Insights into the Role of Nanorod-Shaped MnO2 and CeO2 in a Plasma Catalysis System for Methanol Oxidation
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Preparation and Evaluation
2.2. Catalyst Characterization
2.3. Investigation of the Ces for CO2 c
- (1)
- Methanol molecules
- (2)
- Active oxygen species in the catalysts
- (3)
- Short-lived oxygen species
- (4)
- Long-lived oxygen species
2.4. Raman Analysis of the Reactive Oxygen Species (ROS)
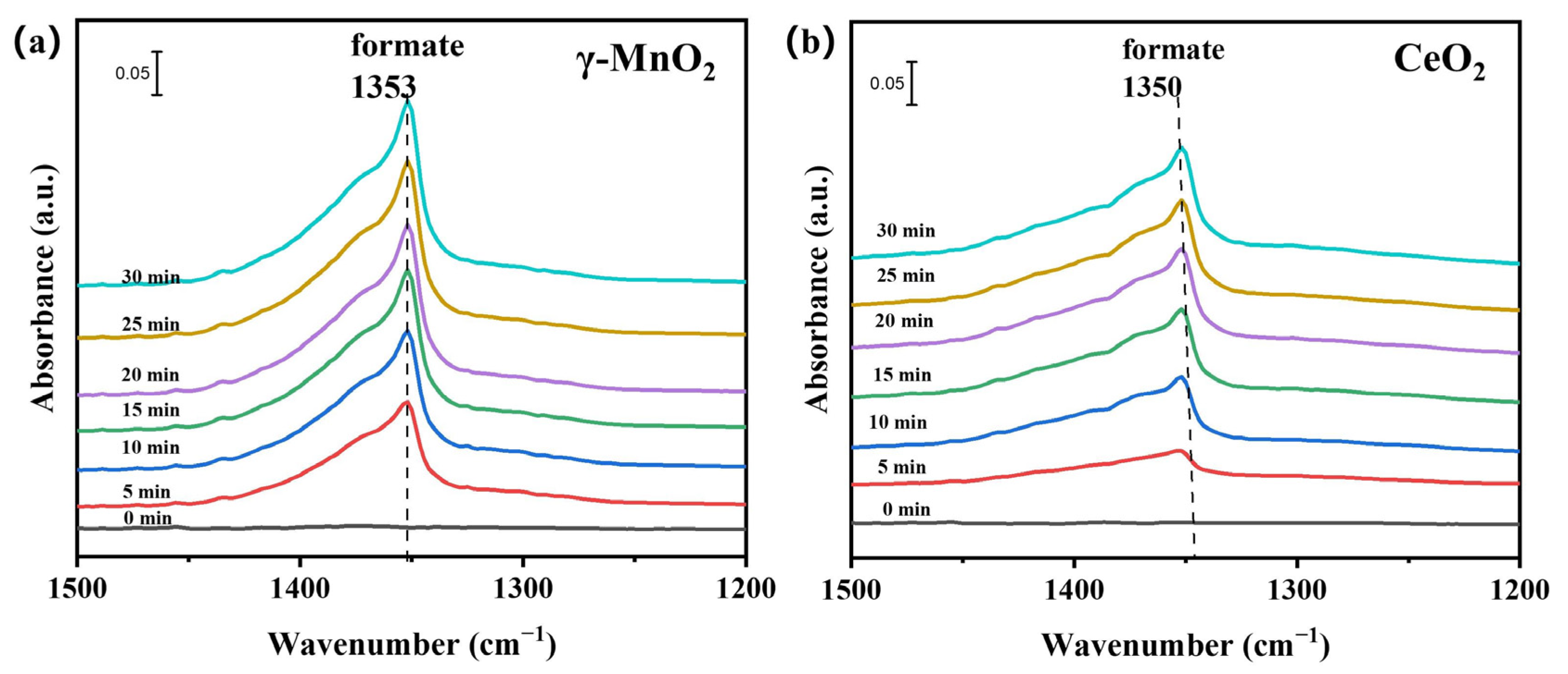
2.5. Investigation of the Reaction Pathway of Methanol Oxidation
3. Results and Discussion
3.1. Catalyst Characterization
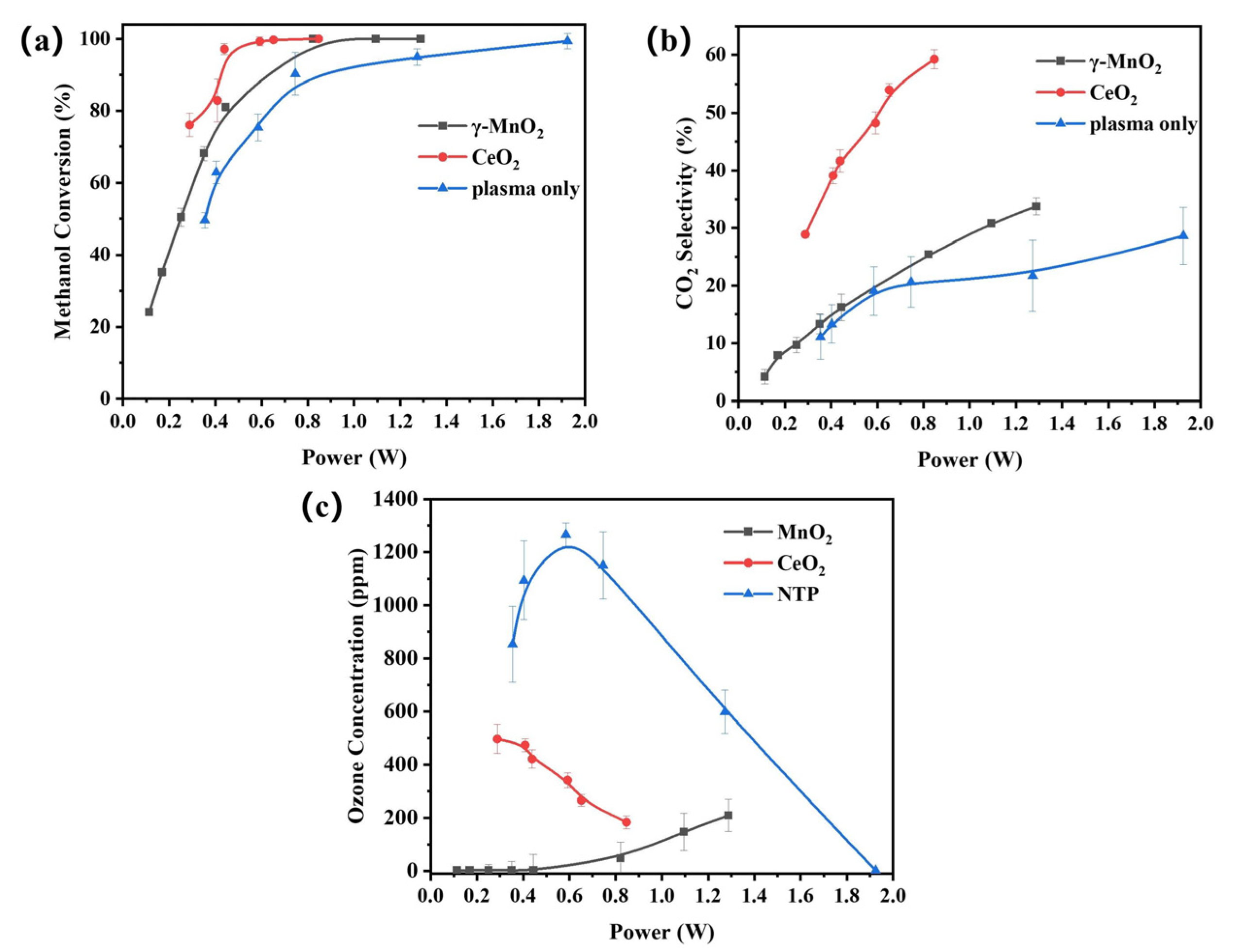
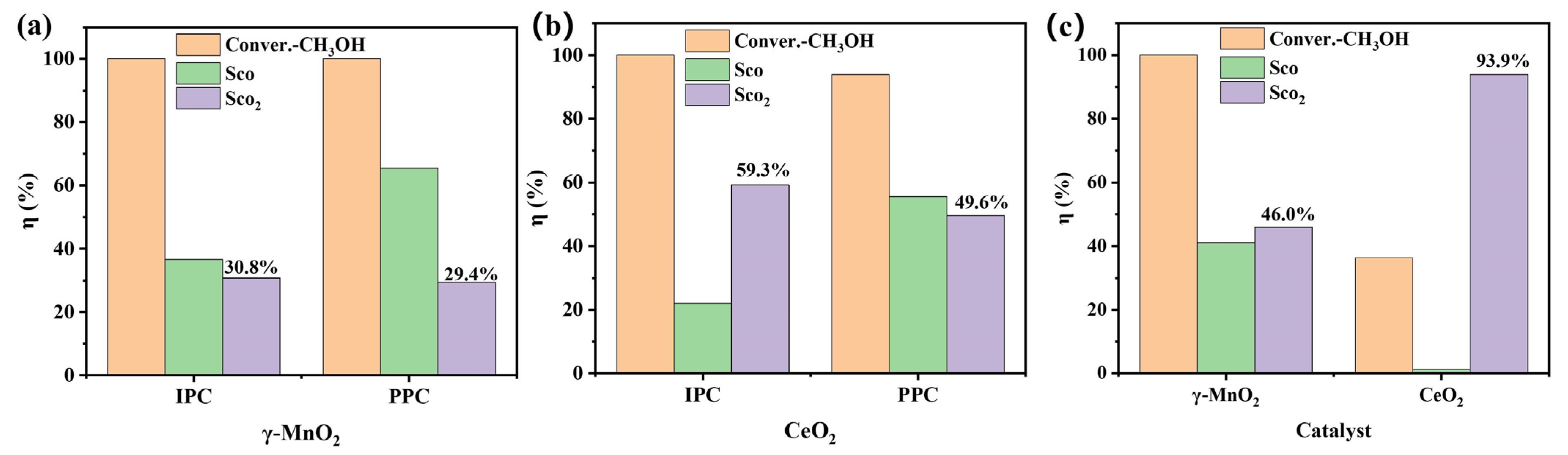
3.2. Plasma Catalysis Performance Evaluation
4. Mechanistic Studies on the Roles of the Catalysts
4.1. Electrical Discharge Characteristics
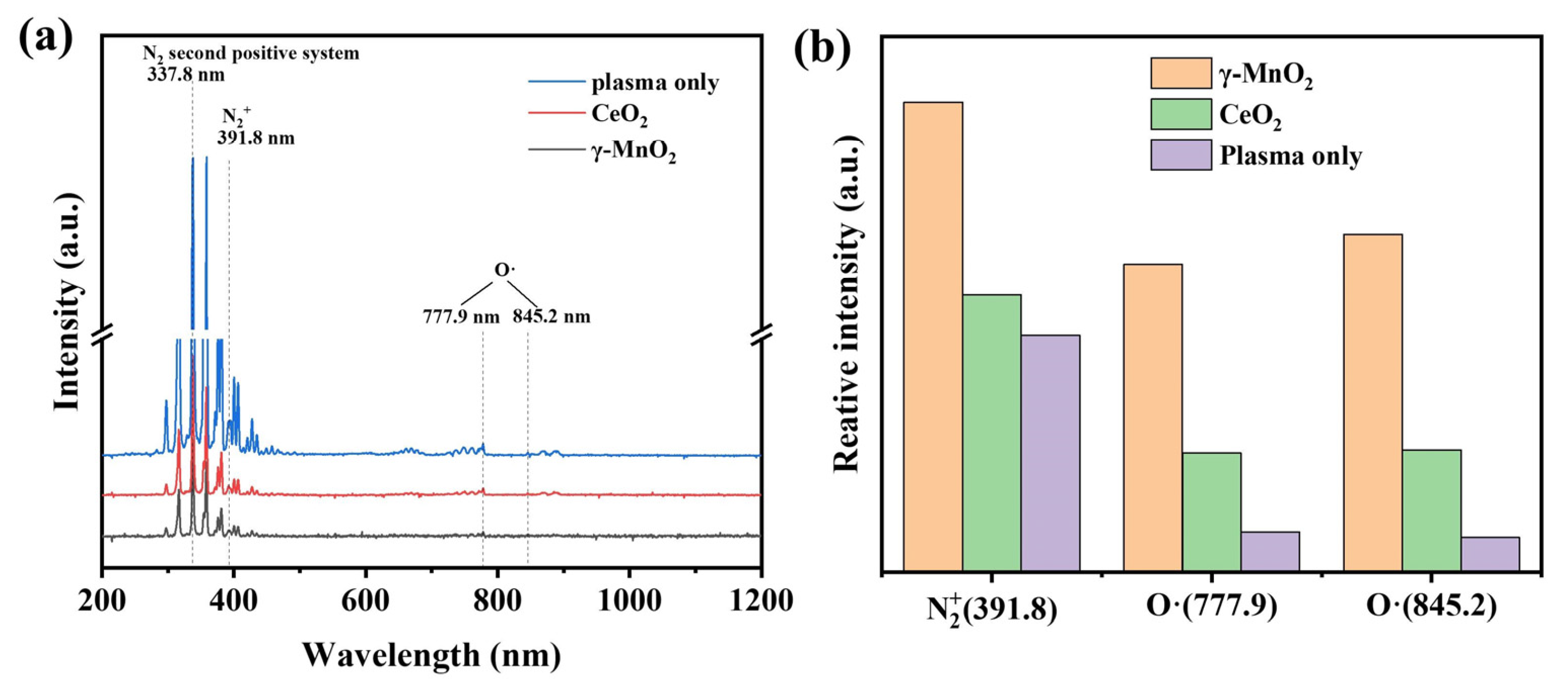
4.2. Active Species in the Plasma
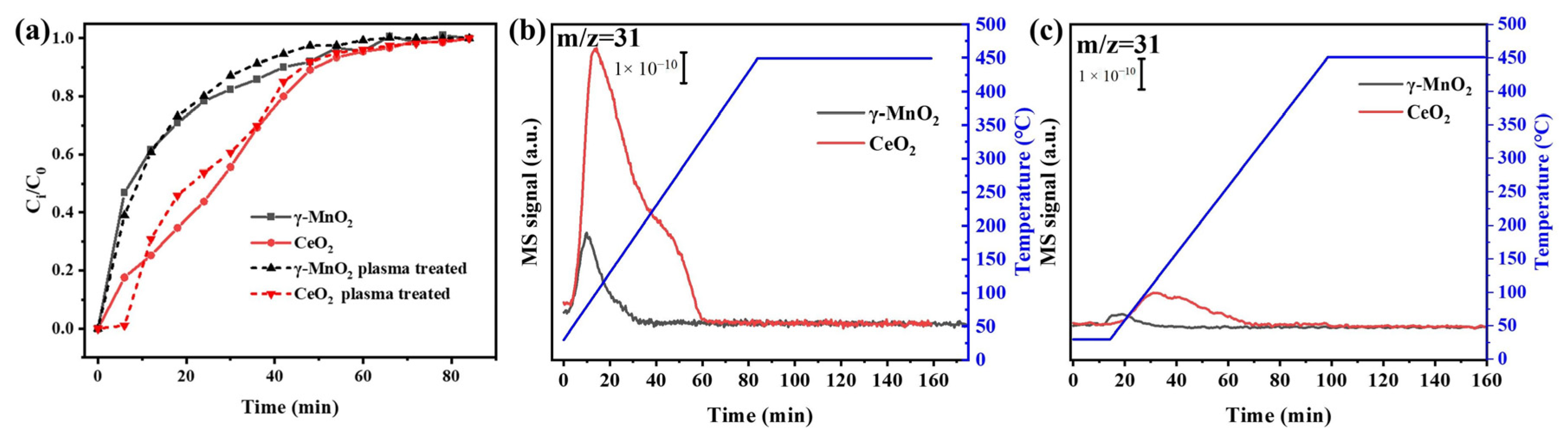
4.3. Methanol Adsorption and Activation on the Catalysts
4.4. Oxygen Species for CO2 Formation
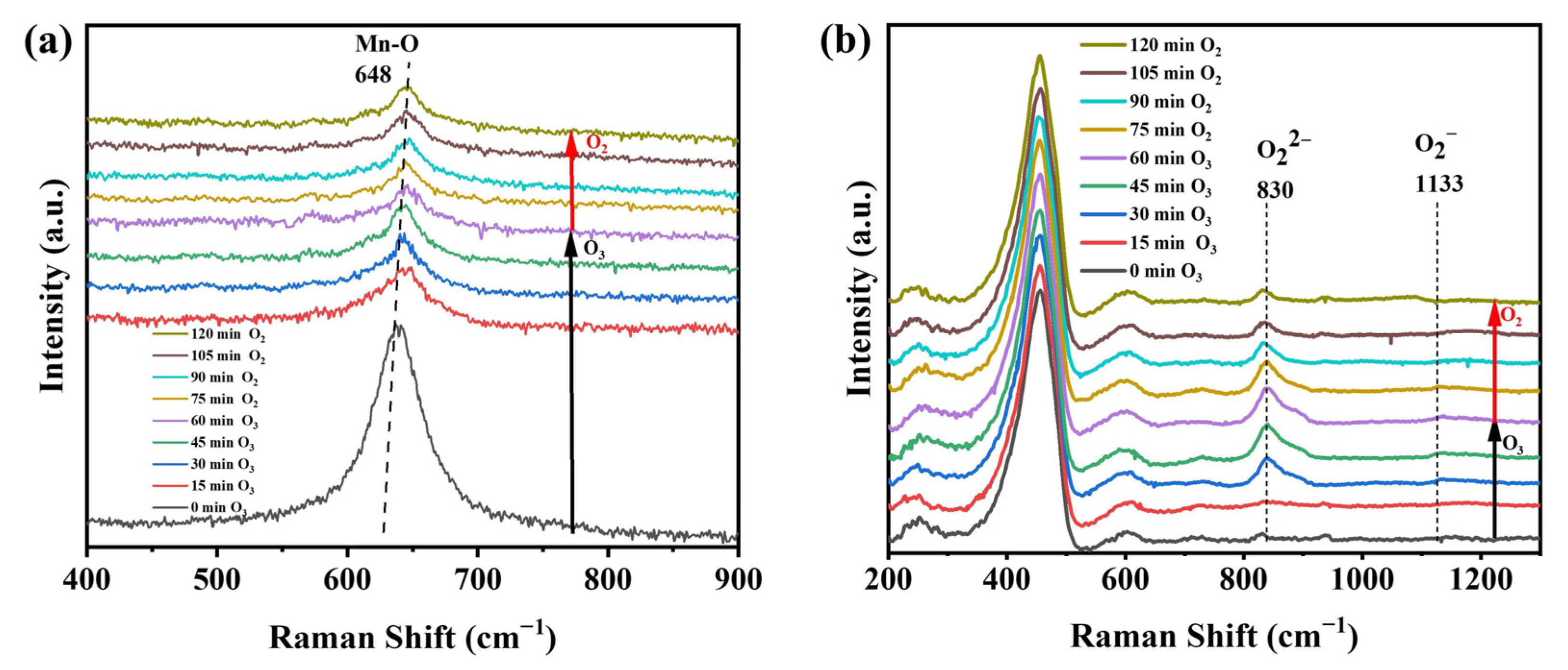
4.5. In situ Raman Analysis of ROS
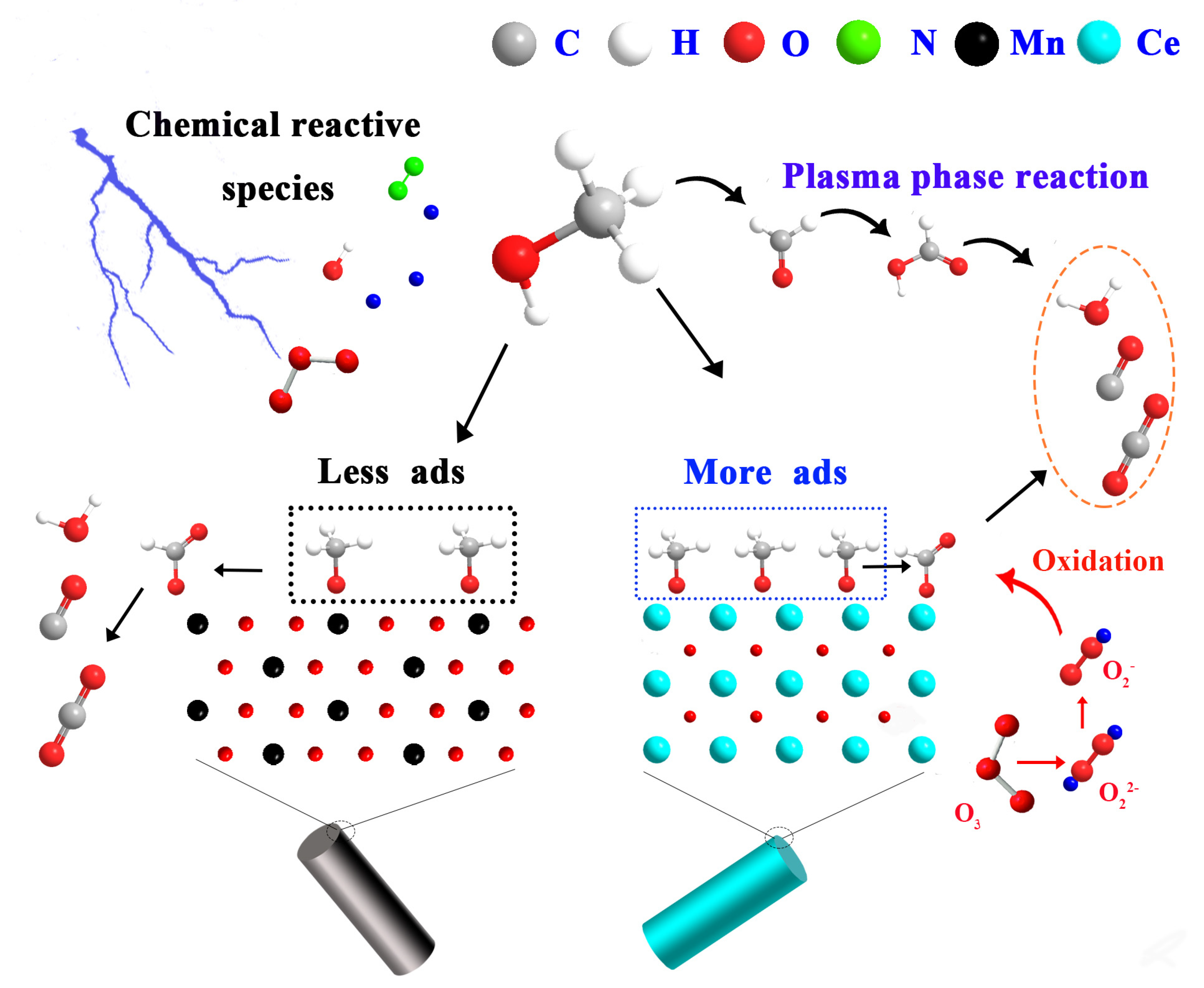
5. Reaction Pathway
5.1. Reactions in the Gas Phase
5.2. Reactions on the Surfaces of the Catalysts
5.3. Deduction of the Reaction Pathway
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cohen, A.J.; Brauer, M.; Burnett, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Sherrill, D.; Annesi-Maesano, I. Estimating the Health Effects of Exposure to Multi-Pollutant Mixture. Ann. Epidemiol. 2012, 22, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: A review. Appl. Catal. B Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef]
