TiO2 Nanoparticles Loaded with Polygonum cuspidatum Extract for Wound Healing Applications: Exploring Their Hemolytic, Antioxidant, Cytotoxic, and Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of TiO2-Loaded Extract NPs from P. cuspidatum Hydroalcoholic Extract
2.3. Antioxidant Assay
2.3.1. DPPH Radical Scavenging Activity
2.3.2. ABTS+ Radical Scavenging Activity
2.4. Hemolytic Assay
2.5. Resveratrol Release Assay
2.6. Antibacterial Assay
2.6.1. Bacterial Strains
2.6.2. Microdilution Assay
2.6.3. Antifungal Assay
2.7. In Vitro Cytotoxic Assay
2.8. Statistical Analysis
3. Results and Discussion
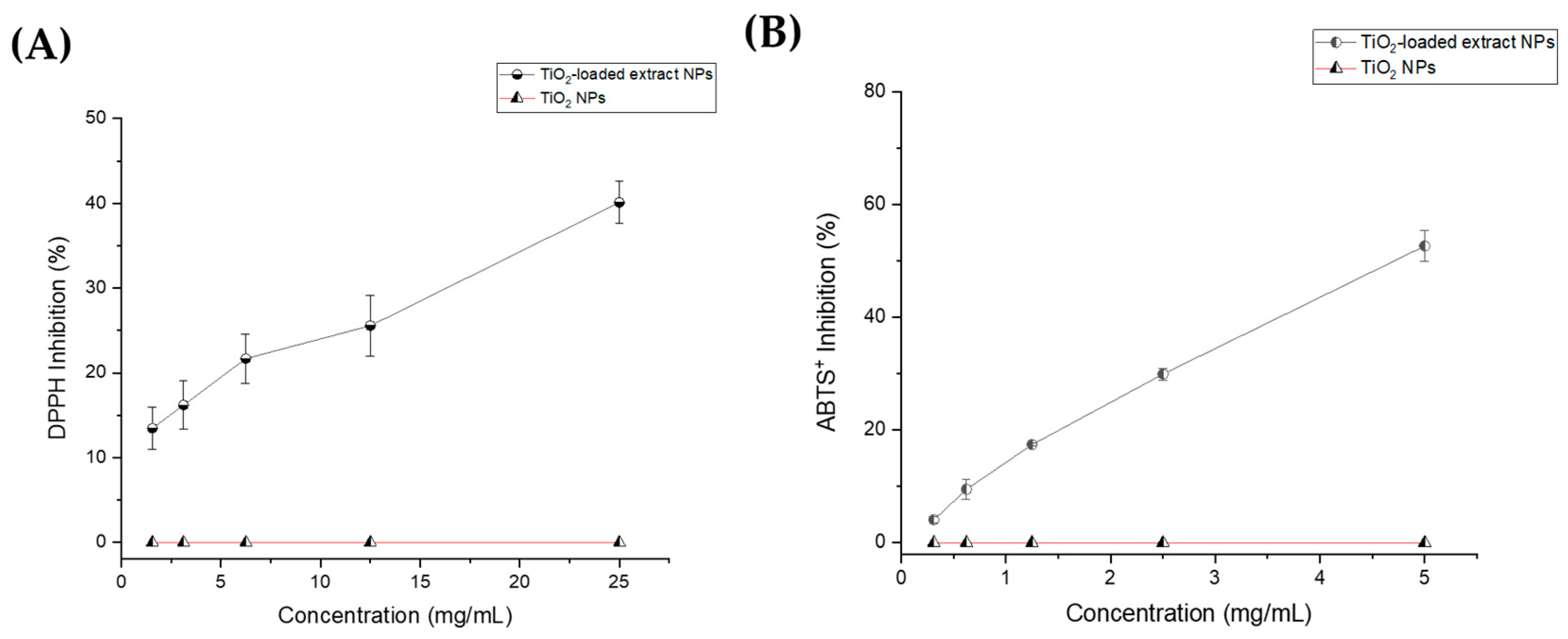
3.1. Antioxidant Activity
3.2. Hemolytic Actiivit
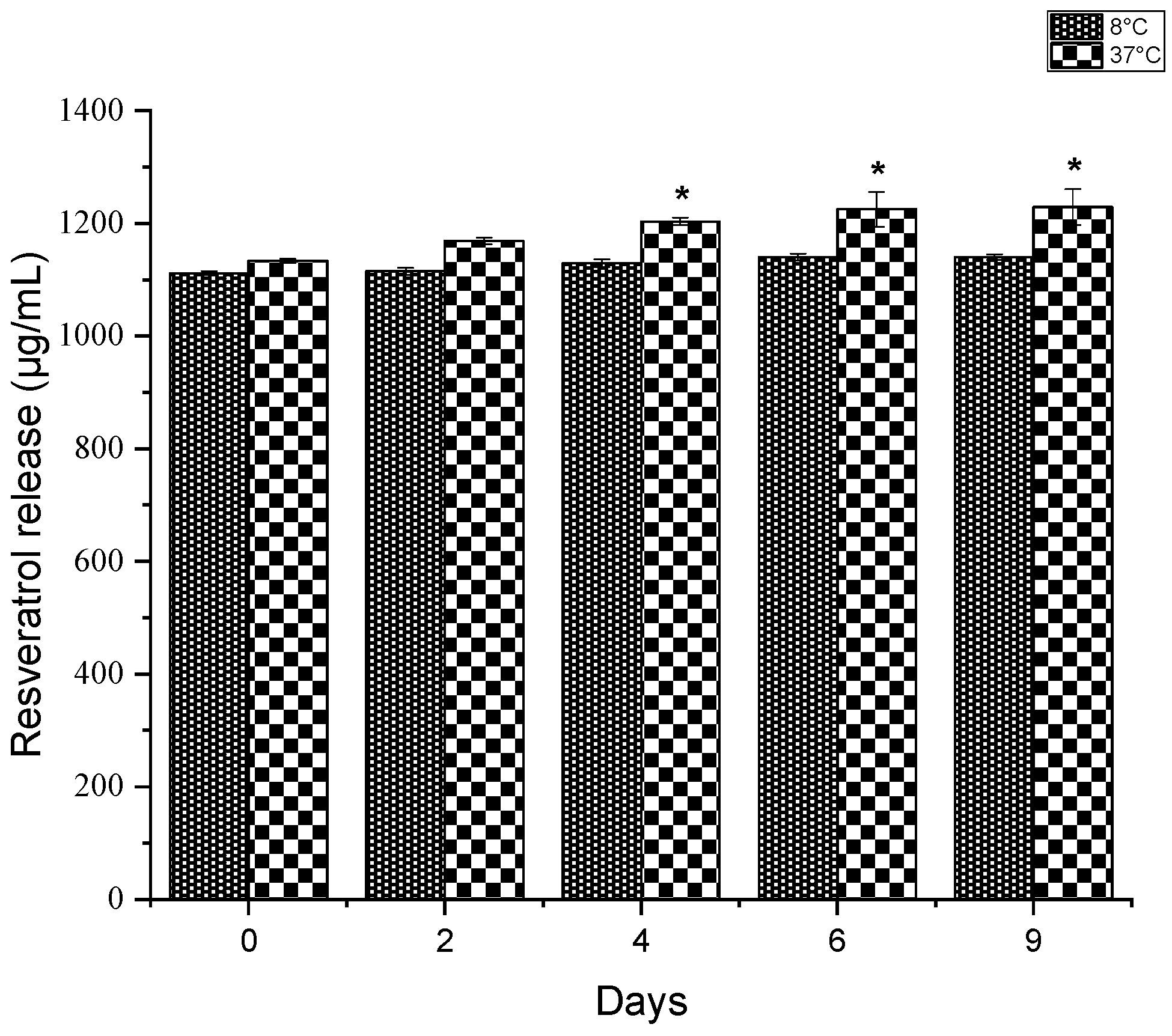
3.3. Resveratrol Release
3.4. Antibacterial Activity
3.5. Antifungal Activity
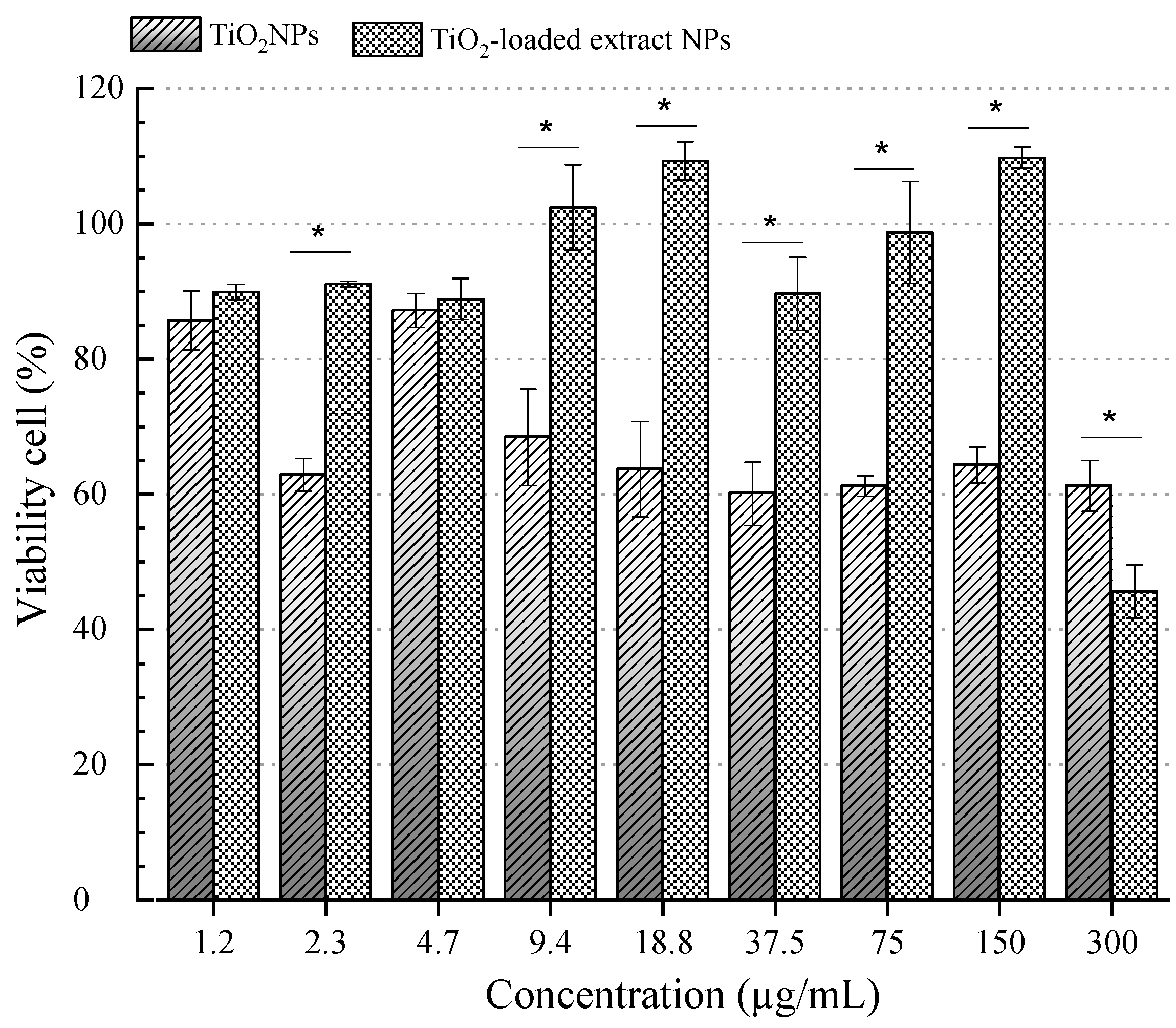
3.6. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Konyalioglu, S.; Armagan, G.; Yalcin, A.; Atalayin, C.; Dagci, T. Effects of resveratrol on hydrogen peroxide-induced oxidative stress in embryonic neural stem cells. Neural. Regen. Res. 2013, 8, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Fletes-Vargas, G.; Rodríguez-Preciado, S.Y.; Díaz-Zaragoza, M.; Rodríguez-Rodríguez, R. Natural Hydrogels as Wound Dressing for Skin Wound-Healing Applications. In Interaction of Nanomaterials With Living Cells; Sheikh, F.A., Majeed, S., Beigh, M.A., Eds.; Springer Nature: Singapore, 2023; pp. 439–469. [Google Scholar]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Ke, J.; Li, M.T.; Xu, S.; Ma, J.; Liu, M.Y.; Han, Y. Advances for pharmacological activities of Polygonum cuspidatum—A review. Pharm. Biol. 2023, 61, 177–188. [Google Scholar] [CrossRef]
- Anupong, W.; On-uma, R.; Jutamas, K.; Salmen, S.H.; Alharbi, S.A.; Joshi, D.; Jhanani, G.K. Antibacterial, antifungal, antidiabetic, and antioxidant activities potential of Coleus aromaticus synthesized titanium dioxide nanoparticles. Environ. Res. 2023, 216, 114714. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, J. Resveratrol alleviates Staphylococcus aureus pneumonia by inhibition of the NLRP3 inflammasome. Exp. Ther. Med. 2017, 14, 6099–6104. [Google Scholar] [CrossRef]
