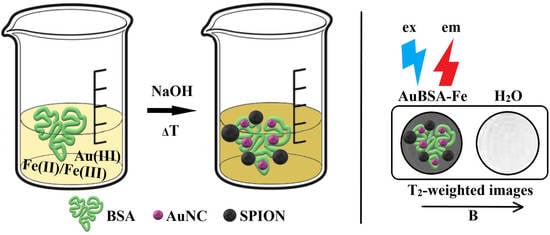
Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals for Syntheses
2.2. Chemicals for Alamar Blue Assay
2.3. Syntheses of AuBSA and AuBSA-Fe—Their Purification, Concentrate Formation, and Storage
2.4. Characterization Techniques
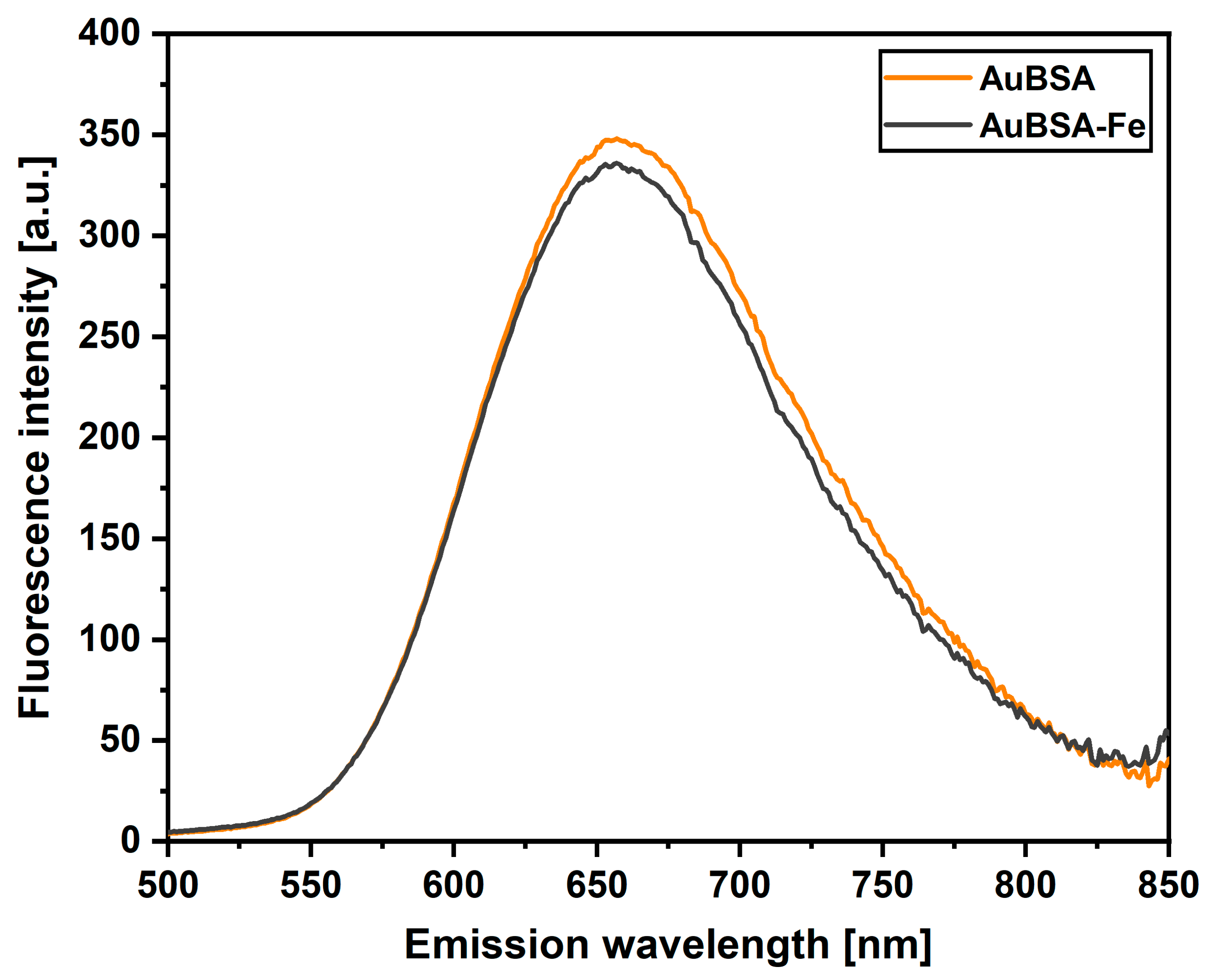
2.4.1. Fluorescence Spectroscopy
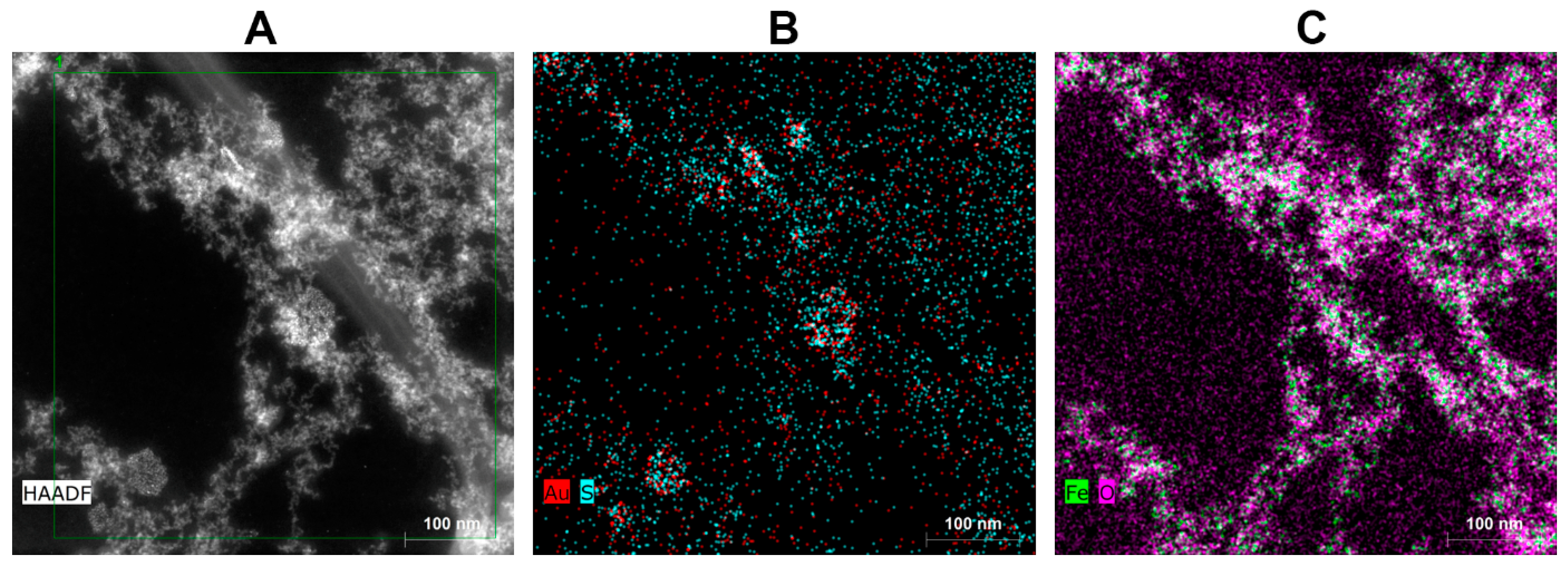
2.4.2. HR-TEM, STEM, and EDS
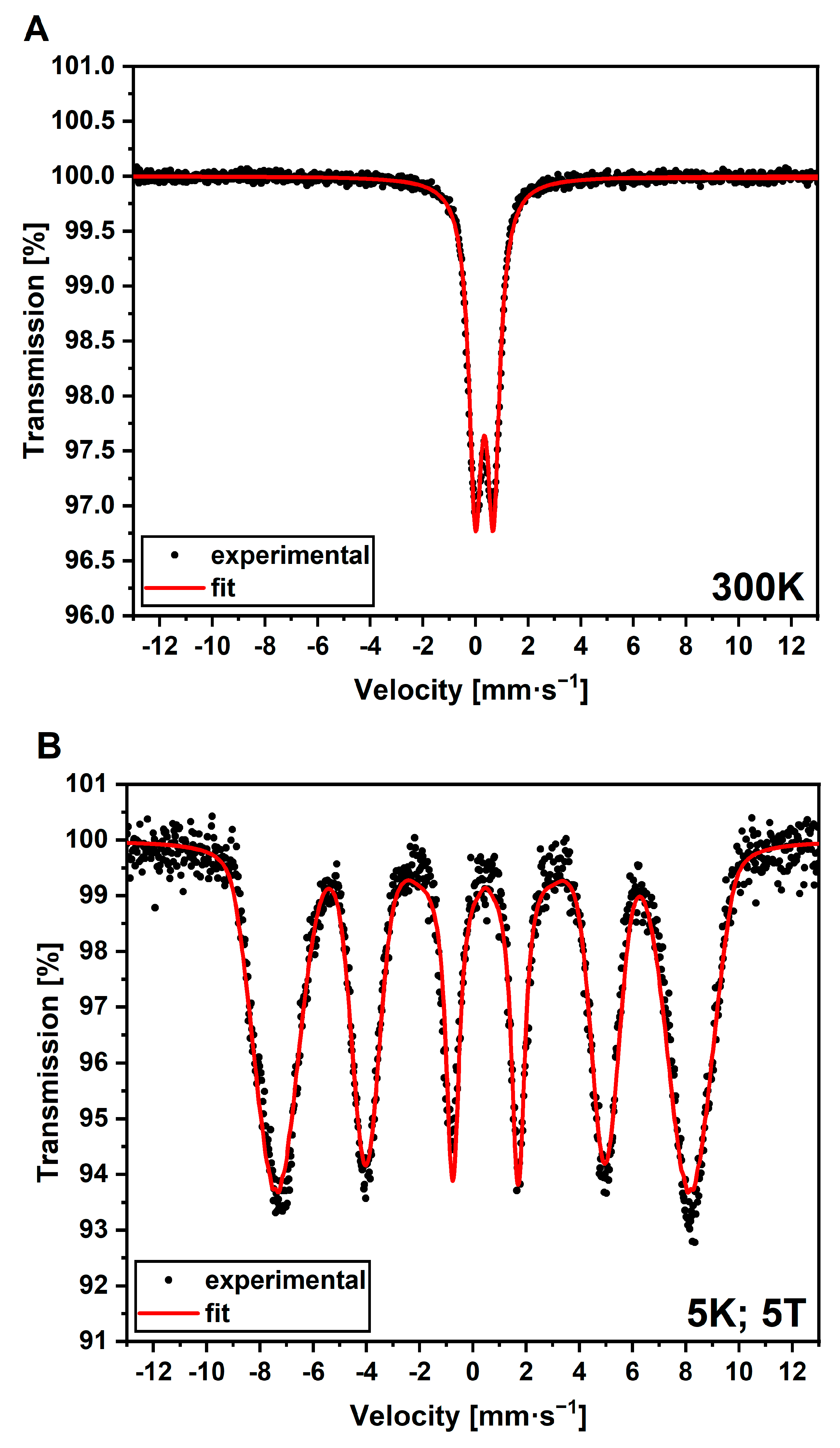
2.4.3. Mössbauer Spectroscopy
2.4.4. XPS
2.4.5. ICP-MS
2.4.6. MR Relaxometry and Imaging
2.4.7. Alamar Blue Assay (Resazurin Assay)
3. Results and Discussion
3.1. Luminescent Properties of AuBSA-Fe in Comparison to AuBSA
3.2. Investigation of Morphology and Particle Size Distribution in Luminescent AuBSA-Fe
3.3. Evidence of SPIONs in Luminescent AuBSA-Fe via Mössbauer Spectroscopy
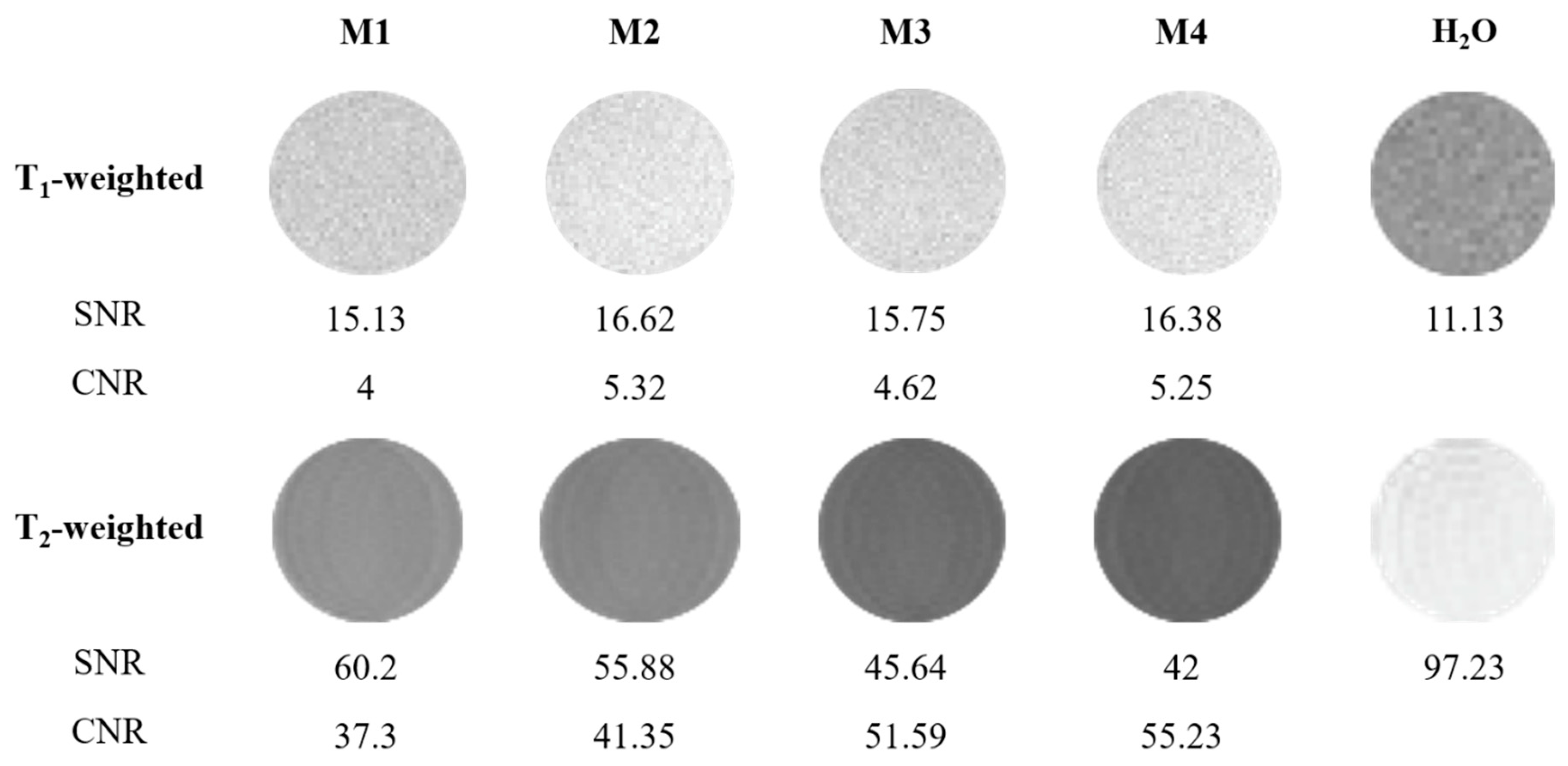
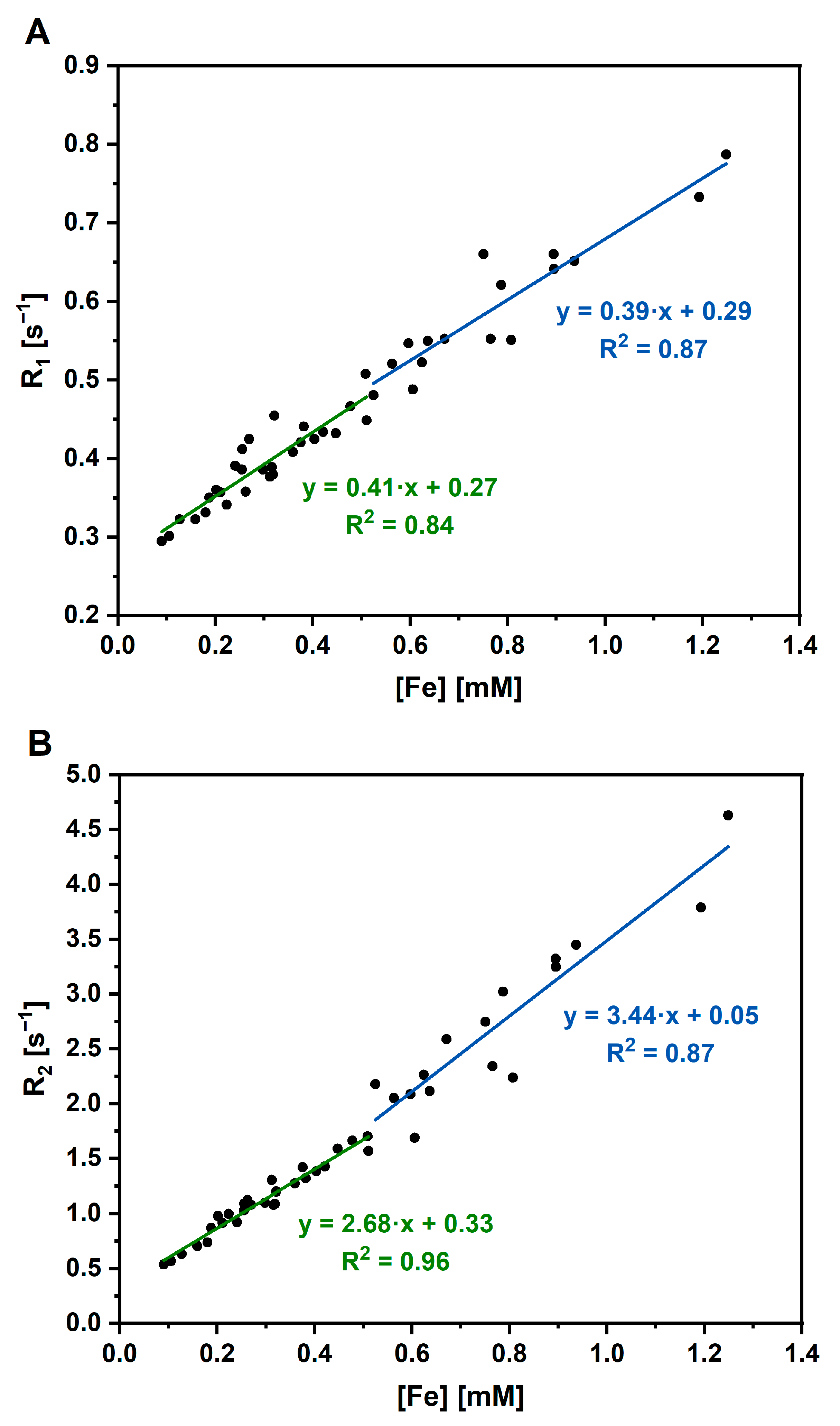
3.4. Application of Luminescent AuBSA-Fe as MRI Contrast Agents
3.5. Stability and Biocompatibility of AuBSA-Fe Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of Nanoparticles in Biomedical Imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Nienhaus, K.; Wang, H.; Nienhaus, G.U. Nanoparticles for Biomedical Applications: Exploring and Exploiting Molecular Interactions at the Nano-Bio Interface. Mater. Today Adv. 2020, 5, 100036. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Zhang, C.; Jia, Y.; Luo, Y.; Gao, X. AuGd Integrated Nanoprobes for Optical/MRI/CT Triple-Modal in Vivo Tumor Imaging. Nanoscale 2017, 9, 4620–4628. [Google Scholar] [CrossRef]
- Pan, U.N.; Khandelia, R.; Sanpui, P.; Das, S.; Paul, A.; Chattopadhyay, A. Protein-Based Multifunctional Nanocarriers for Imaging, Photothermal Therapy, and Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 19495–19501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Du, T.; ur Rehman, F.; Lai, L.; Liu, X.; Jiang, X.; Li, X.; Chen, Y.; Zhang, H.; Sun, Y.; et al. Biosynthesized Gold Nanoclusters and Iron Complexes as Scaffolds for Multimodal Cancer Bioimaging. Small 2016, 12, 6255–6265. [Google Scholar] [CrossRef]