- Karuppiah, J.; Linga Reddy, E.; Manoj Kumar Reddy, P.; Ramaraju, B.; Karvembu, R.; Subrahmanyam, C. Abatement of mixture of volatile organic compounds (VOCs) in a catalytic non-thermal plasma reactor. J. Hazard. Mater. 2012, 237–238, 283–289. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, X.; Thevenet, F.; Rousseau, A. Dynamic probing of plasma-catalytic surface processes: Oxidation of toluene on CeO2. Plasma Process. Polym. 2017, 14, 1600114. [Google Scholar] [CrossRef]
- Pan K, L.; Chang M, B. Plasma catalytic oxidation of toluene over double perovskite-type oxide via packed-bed DBD. Environ. Sci. Pollut. Res. 2019, 26, 12948–12962. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, Y.; Tanaya, H.; Theerthagiri, J.; Jun, S.; Min, A.; Kim, G.; Chul, H.; Yong, M. Pulsed laser-driven green synthesis of trimetallic AuPtCu nanoalloys for formic acid electro-oxidation in acidic environment. Fuel 2023, 332, 126164. [Google Scholar] [CrossRef]
- Babu, M.; Rhan, A.; Ramakrishan, S.; Logeshwaran, N.; Kumar, S.; Joo, H.; Jin, D. Integrating the essence of metal organic framework-derived ZnCoTe–N–C/MoS2 cathode and ZnCo-NPS-N-CNT as anode for high-energy density hybrid supercapacitors. Compos. Part B 2022, 247, 110339. [Google Scholar]
