Abstract
Background: The Er:YAG laser has gained attention in dentistry for its potential to enhance microbial disinfection through targeted photothermal and photoacoustic mechanisms. Objective: This systematic review aimed to evaluate the antibacterial and bactericidal efficacy of Er:YAG laser therapy across clinically relevant oral pathogens in in vitro models. Methods: Following the PRISMA 2020 guidelines, a systematic search of PubMed, Embase, Scopus, and the Cochrane Library was conducted for studies published between 2015 and 2025. The review protocol was registered with PROSPERO (CRD420251031368). Eligibility criteria included in vitro or animal studies assessing the bactericidal effects of the Er:YAG laser on oral bacteria or fungi, either alone or in combination with chemical disinfectants. Study selection, data extraction, and quality assessment were conducted independently by multiple reviewers. Results: Ten in vitro studies met inclusion criteria. The Er:YAG laser demonstrated significant antibacterial effects against Enterococcus faecalis, Streptococcus mutans, Porphyromonas gingivalis, Candida albicans, and other species. Greater bacterial reduction was consistently observed when the laser was combined with adjunctive irrigants such as sodium hypochlorite or hydrogen peroxide. The laser was effective in reducing biofilm biomass and viable counts, particularly in complex anatomical settings. Most studies were rated as low risk of bias. Conclusions: Er:YAG laser therapy is a promising adjunctive tool for microbial disinfection in dentistry, particularly in challenging anatomical sites. Further well-designed in vivo and clinical studies are needed to confirm its efficacy and determine optimal treatment parameters.
1. Introduction
Lasers have become increasingly prominent in modern dental practice, where innovative technologies are continually sought to enhance both clinical outcomes and patient comfort [,,,,,]. Among these technologies, the Erbium-doped Yttrium–Aluminum–Garnet (Er:YAG) laser has drawn attention due to its unique photothermal properties, which enable simultaneous ablation of dental hard tissues and microbial reduction [,]. Unlike conventional approaches that rely heavily on chemical irrigants or mechanical instrumentation for disinfection, the Er:YAG laser delivers energy precisely to targeted sites, potentially improving penetration into complex anatomical areas such as dentinal tubules or periodontal pockets [,]. This targeted energy release may offer improved bactericidal effects, thereby reducing the likelihood of persistent or recurrent infections [,,,,].
Despite the proven efficacy of traditional non-laser alternatives, such as sodium hypochlorite, chlorhexidine, or hydrogen peroxide, their limitations have become more evident in recent years [,,,,]. Chemical irrigants, for instance, can pose significant drawbacks including unpleasant taste, cytotoxicity to oral tissues if overextended, and potential allergies or sensitivities in some patients []. Furthermore, the stability and activity of these chemicals can be adversely affected by organic matter or certain pH conditions, ultimately undermining their antimicrobial performance []. However, their limitations have become more apparent in recent years [,]. These include unpleasant taste, cytotoxicity when extruded beyond the apex, and potential allergic reactions or sensitivities in susceptible individuals []. Moreover, their antimicrobial effectiveness may be compromised by interactions with organic matter, suboptimal pH conditions, or dentin buffering, which can deactivate active components []. Mechanical instrumentation, although indispensable, also has inherent limits; endodontic files and manual scraping do not always reach the intricate branching of root canal systems or the more inaccessible areas of subgingival biofilms [,,]. This leaves behind microbial reservoirs that can perpetuate infection and compromise long-term treatment success [,,,,,]. In response to these concerns, the Er:YAG laser has been proposed as an adjunct or alternative that might circumvent some of these shortcomings [,,,]. Its ability to create micro-explosions of steam within hard and soft tissues not only disrupts resistant biofilms but also preserves healthy tissue integrity []. In response, various laser systems have been introduced to augment decontamination efforts. Diode lasers (810–980 nm) are commonly used for soft tissue decontamination and are effective against pigmented bacteria due to their absorption by melanin and hemoglobin, though they lack ablative capability on hard tissues []. Nd:YAG lasers (1064 nm) offer deeper penetration into soft tissues and have demonstrated bactericidal effects, particularly in periodontal applications []. CO2 lasers (10,600 nm) provide strong soft tissue ablation and hemostasis but are less commonly used for deep bacterial elimination due to their superficial absorption profile []. Er:YAG lasers, operating at 2940 nm, exhibit high absorption in water and hydroxyapatite, making them uniquely suited for both hard and soft tissue applications, including smear layer removal and biofilm disruption [].
The Er:YAG laser eliminates bacteria through a combination of photothermal and photomechanical effects []. Its 2940 nm wavelength is highly absorbed by water and hydroxyapatite, allowing for efficient energy transfer to both the irrigant and dentin []. This interaction generates cavitation bubbles and shock waves within the irrigant, a phenomenon known as photoacoustic streaming, which enhances the fluid’s penetration into complex canal anatomies and dentinal tubules []. Additionally, the laser’s ability to ablate the smear layer exposes dentinal tubule openings, facilitating deeper disinfectant infiltration []. The resulting high-energy microenvironment disrupts bacterial biofilms, damages cell walls, and improves the bactericidal effectiveness of irrigants like sodium hypochlorite []. Collectively, these effects contribute to the Er:YAG laser’s potent antimicrobial action, especially in areas inaccessible to traditional instruments.
However, questions remain about the generalizability of these observed antimicrobial effects, given the variety of laser parameters, bacterial strains, and study designs reported in the literature [,,,,]. Therefore, a systematic evaluation of these studies is vital to clarify the precise bactericidal capabilities of Er:YAG lasers and to determine how factors such as energy output, wavelength, exposure time, and adjunctive chemical agents might optimize clinical outcomes.