- Pahari, S.K.; Olszakier, S.; Kahn, I.; Amirav, L. Magneto-Fluorescent Yolk–Shell Nanoparticles. Chem. Mater. 2018, 30, 775–780. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Che, Y.; Liao, X.; Jiang, Y. A Type of Novel Fluorescent Magnetic Carbon Quantum Dots for Cells Imaging and Detection. J. Biomed. Mater. Res. A 2015, 103, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, Y.; Song, Q. Gold Nanoclusters Decorated with Magnetic Iron Oxide Nanoparticles for Potential Multimodal Optical/Magnetic Resonance Imaging. J. Mater. Chem. C Mater. 2015, 3, 5910–5917. [Google Scholar] [CrossRef]
- Huang, C.-L.; Hsieh, W.-J.; Lin, C.-W.; Yang, H.-W.; Wang, C.-K. Multifunctional Liposomal Drug Delivery with Dual Probes of Magnetic Resonance and Fluorescence Imaging. Ceram. Int. 2018, 44, 12442–12450. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Aboutalebi, F.; Mirahmadi-Zare, S.Z.; Hadadzadeh, H.; Nasr-Esfahani, M.-H. Selective Chemotherapy and Imaging of Colorectal and Breast Cancer Cells by a Modified MUC-1 Aptamer Conjugated to a Poly(Ethylene Glycol)-Dimethacrylate Coated Fe3O4–AuNCs Nanocomposite. New J. Chem. 2019, 43, 238–248. [Google Scholar] [CrossRef]
- Sheng, J.; Jiang, X.; Wang, L.; Yang, M.; Liu, Y.-N. Biomimetic Mineralization Guided One-Pot Preparation of Gold Clusters Anchored Two-Dimensional MnO2 Nanosheets for Fluorometric/Magnetic Bimodal Sensing. Anal. Chem. 2018, 90, 2926–2932. [Google Scholar] [CrossRef]
- Xu, Y.; Palchoudhury, S.; Qin, Y.; Macher, T.; Bao, Y. Make Conjugation Simple: A Facile Approach to Integrated Nanostructures. Langmuir 2012, 28, 8767–8772. [Google Scholar] [CrossRef]