- Babu, M.; Kim, A.A.; Chandra, P.; Jin, D.; Joo, H. Assembling zinc cobalt hydroxide / ternary sulfides heterostructure and iron oxide nanorods on three-dimensional hollow porous carbon nanofiber as high energy density hybrid supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar]
- Chen, X.; Yu, E.; Cai, S.; Yu, E.; Jia, H.; Cai, S.; Liang, P.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, X.; Qin, R.; Zeng, Y.; Qu, R.; Zheng, C.; Tu, X. Plasma-catalytic removal of formaldehyde over Cu-Ce catalysts in a dielectric barrier discharge reactor. Appl. Catal. B Environ. 2015, 170, 293–300. [Google Scholar] [CrossRef]
- Babu, M.; Shin, M.; Joo, H. Polyaniline-silver-manganese dioxide nanorod ternary composite for asymmetric supercapacitor with remarkable electrochemical performance. Int. J. Hydrogen Energy 2020, 46, 474–485. [Google Scholar]
- Poudel M, B.; Kim H, J. Synthesis of high-performance nickel hydroxide nanosheets/gadolinium doped-α-MnO2 composite nanorods as cathode and Fe3O4/GO nanospheres as anode for an all-solid-state asymmetric supercapacitor. J. Energy Chem. 2022, 64, 475–484. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache N, B.; Parvulescu V, I.; Subrahmanyam, C.; Renken, A.; Kiwi-Minsker, L. Improved performance of non-thermal plasma reactor during decomposition of trichloroethylene: Optimization of the reactor geometry and introduction of catalytic electrode. Appl. Catal. B Environ. 2007, 74, 270–277. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Wang, J. Methanol plasma-catalytic oxidation over CeO2 catalysts: Effect of ceria morphology and reaction mechanism. Chem. Eng. J. 2019, 369, 233–244. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Wang, H.; Liu, Z.; Wu, Z. In-plasma catalytic degradation of toluene over different MnO2 polymorphs and study of reaction mechanism. Chin. J. Catal. 2017, 38, 793–803. [Google Scholar] [CrossRef]
- Wang, B.; Chi, C.; Xu, M.; Wang, C.; Meng, D. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge. Chem. Eng. J. 2017, 322, 679–692. [Google Scholar] [CrossRef]
- Qin, C.; Huang, X.; Zhao, J.; Huang, J.; Kang, Z.; Dang, X. Removal of toluene by sequential adsorption-plasma oxidation: Mixed support and catalyst deactivation. J. Hazard. Mater. 2017, 334, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gorky, F.; Best, A.; Jasinski, J.; Allen B, J.; Alba-Rubio A, C.; Carreon M, L. Plasma catalytic ammonia synthesis on Ni nanoparticles: The size effect. J. Catal. 2021, 393, 369–380. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C. 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Chen, B.; Wu, B.; Yu, L.; Crocker, M.; Shi, C. Investigation into the Catalytic Roles of Various Oxygen Species over Different Crystal Phases of MnO2 for C6H6 and HCHO Oxidation. ACS Catal. 2020, 10, 6176–6187. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Meng, D.; Guo, Y.; Guo, Y.; Lu, G. Effect of Ceria Crystal Plane on the Physicochemical and Catalytic Properties of Pd/Ceria for CO and Propane Oxidation. ACS Catal. 2016, 6, 2265–2279. [Google Scholar] [CrossRef]