- Hadzik, J.; Choromańska, A.; Karolewicz, B.; Matkowski, A.; Dominiak, M.; Złocińska, A.; Nawrot-Hadzik, I. Oral Wound Healing Potential of Polygoni Cuspidati Rhizoma et Radix Decoction-In Vitro Study. Pharmaceuticals 2023, 16, 267. [Google Scholar] [CrossRef]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef]
- Kant, V.; Kumari, P.; Jitendra, D.K.; Ahuja, M.; Kumar, V. Nanomaterials of Natural Bioactive Compounds for Wound Healing: Novel Drug Delivery Approach. Curr. Drug Deliv. 2021, 18, 1406–1425. [Google Scholar] [CrossRef]
- Jia, Y.; Shao, J.H.; Zhang, K.W.; Zou, M.L.; Teng, Y.Y.; Tian, F.; Chen, M.N.; Chen, W.W.; Yuan, Z.D.; Wu, J.J.; et al. Emerging Effects of Resveratrol on Wound Healing: A Comprehensive Review. Molecules 2022, 27, 6736. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Alasiri, A.S.; Ahmad, J.; Alqahtani, A.A.; Abdullah, M.M.; Abdel-Wahab, B.A.; Pathak, K.; Saikia, R.; Das, A.; Sarma, H.; et al. Green Synthesis of Titanium Dioxide Nanoparticles Using Ocimum sanctum Leaf Extract: In Vitro Characterization and Its Healing Efficacy in Diabetic Wounds. Molecules 2022, 27, 7712. [Google Scholar] [CrossRef] [PubMed]
- Fletes-Vargas, G.; Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Yáñez-Sánchez, I.; Gálvez-Gastelum, F.J.; Carneiro, G.; Pérez-Larios, A. Physical chitosan hydrogels loaded with TiO2 nanoparticles/resveratrol hydroalcoholic extract: Hemocompatibility, swelling, microstructural, and EDX mapping analysis. Mater. Lett. 2024, 374, 137180. [Google Scholar] [CrossRef]
- Fletes-Vargas, G.; Rodríguez-Rodríguez, R.; Pacheco, N.; Pérez-Larios, A.; Espinosa-Andrews, H. Evaluation of the Biological Properties of an Optimized Extract of Polygonum cuspidatum Using Ultrasonic-Assisted Extraction. Molecules 2023, 28, 4079. [Google Scholar] [CrossRef]
