Biological Applications of Severely Plastically Deformed Nano-Grained Medical Devices: A Review
Abstract
1. Introduction
- Greater strains are imposed on the material;
- More hydrostatic pressure is applied;
- Material free flow is prevented;
- The dimensions of the material are not changed significantly during or after the process;
- Materials with micro- and/or nanostructured and high-angle grain boundaries are produced;
- Micro-structured and/or nanostructured homogeneous materials with uniform properties are fabricated;
- There are no pores, mechanical defects, or cracks in the final material [24].
2. Main SPD Processes
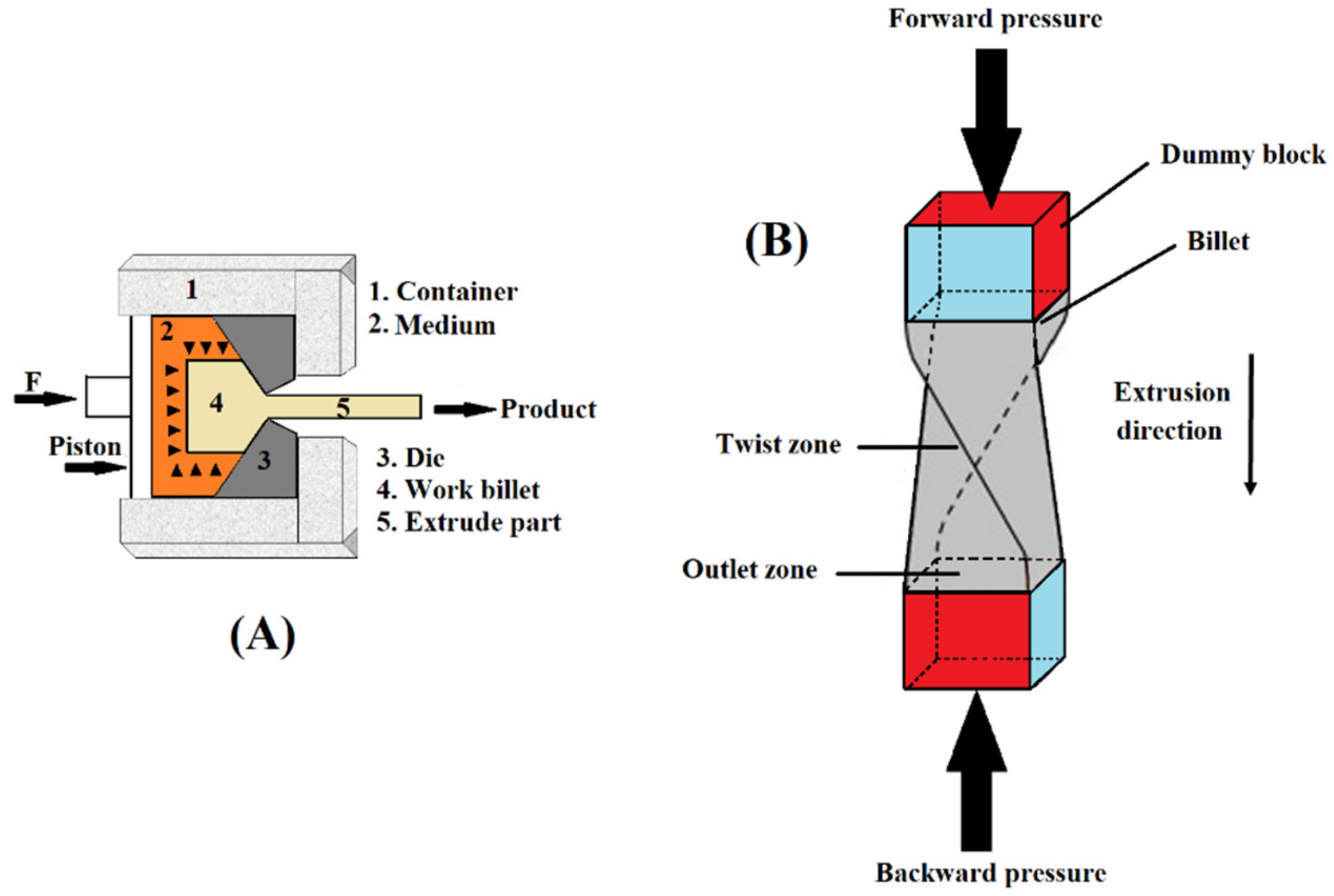
2.1. Equal-Channel Angular Press (ECAP) Process
2.2. High-Pressure Torsion (HPT) Process
2.3. Hydrostatic Extrusion (HE)
2.4. Twist Extrusion (TE)
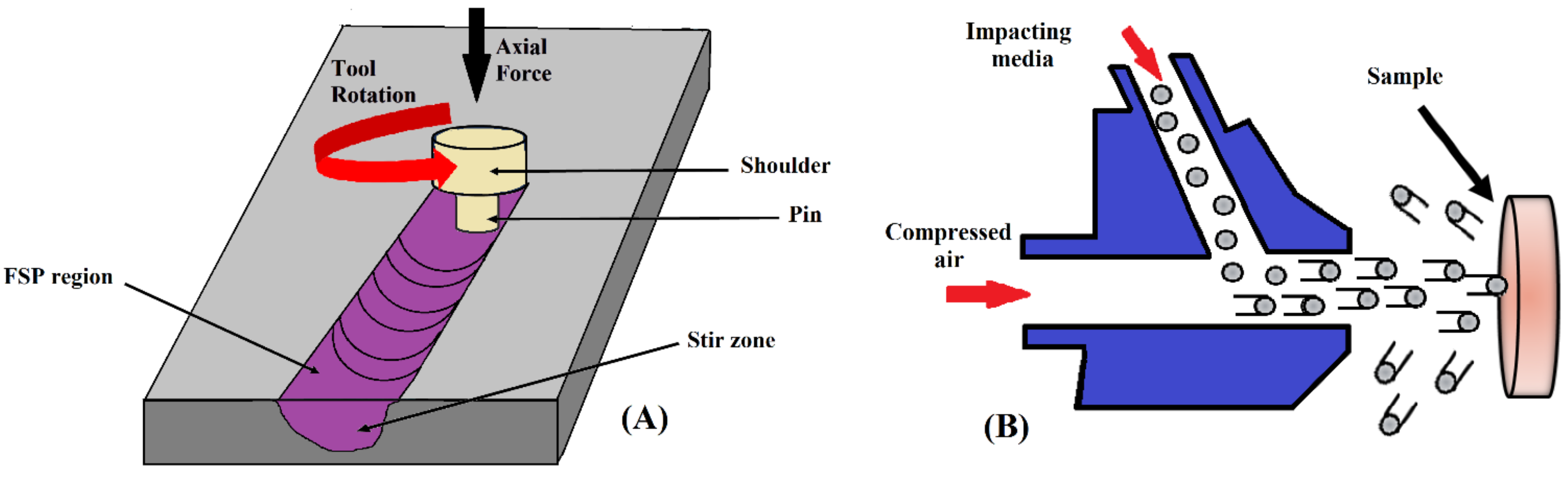
2.5. Friction Stir Processing (FSP)
2.6. Severe Shot Peening (SSP)
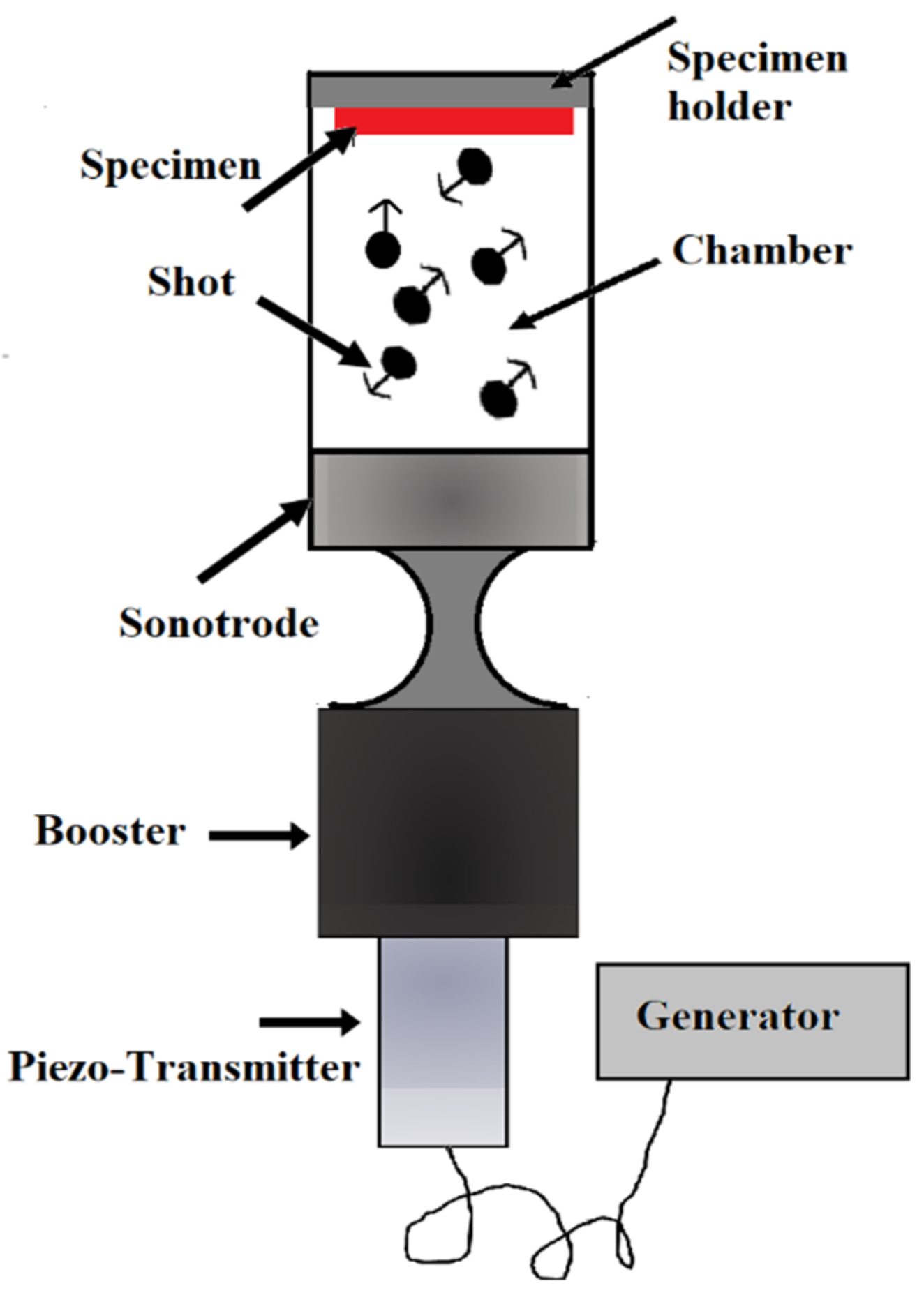
2.7. Ultrasonic Shot Peening (USSP)
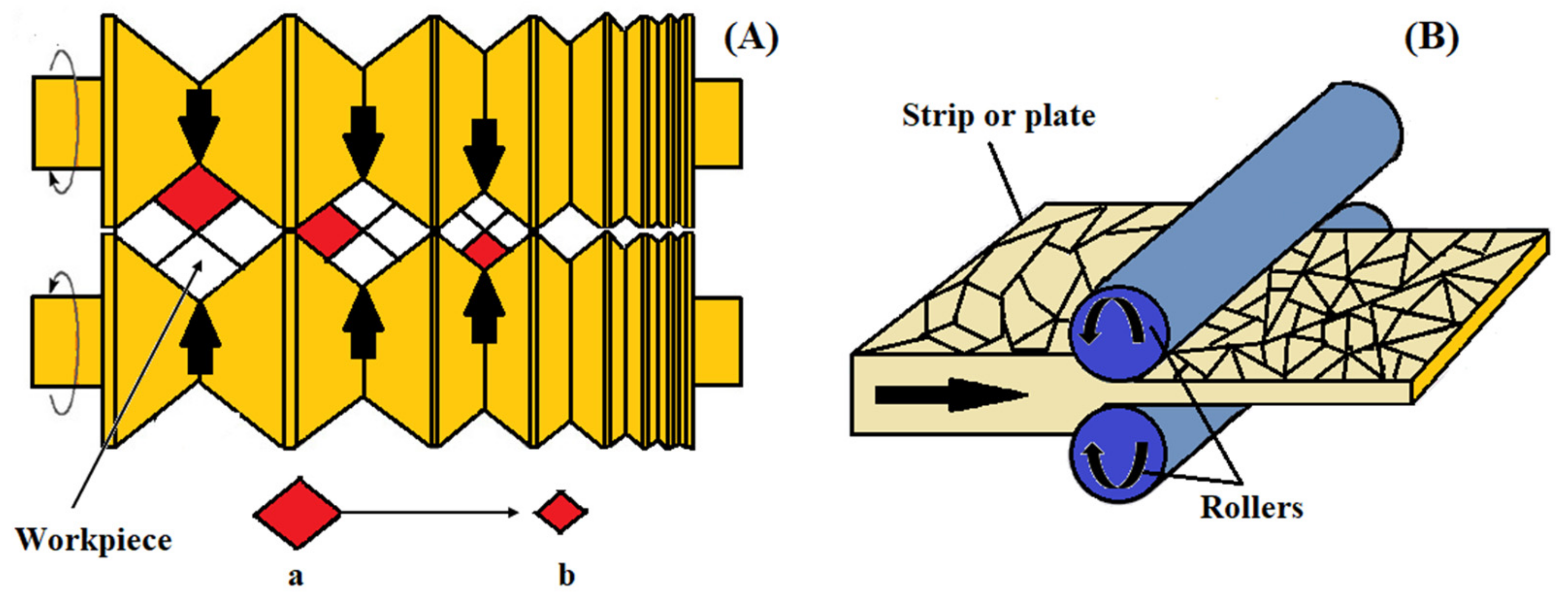
2.8. Warm Continuous Multidirectional Rolling
- Continuous rolling (coil to coil).
- Rolling in a controlled temperature via on-line heating.
- Two-tandem rolling in the vertical and horizontal directions.
- Rolling in multiple directions with square and oval grooves.
2.9. Warm Multi-Pass Caliber Rolling
2.10. Cold-Rolling Process
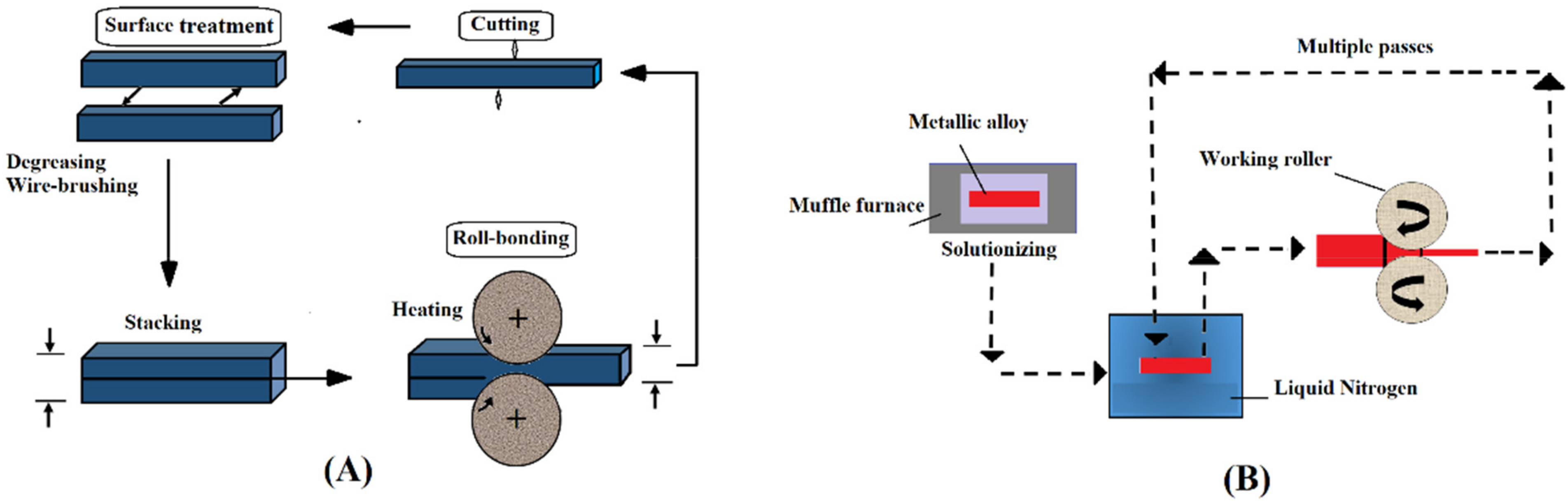
2.11. Accumulative Roll-Bonding Process (ARB)
2.12. Cryo-Rolling (CR)
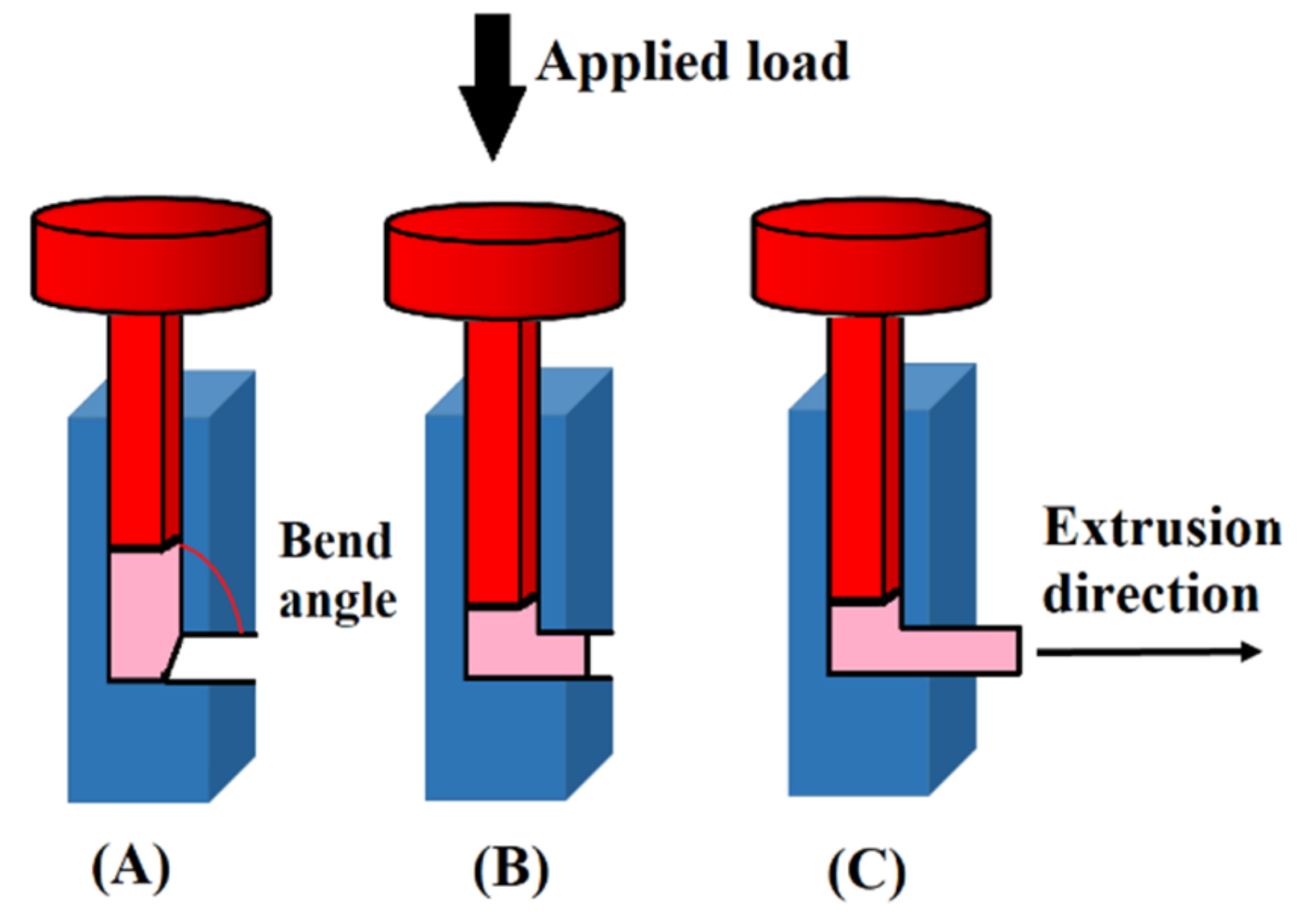
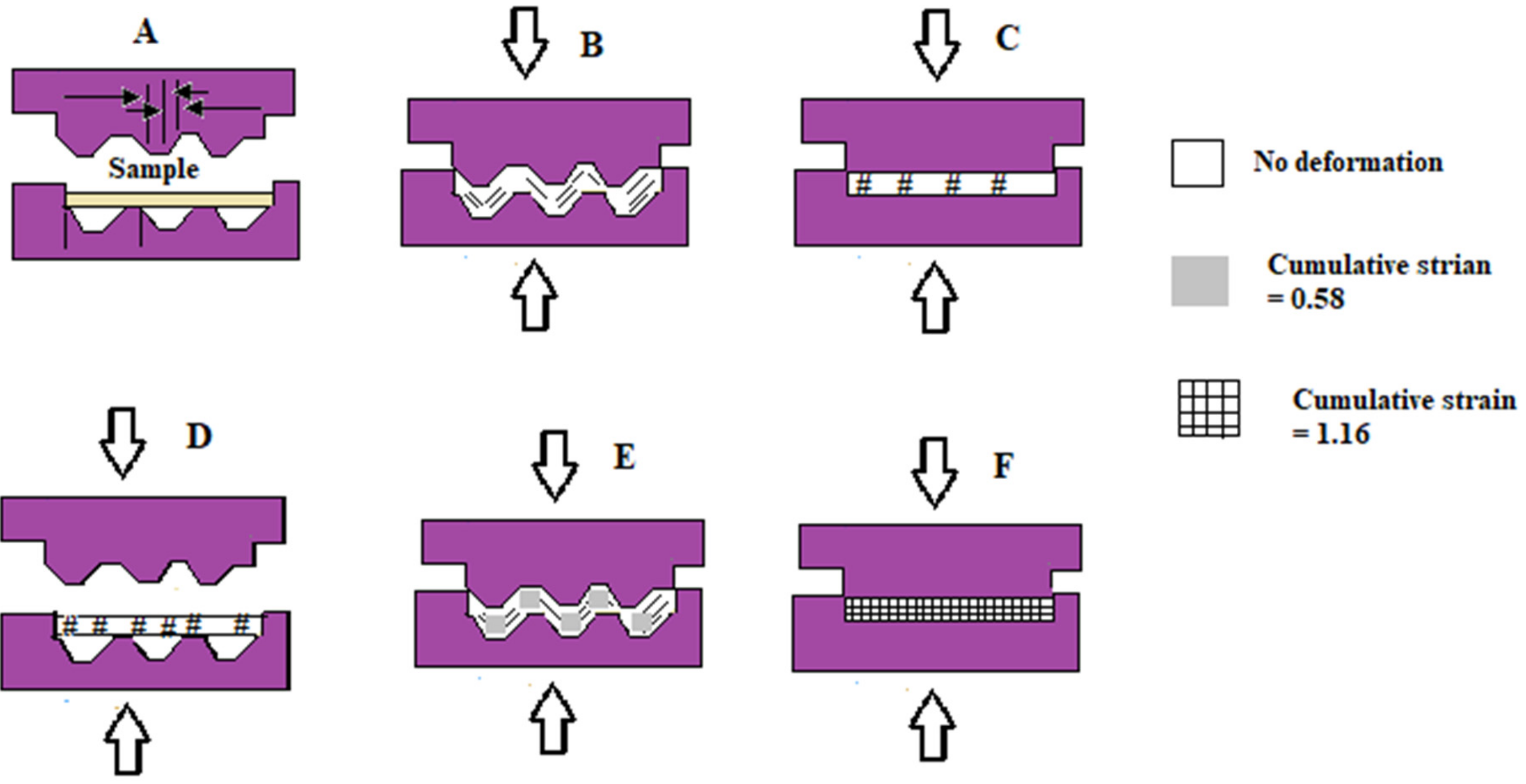
2.13. Constrained Groove Pressing (CGP)
3. Metallic Biomaterials for Medical Implants
3.1. Ultra-Fine-Grained Titanium
3.2. Ultra-Fine-Grained Stainless Steel
3.3. Ultra-Fine-Grained Magnesuim
4. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Ni, S.; Liao, X.; Song, M.; Zhu, Y. Structural evolutions of metallic materials processed by severe plastic deformation. Mater. Sci. Eng. 2018, 133, 1–59. [Google Scholar] [CrossRef]
- Ratna Sunil, B.; Thirugnanam, A.; Chakkingal, U.; Sampath Kumar, T. Nano and ultra fine grained metallic biomaterials by severe plastic deformation techniques. Mater. Technol. 2016, 31, 743–755. [Google Scholar] [CrossRef]
- Tevlek, A.; Aydin, H.M.; Maleki, E.; Varol, R.; Unal, O. Effects of severe plastic deformation on pre-osteoblast cell behavior and proliferation on AISI 304 and Ti-6Al-4V metallic substrates. Surf. Coat. Technol. 2019, 366, 204–213. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Elias, C.N.; Meyers, M.A.; Valiev, R.Z.; Monteiro, S.N. Ultrafine grained titanium for biomedical applications: An overview of performance. J. Mater. Res. Technol. 2013, 2, 340–350. [Google Scholar] [CrossRef]
- Wang, K.; Wang, D.; Han, F. Effect of crystalline grain structures on the mechanical properties of twinning-induced plasticity steel. Acta Mech. Sin. 2016, 32, 181–187. [Google Scholar] [CrossRef]
- Dasgupta, S.; Tarafder, S.; Bandyopadhyay, A.; Bose, S. Effect of grain size on mechanical, surface and biological properties of microwave sintered hydroxyapatite. Mater. Sci. Eng. 2013, 33, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Deng, C.; Chen, X.; Zhao, X.; Chen, Y.; Fan, Y.; Zhang, X. Mechanical and biological properties of the micro-/nano-grain functionally graded hydroxyapatite bioceramics for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2015, 48, 1–11. [Google Scholar] [CrossRef]
- An, B.; Li, Z.; Diao, X.; Xin, H.; Zhang, Q.; Jia, X.; Wu, Y.; Li, K.; Guo, Y. In vitro and in vivo studies of ultrafine-grain Ti as dental implant material processed by ECAP. Mater. Sci. Eng. 2016, 67, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hoshi, N.; Hayakawa, T.; Ohkubo, C.; Miura, H.; Kimoto, K. Mechanical properties and biological responses of ultrafine-grained pure titanium fabricated by multi-directional forging. Mater. Sci. Eng. 2019, 245, 30–36. [Google Scholar] [CrossRef]
- Bagherifard, S.; Ghelichi, R.; Khademhosseini, A.; Guagliano, M. Cell response to nanocrystallized metallic substrates obtained through severe plastic deformation. ACS Appl. Mater. Interfaces 2014, 6, 7963–7985. [Google Scholar] [CrossRef]