This review is novel in its exclusive focus on in vitro microbiological outcomes related to Er:YAG laser use in dentistry. This review aims to synthesize the current evidence on the antibacterial efficacy of Er:YAG lasers in dentistry. By collating and critically evaluating findings from diverse experimental protocols, we seek to identify both the strengths of Er:YAG therapy and the gaps in our understanding. Ultimately, the insights gained here will help inform clinical decisions and guide future research into refining laser-based approaches for effective infection control. While previous reviews have addressed general clinical applications of lasers in dentistry, our review is distinct in its exclusive focus on microbiological outcomes derived from in vitro studies using the Er:YAG laser. We systematically evaluate its antibacterial and bactericidal performance across diverse pathogens, surface types, and anatomical models, with a dedicated quality assessment framework adapted to laser-based microbiological research. This approach provides a more mechanistic and pathogen-specific synthesis of the evidence, which has been lacking in the prior literature.
2. Materials and Methods
2.1. Focused Question
A systematic review was conducted using the PICO framework [], structured as follows: In patients with microbial infections (Population), does treatment with the Er:YAG laser (Intervention), compared to conventional antimicrobial therapies, alternative laser modalities, or no laser treatment (Comparison), lead to superior bacterial eradication or reduction in microbial load (Outcome)?
2.2. Search Strategy
This systematic review, registered with PROSPERO [] (ID: CRD420251031368), was designed and executed in accordance with the PRISMA 2020 guidelines for transparent and structured reporting of systematic reviews []. A thorough and methodical literature search was conducted across major electronic databases: PubMed/Medline, Embase, Scopus, and the Cochrane Library, to identify relevant studies evaluating the antibacterial and bactericidal effects of the Er:YAG laser. The full search protocol is illustrated in Figure 1. Three independent reviewers carried out database queries using a predefined set of search terms tailored to microbiological outcomes associated with Er:YAG laser application. Language filters were applied to limit inclusion to studies published in English between 1 January 2015 and 3 March 2025. The selection process began with title and abstract screening to assess potential eligibility based on established inclusion criteria (outlined in Table 1), followed by an in-depth full-text review independently conducted by two authors. To enhance the comprehensiveness of the review, a snowballing strategy was also implemented wherein the reference lists of included articles were examined for additional relevant studies. The overarching aim of this review was to synthesize microbiological evidence regarding the efficacy of Er:YAG laser treatment in reducing or eradicating bacterial pathogens, whether as a monotherapy or as an adjunct to conventional antimicrobial approaches. All final inclusions were determined based on rigorous adherence to predefined eligibility parameters.
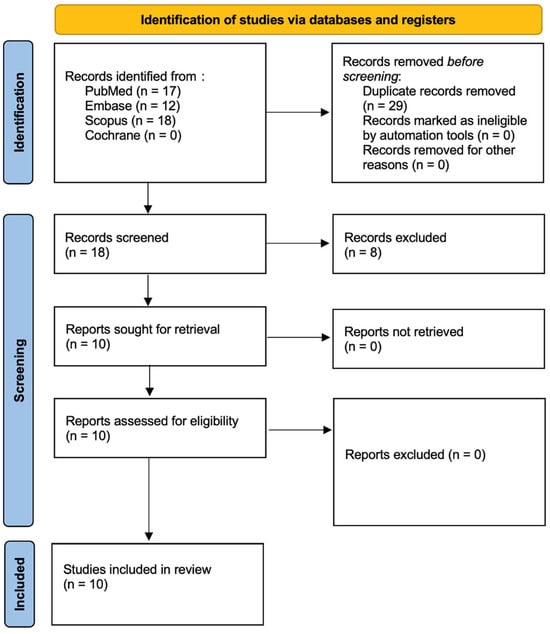
Figure 1.
PRISMA 2020 flow diagram.

Table 1.
Search syntax used in the study.
2.3. Study Selection Process
To maintain methodological robustness and minimize potential bias, all identified records were subjected to a rigorous, independent screening process conducted by multiple reviewers. Titles and abstracts were systematically assessed for alignment with the predefined inclusion criteria. In cases of disagreement, reviewers engaged in consensus discussions to resolve discrepancies and ensure consistent decision-making. The selection criteria are as follows:
2.3.1. Inclusion Criteria
- Experimental studies investigating the antimicrobial or bactericidal effects of the Er:YAG laser, conducted either in vitro or in animal models.
- Studies assessing microbial susceptibility to Er:YAG laser treatment were included only if they involved clinically significant oral pathogens (e.g., Gram-positive or Gram-negative bacteria and fungi such as E. faecalis, S. mutans, P. gingivalis, C. albicans) relevant to dental infections.
- Investigations exploring potential synergistic effects when Er:YAG laser therapy is combined with conventional antimicrobial agents.
- Studies employing controlled experimental designs, including comparisons with untreated groups, placebo interventions, or other antimicrobial technologies.
- Studies that clearly state the bacteria the Er:YAG laser was tested on.
- Research directly comparing the efficacy of Er:YAG laser treatment with that of standard antimicrobial therapies in terms of microbial load reduction or eradication.
- Studies incorporating follow-up assessments to evaluate the durability of antimicrobial effects and any recurrence of microbial growth post-treatment.
2.3.2. Exclusion Criteria
- Non-scholarly publications, including conference abstracts, case reports, editorials, opinion articles, book chapters, and unpublished theses.
- Studies not published in peer-reviewed journals or lacking sufficient scientific rigor.