- Meng, L.; Ma, X.; Jiang, S.; Ji, G.; Han, W.; Xu, B.; Tian, J.; Tian, W. High-Efficiency Fluorescent and Magnetic Multimodal Probe for Long-Term Monitoring and Deep Penetration Imaging of Tumors. J. Mater. Chem. B 2019, 7, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Tan, J.-E.; Tian, Y.; Huang, S.; Sun, P.-H.; Wang, M.; Han, Y.-J.; Li, H.-S.; Wu, H.-B.; Zhang, X.-M.; et al. Multifunctional Superparamagnetic Nanoparticles Conjugated with Fluorescein-Labeled Designed Ankyrin Repeat Protein as an Efficient HER2-Targeted Probe in Breast Cancer. Biomaterials 2017, 147, 86–98. [Google Scholar] [CrossRef]
- Le, W.; Cui, S.; Chen, X.; Zhu, H.; Chen, B.; Cui, Z. Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents. Nanomaterials 2016, 6, 65. [Google Scholar] [CrossRef]
- Liang, G.; Xiao, L. Gd 3+-Functionalized Gold Nanoclusters for Fluorescence–Magnetic Resonance Bimodal Imaging. Biomater. Sci. 2017, 5, 2122–2130. [Google Scholar] [CrossRef]
- Dong, D.; Jing, X.; Zhang, X.; Hu, X.; Wu, Y.; Duan, C. Gadolinium(III)–Fluorescein Complex as a Dual Modal Probe for MRI and Fluorescence Zinc Sensing. Tetrahedron 2012, 68, 306–310. [Google Scholar] [CrossRef]
- Guan, S.; Liang, R.; Li, C.; Wei, M. A Supramolecular Material for Dual-Modal Imaging and Targeted Cancer Therapy. Talanta 2017, 165, 297–303. [Google Scholar] [CrossRef]
- Andrýsková, P.; Šišková, K.M.; Michetschlägerová, Š.; Jiráková, K.; Kubala, M.; Jirák, D. The Effect of Fatty Acids and BSA Purity on Synthesis and Properties of Fluorescent Gold Nanoclusters. Nanomaterials 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Hsu, N.-Y.; Lin, Y.-W. Microwave-Assisted Synthesis of Bovine Serum Albumin–Gold Nanoclusters and Their Fluorescence-Quenched Sensing of Hg2+ Ions. New J. Chem. 2016, 40, 1155–1161. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Y.; Zheng, B.; Yuan, H.; Guo, Y.; Xiao, D.; Choi, M.M.F. Microwave-Assisted Synthesis of BSA-Stabilized and HSA-Protected Gold Nanoclusters with Red Emission. J. Mater. Chem. 2012, 22, 1000–1005. [Google Scholar] [CrossRef]
