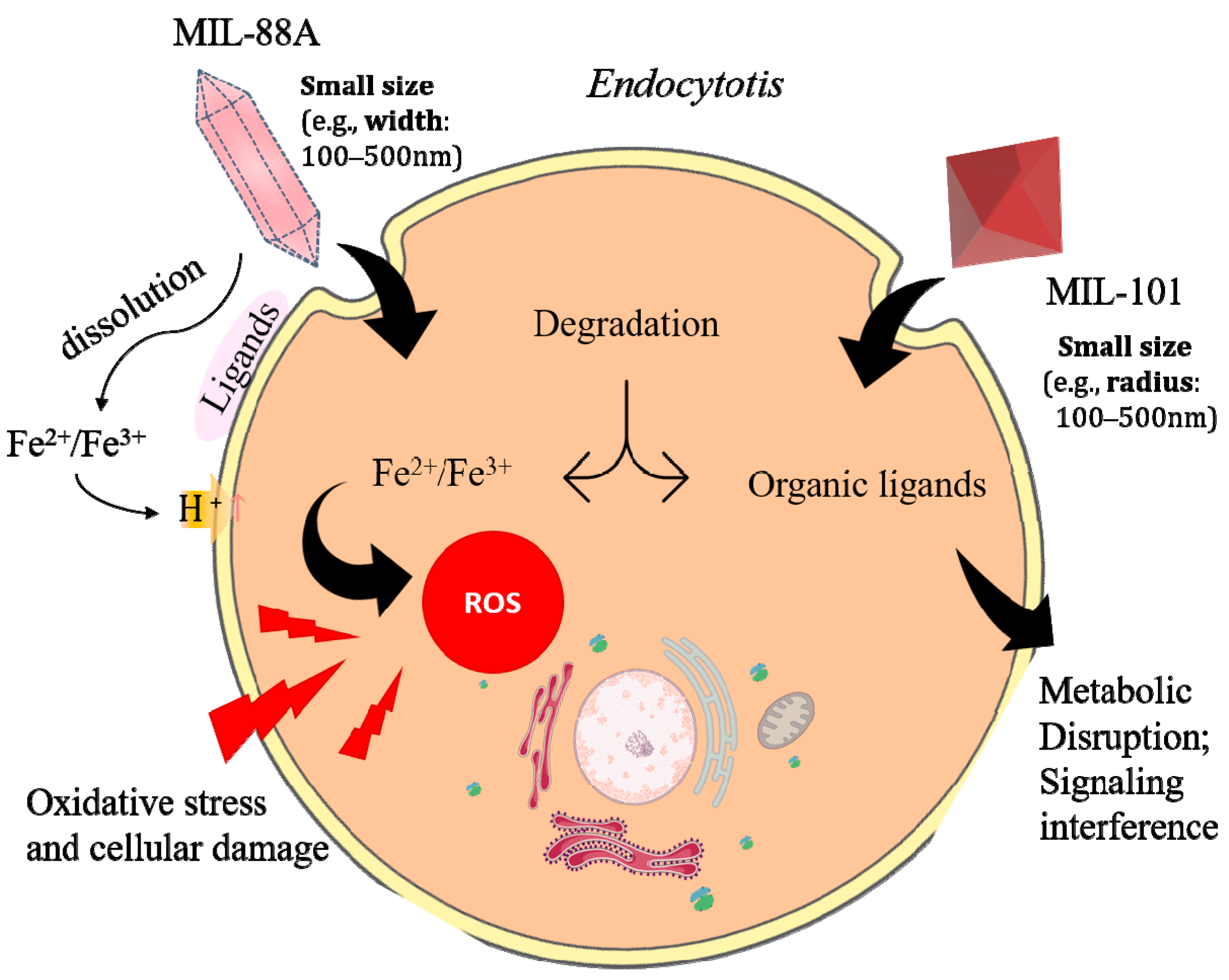
3.3. Toxicity Evalution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Wang, Q.; Li, Y.; Serrano-Lotina, A.; Han, W.; Portela, R.; Wang, R.; Bañares, M.A.; Yeung, K.L. Operando investigation of toluene oxidation over 1D Pt@ CeO2 derived from Pt cluster-containing MOF. J. Am. Chem. Soc. 2020, 143, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.W. Metal–organic frameworks for biomedical applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 190, 114496. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lepoitevin, M.; Serre, C. Iron-MOFs for Biomedical Applications. Adv. Healthc. Mater. 2025, 14, 2402630. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Cunha, D.; Imbuluzqueta, E.; Ragon, F.; Serre, C.; Blanco-Prieto, M.J.; Horcajada, P. Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B 2014, 2, 262–271. [Google Scholar] [CrossRef]
- Chen, G.; Leng, X.; Luo, J.; You, L.; Qu, C.; Dong, X.; Huang, H.; Yin, X.; Ni, J. In vitro toxicity study of a porous iron (III) metal‒organic framework. Molecules 2019, 24, 1211. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, M.; Qiu, T.; Wang, Q.; Chen, Z.; Deng, M.; Yang, Y.; Yang, Y.; Li, W. Ling, Gelothermal Synthesis of Monodisperse MIL-88A Nanoparticles with Tunable Sizes and Metal Centers for Potential Bioapplications. Small 2023, 19, 2301894. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorbeh, F.; Shahhosseini, S.; Dadashzadeh, S.; Asadian, E.; Mosayebnia, M.; Siavashy, S.J.H. An investigation of affecting factors on MOF characteristics for biomedical applications: A systematic review. Heliyon 2021, 7, e06914. [Google Scholar] [CrossRef]
- Hao, F.; Yan, Z.Y.; Yan, X.P. Recent advances in research on the effect of physicochemical properties on the cytotoxicity of metal–organic frameworks. Small Sci. 2022, 2, 2200044. [Google Scholar] [CrossRef]
- Far, B.F.; AdibAmini, S.; Pourmolaei, A. Cytotoxicity and Biocompatibility of Metal-Organic Frameworks. In Logic for Metal–Organic Framework Selection: MOFs for Biomedical Applications; ACS Publications: Washington, DC, USA, 2024; pp. 69–105. [Google Scholar]
- Zhang, H.; Ma, W.; Wang, Z.; Wu, X.; Zhang, H.; Fang, W.; Yan, R.; Jin, Y. Self-supply oxygen ROS reactor via fenton-like reaction and modulating glutathione for Amplified cancer therapy effect. Nanomaterials 2022, 12, 2509. [Google Scholar] [CrossRef]
- Yang, B.; Ding, L.; Yao, H.; Chen, Y.; Shi, J. A metal-organic framework (MOF) Fenton nanoagent-enabled nanocatalytic cancer therapy in synergy with autophagy inhibition. Adv. Mater. 2020, 32, 1907152. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, R.K.; Akhbari, K. Biocompatible MIL-101 (Fe) as a smart carrier with high loading potential and sustained release of curcumin. Inorg. Chem. 2020, 59, 3570–3578. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Li, B.; He, J.; Duan, D.; Shao, D.; Nie, M. Fe-MIL-101 exhibits selective cytotoxicity and inhibition of angiogenesis in ovarian cancer cells via downregulation of MMP. Sci. Rep. 2016, 6, 26126. [Google Scholar] [CrossRef] [PubMed]