- Yu, L.; Peng, R.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Ag supported on CeO2 with different morphologies for the catalytic oxidation of HCHO. Chem. Eng. J. 2018, 334, 2480–2487. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B Environ. 2020, 260, 118150. [Google Scholar] [CrossRef]
- Li, W.; Gibbs G, V.; Oyama S, T. Mechanism of ozone decomposition on a manganese oxide catalyst. 1. In situ Raman spectroscopy and Ab initio molecular orbital calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Jarrige, J.; Vervisch, P. Plasma-enhanced catalysis of propane and isopropyl alcohol at ambient temperature on a MnO2-based catalyst. Appl. Catal. B Environ. 2009, 90, 74–82. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, X.; He, J.; Ou, W.; Ye, D. Effect of manganese oxide catalyst on the dielectric barrier discharge decomposition of toluene. Catal. Today 2010, 153, 176–183. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Wang, Z.; Zhou, Y.; Wu, Z. A novel hybrid Bi2MoO6-MnO2 catalysts with the superior plasma induced pseudo photocatalytic-catalytic performance for ethyl acetate degradation. Appl. Catal. B Environ. 2019, 254, 339–350. [Google Scholar] [CrossRef]
- Walkenhorst, A.; Schmitt, M.; Adrian, H.; Petersen, K. CeO2: An alternative insulator material for superconducting field effect devices. Appl. Phys. Lett. 1994, 64, 1871–1873. [Google Scholar] [CrossRef]