- Ostruszka, R.; Zoppellaro, G.; Tomanec, O.; Pinkas, D.; Filimonenko, V.; Šišková, K. Evidence of Au(II) and Au(0) States in Bovine Serum Albumin-Au Nanoclusters Revealed by CW-EPR/LEPR and Peculiarities in HR-TEM/STEM Imaging. Nanomaterials 2022, 12, 1425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure-Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef] [PubMed]
- Babes, L.; Denizot, B.; Tanguy, G.; le Jeune, J.J.; Jallet, P. Synthesis of Iron Oxide Nanoparticles Used as MRI Contrast Agents: A Parametric Study. J. Colloid Interface Sci. 1999, 212, 474–482. [Google Scholar] [CrossRef]
- Bajaj, A.; Samanta, B.; Yan, H.; Jerry, D.J.; Rotello, V.M. Stability, Toxicity and Differential Cellular Uptake of Protein Passivated-Fe3O4 Nanoparticles. J. Mater. Chem. 2009, 19, 6328. [Google Scholar] [CrossRef]
- Li, D.; Hua, M.; Fang, K.; Liang, R. BSA Directed-Synthesis of Biocompatible Fe3O4 Nanoparticles for Dual-Modal T1 and T2 MR Imaging in Vivo. Anal. Methods 2017, 9, 3099–3104. [Google Scholar] [CrossRef]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine Serum Albumin (BSA) Coated Iron Oxide Magnetic Nanoparticles as Biocompatible Carriers for Curcumin-Anticancer Drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Wei, Y.; Zhao, H.; Tao, T.; Wang, H.; Wang, Z.; Du, J.; Wang, H.; Qian, J.; et al. In Situ One-Pot Synthesis of Fe2O3@BSA Core-Shell Nanoparticles as Enhanced T1-Weighted Magnetic Resonance Imagine Contrast Agents. ACS Appl. Mater. Interfaces 2020, 12, 56701–56711. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Chang, Y.; Zhao, L.; Zhang, K.; Zhao, Y.; Gao, F.; Gao, X. Ultrasmall Superparamagnetic Iron Oxide Nanoparticle for T2-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 28959–28966. [Google Scholar] [CrossRef]