- Gleiter, H. Nanocrystalline materials. In Advanced Structural and Functional Materials; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–37. [Google Scholar]
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.; Chen, H.; Yao, M.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef]
- Chattopadhyay, R. Surface Wear: Analysis, Treatment, and Prevention; ASM Iinternational: Portland, OR, USA, 2001. [Google Scholar]
- Truong, V.K.; Rundell, S.; Lapovok, R.; Estrin, Y.; Wang, J.Y.; Berndt, C.C.; Barnes, D.G.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. Effect of ultrafine-grained titanium surfaces on adhesion of bacteria. Appl. Microbiol. Biotechnol. 2009, 83, 925–937. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zechetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef]
- Nouri, A.; Wen, C. Introduction to surface coating and modification for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–60. [Google Scholar]
- Valiev, R.Z.; Prokofiev, E.A.; Kazarinov, N.A.; Raab, G.I.; Minasov, T.B.; Stráský, J. Developing nanostructured Ti alloys for innovative implantable medical devices. Materials 2020, 13, 967. [Google Scholar] [CrossRef]
- Unal, O.; Varol, R. Surface severe plastic deformation of AISI 304 via conventional shot peening, severe shot peening and repeening. Appl. Surf. Sci. 2015, 351, 289–295. [Google Scholar] [CrossRef]
- Faraji, G.; Kim, H. Review of principles and methods of severe plastic deformation for producing ultrafine-grained tubes. Mater. Sci. Technol. 2017, 33, 905–923. [Google Scholar] [CrossRef]
- Bridgman, P. The effect of pressure on the tensile properties of several metals and other materials. J. Appl. Phys. 1953, 24, 560–570. [Google Scholar] [CrossRef]
- Faraji, G.; Mashhadi, M.M.; Kim, H.S. Tubular channel angular pressing (TCAP) as a novel severe plastic deformation method for cylindrical tubes. Mater. Lett. 2011, 65, 3009–3012. [Google Scholar] [CrossRef]
- Faraji, G.; Kim, H.S.; Kashi, H.T. Severe Plastic Deformation: Methods, Processing and Properties; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Rosochowski, A. Processing metals by severe plastic deformation. Proc. Solid State Phenom. 2005, 101, 13–22. [Google Scholar] [CrossRef]
- Sergueeva, A.V.; Stolyarov, V.V.; Valiev, R.Z.; Mukherjee, A.K. Advanced mechanical properties of pure titanium with ultrafine grained structure. Scr. Mater. 2001, 45, 747–752. [Google Scholar] [CrossRef]
- Krawczynska, A.T.; Chrominski, W.; Ura-Binczyk, E.; Kulczyk, M.; Lewandowska, M. Mechanical properties and corrosion resistance of ultrafine grained austenitic stainless steel processed by hydrostatic extrusion. Mater. Des. 2017, 136, 34–44. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Chen, W.; Qin, J.; Fouse, J. Effects of rolling temperature and subsequent annealing on mechanical properties of ultrafine-grained Cu–Zn–Si alloy. Mater. Charact. 2015, 106, 100–107. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, Y.; Gui, Y.; Zhu, M. Corrosion behaviour of nanocrystalline 304 stainless steel prepared by equal channel angular pressing. Corros. Sci. 2012, 54, 60–67. [Google Scholar] [CrossRef]
- Pisarek, M.; Kędzierzawski, P.; Janik-Czachor, M.; Kurzydłowski, K. The effect of hydrostatic extrusion on resistance of 316 austenitic stainless steel to pit nucleation. Electrochem. Commun. 2007, 9, 2463–2466. [Google Scholar] [CrossRef]
- He, Y.; Pan, Q.; Qin, Y.; Liu, X.; Li, W. Microstructure and mechanical properties of ultrafine grain ZK60 alloy processed by equal channel angular pressing. J. Mater. Sci. 2010, 45, 1655–1662. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Chai, L.; Li, J.; Lu, C.; Godbole, A.; Wang, H.; Kong, C. High thermal stability and excellent mechanical properties of ultrafine-grained high-purity copper sheets subjected to asymmetric cryorolling. Mater. Charact. 2019, 153, 34–45. [Google Scholar] [CrossRef]
- Segal, V. modes and processes of severe plastic deformation (SPD). Materials 2018, 11, 1175. [Google Scholar] [CrossRef]