- Van Laer, K.; Bogaerts, A. How bead size and dielectric constant affect the plasma behaviour in a packed bed plasma reactor: A modelling study. Plasma Sources Sci. Technol. 2017, 26, 85007. [Google Scholar] [CrossRef]
- Wang, Y.; Craven, M.; Yu, X.; Ding, J.; Bryant, P.; Huang, J.; Tu, X. Plasma-Enhanced Catalytic Synthesis of Ammonia over a Ni/Al2O3 Catalyst at Near-Room Temperature: Insights into the Importance of the Catalyst Surface on the Reaction Mechanism. ACS Catal. 2019, 9, 10780–10793. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, Z.; Nikiforov, A.; Chen, J.; Wang, J.; Zhao, L.; Zhang, X. The influence of relative humidity on double dielectric barrier discharge plasma for chlorobenzene removal. J. Clean. Prod. 2021, 288, 125502. [Google Scholar] [CrossRef]
- Yi, Y.; Li, S.; Cui, Z. Selective oxidation of CH4 to CH3OH through plasma catalysis: Insights from catalyst characterization and chemical kinetics modelling. Appl. Catal. B Environ. 2021, 296, 120384. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Yang, L.; He, G.; Zhang, C.; Zhang, R.; He, H. Oxygen Vacancies Induced by Transition Metal Doping in γ-MnO2 for Highly Efficient Ozone Decomposition. Environ. Sci. Technol. 2018, 52, 12685–12696. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Ganduglia-Pirovano, M.V.; Hess, C. Experimental and Theoretical Study on the Nature of Adsorbed Oxygen Species on Shaped Ceria Nanoparticles. J. Phys. Chem. Lett. 2018, 9, 6593–6598. [Google Scholar] [CrossRef] [PubMed]