- Li, H.; Yan, K.; Shang, Y.; Shrestha, L.; Liao, R.; Liu, F.; Li, P.; Xu, H.; Xu, Z.; Chu, P.K. Folate-Bovine Serum Albumin Functionalized Polymeric Micelles Loaded with Superparamagnetic Iron Oxide Nanoparticles for Tumor Targeting and Magnetic Resonance Imaging. Acta Biomater. 2015, 15, 117–126. [Google Scholar] [CrossRef]
- Vismara, E.; Bongio, C.; Coletti, A.; Edelman, R.; Serafini, A.; Mauri, M.; Simonutti, R.; Bertini, S.; Urso, E.; Assaraf, Y.; et al. Albumin and Hyaluronic Acid-Coated Superparamagnetic Iron Oxide Nanoparticles Loaded with Paclitaxel for Biomedical Applications. Molecules 2017, 22, 1030. [Google Scholar] [CrossRef]
- An, L.; Yan, C.; Mu, X.; Tao, C.; Tian, Q.; Lin, J.; Yang, S. Paclitaxel-Induced Ultrasmall Gallic Acid-Fe@BSA Self-Assembly with Enhanced MRI Performance and Tumor Accumulation for Cancer Theranostics. ACS Appl. Mater. Interfaces 2018, 10, 28483–28493. [Google Scholar] [CrossRef]
- Tian, Q.; An, L.; Tian, Q.; Lin, J.; Yang, S. Ellagic Acid-Fe@BSA Nanoparticles for Endogenous H2S Accelerated Fe(III)/Fe(II) Conversion and Photothermal Synergistically Enhanced Chemodynamic Therapy. Theranostics 2020, 10, 4101–4115. [Google Scholar] [CrossRef]
- Harini, G.; Balasurya, S.; Khan, S.S. Recent Advances on Gadolinium-Based Nano-Photocatalysts for Environmental Remediation and Clean Energy Production: Properties, Fabrication, Defect Engineering and Toxicity. J. Clean Prod. 2022, 345, 131139. [Google Scholar] [CrossRef]
- Gao, F.; Qu, H.; Duan, Y.; Wang, J.; Song, X.; Ji, T.; Cao, L.; Nie, G.; Sun, S. Dopamine Coating as a General and Facile Route to Biofunctionalization of Superparamagnetic Fe3O4 Nanoparticles for Magnetic Separation of Proteins. RSC Adv. 2014, 4, 6657. [Google Scholar] [CrossRef]
- Nosrati, H.; Davaran, S.; Kheiri Manjili, H.; Rezaeejam, H.; Danafar, H. Bovine Serum Albumin Stabilized Iron Oxide and Gold Bimetallic Heterodimers: Synthesis, Characterization and Stereological Study. Appl. Organomet. Chem. 2019, 33, e5155. [Google Scholar] [CrossRef]