- Pourbashiri, M.; Poletti, C.; Sedighi, M.; Sommitsch, C. Strengthening mechanisms of Al wires processed by equal channel angular torsion drawing. Mater. Sci. Technol. 2020, 36, 65–82. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Zhang, Y.; Li, M. High tensile ductility and strength in a gradient structured Zr. Mater. Lett. 2018, 228, 500–503. [Google Scholar] [CrossRef]
- Patel, P.; Mohanan, M.; Sarvaiya, V.; Acharya, V.; Basa, D.K.; Chaudhury, S.K. Design, fabrication of equal channel angular extrusion process and its effect on microstructure and hardness of Al and Cu alloys. Trans. Indian Inst. Met. 2018, 71, 2605–2614. [Google Scholar] [CrossRef]
- Verlinden, B. Severe plastic deformation of metals. Metall. Mater. Eng. 2018. [Google Scholar] [CrossRef]
- Rosochowski, A. Severe Plastic Deformation of Metals. U.S. Patent Application No. 20090126444, 21 January 2014. [Google Scholar]
- Nishida, Y.; Arima, H.; Kim, J.-C.; Ando, T. Rotary-die equal-channel angular pressing of an Al–7 mass% Si–0.35 mass% Mg alloy. Scr. Mater. 2001, 45, 261–266. [Google Scholar] [CrossRef]
- Mesbah, M.; Faraji, G.; Bushroa, A.R. Characterization of nanostructured pure aluminum tubes produced by tubular channel angular pressing (TCAP). Mater. Sci. Eng. A 2014, 590, 289–294. [Google Scholar] [CrossRef]
- Mora-Sanchez, H.; Sabirov, I.; Monclus, M.; Matykina, E.; Molina-Aldareguia, J. Ultra-fine grained pure Titanium for biomedical applications. Mater. Technol. 2016, 31, 756–771. [Google Scholar] [CrossRef]
- Bridgman, P. Flow phenomena in heavily stressed metals. J. Appl. Phys. 1937, 8, 328–336. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. Scaling-up of high pressure torsion using ring shape. Mater. Trans. 2009, 50, 92–95. [Google Scholar] [CrossRef]
- Edalati, K.; Lee, S.; Horita, Z. Continuous high-pressure torsion using wires. J. Mater. Sci. 2012, 47, 473–478. [Google Scholar] [CrossRef]
- Semenov, V.; Huang, S.-J.; Tontchev, N.; Valiev, R.; Belov, P.; Bogale, D.; Wang, A. Corrosion behavior of commercially-pure titamium with different microstructures. Mater. Sci. Non-Equilib. Phase Transform. 2017, 3, 167–171. [Google Scholar]
- Zhengjie, L.; Liqiang, W.; Kelvin, W.; Jining, Q. The ultrafine-grained titanium and biomedical titanium alloys processed by severe plastic deformation (SPD). SOJMSE 2013, 1, 1. [Google Scholar]
- Garbacz, H.; Topolski, K.; Motyka, M. Hydrostatic extrusion. In Nanocrystalline Titanium; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–53. [Google Scholar]
- Pugh, H.L.D. Mechanical Behaviour of Materials under Pressure; Elsevier: Amsterdam, The Netherlands, 1973. [Google Scholar]
- Topolski, K.; Pachla, W.; Garbacz, H. Progress in hydrostatic extrusion of titanium. J. Mater. Sci. 2013, 48, 4543–4548. [Google Scholar] [CrossRef]
- Kulagin, R.; Latypov, M.I.; Kim, H.S.; Varyukhin, V.; Beygelzimer, Y. Cross flow during twist extrusion: Theory, experiment, and application. Met. Mater. Trans. A 2013, 44, 3211–3220. [Google Scholar] [CrossRef]
- Varyukhin, V.; Beygelzimer, Y.; Kulagin, R.; Prokof’eva, O.; Reshetov, A. Twist extrusion: Fundamentals and applications. Mater. Sci. Forum 2011, 667, 31–37. [Google Scholar] [CrossRef]
- Beygelzimer, Y.; Varyukhin, V.; Synkov, S.; Orlov, D. Useful properties of twist extrusion. Mater. Sci. Eng. A 2009, 503, 14–17. [Google Scholar] [CrossRef]
- Hannard, F.; Castin, S.; Maire, E.; Mokso, R.; Pardoen, T.; Simar, A. Ductilization of aluminium alloy 6056 by friction stir processing. Acta Mater. 2017, 130, 121–136. [Google Scholar] [CrossRef]
- Bagherifard, S.; Hickey, D.J.; Fintová, S.; Pastorek, F.; Fernandez-Pariente, I.; Bandini, M.; Webster, T.J.; Guagliano, M. Effects of nanofeatures induced by severe shot peening (SSP) on mechanical, corrosion and cytocompatibility properties of magnesium alloy AZ31. Acta Biomater. 2018, 66, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S.; Guagliano, M. Fatigue behavior of a low-alloy steel with nanostructured surface obtained by severe shot peening. Eng. Fract. Mech. 2012, 81, 56–68. [Google Scholar] [CrossRef]
- Guagliano, M. Relating Almen intensity to residual stresses induced by shot peening: A numerical approach. J. Mater. Process. Technol. 2001, 110, 277–286. [Google Scholar] [CrossRef]
