Transparent 3-Layered Bacterial Nanocellulose as a Multicompartment and Biomimetic Scaffold for Co-Culturing Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemical Origins
2.2. Multilayered BNC Fabrication
2.3. Thickness and Transparency Measurement
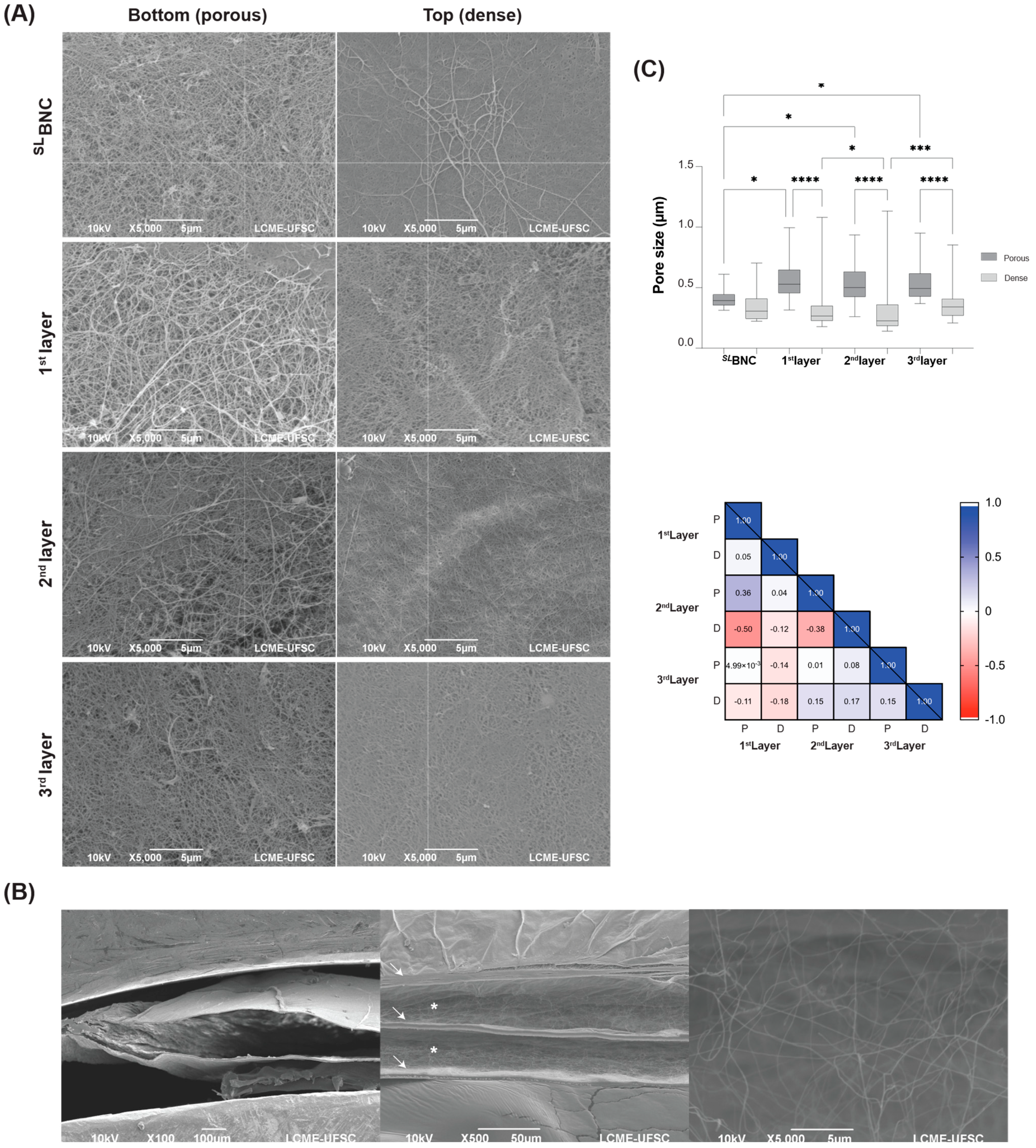
2.4. Pore Size Analysis
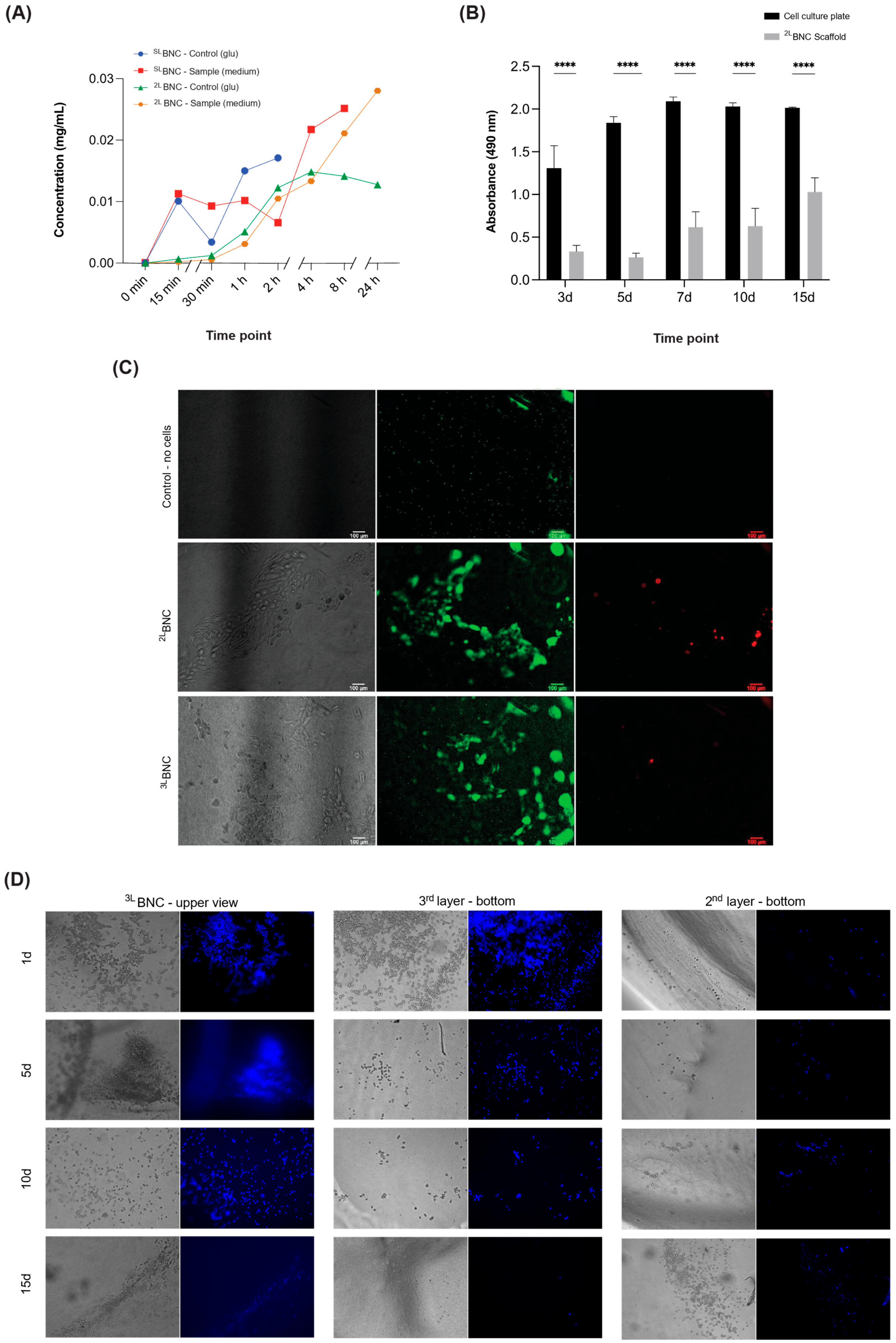
2.5. Nutrient Transport
2.6. Rheological Analysis
2.7. Standardization of Cell Culture Protocol
2.8. Cell Viability
2.9. Triple Co-Culturing into 3LBNC Scaffolds
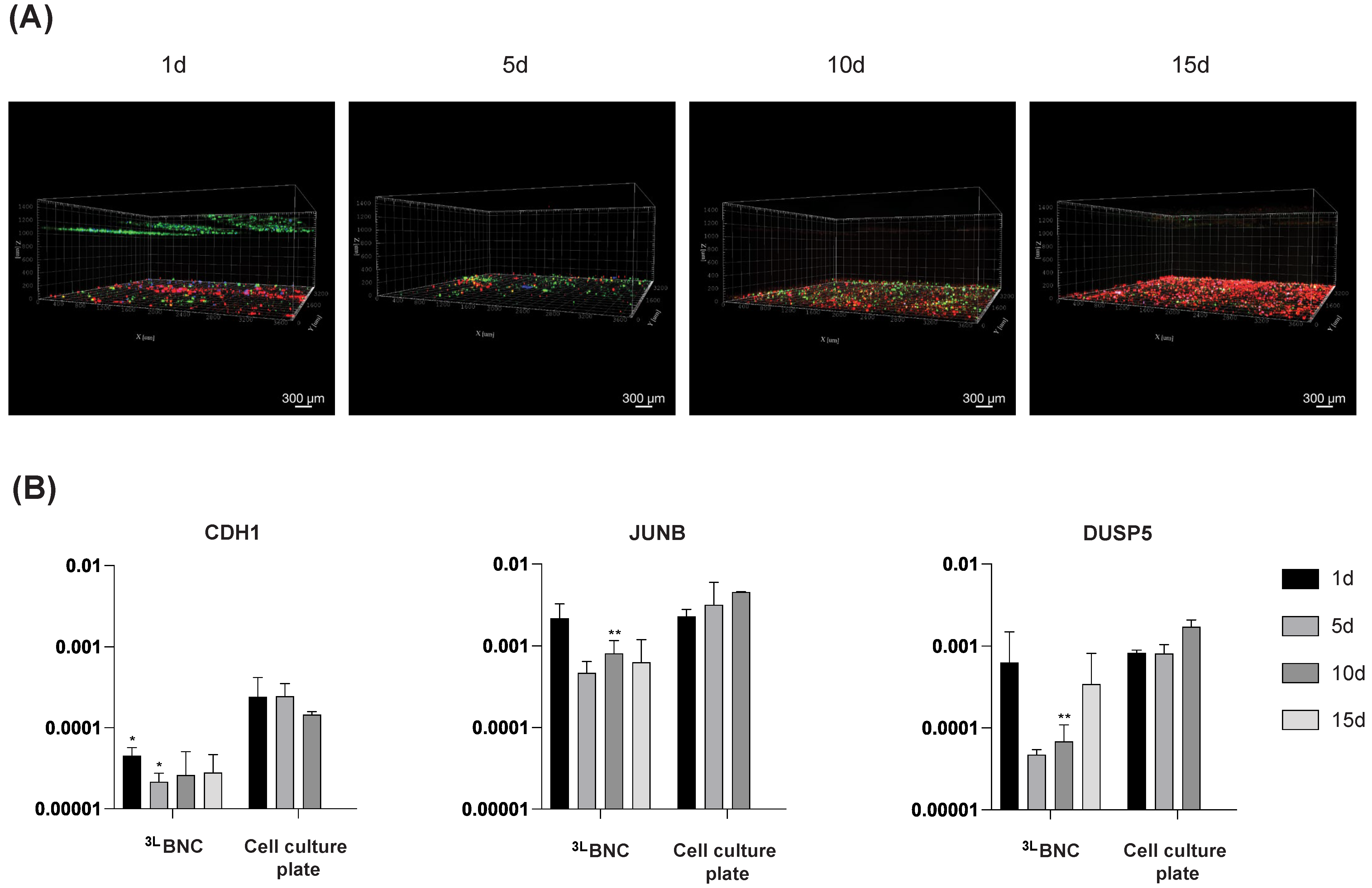
2.10. Confocal Microscopy
2.11. RNA Quantification by qPCR
2.12. Statistical Analysis
3. Results
3.1. Physicochemical Properties of the 3LBNC Scaffold