- Harling, A.M.; Glover, D.J.; Whitehead, J.C.; Zhang, K. The role of ozone in the plasma-catalytic destruction of environmental pollutants. Appl. Catal. B Environ. 2009, 90, 157–161. [Google Scholar] [CrossRef]
- Tou, A.; Kim, H.H.; Einaga, H.; Teramoto, Y.; Ogata, A. Ozone-assisted catalysis of CO: In situ Fourier transform IR evidence of the cooperative effect of a bimetallic Ag-Pd catalyst. Chem. Eng. J. 2019, 355, 380–389. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Bosco, M.V.; Bonivardi, A.; Busnengo, H.F.; Ganduglia-Pirovano, M.V. Insights into the Nature of Formate Species in the Decomposition and Reaction of Methanol over Cerium Oxide Surfaces: A Combined Infrared Spectroscopy and Density Functional Theory Study. J. Phys. Chem. C 2015, 119, 21452–21464. [Google Scholar] [CrossRef]
- Li, C.; Domen, K.; Maruya, K.I.; Onishi, T. Spectroscopic identification of adsorbed species derived from adsorption and decomposition of formic acid, methanol, and formaldehyde on cerium oxide. J. Catal. 1990, 125, 445–455. [Google Scholar] [CrossRef]
- Trinh, H.Q.; Mok, Y.S. Plasma-catalytic oxidation of acetone in annular porous monolithic ceramic-supported catalysts. Chem. Eng. J. 2014, 251, 199–206. [Google Scholar] [CrossRef]
- Chan-chan, L.H.; González-garcía, G.; Vargas-coronado, R.F.; Cervantes-uc, J.M.; Hernández-sánchez, F.; Marcos-fernandez, A.; Cauich-rodríguez, J.V. Characterization of model compounds and poly (amide-urea) urethanes based on amino acids by FTIR, NMR and other analytical techniques. Eur. Polym. J. 2017, 92, 27–39. [Google Scholar] [CrossRef]