- Chaise, T.; Li, J.; Nélias, D.; Kubler, R.; Taheri, S.; Douchet, G.; Robin, V.; Gilles, P. Modelling of multiple impacts for the prediction of distortions and residual stresses induced by ultrasonic shot peening (USP). J. Mater. Process. Technol. 2012, 212, 2080–2090. [Google Scholar] [CrossRef]
- Liu, N.; Chen, L.; Fu, Y.; Zhang, Y.; Tan, T.; Yin, F.; Liang, C. Interfacial characteristic of multi-pass caliber-rolled Mg/Al compound castings. J. Mater. Process. Technol. 2019, 267, 196–204. [Google Scholar] [CrossRef]
- Krállics, G.; Gubicza, J.; Bezi, Z.; Barkai, I. Manufacturing of ultrafine-grained titanium by caliber rolling in the laboratory and in industry. J. Mater. Process. Technol. 2014, 214, 1307–1315. [Google Scholar] [CrossRef]
- Torizuka, S.; Muramatsu, E.; Komatsu, T.; Nagayama, S. Production processes for nanostructured wires, bars and strips. In Nanostructured Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2011; pp. 715–746. [Google Scholar]
- Lee, T.; Shih, D.S.; Lee, Y.; Lee, C.S. Manufacturing ultrafine-grained Ti-6Al-4V bulk rod using multi-pass caliber-rolling. Metals 2015, 5, 777–789. [Google Scholar] [CrossRef]
- Aanestad, A. Simulation of cold rolling. Mater. Sci. Technol. 1986, 2, 620–624. [Google Scholar] [CrossRef]
- Singh, M.; Singh, A.K. Performance investigation of magnetorheological finishing of rolls surface in cold rolling process. J. Manuf. Process. 2019, 41, 315–329. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Zhang, H.; Shoji, T. Effect of cold rolling on the fretting wear behavior and mechanism in Inconel 600 alloy. Tribol. Trans. 2016, 59, 923–931. [Google Scholar] [CrossRef]
- Sedighi, M.; Mahmoodi, M. Pressure distribution in cold rolling of turbo-engine thin compressor blades. Mater. Manuf. Process. 2012, 27, 401–405. [Google Scholar] [CrossRef]
- Zarate, L.E.; Bittencout, F.R. Representation and control of the cold rolling process through artificial neural networks via sensitivity factors. J. Mater. Process. Technol. 2008, 197, 344–362. [Google Scholar] [CrossRef]
- Lai-Seng, L.; Lenard, J. Study of friction in cold strip rolling. J. Eng. Mater. Technol. 1984, 106, 139–146. [Google Scholar] [CrossRef]
- Etemad, A.; Dini, G.; Schwarz, S. Accumulative roll bonding (ARB)-processed high-manganese twinning induced plasticity (TWIP) steel with extraordinary strength and reasonable ductility. Mater. Sci. Eng. A 2019, 742, 27–32. [Google Scholar] [CrossRef]
- Khdair, A.I.; Fathy, A. Enhanced strength and ductility of Al-SiC nanocomposites synthesized by accumulative roll bonding. J. Mater. Res. Technol. 2020, 9, 478–489. [Google Scholar] [CrossRef]
- Zohoori-Shoar, V.; Eslami, A.; Karimzadeh, F.; Abbasi-Baharanchi, M. Resistance spot welding of ultrafine grained/nanostructured Al 6061 alloy produced by cryorolling process and evaluation of weldment properties. J. Manuf. Process. 2017, 26, 84–93. [Google Scholar] [CrossRef]
- Satish, D.R.; Feyissa, F.; Kumar, D.R. Cryorolling and warm forming of AA6061 aluminum alloy sheets. Mater. Manuf. Process. 2017, 32, 1345–1352. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayaganthan, R. Effect of rolling temperature on microstructure and mechanical properties of 6063 Al alloy. Mater. Sci. Eng. A 2008, 492, 300–305. [Google Scholar] [CrossRef]
- Changela, K.; Krishnaswamy, H.; Digavalli, R.K. Development of combined groove pressing and rolling to produce ultra-fine grained Al alloys and comparison with cryorolling. Mater. Sci. Eng. A 2019, 760, 7–18. [Google Scholar] [CrossRef]
- Krishnaiah, A.; Chakkingal, U.; Venugopal, P. Applicability of the groove pressing technique for grain refinement in commercial purity copper. Mater. Sci. Eng. A 2005, 410, 337–340. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Kazeminezhad, M. The effect of constrained groove pressing on grain size, dislocation density and electrical resistivity of low carbon steel. Mater. Des. 2011, 32, 3280–3286. [Google Scholar] [CrossRef]
- Shin, D.H.; Park, J.-J.; Kim, Y.-S.; Park, K.-T. Constrained groove pressing and its application to grain refinement of aluminum. Mater. Sci. Eng. A 2002, 328, 98–103. [Google Scholar] [CrossRef]