- Articles written in languages other than English.
- Redundant publications, such as duplicate reports or multiple articles derived from the same study population without presenting new or distinct data.
- Research unrelated to the treatment of infectious diseases or focused on non-infectious conditions.
- Studies that do not include a comparison or control group to contextualize antimicrobial outcomes.
- Investigations in which the Er:YAG laser is not used as an antimicrobial therapeutic modality.
- Studies using other laser types or technologies without direct evaluation of Er:YAG laser efficacy.
- Research addressing irrelevant pathogens or general microbiological studies without specific outcomes related to bacterial or fungal eradication.
- In vitro studies conducted under highly artificial conditions that limit translational or clinical relevance.
This meticulous, multi-step evaluation, aligned with the PRISMA 2020 guidelines, was designed to uphold transparency and reproducibility []. Only studies demonstrating clear relevance and methodological soundness were selected for inclusion. This stringent selection framework was implemented to produce a credible and evidence-based synthesis of current microbiological data on the antibacterial and bactericidal performance of the Er:YAG laser in the context of infection management.
2.4. Risk of Bias in Individual Studies
To ensure objectivity and reduce the risk of selection bias during the screening phase, all titles and abstracts identified through the search strategy were independently assessed by multiple reviewers. The level of inter-reviewer agreement was quantified using Cohen’s kappa statistic (κ = 0.82), providing a standardized measure of consistency in decision-making []. Discrepancies regarding study inclusion were resolved through structured discussions until a unanimous consensus was achieved. This systematic and collaborative method was employed to uphold the methodological rigor of the review and maintain the integrity of the study selection process.
2.5. Quality Assessment
The methodological quality of the included studies was independently assessed by three reviewers, focusing on key elements relevant to the design, execution, and reporting of Er:YAG laser interventions. A structured scoring system was applied to evaluate risk of bias, with each study receiving 1 point for meeting a criterion (“yes”) and 0 points for not meeting it (“no”), based on nine predefined items:
- (1)
- Clear reporting of Er:YAG laser operating parameters (e.g., energy settings, frequency, pulse duration);
- (2)
- Identification of the laser device or manufacturer;
- (3)
- Detailed description of the irradiation protocol, including exposure time and treatment area;
- (4)
- Provision of full technical specifications such as wavelength, spot size, energy fluence, and repetition rate;
- (5)
- Use of dosimetric validation tools such as a power meter;
- (6)
- Inclusion of an appropriate control group (e.g., untreated, placebo, or comparative intervention);
- (7)
- Use of valid statistical analysis for microbiological outcomes;
- (8)
- Transparency in outcome reporting, with no selective or missing data;
- (9)
- Absence of conflicts of interest or undue influence from funding sources.
Each study was assigned a total score out of nine, with risk of bias categorized as high (0–3 points), moderate (4–6 points), or low (7–9 points). Final quality judgments were made in alignment with the guidance provided by the Cochrane Handbook for Systematic Reviews of Interventions []. The results of this assessment are presented in Table 2.

Table 2.
The results of the quality assessment and risk of bias across the studies.
2.6. Data Extraction
Following consensus on the final selection of studies for inclusion, two reviewers independently performed data extraction using a standardized protocol to ensure consistency and accuracy. Extracted data included bibliographic information (first author, year of publication), study design, microbial species or strains investigated, composition of experimental and control groups, duration of follow-up (if applicable), primary and secondary outcome measures, detailed technical specifications of the Er:YAG laser (e.g., energy settings, pulse duration, wavelength), any adjunctive treatments used, and relevant procedural parameters such as treatment duration and exposure conditions.
3. Results
3.1. Study Selection
In accordance with the PRISMA 2020 guidelines [], the study selection process is illustrated in Figure 1. The initial database search yielded 47 records, which were narrowed down to 10 unique studies after duplicate removal. Title and abstract screening confirmed the relevance of these articles, all of which proceeded to full-text review. No studies were excluded at this stage, resulting in a final inclusion of 10 publications spanning the last decade. These studies were deemed suitable for synthesis based on their investigation of the antibacterial and bactericidal efficacy of the Er:YAG laser. A detailed overview of each study’s design, methodology, and outcomes is presented in Table 3. The search identified only in vitro studies. More in vivo studies are needed.

Table 3.
A general overview of the studies.
3.2. Data Presentation
A comprehensive summary of the findings from the 10 included studies is provided in Table 4, Table 5 and Table 6, offering a structured and accessible presentation of key outcomes, methodological features, and microbiological evidence related to the antibacterial and bactericidal effects of the Er:YAG laser.

Table 4.
A summary of the molecular aspects of the Er:YAG laser.

Table 5.
Main outcomes and details from each study.

Table 6.
Characteristics of lights sources used.
3.3. Overview of Study Characteristics
Table 4 presents the fundamental characteristics of the studies included in this review, emphasizing variations in experimental design, targeted microbial species, Er:YAG laser treatment parameters, and the criteria used to assess antibacterial and bactericidal outcomes.
3.4. Characteristics of Light Sources Used in PDT
Table 6 presents the key physical properties of the light sources utilized in the studies meeting the inclusion criteria.