- Serag, E.; El-Fakharany, E.M.; Hammad, S.F.; El-Khouly, M.E. Metal–organic framework MIL-101 (Fe) functionalized with folic acid as a multifunctional nanocarrier for targeted chemotherapy–photodynamic therapy. Biomater. Sci. 2025, 13, 2351–2367. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, G.; Dorożyński, P.; Gil, B.; Roth, W.J.; Strzempek, M.; Marszałek, B.; Węglarz, W.P.; Menaszek, E.; Strzempek, W.; Kulinowski, P. Iron-based metal-organic frameworks as a theranostic carrier for local tuberculosis therapy. Pharm. Res. 2018, 35, 144. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Barroso, N.; Dutta, S.; Andreo, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S.; Wuttke, S. Guest-induced breathing mediated selective alcohol recovery from water by MIL-88A (Fe). J. Mater. Chem. A 2023, 11, 21300–21311. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Zhang, J.; Zhang, D.; Zhou, X.; Xiong, J. Breathable metal–organic framework enhanced humidity-responsive nanofiber actuator with autonomous triboelectric perceptivity. ACS Nano 2023, 17, 17920–17930. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Alalm, M.G.; Kaliaguine, S.; Boffito, D.C. Revisiting the MIL-101 metal–organic framework: Design, synthesis, modifications, advances, and recent applications. J. Mater. Chem. A 2021, 9, 22159–22217. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, E.; Zebarjad, S.M.; Hosseini, H.R.M. Chagnon, Preparation, optimization and evolution of the kinetic mechanism of an Fe-MIL-88A metal–organic framework. CrystEngComm 2019, 21, 544–553. [Google Scholar] [CrossRef]
- Benítez, A.; Amaro-Gahete, J.; Esquivel, D.; Romero-Salguero, F.J.; Morales, J.; Caballero, Á. MIL-88A metal-organic framework as a stable sulfur-host cathode for long-cycle Li-S batteries. Nanomaterials 2020, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Van Vleet, M.J.; Weng, T.; Li, X.; Schmidt, J. In situ, time-resolved, and mechanistic studies of metal–organic framework nucleation and growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef]
- Liu, Z.; Navas, J.L.; Han, W.; Ibarra, M.R.; Kwan, J.K.C.; Yeung, K.L. Gel transformation as a general strategy for fabrication of highly porous multiscale MOF architectures. Chem. Sci. 2023, 14, 7114–7125. [Google Scholar] [CrossRef]
- Liu, Z.; Marquina, C.; Han, W.; Kwan, J.K.; Ibarra, M.R.; Yeung, K.L. Insight into the molecular mechanism of organic pollutants’ adsorption on magnetic ZIF-8 synthesized via a transformational route. Sep. Purif. Technol. 2024, 356, 130006. [Google Scholar] [CrossRef]
- Carpenter, B.P.; Talosig, A.R.; Rose, B.; Di Palma, G.; Patterson, J.P. Understanding and controlling the nucleation and growth of metal–organic frameworks. Chem. Soc. Rev. 2023, 52, 6918–6937. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Self-assembled core–shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ahn, E.-Y.; Park, Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef]