- Gupta, A.K.; Maddukuri, T.S.; Singh, S.K. Constrained groove pressing for sheet metal processing. Prog. Mater. Sci. 2016, 84, 403–462. [Google Scholar] [CrossRef]
- Salvati, E.; Zhang, H.; Fong, K.S.; Song, X.; Korsunsky, A.M. Separating plasticity-induced closure and residual stress contributions to fatigue crack retardation following an overload. J. Mech. Phys. Solids 2017, 98, 222–235. [Google Scholar] [CrossRef]
- Fong, K.S.; Tan, M.J.; Chua, B.W.; Atsushi, D. Enabling wider use of Magnesium Alloys for lightweight applications by improving the formability by Groove Pressing. Procedia CIRP 2015, 26, 449–454. [Google Scholar] [CrossRef]
- Mishnaevsky Jr, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’yov, A.V.; Korotitskiy, A.; Gutmanas, E. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Elias, C.N.; Fernandes, D.J.; de Souza, F.M.; dos Santos Monteiro, E.; de Biasi, R.S. Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J. Mater. Res. Technol. 2019, 8, 1060–1069. [Google Scholar] [CrossRef]
- Morais, L.S.; Serra, G.G.; Muller, C.A.; Andrade, L.R.; Palermo, E.F.; Elias, C.N.; Meyers, M. Titanium alloy mini-implants for orthodontic anchorage: Immediate loading and metal ion release. Acta Biomater. 2007, 3, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, D.; Yamamoto, A.; Wieciński, P.; Garbacz, H. Biological behavior of titanium processed by severe plastic deformation. Appl. Surf. Sci. 2019, 472, 54–63. [Google Scholar] [CrossRef]
- Nie, F.; Zheng, Y.; Wei, S.; Wang, D.; Yu, Z.; Salimgareeva, G.; Polyakov, A.; Valiev, R. In vitro and in vivo studies on nanocrystalline Ti fabricated by equal channel angular pressing with microcrystalline CP Ti as control. J. Biomed. Mater. Res. A 2013, 101, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kim, Y.-J.; Park, C.H.; Lee, D.-H.; Ko, Y.G.; Jang, J.-H.; Lee, C.S. Enhanced osteoblast response to an equal channel angular pressing-processed pure titanium substrate with microrough surface topography. Acta Biomater. 2009, 5, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.A.G.; Junior, A.M.J.; Roche, V.; Lepretre, J.-C.; Afonso, C.R.M.; Travessa, D.N.; Asato, G.H.; Bolfarini, C.; Botta, W.J. Severe plastic deformation and different surface treatments on the biocompatible Ti13Nb13Zr and Ti35Nb7Zr5Ta alloys: Microstructural and phase evolutions, mechanical properties, and bioactivity analysis. J. Alloy. Compd. 2020, 812, 152116. [Google Scholar] [CrossRef]
- Hernández-López, J.; Conde, A.; De Damborenea, J.; Arenas, M. Correlation of the nanostructure of the anodic layers fabricated on Ti13Nb13Zr with the electrochemical impedance response. Corros. Sci. 2015, 94, 61–69. [Google Scholar] [CrossRef]
- Hazan, R.; Brener, R.; Oron, U. Bone growth to metal implants is regulated by their surface chemical properties. Biomaterials 1993, 14, 570–574. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, B.; Tang, C.; Wu, Y.; Sun, P.; Zhang, X.; Jia, Y. Increased osteoblast function in vitro and in vivo through surface nanostructuring by ultrasonic shot peening. Int. J. Nanomed. 2015, 10, 4593. [Google Scholar]
- Faghihi, S.; Azari, F.; Zhilyaev, A.P.; Szpunar, J.A.; Vali, H.; Tabrizian, M. Cellular and molecular interactions between MC3T3-E1 pre-osteoblasts and nanostructured titanium produced by high-pressure torsion. Biomaterials 2007, 28, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, S.; Zhilyaev, A.P.; Szpunar, J.A.; Azari, F.; Vali, H.; Tabrizian, M. Nanostructuring of a titanium material by high-pressure torsion improves pre-osteoblast attachment. Adv. Mater. 2007, 19, 1069–1073. [Google Scholar] [CrossRef]
- Baek, S.M.; Shin, M.H.; Moon, J.; Jung, H.S.; Hwang, W.; Yeom, J.T.; Hahn, S.K.; Kim, H.S. Superior pre-osteoblast cell response of etched ultrafine-grained titanium with a controlled crystallographic orientation. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Balakrishnan, A.; Lee, B.; Kim, W.; Smetana, K.; Park, J.K.; Panigrahi, B. In vitro biocompatibility of equal channel angular processed (ECAP) titanium. Biomed. Mater. 2007, 2, S117. [Google Scholar] [CrossRef]