- Nosrati, H.; Baghdadchi, Y.; Abbasi, R.; Barsbay, M.; Ghaffarlou, M.; Abhari, F.; Mohammadi, A.; Kavetskyy, T.; Bochani, S.; Rezaeejam, H.; et al. Iron Oxide and Gold Bimetallic Radiosensitizers for Synchronous Tumor Chemoradiation Therapy in 4T1 Breast Cancer Murine Model. J. Mater. Chem. B 2021, 9, 4510–4522. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K.; Spieles, M. Fluorescence Quantum Yields of a Series of Red and Near-Infrared Dyes Emitting at 600−1000 nm. Anal. Chem. 2011, 83, 1232–1242. [Google Scholar] [CrossRef]
- Procházka, V.; Novák, P.; Stejskal, A. Department of Experimental Physics. Mössbauer Spectrometers OLTWINS. Available online: http://oltwins.upol.cz/ (accessed on 6 February 2023).
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-Friendly Software for Mössbauer Spectrum Analysis. J. Radioanal. Nucl. Chem. Artic. 1996, 210, 105–118. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, C.; Razzaque, S.; Hussain, I.; Lu, Q.-W.; Tan, B. Synthesis of Water-Soluble and Highly Fluorescent Gold Nanoclusters for Fe3+ Sensing in Living Cells Using Fluorescence Imaging. J. Mater. Chem. B 2017, 5, 5608–5615. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Jiang, H.; Wang, X. Selective and Sensitive Detection of Fe3+ Ion in Drinking Water Using L-Glutathione Stabilized Red Fluorescent Gold Nanoclusters. J. Nanosci. Nanotechnol. 2016, 16, 12179–12186. [Google Scholar] [CrossRef]
- Ungor, D.; Csapó, E.; Kismárton, B.; Juhász, Á.; Dékány, I. Nucleotide-Directed Syntheses of Gold Nanohybrid Systems with Structure-Dependent Optical Features: Selective Fluorescence Sensing of Fe3+ Ions. Colloids Surf B Biointerfaces 2017, 155, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Šišková, K.; Machala, L.; Tuček, J.; Kašlík, J.; Mojzeš, P.; Zbořil, R. Mixtures of L-Amino Acids as Reaction Medium for Formation of Iron Nanoparticles: The Order of Addition into a Ferrous Salt Solution Matters. Int. J. Mol. Sci. 2013, 14, 19452–19473. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, G.J.; Henkelman, R.M. Gd-DTPA Relaxivity Depends on Macromolecular Content. Magn. Reson. Med. 2000, 44, 665–667. [Google Scholar] [CrossRef]