4. Discussion
4.1. Results in the Context of Other Evidence
The Er:YAG laser consistently demonstrates significant antibacterial efficacy against a broad range of oral pathogens, including E. faecalis, S. mutans, P. gingivalis, and C. albicans [,,,,,,,,,]. When combined with chemical irrigants or adjunctive agents, it often produces synergistic or additive bactericidal effects, outperforming either modality used alone [,,]. Optimal laser parameters such as energy output, frequency, and pulse duration—are crucial for maximizing bacterial reduction while preserving healthy tissue [,,]. The Er:YAG laser efficiently removes biofilms from titanium surfaces with minimal or no damage, thus maintaining implant structural integrity [,,]. Photoacoustic streaming with the Er:YAG laser enhances disinfection in complex anatomies, including root canals and peri-implant spaces [,,]. Its antibacterial efficacy can be comparable or superior to conventional approaches like ultrasonic irrigation, sodium hypochlorite, or chlorhexidine [,,]. Low-concentration antiseptics combined with the Er:YAG laser have been shown to reduce microbial loads below detectable limits in some protocols []. The laser proves effective against both planktonic cells and mature biofilms, highlighting its capacity to eliminate resilient microbial communities [,]. Er:YAG treatment can also improve or maintain surface wettability and topography, which is beneficial for subsequent clinical procedures [,]. Overall, the Er:YAG laser emerges as a versatile, minimally invasive option that can complement or substitute traditional disinfection methods, though further standardized and clinically oriented studies are required to validate these findings [,,,,].
The results of this systematic review corroborate a considerable body of prior investigations, all emphasizing the pronounced bactericidal potency of Er:YAG lasers across endodontic and periodontal applications []. Numerous studies offer compelling evidence for this laser’s ability to significantly diminish microbial populations in various clinical contexts, often outperforming alternative modalities or traditional approaches. Sebbane et al. demonstrated that Er:YAG laser irradiation, delivered via a novel side-firing spiral Endo tip, led to a remarkable reduction in Enterococcus faecalis biofilm within root canals. The most robust antibacterial impact occurred when 17% EDTA was employed, followed by a final rinse with 2.5% NaOCl []. Moritz et al. similarly found that Er:YAG, Nd:YAG, and Ho:YAG lasers significantly decreased bacterial counts in infected root canals; notably, the Er:YAG laser achieved the highest average bacterial eradication rate, registering at 99.64% []. Further supporting these findings, Bao et al. reported that Er:YAG laser-activated irrigation methods, including PIPS and SWEEPS, were superior to conventional needle irrigation, passive ultrasonic irrigation, and sonic-powered irrigation for removing multispecies biofilms from both apical artificial grooves and dentinal tubules []. Yang et al. observed that supplementing Er:YAG laser treatment with photodynamic therapy substantially enhanced its bactericidal effect against E. faecalis, reaching disinfection outcomes comparable to the Er:YAG laser combined with NaOCl []. Meanwhile, Ando et al. revealed that the Er:YAG laser exerted a potent bactericidal effect on Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans under in vitro conditions, with significant bacterial reduction seen at relatively low energy levels, starting from 0.3 J/cm2 [,,,,]. Kranendonk et al. further reinforced the laser’s efficacy by showing that Nd:YAG irradiation destroyed all viable cells of six periodontal pathogens within a 15 s exposure, indicating complete bactericidal action in vitro []. In addition to these findings, several reports have verified that combining Er:YAG laser irradiation with adjunctive chemical irrigants, such as sodium hypochlorite or hydrogen peroxide, can yield synergistic antibacterial effects—consistent with the present review’s observation that multimodal disinfection regimens frequently surpass monotherapies []. This heightened effectiveness appears closely tied to the laser’s photothermal properties, which compromise microbial cell walls and simultaneously promote deeper irrigant penetration into anatomically intricate areas [].
Investigations into other laser systems, such as Er-, Cr:YSGG and diode lasers, suggest that while these alternatives can achieve clinically meaningful disinfection, Er:YAG lasers frequently deliver superior biofilm disruption and penetration depth, especially in complex clinical scenarios like narrow root canals or around implant surfaces []. Such advantages resonate with our findings that the Er:YAG laser can substantially lower microbial loads across a spectrum of pathogens—including E. faecalis, S. mutans, and C. albicans, without causing deleterious effects on tooth or implant structures []. Concurrent research on photoacoustic streaming reveals that specific the Er:YAG laser parameter selections can further optimize fluid dynamics, facilitating the removal of biofilms from difficult-to-access regions. These discoveries underscore the critical need to fine-tune energy settings and pulse durations for maximum clinical benefit []. Nevertheless, certain inconsistencies in outcomes across different studies may stem from methodological variations, including laser parameters, treatment duration, and the bacterial or fungal strains targeted []. Looking ahead, comprehensive in vivo investigations—particularly multicenter randomized controlled trials—are essential for standardizing Er:YAG laser protocols and confirming their meaningful influence on sustained treatment success []. As the literature on Er:YAG use continues to expand, it is plausible that clinicians will increasingly incorporate this technology into conventional therapeutic strategies, capitalizing on its robust antibacterial effects, efficient disruption of biofilms, and preservation of healthy tissue [,,]. By integrating these evidence-based insights, the dental community can refine clinical protocols and ultimately enhance patient outcomes in both endodontic and periodontal care.