- Bhatnagar, A.S.; Prasanth Kumar, P.K.; Hemavathy, J.; Gopala Krishna, A.G. Fatty Acid Composition, Oxidative Stability, and Radical Scavenging Activity of Vegetable Oil Blends with Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 991–999. [Google Scholar] [CrossRef]
- Scalise, M.L.; Arrúa, E.C.; Rial, M.S.; Esteva, M.I.; Salomon, C.J.; Fichera, L.E. Promising Efficacy of Benznidazole Nanoparticles in Acute Trypanosoma cruzi Murine Model: In-Vitro and In-Vivo Studies. Am. J. Trop. Med. Hyg. 2016, 95, 388–393. [Google Scholar] [CrossRef]
- Travnickova, E.; Mikula, P.; Oprsal, J.; Bohacova, M.; Kubac, L.; Kimmer, D.; Soukupova, J.; Bittner, M. Resazurin assay for assessment of antimicrobial properties of electrospun nanofiber filtration membranes. AMB Express 2019, 9, 183. [Google Scholar] [CrossRef]
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Chu, G.C.; Chao, T.; Heard, T.C.; Gómez, B.I.; Sousse, L.E.; Natesan, S.; Christy, R.J. ASCs derived from burn patients are more prone to increased oxidative metabolism and reactive oxygen species upon passaging. Stem Cell Res. Ther. 2021, 12, 270. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. The Effects of Acyl Chain Length on Antioxidant Efficacy of Mono- and Multi-Acylated Resveratrol: A Comparative Assessment. Molecules 2022, 27, 1001. [Google Scholar] [CrossRef] [PubMed]
- Yedgar, S.; Barshtein, G.; Gural, A. Hemolytic Activity of Nanoparticles as a Marker of Their Hemocompatibility. Micromachines 2022, 13, 2091. [Google Scholar] [CrossRef]
- Fröhlich, E. Hemocompatibility of inhaled environmental nanoparticles: Potential use of in vitro testing. J. Hazard. Mater. 2017, 336, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Chakraborty, A.; Mukherjee, A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J. Appl. Toxicol. 2013, 33, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical use of resveratrol: Technological aspects. Pharmacia 2020, 67, 89–94. [Google Scholar] [CrossRef]
