PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanosphere Preparation
2.3. Nanosphere Characterization
2.4. Cells Isolation, Differentiation and Exposure
2.4.1. Cellular Viability Assay
2.4.2. Nanosphere Cell Uptake Quantification
2.4.3. Real-Time Quantitative PCR (RT-qPCR)
2.5. Confocal Microscopy
2.6. Statistics
3. Results and Discussion
3.1. Characteristics of PLGA Nanospheres
3.2. Nanosphere Loading Efficiency
3.3. Cellular Uptake of Nanospheres
3.3.1. Internalization
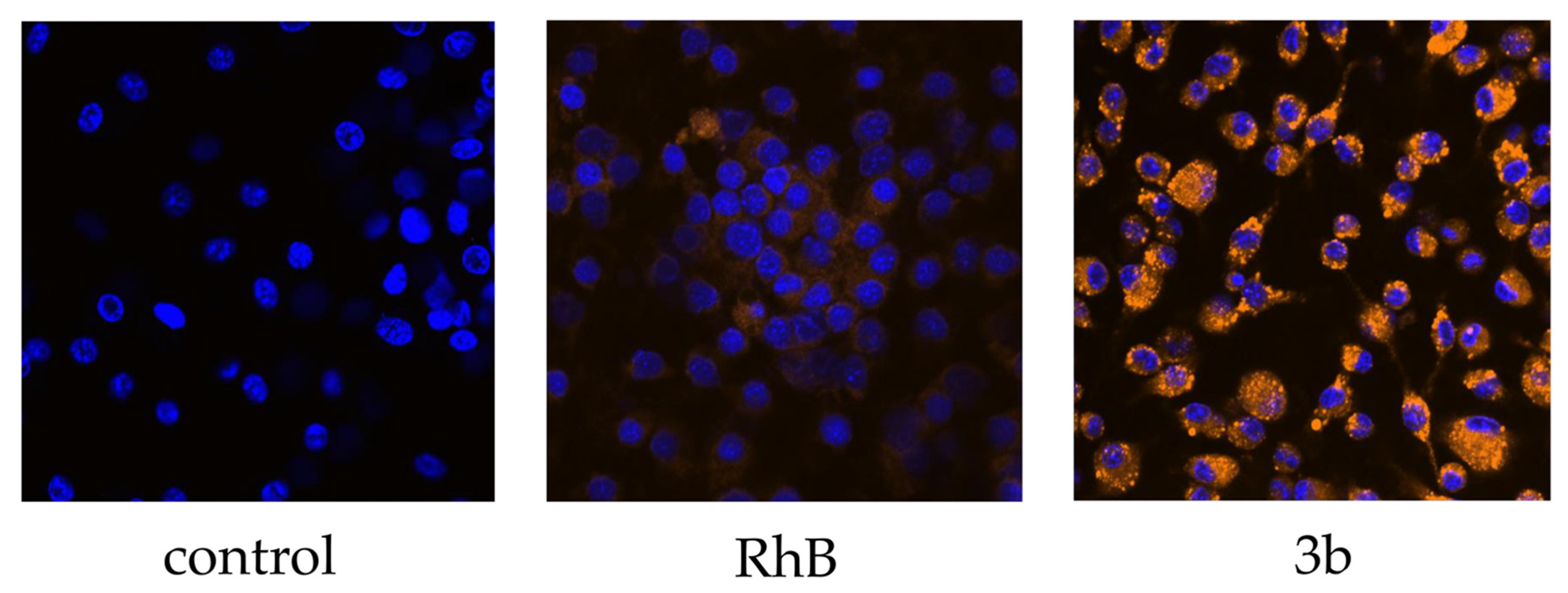
3.3.2. Confocal Microscopy
3.4. Inflammatory Effect
3.5. Effect of Nanospheres on Cellular Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, S.; Plüddemann, A. The Mononuclear Phagocytic System. Generation of Diversity. Front. Immunol. 2019, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Sitia, G.; Iannacone, M.; Aiolfi, R.; Isogawa, M.; van Rooijen, N.; Scozzesi, C.; Bianchi, M.E.; von Andrian, U.H.; Chisari, F.V.; Guidotti, L.G. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 2011, 7, e1002061. [Google Scholar] [CrossRef]
- Li, P.; He, K.; Li, J.; Liu, Z.; Gong, J. The role of Kupffer cells in hepatic diseases. Mol. Immunol. 2017, 85, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Xie, Y.; Tolmeijer, S.; Oskam, J.M.; Tonkens, T.; Meijer, A.H.; Schaaf, M.J.M. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis. Models Mech. 2019, 12, dmm037887. [Google Scholar] [CrossRef]
- Sica, A.; Erreni, M.; Allavena, P.; Porta, C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015, 72, 4111–4126. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.L.; Weiss, R.E. Steroid-induced diabetes: A clinical and molecular approach to understanding and treatment. Diabetes Metab. Res. Rev. 2014, 30, 96–102. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, D.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Diseases. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Wang, M.T.; Jin, Y.; Yang, Y.X.; Zhao, C.Y.; Yang, H.Y.; Xu, X.F.; Qin, X.; Wang, Z.D.; Zhang, Z.R.; Jian, Y.L.; et al. In vivo biodistribution, anti-inflammatory, and hepatoprotective effects of liver targeting dexamethasone acetate loaded nanostructured lipid carrier system. Int. J. Nanomed. 2010, 5, 487–497. [Google Scholar] [CrossRef][Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.; Hillaireau, H.; Vergnaud, J.; Tsapis, N.; Pallardy, M.; Kerdine-Römer, S.; Fattal, E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int. J. Pharm. 