Laser-activated antibacterial nanoparticles (NPs) have recently emerged as a promising adjunctive strategy for oral bacterial eradication. These nanoparticles—often composed of gold, silver, zinc oxide, titanium dioxide, or graphene-based composites—can be functionalized with photosensitizers or antimicrobial agents and activated by specific laser wavelengths to exert targeted antimicrobial effects [,,]. Upon laser irradiation, these NPs can generate localized heat (photothermal effect), reactive oxygen species (photodynamic effect), or even release embedded bactericidal agents, resulting in enhanced biofilm disruption and microbial death [,,]. This approach offers multiple advantages: improved specificity, deeper penetration into infected tissues, and reduced collateral damage to host cells compared to systemic antimicrobials []. In the context of dental infections, laser-activated NPs have shown efficacy against key pathogens such as Streptococcus mutans, Porphyromonas gingivalis, and Enterococcus faecalis, both in planktonic and biofilm states [,,]. Notably, gold and graphene oxide nanoparticles combined with diode or Er:YAG lasers have demonstrated synergistic antibacterial activity, particularly through enhanced photothermal destruction and improved permeability of bacterial membranes [,,,]. Despite their potential, clinical translation of this technique is still limited due to concerns about long-term biocompatibility, toxicity, and regulatory approval pathways [,]. Further studies are needed to optimize NP formulations, laser parameters, and delivery systems to ensure safety and reproducibility in vivo.
4.2. Limitations of the Evidence
The current body of research is hindered by substantial variability in both study designs and methodologies, making it difficult to derive clear, universally applicable conclusions. Much of the work has been conducted using in vitro models that do not fully capture the complexity of human biology or real-world conditions, thereby limiting the practical relevance of the outcomes. Furthermore, the lack of standardized protocols—particularly concerning characterization of particles, exposure scenarios, and assessment parameters, leads to inconsistent results that are often impossible to compare directly. Short-term experimental setups further constrain understanding of any long-term impacts, as they offer limited insight into potential chronic or cumulative effects. In addition, restricting the review to English-language publications and omitting the gray literature may introduce a selection bias that excludes potentially significant data. Collectively, these factors emphasize an urgent need for broader, more rigorous, and standardized investigations that encompass long-term observations and more accurately reflect real-life exposures.
4.3. Limitations of the Review Process
Despite yielding informative insights, this systematic review faces several inherent limitations. First, the predominance of in vitro experimental designs restricts the direct clinical applicability of the findings, as laboratory conditions do not fully capture the complexities of in vivo environments. While this does not limit the validity of our conclusion, more experimental in vivo evidence must be collected. Second, the studies included exhibit notable heterogeneity in laser parameters, treatment durations, and microbial species, complicating direct comparisons and meta-analyses. Third, the exclusive focus on English-language publications and peer-reviewed articles raises the possibility of publication bias, thereby potentially excluding relevant data reported in other languages or in the gray literature. Additionally, many studies have relatively short follow-up periods, limiting insights into longer-term outcomes and potential microbial recolonization. These methodological and reporting variations underscore the need for more standardized protocols, multicenter trials, and extended observation periods to better validate and generalize the antibacterial efficacy of Er:YAG laser treatment.
4.4. Implications for Practice, Policy, and Future Research
Er:YAG laser therapy shows considerable promise for enhancing disinfection in dental procedures, offering a minimally invasive yet highly targeted approach to bacterial and biofilm reduction. Clinically, these findings support the integration of Er:YAG lasers as either a standalone or adjunctive method, particularly in challenging areas such as root canal systems, subgingival regions, and peri-implant spaces. However, one practical concern is the higher upfront cost of laser devices compared to conventional chemical irrigants. This may initially increase the cost of treatment, particularly in practices without existing laser infrastructure. Nevertheless, the long-term sustainability of Er:YAG laser therapy lies in its potential to reduce treatment failures, minimize reinfection, and decrease the need for retreatment, outcomes that could offset the initial investment. Moreover, laser treatment often results in less procedural discomfort and faster healing, potentially enhancing patient satisfaction and reducing chair time. From a sustainability standpoint, the Er:YAG laser offers an eco-friendlier alternative by reducing the volume and disposal burden of chemical irrigants such as sodium hypochlorite and chlorhexidine, which carry cytotoxic and environmental risks. While we do not propose completely replacing chemical agents, laser technology may reduce our dependence on them and mitigate their drawbacks when used in conjunction with low-concentration adjuncts. From a policy perspective, adopting standardized protocols and evidence-based guidelines for Er:YAG laser use could streamline training, ensure consistent treatment outcomes, and foster greater acceptance among practitioners. Additionally, policymakers and professional organizations may consider promoting reimbursement models or financial incentives to encourage broader adoption, given the laser’s potential to improve long-term treatment success and minimize reliance on chemical irrigants. Future research must emphasize well-designed, multicenter clinical trials to confirm and extend current in vitro findings, clarify optimal energy settings, and determine the most effective adjunctive irrigants or antimicrobial agents. Investigations into patient-centered outcomes, cost-effectiveness, and long-term stability of results would further elucidate Er:YAG laser therapy’s role in mainstream dental practice. Rigorous studies examining its impact on microbial resistance, as well as the compatibility of various laser parameters with innovative materials and restoration techniques, will also be crucial in refining this technology for broader, more impactful clinical implementation. Specific unresolved questions that future clinical trials should address include: what are the optimal Er:YAG laser parameters (e.g., energy output, pulse duration, frequency) that maximize bacterial eradication while preserving tissue integrity; how does Er:YAG laser disinfection compare with standard chemical irrigants (e.g., sodium hypochlorite, chlorhexidine) in terms of clinical efficacy, safety, and long-term outcomes; can Er:YAG laser treatment reduce the incidence of reinfection or microbial recolonization in root canal or peri-implant therapy; what is the clinical effectiveness of Er:YAG lasers in challenging anatomical sites, such as curved root canals or peri-implant defects; what are the patient-centered outcomes (e.g., post-operative discomfort, healing time, satisfaction) associated with Er:YAG laser use compared to conventional treatments; and are there cost-effectiveness or workflow benefits that support broader clinical adoption of laser-based disinfection.