- Soldati, P.P.; Polonini, H.C.; Paes, C.Q.; Restrepob, J.A.S.; Creczynksi-Pasa, T.B.; Chaves, M.G.A.M.; Brandão, M.A.F.; Pittella, F.; Raposo, N.R.B. Controlled release of resveratrol from lipid nanoparticles improves antioxidant effect. IFAC-Pap. 2018, 51, 16–21. [Google Scholar] [CrossRef]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J.M. Wound healing and microbiome, an unexpected relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S3), 7–15. [Google Scholar] [CrossRef]
- White, E.K.; Grice, E.A. The Wound Microbiome. Cold Spring Harb. Perspect. Biol. 2023, 15, a041218. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, J.; Qin, S.; Zhou, Z.; Xu, Y.; Liu, C. Advances and Challenges in Immune-Modulatory Biomaterials for Wound Healing Applications. Pharmaceutics 2024, 16, 990. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Lin, Y.; Zhao, X.; Chen, H.; Zhong, Y.; Jiang, W.; Wu, X.; Lin, X. Unveiling the antibacterial mechanism of resveratrol against Aeromonas hydrophila through proteomics analysis. Front. Cell. Infect. Microbiol. 2024, 14, 1378094. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008, 109, 530–537. [Google Scholar] [CrossRef]
- Su, P.W.; Yang, C.H.; Yang, J.F.; Su, P.Y.; Chuang, L.Y. Antibacterial Activities and Antibacterial Mechanism of Polygonum cuspidatum Extracts against Nosocomial Drug-Resistant Pathogens. Molecules 2015, 20, 11119–11130. [Google Scholar] [CrossRef]
- Üstündağ, H.; Kara, A.; Doğanay, S.; Kurt, N.; Erbaş, E.; Kalindemirtaş, F.D.; Kariper, İ.A. Molecular mechanisms of resveratrol and its silver nanoparticle conjugate in addressing sepsis-induced lung injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 6249–6261. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Grice, E.A. Fungi in the Wound Microbiome. Adv. Wound Care 2018, 7, 247–255. [Google Scholar] [CrossRef]