2015, 482, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C. Nanotechnology for drug delivery: The perfect partnership. Expert Opin. Drug Deliv. 2008, 5, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoon, H.Y.; Kim, J.; Yang, S.; Lee, J.; Choi, J.W.; Moon, Y.; Kim, J.; Lim, S.; Shim, M.K.; et al. Doxorubicin-Loaded PLGA Nanoparticles for Cancer Therapy: Molecular Weight Effect of PLGA in Doxorubicin Release for Controlling Immunogenic Cell Death. Pharmaceutics 2020, 12, 18. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Sergienko, K.V.; Kolmakova, A.A.; Konushkin, S.V.; Baikin, A.S.; Kolmakov, A.G.; Sevostyanov, M.A.; Kulikov, A.V.; Ivanov, V.E.; Belosludtsev, K.N.; et al. Development of a Biocompatible PLGA Polymers Capable to Release Thrombolytic Enzyme Prourokinase. J. Biomater. Sci. Polym. Ed. 2020, 31, 1405–1420. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Baikin, A.S.; Sergienko, K.V.; Shatova, L.A.; Kirsankin, A.A.; Baymler, I.V.; Shkirin, A.V.; Gudkov, S.V. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase. React. Funct. Polym. 2020, 150, 9. [Google Scholar] [CrossRef]
- Zhou, J.; Walker, J.; Ackermann, R.; Olsen, K.; Hong, J.K.Y.; Wang, Y.; Schwendeman, S.P. Effect of Manufacturing Variables and Raw Materials on the Composition-Equivalent PLGA Microspheres for 1-Month Controlled Release of Leuprolide. Mol. Pharm. 2020, 17, 1502–1515. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Snejdrova, E.; Podzimek, S.; Martiska, J.; Holas, O.; Dittrich, M. Branched PLGA derivatives with tailored drug delivery properties. Acta Pharm. 2020, 70, 63–75. [Google Scholar] [CrossRef]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev. Ind. Pharm. 1999, 25, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Further application of a modified spontaneous emulsification solvent diffusion method to various types of PLGA and PLA polymers for preparation of nanoparticles. Powder Technol. 2000, 107, 137–143. [Google Scholar] [CrossRef]
- Weischenfeldt, J.; Porse, B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot5080. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef]