- Bjørnerud, A.; Johansson, L.O.; Briley-Saebø, K.; Ahlström, H.K. Assessment of T1 and T2* Effects in Vivo and Ex Vivo Using Iron Oxide Nanoparticles in Steady State-Dependence on Blood Volume and Water Exchange. Magn. Reson. Med. 2002, 47, 461–471. [Google Scholar] [CrossRef]
- Van Osch, M.J.P.; Vonken, E.P.A.; Viergever, M.A.; van der Grond, J.; Bakker, C.J.G. Measuring the Arterial Input Function with Gradient Echo Sequences. Magn. Reson. Med. 2003, 49, 1067–1076. [Google Scholar] [CrossRef]
- Zhao, J.M.; Clingman, C.S.; Närväinen, M.J.; Kauppinen, R.A.; van Zijl, P.C.M. Oxygenation and Hematocrit Dependence of Transverse Relaxation Rates of Blood at 3T. Magn. Reson. Med. 2007, 58, 592–597. [Google Scholar] [CrossRef]
- Calamante, F.; Connelly, A.; van Osch, M.J.P. Nonlinear ΔR2* Effects in Perfusion Quantification Using Bolus-Tracking MRI. Magn. Reson. Med. 2009, 61, 486–492. [Google Scholar] [CrossRef]
- Patil, V.; Jensen, J.H.; Johnson, G. Intravascular Contrast Agent T2* Relaxivity in Brain Tissue. NMR Biomed. 2013, 26, 392–399. [Google Scholar] [CrossRef]
- Ta, H.T.; Li, Z.; Wu, Y.; Cowin, G.; Zhang, S.; Yago, A.; Whittaker, A.K.; Xu, Z.P. Effects of Magnetic Field Strength and Particle Aggregation on Relaxivity of Ultra-Small Dual Contrast Iron Oxide Nanoparticles. Mater. Res. Express 2017, 4, 116105. [Google Scholar] [CrossRef]
- Weisskoff, R.; Zuo, C.S.; Boxerman, J.L.; Rosen, B.R. Microscopic Susceptibility Variation and Transverse Relaxation: Theory and Experiment. Magn. Reson. Med. 1994, 31, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Goegan, P.; Johnson, G.; Vincent, R. Effects of Serum Protein and Colloid on the AlamarBlue Assay in Cell Cultures. Toxicol. In Vitr. 1995, 9, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Funk, D.; Schrenk, H.