- Shevelev, A.B.; La Porta, N.; Isakova, E.P.; Martens, S.; Biryukova, Y.K.; Belous, A.S.; Sivokhin, D.A.; Trubnikova, E.V.; Zylkova, M.V.; Belyakova, A.V.; et al. In Vivo Antimicrobial and Wound-Healing Activity of Resveratrol, Dihydroquercetin, and Dihydromyricetin against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans. Pathogens 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Kim, Y.-H. Anticandidal effect of Polygonum cuspidatum on C. albicans biofilm formation. J. Physiol. Pathol. Korean Med. 2012, 26, 74–80. [Google Scholar]
- Collado-González, M.; Guirao-Abad, J.P.; Sánchez-Fresneda, R.; Belchí-Navarro, S.; Argüelles, J.-C. Resveratrol lacks antifungal activity against Candida albicans. World J. Microbiol. Biotechnol. 2012, 28, 2441–2446. [Google Scholar] [CrossRef]
- Salazar, J.; Carmona, T.; Zacconi, F.C.; Venegas-Yazigi, D.; Cabello-Verrugio, C.; Choi, W.I.; Vilos, C. The Human Dermis as a Target of Nanoparticles for Treating Skin Conditions. Pharmaceutics 2023, 15, 10. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Jin, C.Y.; Zhu, B.S.; Wang, X.F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, J.; Liu, X.; Gao, G.; Huang, J.; Li, G. Electrochemical sensing DNA damage with nano-titanium dioxide and repair with a medicinal herb species resveratrol. J. Biotechnol. 2007, 127, 653–656. [Google Scholar] [CrossRef]
- Giovannelli, L.; Pitozzi, V.; Jacomelli, M.; Mulinacci, N.; Laurenzana, A.; Dolara, P.; Mocali, A. Protective Effects of Resveratrol Against Senescence-Associated Changes in Cultured Human Fibroblasts. J. Gerontol. Ser. A 2010, 66A, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kaleci, B.; Koyuturk, M. Efficacy of resveratrol in the wound healing process by reducing oxidative stress and promoting fibroblast cell proliferation and migration. Dermatol. Ther. 2020, 33, e14357. [Google Scholar] [CrossRef] [PubMed]

| P. cuspidatum Extract | TiO2-Loaded Extract NPs | TiO2 NPs | |||
|---|---|---|---|---|---|
| (Resveratrol mg/mL) | ROH (%) | (mg/mL) | ROH (%) | (mg/mL) | ROH (%) |
| 10 | 42.3 ± 4.3 a | 20 | 3.9 ± 0.6 a | 20 | 15.6 ± 4.6 a |
| 5 | 24.8 ± 1.1 b | 10 | 3.2 ± 1.3 a | 10 | 7.96 ± 1.3 b |
| 2.5 | 7.5 ± 1.2 c | 5 | 2.0 ± 0.6 a | 5 | 5.9 ± 0.9 b |