5. Conclusions
This review confirms the in vitro antibacterial efficacy of the Er:YAG laser against key oral pathogens, including E. faecalis, S. mutans, P. gingivalis, and C. albicans, particularly in complex anatomical sites such as root canals and peri-implant regions. The laser’s ability to disrupt biofilms while preserving the integrity of dentin, enamel, and titanium supports its value in minimally invasive decontamination. To enable clinical adoption, future research should focus on in vivo trials, parameter standardization, and comparative studies with conventional methods. Investigating synergies with irrigants or photodynamic agents, as well as patient-centered outcomes, will be essential to guide evidence-based use. Looking ahead, the Er:YAG laser holds promise for broader use in conservative endodontics, peri-implant care, and periodontal therapy, especially when integrated with emerging techniques like nanoparticle-assisted disinfection. While diode lasers may offer a cost-effective alternative for soft tissue decontamination, their lower wavelength and lack of hard tissue interaction limit their use in root canal or mineralized tissue procedures. Diodes are unlikely to replace Er:YAG lasers but may serve as complementary tools, selected based on target tissue and treatment goals. In conclusion, the Er:YAG laser represents a versatile and effective antibacterial tool with expanding potential in dental practice. Continued high-quality research is needed to define its optimal clinical role and integrate it safely into routine protocols.
Author Contributions
Conceptualization, J.F.-R. and R.W.; methodology, J.F.-R., D.S. and R.W.; software, J.F.-R.; formal analysis, J.F.-R., A.K.-K., D.S. and R.W.; investigation, J.F.-R., D.S. and R.W.; writing—original draft preparation, J.F.-R., D.S. and R.W.; writing—review and editing, J.F.-R., A.K.-K., D.S. and and R.W.; supervision, D.S. and R.W.; funding acquisition, D.S., R.W. and A.K.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sachelarie, L.; Cristea, R.; Burlui, E.; Hurjui, L.L. Laser Technology in Dentistry: From Clinical Applications to Future Innovations. Dent. J. 2024, 12, 420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liaqat, S.; Qayyum, H.; Rafaqat, Z.; Qadir, A.; Fayyaz, S.; Khan, A.; Jabeen, H.; Muhammad, N.; Khan, M.A. Laser as an innovative tool, its implications and advances in dentistry: A systematic review. J. Photochem. Photobiol. 2022, 12, 100148. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Trilli, I.; Piras, F.; Ciocia, A.M.; Inchingolo, A.D.; Mancini, A.; Hazballa, D.; Di Venere, D.; Inchingolo, F.; et al. Therapeutic and adverse effects of lasers in dentistry: A systematic review. Photonics 2023, 10, 650. [Google Scholar] [CrossRef]
- Ting, M.; Alluri, L.S.C.; Sulewski, J.G.; Suzuki, J.B.; da Silva, A.P.B. Laser treatment of peri-implantitis: A systematic review of radiographic outcomes. Dent. J. 2022, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.M.; Halawani, M.; Alarawi, H.A.; Alzaidi, A.M.; Alhogail, L.H.; Alsobhi, S.W.O.; Alamrai, A.H.M.; Alrashedi, J.A.M.; AlRashidi, A.N.B.; Al-Ghamdi, T.A.A. The Use of Laser Technology in Dentistry: A Comprehensive Review. Migr. Lett. 2022, 19, 577–586. [Google Scholar]
- Everett, J.D.; Rossmann, J.A.; Kerns, D.G.; Al-Hashimi, I. Laser-Assisted Non-Surgical Periodontal Therapy: A Double-Blind, Randomized Clinical Trial. Open Dent. J. 2017, 11, 79–90. [Google Scholar] [CrossRef]
- Almoharib, H. Erbium-Doped Yttrium Aluminium Garnet (Er:YAG) Lasers in the Treatment of Peri-Implantitis. Cureus 2025, 17, e78279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laky, M.; Toth, P.; Laky, B.; Vaskovich, T.; Kurzmann, C.; Arslan, M.; Nguyen, M.; Rausch-Fan, X.; Moritz, A.; Shokoohi-Tabrizi, H.A. Optimized Erbium-Doped Yttrium Aluminum Garnet (Er:YAG) Laser Parameters for the Removal of Dental Ceramic Restorations. Materials 2023, 16, 5835. [Google Scholar] [CrossRef]
- Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Tkaczyk, M.; Grzech-Leśniak, K.; Zawilska, A.; Wiench, R. Enhancing Root Canal Disinfection with Er:YAG Laser: A Systematic Review. Dent. J. 2025, 13, 101. [Google Scholar] [CrossRef]
- Deeb, J.G.; Grzech-Leśniak, K.; Weaver, C.; Matys, J.; Bencharit, S. Retrieval of Glass Fiber Post Using Er:YAG Laser and Conventional Endodontic Ultrasonic Method: An In Vitro Study. J. Prosthodont. 2019, 28, 1024–1028. [Google Scholar] [CrossRef]