-H.; Frei, E. Serum Albumin Leads to False-Positive Results in the XTT and the MTT Assay. Biotechniques 2007, 43, 178–186. [Google Scholar] [CrossRef]
- Neufeld, B.H.; Tapia, J.B.; Lutzke, A.; Reynolds, M.M. Small Molecule Interferences in Resazurin and MTT-Based Metabolic Assays in the Absence of Cells. Anal. Chem. 2018, 90, 6867–6876. [Google Scholar] [CrossRef] [PubMed]

| Sample | Z-Average [nm] | PDI |
|---|---|---|
| AuBSA | 23.9 ± 10.8 | 0.4 ± 0.1 |
| AuBSA-Fe | 71.2 ± 8.0 | 1.0 ± 0.0 |
| Fe Concentration [mM] | Relaxivity r1 [L·mmol−1·s−1] | Relaxivity r2 [L·mmol−1·s−1] |
|---|---|---|
| <0.52 | 0.41 ± 0.04 | 2.68 ± 0.11 |
| ≥0.52 | 0.39 ± 0.04 | 3.44 ± 0.36 |
| AuBSA-Fe | Average Viability [%] |
|---|---|
| Iron concentration < 0.52 mM | 78 ± 3 |
| Iron concentration ≥ 0.52 mM | 80 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostruszka, R.; Půlpánová, D.; Pluháček, T.; Tomanec, O.; Novák, P.; Jirák, D.; Šišková, K. Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent. Nanomaterials 2023, 13, 1027. https://doi.org/10.3390/nano13061027
Ostruszka R, Půlpánová D, Pluháček T, Tomanec O, Novák P, Jirák D, Šišková K. Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent. Nanomaterials. 2023; 13(6):1027. https://doi.org/10.3390/nano13061027
Chicago/Turabian StyleOstruszka, Radek, Denisa Půlpánová, Tomáš Pluháček, Ondřej Tomanec, Petr Novák, Daniel Jirák, and Karolína Šišková. 2023. "Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent" Nanomaterials 13, no. 6: 1027. https://doi.org/10.3390/nano13061027
APA StyleOstruszka, R., Půlpánová, D., Pluháček, T., Tomanec, O., Novák, P., Jirák, D., & Šišková, K. (2023). Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent. Nanomaterials, 13(6), 1027. https://doi.org/10.3390/nano13061027