- Bindu, S.; Sanosh, K.; Smetana, K.; Balakrishnan, A.; Kim, T. An in vivo evaluation of ultra-fine grained titanium implants. J. Mater. Sci. Nanotechnol. 2009, 25, 556. [Google Scholar]
- Ozaltin, K.; Panigrahi, A.; Chrominski, W.; Bulutsuz, A.; Kulczyk, M.; Zehetbauer, M.J.; Lewandowska, M. Microstructure and texture evolutions of biomedical Ti-13Nb-13Zr alloy processed by hydrostatic extrusion. Met. Mater. Trans. A 2017, 48, 5747–5755. [Google Scholar] [CrossRef]
- Serra, G.; Morais, L.; Elias, C.N.; Semenova, I.P.; Valiev, R.; Salimgareeva, G.; Pithon, M.; Lacerda, R. Nanostructured severe plastic deformation processed titanium for orthodontic mini-implants. Mater. Sci. Eng. 2013, 33, 4197–4202. [Google Scholar] [CrossRef]
- Oliveira, D.; Prokofiev, E.; Sanches, L.; Polyakova, V.; Valiev, R.; Botta, W.; Junior, A.; Bolfarini, C. Surface chemical treatment of ultrafine-grained Ti–6Al–7Nb alloy processed by severe plastic deformation. J. Alloys Compd. 2015, 643, S241–S245. [Google Scholar] [CrossRef]
- Pachla, W.; Kulczyk, M.; Przybysz, S.; Skiba, J.; Wojciechowski, K.; Przybysz, M.; Topolski, K.; Sobolewski, A.; Charkiewicz, M. Effect of severe plastic deformation realized by hydrostatic extrusion and rotary swaging on the properties of CP Ti grade 2. J. Mater. Process. Technol. 2015, 221, 255–268. [Google Scholar] [CrossRef]
- Kim, T.N.; Balakrishnan, A.; Lee, B.; Kim, W.; Dvorankova, B.; Smetana, K.; Park, J.K.; Panigrahi, B. In vitro fibroblast response to ultra fine grained titanium produced by a severe plastic deformation process. J. Mater. Sci. Mater. Med. 2008, 19, 553. [Google Scholar] [CrossRef]
- Reshadi, F.; Khorasani, S.; Faraji, G. Surface characterization of nanostructured commercially pure titanium modified by sandblasting and acid-etching for implant applications. Proc. Inst. Mech. Eng. 2020, 234, 414–423. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Hajizadeh, K.; Hadjizadeh, A.; Shakeri, M.; Alamdari, S.G.; Masoudfar, S.; Aghaie, E.; Javidi, M.; Zdunek, J.; Kurzydlowski, K. Electrochemical and cellular behavior of ultrafine-grained titanium in vitro. Mater. Sci. Eng. 2014, 39, 299–304. [Google Scholar] [CrossRef]
- Zheng, C.; Nie, F.; Zheng, Y.; Cheng, Y.; Wei, S.; Valiev, R. Enhanced in vitro biocompatibility of ultrafine-grained titanium with hierarchical porous surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Jindal, S.; Bansal, R.; Singh, B.P.; Pandey, R.; Narayanan, S.; Wani, M.R.; Singh, V. Enhanced osteoblast proliferation and corrosion resistance of commercially pure titanium through surface nanostructuring by ultrasonic shot peening and stress relieving. J. Oral. Implantol. 2014, 40, 347–355. [Google Scholar] [CrossRef]
- Nunes, A.R.V.; Borborema, S.; Araújo, L.S.; Dille, J.; Malet, L.; de Almeida, L.H. Production, microstructure and mechanical properties of cold-rolled Ti-Nb-Mo-Zr alloys for orthopedic applications. J. Alloys Compd. 2018, 743, 141–145. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Molotnikov, A.; Lapovok, R.; Zeller, R.; Berner, S.; Habersetzer, P.; Dalla Torre, F. Microstructure and mechanical properties of Ti–15Zr alloy used as dental implant material. J. Mech. Behav. Biomed. Mater. 2016, 62, 384–398. [Google Scholar] [CrossRef]
- Lan, C.; Wu, Y.; Guo, L.; Chen, H.; Chen, F. Microstructure, texture evolution and mechanical properties of cold rolled Ti-32.5 Nb-6.8 Zr-2.7 Sn biomedical beta titanium alloy. J. Mater. Sci. Nanotechnol. 2018, 34, 788–792. [Google Scholar] [CrossRef]
- Hajizadeh, K.; Maleki-Ghaleh, H.; Arabi, A.; Behnamian, Y.; Aghaie, E.; Farrokhi, A.; Hosseini, M.; Fathi, M. Corrosion and biological behavior of nanostructured 316L stainless steel processed by severe plastic deformation. Surf. Interface Anal. 2015, 47, 978–985. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R. Metals for biomedical applications. Biomed. Eng. Theory Appl. 2011, 1, 411–430. [Google Scholar]