- Li, F.J.; Zhu, A.P.; Song, X.L.; Ji, L.J.; Wang, J. The internalization of fluorescence-labeled PLA nanoparticles by macrophages. Int. J. Pharm. 2013, 453, 506–513. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Tammam, S.N.; Azzazy, H.M.; Lamprecht, A. Biodegradable Particulate Carrier Formulation and Tuning for Targeted Drug Delivery. J. Biomed. Nanotechnol. 2015, 11, 555–577. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Alshamsan, A. Nanoprecipitation is more efficient than emulsion solvent evaporation method to encapsulate cucurbitacin I in PLGA nanoparticles. Saudi Pharm. J. 2014, 22, 219–222. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharm. Rev 2001, 53, 283–318. [Google Scholar]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]
- Park, S.J. Protein-Nanoparticle Interaction: Corona Formation and Conformational Changes in Proteins on Nanoparticles. Int. J. Nanomed. 2020, 15, 5783–5802. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Peters, F.M.; Warzecha, K.T.; Bienert, M.; van Bloois, L.; Trautwein, C.; Lammers, T.; Tacke, F. Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1209–1220. [Google Scholar] [CrossRef]
- Hyrsova, L.; Smutny, T.; Carazo, A.; Moravcik, S.; Mandikova, J.; Trejtnar, F.; Gerbal-Chaloin, S.; Pavek, P. The pregnane X receptor down-regulates organic cation transporter 1 (SLC22A1) in human hepatocytes by competing for (“squelching”) SRC-1 coactivator. Br. J. Pharm. 2016, 173, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Smutny, T.; Nova, A.; Drechslerová, M.; Carazo, A.; Hyrsova, L.; Hrušková, Z.R.; Kuneš, J.; Pour, M.; Špulák, M.; Pavek, P. 2-(3-Methoxyphenyl)quinazoline Derivatives: A New Class of Direct Constitutive Androstane Receptor (CAR) Agonists. J. Med. Chem. 2016, 59, 4601–4610. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Utsumi, T.; Seo, Y.E.; Deng, Y.; Satoh, A.; Saltzman, W.M.; Iwakiri, Y. Cellular distribution of injected PLGA-nanoparticles in the liver. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, S.; Wang, Y.; Bao, W.; Zhou, Y.; Dang, W.; Wang, X.; Li, H.; Cao, X.; You, Y.; et al. BIG1 controls macrophage pro-inflammatory responses through ARF3-mediated PI(4,5)P2 synthesis. Cell Death Dis. 2020, 11, 374. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, W.T.; Qiu, Y.; Ju, M.J.; Yang, C.; Tu, G.W.; Luo, Z. Cyclic helix B peptide alleviates sepsis-induced acute lung injury by downregulating NLRP3 inflammasome activation in alveolar macrophages. Int. Immunopharmacol. 2020, 88, 106849. [Google Scholar] [CrossRef] [PubMed]
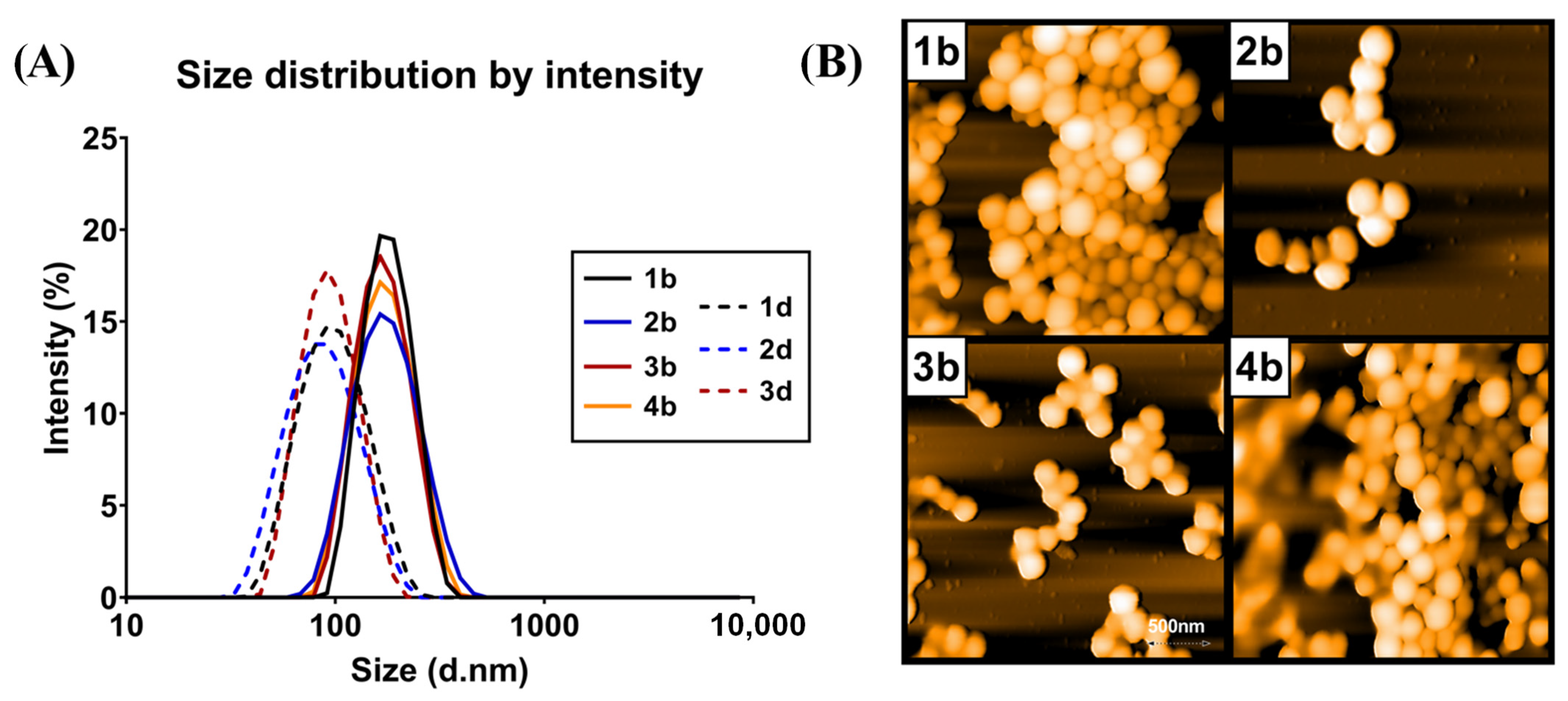



| Batch No. | Polymer | Preparation Method | Fluorescent Tracer | Size (nm) ± SD | PDI ± SD | Zeta Potential (mV) ± SD |
|---|---|---|---|---|---|---|
| 1a | PLGA 50:50 | NPM | - | 157.5 ± 3.7 | 0.06 ± 0.02 | −27 ± 3 |