- Preissig, J.; Hamilton, K.; Markus, R. Current Laser Resurfacing Technologies: A Review that Delves Beneath the Surface. Semin. Plast. Surg. 2012, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Walia, V.; Goswami, M.; Mishra, S.; Walia, N.; Sahay, D. Comparative Evaluation of the Efficacy of Chlorhexidine, Sodium Hypochlorite, the Diode Laser and Saline in Reducing the Microbial Count in Primary Teeth Root Canals—An In Vivo Study. J. Lasers Med. Sci. 2019, 10, 268–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddy, N.; Deeb, J.G.; Kitten, T.; Carrico, C.K.; Grzech-Leśniak, K. The In Vitro Effect of Laser Irradiation (Er:YAG and CO2) and Chemical Reagents (Hydrogen Peroxide, Sodium Hypochlorite, Chlorhexidine, or Sodium Fluoride) Alone or in Combination on Reducing Root Caries Bacteria. Int. J. Mol. Sci. 2022, 23, 15732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zheng, X.; Liang, Y.; Zhang, C.; Fan, B.; Liang, J.; Ling, J.; Bian, Z.; Yu, Q.; Hou, B.; et al. Expert consensus on irrigation and intracanal medication in root canal therapy. Int. J. Oral Sci. 2024, 16, 23. [Google Scholar] [CrossRef]
- Rotundo, R.; Nieri, M.; Cairo, F.; Franceschi, D.; Mervelt, J.; Bonaccini, D.; Esposito, M.; Pini-Prato, G. Lack of adjunctive benefit of Er:YAG laser in non-surgical periodontal treatment: A randomized split-mouth clinical trial. J. Clin. Periodontol. 2010, 37, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Bajrami, D.; Hoxha, V.; Gorduysus, O.; Muftuoglu, S.; Zeybek, N.D.; Küçükkaya, S. Cytotoxic effect of endodontic irrigants in vitro. Med. Sci. Monit. Basic Res. 2014, 20, 22–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arias, A.; Peters, O.A. Present status and future directions: Canal shaping. Int. Endod. J. 2022, 55 (Suppl. S3), 637–655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ginjeira, A.; Baruwa, A.O.; Baumotte, K. Evaluation and Comparison of Manual and Mechanical Endodontic Instrumentation Completed by Undergraduate Dental Students on Endodontic Blocks. Dent. J. 2024, 12, 363. [Google Scholar] [CrossRef]
- Dembicka-Mączka, D.; Kępa, M.; Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Matys, J.; Grzech-Leśniak, K.; Wiench, R. Evaluation of the Disinfection Efficacy of Er: YAG Laser Light on Single-Species Candida Biofilms—An In Vitro Study. Dent. J. 2025, 13, 88. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Shi, L.; Zhang, F.; Zheng, S. Comparison of clinical parameters, microbiological effects and calprotectin counts in gingival crevicular fluid between Er: YAG laser and conventional periodontal therapies: A split-mouth, single-blinded, randomized controlled trial. Medicine 2017, 96, e9367. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Kapłon, K.; Kotucha, K.; Moś, M.; Skaba, D.; Kawczyk-Krupka, A.; Wiench, R. Hypocrellin-Mediated PDT: A Systematic Review of Its Efficacy, Applications, and Outcomes. Int. J. Mol. Sci. 2025, 26, 4038. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Do, Q.L.; Gaudin, A. The Efficiency of the Er: YAG Laser and PhotonInduced Photoacoustic Streaming (PIPS) as an Activation Method in Endodontic Irrigation: A Literature Review. J. Lasers Med. Sci. 2020, 11, 316–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Li, Y.; Meng, Q.; Meng, J.; Mei, M.L. Effect of Er:YAG laser irrigation with different etching modes on the push-out bond strength of fiber posts to the root dentine. Lasers Med. Sci. 2022, 37, 2687–2696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbero-Navarro, I.; Sofian-Pauliuc, I.; Irigoyen-Camacho, M.E.; Zepeda-Zepeda, M.A.; Ribas-Perez, D.; Castaño-Seiquer, A.L. Evaluating the Preventive and Therapeutic Roles of Active Irrigation Systems in Root Canal Treatment: A Narrative Review and Critical Appraisal of Theory and Methodology. Dent. J. 2024, 13, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, A.; Huang, L.; Li, B.; Huang, Y.; Zhou, X.; Zhang, X.; Gong, Q. Micro-CT Evaluation of Different Root Canal Irrigation Protocols on the Removal of Accumulated Hard Tissue Debris: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6053. [Google Scholar] [CrossRef]
- Ballal, N.V.; Gandhi, P.; Shenoy, P.A.; Dummer, P.M.H. Evaluation of various irrigation activation systems to eliminate bacteria from the root canal system: A randomized controlled single blinded trial. J. Dent. 2020, 99, 103412. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; Shamseer, L.; Tricco, A.C. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst. Rev. 2018, 7, 32. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M. Welch Cochrane Handbook for Systematic Reviews of Interventions, Version 6.4; Cochrane: London, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 20 October 2024).