| 1.25 | 2.3 ± 0.7 d | 2.5 | 1.3 ± 0.2 a | 2.5 | 4.6 ± 0.8 b |
| Bacterial Strain | P. cuspidatum Extract (Resveratrol mg/mL) | TiO2-Loaded Extract NPs (mg/mL) | TiO2 NPs (mg/mL) |
|---|---|---|---|
| P. aeruginosa | 5 | 50 | ND |
| E. coli | 5 | 50 | ND |
| S. aureus | 5 | 50 | ND |
| S. typhimurium | 5 | 50 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletes-Vargas, G.; Rodríguez-Rodríguez, R.; Pinto, N.V.; Kato, K.C.; Carneiro, G.; Rodrigues, A.P.; Rodrigues-Martins, H.; Espinosa-Andrews, H. TiO2 Nanoparticles Loaded with Polygonum cuspidatum Extract for Wound Healing Applications: Exploring Their Hemolytic, Antioxidant, Cytotoxic, and Antimicrobial Properties. Nanomaterials 2025, 15, 926. https://doi.org/10.3390/nano15120926
Fletes-Vargas G, Rodríguez-Rodríguez R, Pinto NV, Kato KC, Carneiro G, Rodrigues AP, Rodrigues-Martins H, Espinosa-Andrews H. TiO2 Nanoparticles Loaded with Polygonum cuspidatum Extract for Wound Healing Applications: Exploring Their Hemolytic, Antioxidant, Cytotoxic, and Antimicrobial Properties. Nanomaterials. 2025; 15(12):926. https://doi.org/10.3390/nano15120926
Chicago/Turabian StyleFletes-Vargas, Gabriela, Rogelio Rodríguez-Rodríguez, Natalha Vicentina Pinto, Kelly Cristina Kato, Guilherme Carneiro, Ana Paula Rodrigues, Helen Rodrigues-Martins, and Hugo Espinosa-Andrews. 2025. "TiO2 Nanoparticles Loaded with Polygonum cuspidatum Extract for Wound Healing Applications: Exploring Their Hemolytic, Antioxidant, Cytotoxic, and Antimicrobial Properties" Nanomaterials 15, no. 12: 926. https://doi.org/10.3390/nano15120926
APA StyleFletes-Vargas, G., Rodríguez-Rodríguez, R., Pinto, N. V., Kato, K. C., Carneiro, G., Rodrigues, A. P., Rodrigues-Martins, H., & Espinosa-Andrews, H. (2025). TiO2 Nanoparticles Loaded with Polygonum cuspidatum Extract for Wound Healing Applications: Exploring Their Hemolytic, Antioxidant, Cytotoxic, and Antimicrobial Properties. Nanomaterials, 15(12), 926. https://doi.org/10.3390/nano15120926