- Cunha, M.C.P.M.; Weber, M. On the adsorption and reduction of NO− ions at Au and Pt electrodes studied by in situ FTIR spectroscopy. J. Electroanal. Chem. 1996, 414, 163–170. [Google Scholar] [CrossRef]

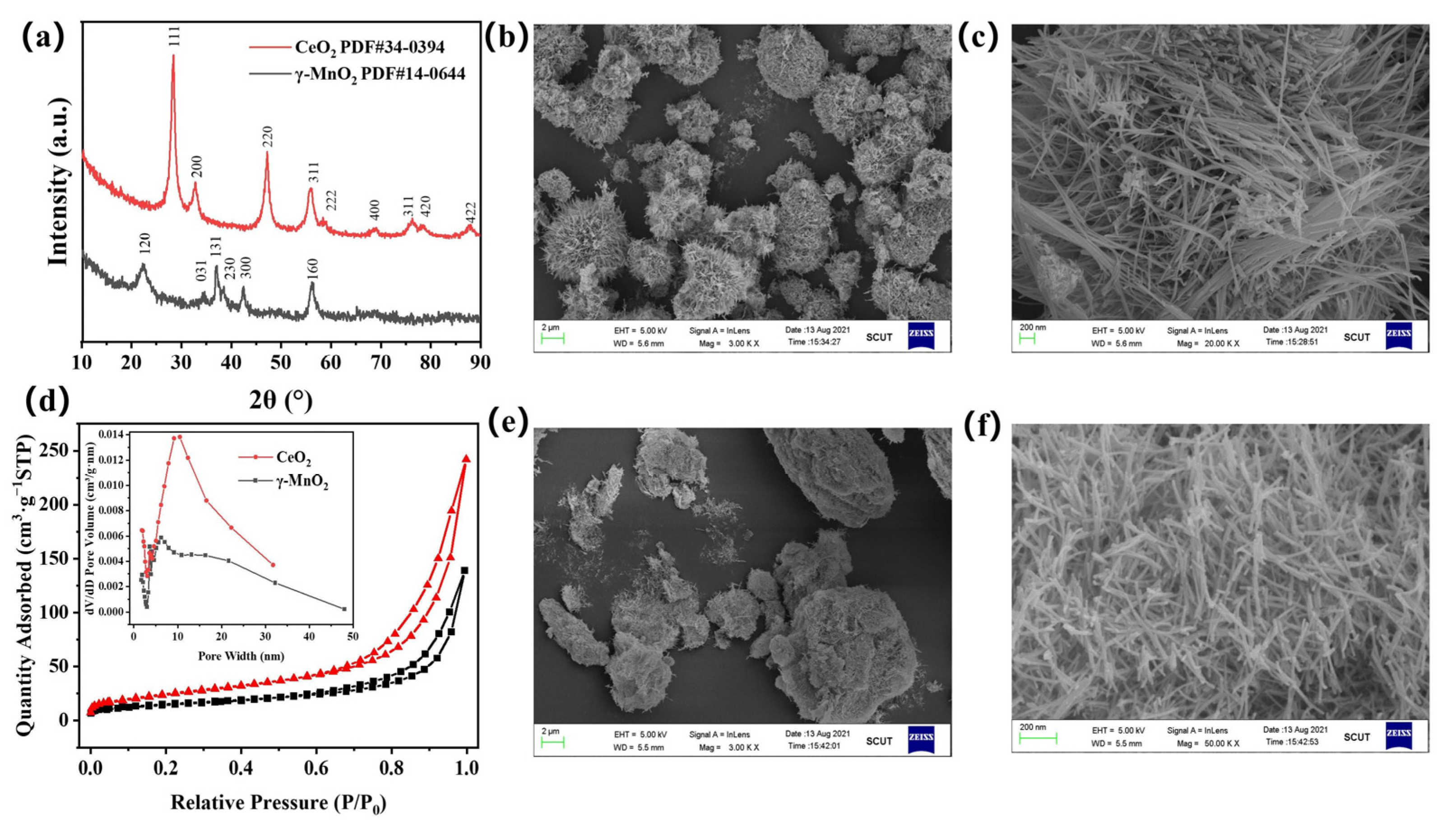

| Sample | Specific Surface Area/(m²·g−1) | Total Pore Volume/(cm3·g−1) | Average Pore Size/(nm) |
|---|---|---|---|
| γ-MnO2 | 61.43 | 0.15 | 15.83 |
| CeO2 | 90.49 | 0.28 | 12.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Chen, G.; Huang, H.; Qin, Y.; Fu, M.; Tu, X.; Ye, D.; Wu, J. Insights into the Role of Nanorod-Shaped MnO2 and CeO2 in a Plasma Catalysis System for Methanol Oxidation. Nanomaterials 2023, 13, 1026. https://doi.org/10.3390/nano13061026
Zhang G, Chen G, Huang H, Qin Y, Fu M, Tu X, Ye D, Wu J. Insights into the Role of Nanorod-Shaped MnO2 and CeO2 in a Plasma Catalysis System for Methanol Oxidation. Nanomaterials. 2023; 13(6):1026. https://doi.org/10.3390/nano13061026
Chicago/Turabian StyleZhang, Guangyi, Gui Chen, Haomin Huang, Yexia Qin, Mingli Fu, Xin Tu, Daiqi Ye, and Junliang Wu. 2023. "Insights into the Role of Nanorod-Shaped MnO2 and CeO2 in a Plasma Catalysis System for Methanol Oxidation" Nanomaterials 13, no. 6: 1026. https://doi.org/10.3390/nano13061026
APA StyleZhang, G., Chen, G., Huang, H., Qin, Y., Fu, M., Tu, X., Ye, D., & Wu, J. (2023). Insights into the Role of Nanorod-Shaped MnO2 and CeO2 in a Plasma Catalysis System for Methanol Oxidation. Nanomaterials, 13(6), 1026. https://doi.org/10.3390/nano13061026