- Shan, X.; Tian, F.; Li, J.; Yang, N.; Wang, Y.; Sun, H. Comparison of Er:YAG laser and ultrasonic in root canal disinfection under minimally invasive access cavity. Lasers Med. Sci. 2022, 37, 3249–3258. [Google Scholar] [CrossRef]
- Alzahrani, K.M.; Alrabiah, M.; AlAali, K.A.; Vohra, F.; Abduljabbar, T. Fracture strength of Er,Yag laser treated PMMA denture-based polymer (DBP) colonized with, C. albicans, S. aureus, S. mutans, and, E. coli. Photodiagn. Photodyn. Ther. 2022, 40, 103074. [Google Scholar] [CrossRef]
- Amid, R.; Kadkhodazadeh, M.; Mojahedi, S.M.; Sarshari, M.G.; Zamani, Z. Physicochemical changes of contaminated titanium discs treated with erbium-doped yttrium aluminum garnet (Er:YAG) laser irradiation or air-flow abrasion: An in vitro study. J. Lasers Med. Sci. 2021, 12, e67. [Google Scholar] [CrossRef] [PubMed]
- Deeb, J.G.; Smith, J.; Belvin, B.R.; Lewis, J.; Grzech-Leśniak, K. Er:YAG laser irradiation reduces microbial viability when used in combination with irrigation with sodium hypochlorite, chlorhexidine, and hydrogen peroxide. Microorganisms 2019, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Grzech-Leśniak, Z.; Pyrkosz, J.; Szwach, J.; Kosidło, P.; Matys, J.; Wiench, R.; Pajączkowska, M.; Nowicka, J.; Dominiak, M.; Grzech-Leśniak, K. Antibacterial effects of Er:YAG laser irradiation on Candida–Streptococcal biofilms. Life 2025, 15, 474. [Google Scholar] [CrossRef]
- Homayouni, A.; Bahador, A.; Moharrami, M.; Pourhajibagher, M.; Rasouli-Ghahroudi, A.A.; Alikhasi, M. Effect of 5 popular disinfection methods on microflora of laboratory-customized implant abutments. Implant Dent. 2019, 28, 437–446. [Google Scholar] [CrossRef]
- Polak, D.; Shani-Kdoshim, S.; Alias, M.; Shapira, L.; Stabholz, A. The in vitro efficacy of biofilm removal from titanium surfaces using Er:YAG laser: Comparison of treatment protocols and ablation parameters. J. Periodontol. 2022, 93, 100–109. [Google Scholar] [CrossRef]
- Seghayer, I.; Lee, A.H.C.; Cheung, G.S.P.; Zhang, C. Effect of passive ultrasonic irrigation, Er,Cr:YSGG laser, and photon-induced photoacoustic streaming against Enterococcus faecalis biofilms in the apical third of root canals. Bioengineering 2023, 10, 490. [Google Scholar] [CrossRef]
- Chohan, H.; Khullar, S.; Patel, R.H.; Rath, J.; Mohammed, S.; Subramani, S.K. Investigations of the Antimicrobial Effectiveness of Different Disinfection Protocols Against Endodontic Pathogens in Root Canal Systems. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S4), S3577–S3579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terlep, S.; Dogsa, I.; Pajk, F.; Stopar, D. Biofilm removal from in vitro narrow geometries using single and dual pulse Er:YAG laser photoacoustic irrigation. Microorganisms 2023, 11, 2102. [Google Scholar] [CrossRef]
- Sebbane, N.; Steinberg, D.; Keinan, D.; Sionov, R.V.; Farber, A.; Sahar-Helft, S. Antibacterial Effect of Er:YAG Laser Irradiation Applied by a New Side-Firing Spiral Tip on Enterococcus faecalis Biofilm in the Tooth Root Canal—An Ex Vivo Study. Appl. Sci. 2022, 12, 12656. [Google Scholar] [CrossRef]
- Moritz, A.; Schoop, U.; Goharkhay, K.; Jakolitsch, S.; Kluger, W.; Wernisch, J.; Sperr, W. The bactericidal effect of Nd:YAG, Ho:YAG, and Er:YAG laser irradiation in the root canal: An in vitro comparison. J. Clin. Laser Med. Surg. 1999, 17, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Liu, H.; Yang, L.; Zhang, L.; Yang, L.; Xiao, N.; Shen, J.; Deng, J.; Shen, Y. In vitro efficacy of Er:YAG laser-activated irrigation versus passive ultrasonic irrigation and sonic-powered irrigation for treating multispecies biofilms in artificial grooves and dentinal tubules: An SEM and CLSM study. BMC Oral Health 2024, 24, 261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, G.; Chen, W. In vitro effects of Er:YAG laser-activated photodynamic therapy on Enterococcus faecalis in root canal treatment. Photodiagn. Photodyn. Ther. 2024, 45, 103992. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Aoki, A.; Watanabe, H.; Ishikawa, I. Bactericidal effect of erbium YAG laser on periodontopathic bacteria. Lasers Surg. Med. 1996, 19, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shimada, K.; Iwasaki, H.; Ito, K. Inhibitory effects of a super pulsed carbon dioxide laser at low energy density on periodontopathic bacteria and lipopolysaccharide in vitro. J. Periodontal. Res. 2005, 40, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Tsui, V.W.; Wong, R.W.; Rabie, A.B. The inhibitory effects of naringin on the growth of periodontal pathogens in vitro. Phytother. Res. 2008, 22, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A.A. Potential applications of Erbium:YAG laser in periodontics. J. Periodontal. Res. 2004, 39, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Andreana, S. The use of diode lasers in periodontal therapy: Literature review and suggested technique. Dent. Today 2005, 24, 132–135. [Google Scholar] [PubMed]
- Kranendonk, A.; van der Reijden, W.; van Winkelhoff, A.; van der Weijden, G. The bactericidal effect of a Genius Nd:YAG laser. Int. J. Dent. Hyg. 2010, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, B.; Wei, Z.; Li, Y.; Yao, Y.; Song, C.; Duan, Y. Discovery of a potent, Highly selective, and In vivo anti-inflammatory Efficacious, P2Y6R antagonist with a novel quinoline-pyrazole scaffold. Eur. J. Med. Chem. 2024, 279, 116890. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, A.O.; Ezzat, S.; Samad, F.A.; Dabbous, O.A.; Dahm, J.; Hamblin, M.R.; Mohamed, T. Studying the viability and growth kinetics of vancomycin-resistant Enterococcus faecalis V583 following femtosecond laser irradiation (420–465 nm). Lasers Med. Sci. 2024, 39, 144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).