| 1b | PLGA 50:50 | NPM | RhB | 171.5 ± 1.4 | 0.10 ± 0.01 | −25 ± 2 |
| 1c | PLGA 50:50 | ESE | - | 91.1 ± 1.2 | 0.09 ± 0.01 | −22 ± 3 |
| 1d | PLGA 50:50 | ESE | RhB | 91.2 ± 5.1 | 0.16 ± 0.03 | −22 ± 1 |
| 2a | PLGA 70:30 | NPM | - | 138.0 ± 6.5 | 0.07 ± 0.03 | −28 ± 2 |
| 2b | PLGA 70:30 | NPM | RhB | 166.7 ± 1.6 | 0.08 ± 0.01 | −29 ± 3 |
| 2c | PLGA 70:30 | ESE | - | 81.4 ± 1.8 | 0.08 ± 0.01 | −24 ± 2 |
| 2d | PLGA 70:30 | ESE | RhB | 81.1 ± 7.9 | 0.07 ± 0.01 | −22 ± 1 |
| 3a | Branched PLGA | NPM | - | 131 ± 6.0 | 0.08 ± 0.01 | −32 ± 2 |
| 3b | Branched PLGA | NPM | RhB | 162.0 ± 9.0 | 0.08 ± 0.01 | −33 ± 1 |
| 3c | Branched PLGA | ESE | - | 97.7 ± 5.7 | 0.10 ± 0.01 | −27 ± 2 |
| 3d | Branched PLGA | ESE | RhB | 89.3 ± 8.6 | 0.08 ± 0.03 | −24 ± 1 |
| 4a | Purasorb 5002 | NPM | - | 145.3 ± 0.8 | 0.1 ± 0.01 | −24 ± 3 |
| 4b | Purasorb 5002 | NPM | RhB | 164.6 ± 5.5 | 0.1 ± 0.01 | −24 ± 1 |
| Batch No. | Polymer | Preparation Method | % Drug Loading Efficiency |
|---|---|---|---|
| 1b | PLGA 50:50 | NPM | 47 ± 4 |
| 1d | PLGA 50:50 | ESE | 28 ± 6 |
| 2b | PLGA 70:30 | NPM | 56 ± 7 |
| 2d | PLGA 70:30 | ESE | 29 ± 3 |
| 3b | Branched PLGA | NPM | 61 ± 11 |
| 3d | Branched PLGA | ESE | 34 ± 4 |
| 4b | Purasorb® | NPM | 18 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boltnarova, B.; Kubackova, J.; Skoda, J.; Stefela, A.; Smekalova, M.; Svacinova, P.; Pavkova, I.; Dittrich, M.; Scherman, D.; Zbytovska, J.; et al. PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System. Nanomaterials 2021, 11, 749. https://doi.org/10.3390/nano11030749
Boltnarova B, Kubackova J, Skoda J, Stefela A, Smekalova M, Svacinova P, Pavkova I, Dittrich M, Scherman D, Zbytovska J, et al. PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System. Nanomaterials. 2021; 11(3):749. https://doi.org/10.3390/nano11030749
Chicago/Turabian StyleBoltnarova, Barbora, Jana Kubackova, Josef Skoda, Alzbeta Stefela, Monika Smekalova, Petra Svacinova, Ivona Pavkova, Milan Dittrich, Daniel Scherman, Jarmila Zbytovska, and et al. 2021. "PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System" Nanomaterials 11, no. 3: 749. https://doi.org/10.3390/nano11030749
APA StyleBoltnarova, B., Kubackova, J., Skoda, J., Stefela, A., Smekalova, M., Svacinova, P., Pavkova, I., Dittrich, M., Scherman, D., Zbytovska, J., Pavek, P., & Holas, O. (2021). PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System. Nanomaterials, 11(3), 749. https://doi.org/10.3390/nano11030749