- Chen, P.; He, M.; Chen, B.; Hu, B. Size-and dose-dependent cytotoxicity of ZIF-8 based on single cell analysis. Ecotoxicol. Environ. Saf. 2020, 205, 111110. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Noga, M.; Michalska, A.; Jurowski, K. The prediction of acute toxicity (LD50) for organophosphorus-based chemical warfare agents (V-series) using toxicology in silico methods, Archives of Toxicology. Arch. Toxicol. 2024, 98, 267–275. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Xu, W.; Zhu, X.; Liang, F.; Yu, Y.; Zheng, Y.; Yao, L.; Zhang, H.; Lin, K. Heterogeneous photocatalysis coupled with Fenton-Like reaction for fluoroquinolone antibiotics degradation by poly (Triazine Imide): From mechanism to application in a continuous flow catalytic system. Chem. Eng. J. 2023, 476, 146856. [Google Scholar] [CrossRef]
- Lagadic-Gossmann, D.; Huc, L.; Lecureur, V. Alterations of intracellular pH homeostasis in apoptosis: Origins and roles. Cell Death Differ. 2004, 11, 953–961. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zha, Y.; Zhong, Z.; Ruan, Y.; Li, Z.; Sun, L.; Hou, S. Improved detection of reactive oxygen species by DCFH-DA: New insight into self-amplification of fluorescence signal by light irradiation. Sens. Actuators B Chem. 2021, 339, 129878. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef]
- Sajid, M. Toxicity of nanoscale metal organic frameworks: A perspective. Environ. Sci. Pollut. Res. 2016, 23, 14805–14807. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Sipos, A.; Kim, K.-J.; Sioutas, C.; Crandall, E.D. Evidence for nanoparticle-induced lysosomal dysfunction in lung adenocarcinoma (A549) cells. Int. J. Mol. Sci. 2019, 20, 5253. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Shang, M.; Niu, S.; Zhang, W.; Zhang, B.; Huang, W.; Wu, T.; Zhang, T.; Tang, M.; et al. Mitophagy–lysosomal pathway is involved in silver nanoparticle-induced apoptosis in A549 cells. Ecotoxicol. Environ. Saf. 2021, 208, 111463. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Deng, H.; Zheng, Y.; Tian, Y.; Zhang, Y.; Garcia, R.M.; Henson Garcia, S.A.; Yeung, K.L. Synthesis, Characterization, and Toxicity Evaluation of Size-Dependent Iron-Based Metal–Organic Frameworks. Nanomaterials 2025, 15, 927. https://doi.org/10.3390/nano15120927
Liu Z, Deng H, Zheng Y, Tian Y, Zhang Y, Garcia RM, Henson Garcia SA, Yeung KL. Synthesis, Characterization, and Toxicity Evaluation of Size-Dependent Iron-Based Metal–Organic Frameworks. Nanomaterials. 2025; 15(12):927. https://doi.org/10.3390/nano15120927
Chicago/Turabian StyleLiu, Zhang, Huaiyu Deng, Yuanzhi Zheng, Yuan Tian, Yanting Zhang, Renz Marion Garcia, Sheena Anne Henson Garcia, and King Lun Yeung. 2025. "Synthesis, Characterization, and Toxicity Evaluation of Size-Dependent Iron-Based Metal–Organic Frameworks" Nanomaterials 15, no. 12: 927. https://doi.org/10.3390/nano15120927
APA StyleLiu, Z., Deng, H., Zheng, Y., Tian, Y., Zhang, Y., Garcia, R. M., Henson Garcia, S. A., & Yeung, K. L. (2025). Synthesis, Characterization, and Toxicity Evaluation of Size-Dependent Iron-Based Metal–Organic Frameworks. Nanomaterials, 15(12), 927. https://doi.org/10.3390/nano15120927