- Sutha, S.; Karunakaran, G.; Rajendran, V. Enhancement of antimicrobial and long-term biostability of the zinc-incorporated hydroxyapatite coated 316L stainless steel implant for biomedical application. Ceram. Int. 2013, 39, 5205–5212. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Idell, Y.; Facco, G.; Kulovits, A.; Shankar, M.; Wiezorek, J. Strengthening of austenitic stainless steel by formation of nanocrystalline γ-phase through severe plastic deformation during two-dimensional linear plane-strain machining. Scr. Mater. 2013, 68, 667–670. [Google Scholar] [CrossRef]
- Yin, F.; Xu, R.; Hu, S.; Zhao, K.; Yang, S.; Kuang, S.; Li, Q.; Han, Q. Enhanced mechanical and biological performance of an extremely fine nanograined 316L stainless steel cell–substrate interface fabricated by ultrasonic shot peening. ACS Biomater. Sci. Eng. 2018, 4, 1609–1621. [Google Scholar] [CrossRef]
- Bagherifard, S.; Hickey, D.J.; de Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef]
- Bahl, S.; Shreyas, P.; Trishul, M.; Suwas, S.; Chatterjee, K. Enhancing the mechanical and biological performance of a metallic biomaterial for orthopedic applications through changes in the surface oxide layer by nanocrystalline surface modification. Nanoscale 2015, 7, 7704–7716. [Google Scholar] [CrossRef]
- Yin, F.; Yang, S.; Hu, S.; Kuang, S.; Han, Q. Enhanced human osteoblast cell functions by “net-like” nanostructured cell-substrate interface in orthopedic applications. Mater. Lett. 2017, 189, 275–278. [Google Scholar] [CrossRef]
- Bagherifard, S.; Slawik, S.; Fernández-Pariente, I.; Pauly, C.; Mücklich, F.; Guagliano, M. Nanoscale surface modification of AISI 316L stainless steel by severe shot peening. Mater. Des. 2016, 102, 68–77. [Google Scholar] [CrossRef]
- Ramalingam, V.V.; Ramasamy, P.; Kovukkal, M.D.; Myilsamy, G. Research and development in magnesium alloys for industrial and biomedical applications: A review. Met. Mater. Int. 2020, 26, 409–430. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, J.; Yu, H.; Chen, C. Degradable magnesium-based alloys for biomedical applications: The role of critical alloying elements. J. Biomater. Appl. 2019, 33, 1348–1372. [Google Scholar] [CrossRef]
- Nayak, S.; Bhushan, B.; Jayaganthan, R.; Gopinath, P.; Agarwal, R.; Lahiri, D. Strengthening of Mg based alloy through grain refinement for orthopaedic application. J. Mech. Behav. Biomed. Mater. 2016, 59, 57–70. [Google Scholar] [CrossRef]
- Saha, P.; Roy, M.; Datta, M.K.; Lee, B.; Kumta, P.N. Effects of grain refinement on the biocorrosion and in vitro bioactivity of magnesium. Mater. Sci. Eng. 2015, 57, 294–303. [Google Scholar] [CrossRef]
- Silva, C.L.; Oliveira, A.C.; Costa, C.G.; Figueiredo, R.B.; de Fátima Leite, M.; Pereira, M.M.; Lins, V.F.; Langdon, T.G. Effect of severe plastic deformation on the biocompatibility and corrosion rate of pure magnesium. J. Mater. Sci. 2017, 52, 5992–6003. [Google Scholar] [CrossRef]
- Dobatkin, S.; Lukyanova, E.; Martynenko, N.; Anisimova, N.Y.; Kiselevskiy, M.; Gorshenkov, M.; Yurchenko, N.Y.; Raab, G.I.; Yusupov, V.; Birbilis, N. Strength, corrosion resistance, and biocompatibility of ultrafine-grained Mg alloys after different modes of severe plastic deformation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 194, 012004. [Google Scholar] [CrossRef]

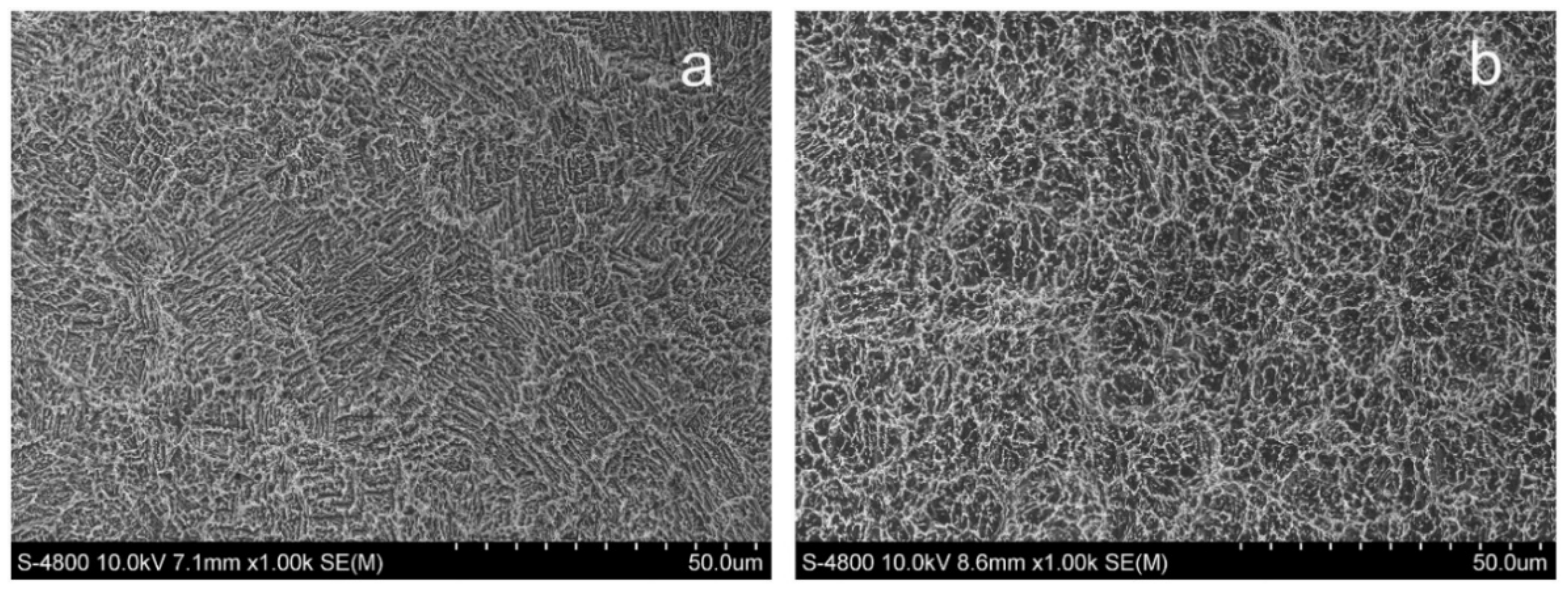
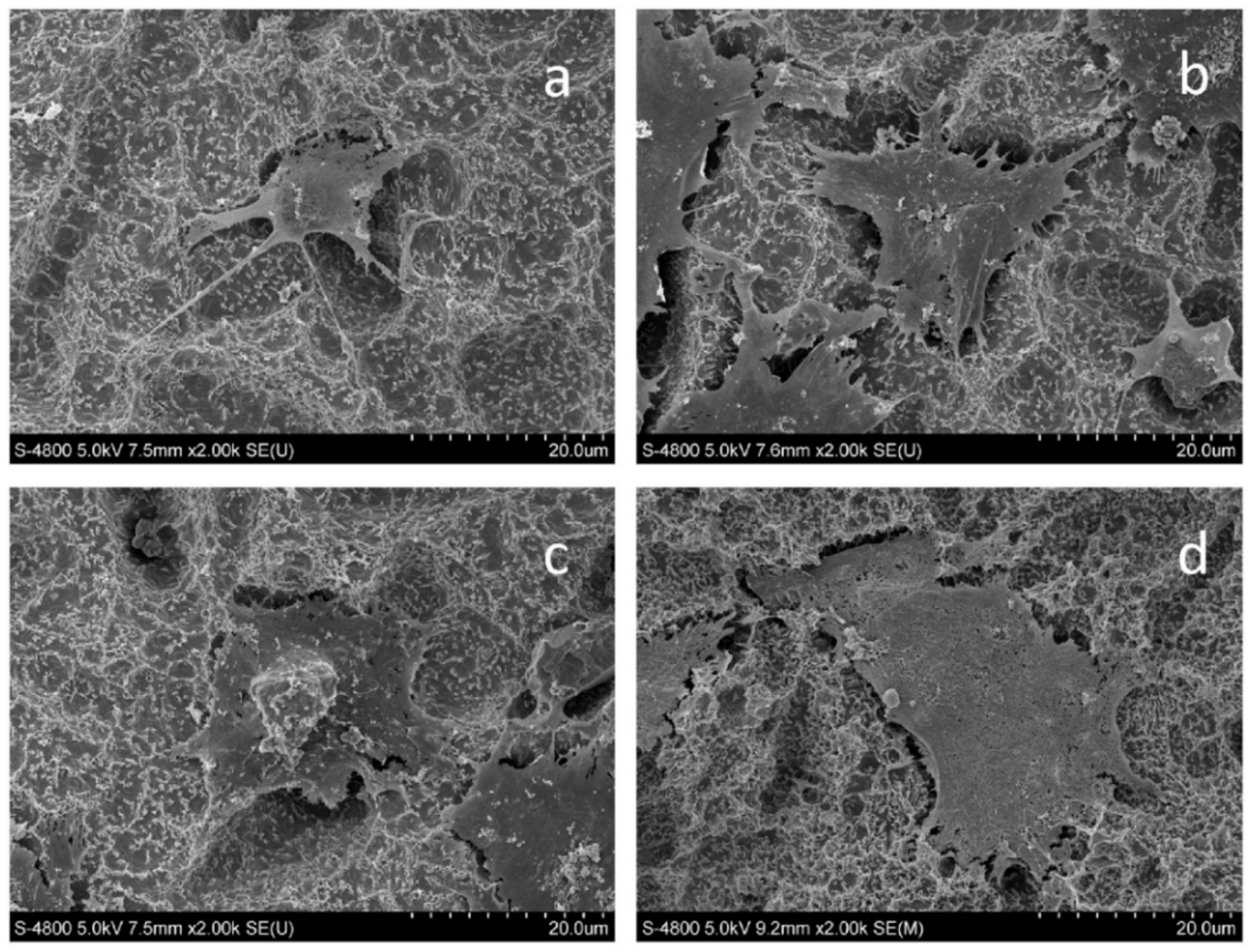
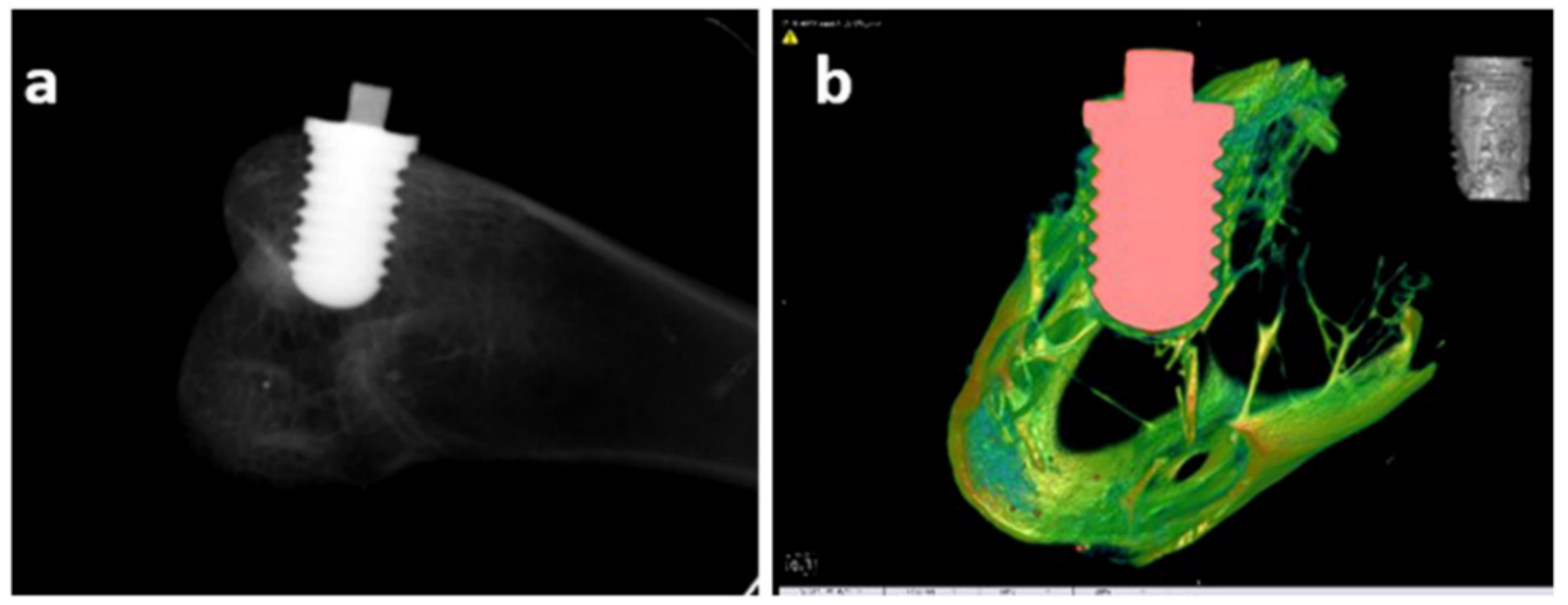
| Metals/Alloys | Benefits | Limitations |
|---|---|---|
| Commercially pure titanium (CP Ti) |
|
|
| Ti–6Al–4V |
|
|
| Stainless steel 316 L |
|
|
| Co–Cr-based |
|
|
| NiTi (Nitinol) |
|
|
| Magnesium-based |
|
|
| Materials/Alloys | SPD Process | Size of Grains/Instrument | Applications | In Vivo/In Vitro | Significant Findings | Ref. |
|---|---|---|---|---|---|---|
| Titanium Grade 2 | Hydrostatic Extrusion (HE) | 92 (nm), TEM | Bone tissues | SaOS-2 cells |
| [83] |
| β-type Ti-13Nb-13Zr (TNZ) | Hydrostatic Extrusion (HE) | 20 (nm) | - | - |
| [96] |
| Ti13Nb13Zr and Ti35Nb7Zr5Ta | High-Pressure Torsion (HPT) | ~203 and ~112 (nm), TEM | - | Osteoblastic cells |
| [86] |
| Nanostructured Ti (nTi) | Equal-Channel Angular Pressing | - | Orthodontics | - |
| [97] |
| Ti–6Al–7Nb | Equal-Channel Angular Pressing | 200 (nm), TEM | Orthopedic implants |
| [98] | |
| Commercially pure (CP) Ti Grade 2 | Cold Hydrostatic Extrusion | <90 (nm), TEM | Surgical osteosynthesis |
| [99] | |
| CP Ti grade 2 and Ti-6A1-4 V | Equal-Channel Angular Pressing | ~23 (nm), TEM | Implants | Mouse fibroblast cell line 3T3 |
| [100] |
| Ti–6Al–4V | Equal-Channel Angular Pressing | ~170 (nm), TEM | Dental implants | MG63 cells |
| [101] |
| CP Ti | Equal-Channel Angular Pressing | 183 (nm), TEM | Implants | Fibroblast cells |
| [102] |
| CP Ti grade 2 and Ti-6A1-4 V | Equal-Channel Angular Pressing | 238 (nm), TEM | Implants | Mouse fibroblast cell line 3T3 |
| [94] |
| CP Ti | Equal-Channel Angular Pressing | 200–300 (nm), SEM | Bone–implant osseointegration | New Zealand rabbits/MC3T3-E1 cells |
| [10] |
| CP Ti grade 2 and Ti-6A1-4 V | Equal-Channel Angular Pressing | 200–300 (nm), SEM | Dental endosseous implants | MC3T3-E1 pre-osteoblast cells |
| [85] |
| Commercial coarse-grained pure titanium | Equal-Channel Angular Pressing | 200 (nm), ESEM | Bone implants | Osteoblast-like cell line MG63 |
| [103] |
| Bulk nanocrystalline Ti bars (Grade 4) | Equal-Channel Angular Pressing | 250 (nm), TEM | Bone implants | Osteoblast cell lines (MG63)/tibia of Beagle dogs |
| [84] |
| CP Ti | High-Pressure Torsion (HPT) | 10–50 (nm), TEM | Bone implants | Mouse pre-osteoblast MC3T3-E1 subclone 14 and fibroblast cell lines from rats |
| [90] |
| CP Ti | Ultrasonic Shot Peening | 14–20 (nm), SEM | Dental and orthopedic implants | Human osteoblast cell line, MG 63 |
| [104] |
| CP Ti | High-Pressure Torsion (HPT) | 10–50 (nm), TEM | Bone implants | Mouse pre-osteoblast MC3T3-E1 |
| [92] |
| CP Ti | Ultrasonic Shot Peening | 57–88 (nm), XRD | Bone implants | MG63 cells/new Zealand White rabbits |
| [89] |
| CP Ti grade 2 | Equal-Channel Angular Pressing | - | Implants | Murine fibroblast cells 3T3/Wistar rats |
| [95] |
| Ti-Nb-Mo-Zr | Cold Rolling | - | Orthopedic implants | - |
| [105] |
| Ti–15Zr | Cold Rolling | 2–5 (µm), SEM | Dental implants | - |
| [106] |
| Ti-32.5Nb-6.8Zr-2.7Sn | Cold Rolling | 200–250 (nm), OM | Bone implants | - |
| [107] |
| Materials/Alloys | SPD Process | Size of Grains/Instrument | Applications | In Vivo/In Vitro | Significant Findings | Ref. |
|---|---|---|---|---|---|---|
| AISI 304 austenitic SS | Severe shot peening (SSP) | Under 300 (nm), FESEM | Implants | MC3T3-E1 pre-osteoblast |
| [3] |
| 316L SS | Severe shot peening | 25 (nm), XRD | Orthopedic implants | Osteoblasts |
| [114] |
| 316L SS | Ultrasonic Shot Peening | 10 (nm), FIB channeling contrast imaging technique | Orthopedic implants | Human osteoblast cells (SaoS-2) |
| [113] |
| 316L SS Sheet | Ultrasonic shot peening | Less than 50 (nm), SEM | Orthopedic implants | MC3T3-E1 subclone 4 |
| [115] |
| 316L SS | Ultrasonic shot peening | 326 (nm), SEM | Orthopedic implants | Human osteoblast cells |
| [116] |
| 316L SS | Equal channel angular pressing | 78 (nm), SEM | Implant | Fibroblast cells |
| [108] |
| 316L SS | Severe shot peening | 100–200 (nm), SEM | Bone implants | Human osteoblasts |
| [117] |
| Materials/Alloys | SPD Process | Size of Grains/Instrument | Applications | In Vivo/In Vitro | Significant Findings | Ref. |
|---|---|---|---|---|---|---|
| WE43 (Mg-Y-Nd-Zr) | ECAP | 0.73 (µm), TEM | Implants | Red blood cells and white blood cells |
| [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalantari, K.; Saleh, B.; Webster, T.J. Biological Applications of Severely Plastically Deformed Nano-Grained Medical Devices: A Review. Nanomaterials 2021, 11, 748. https://doi.org/10.3390/nano11030748
Kalantari K, Saleh B, Webster TJ. Biological Applications of Severely Plastically Deformed Nano-Grained Medical Devices: A Review. Nanomaterials. 2021; 11(3):748. https://doi.org/10.3390/nano11030748
Chicago/Turabian StyleKalantari, Katayoon, Bahram Saleh, and Thomas J. Webster. 2021. "Biological Applications of Severely Plastically Deformed Nano-Grained Medical Devices: A Review" Nanomaterials 11, no. 3: 748. https://doi.org/10.3390/nano11030748
APA StyleKalantari, K., Saleh, B., & Webster, T. J. (2021). Biological Applications of Severely Plastically Deformed Nano-Grained Medical Devices: A Review. Nanomaterials, 11(3), 748. https://doi.org/10.3390/nano11030748