3.2. Cellular Viability and Proliferation of Cells Cultured in Layered BNC Scaffolds
3.3. Triple-Cell Co-Culture in 3LBNC Scaffolds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta 2013, 1832, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Filipe, E.C.; Chitty, J.L.; Cox, T.R. Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 2018, 99, 58–76. [Google Scholar] [CrossRef]
- Armingol, E.; Officer, A.; Harismendy, O.; Lewis, N.E. Deciphering cell–cell interactions and communication from gene expression. Nat. Rev. Genet. 2021, 22, 71–88. [Google Scholar] [CrossRef]
- Herrmann, D.; Conway, J.R.W.; Vennin, C.; Magenau, A.; Hughes, W.G.; Morton, J.P.; Timpson, P. Three-dimensional cancer models mimic cell-matrix interactions in the tumour microenvironment. Carcinogenesis 2014, 35, 1671–1679. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Chen, C.S. Deconstructing Dimensionality. Cell Biol. 2013, 339, 402–405. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Breslin, S.; Driscoll, L.O. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Malhotra, M.; Curtin, C.M.; O’Brien, F.J.; O’Driscoll, C.M. Life in 3D is never flat: 3D models to optimise drug delivery. J. Control. Release 2015, 215, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Monferrer, E.; Martín-Vañó, S.; Carretero, A.; García-Lizarribar, A.; Burgos-Panadero, R.; Navarro, S.; Samitier, J.; Noguera, R. A three-dimensional bioprinted model to evaluate the effect of stiffness on neuroblastoma cell cluster dynamics and behavior. Sci. Rep. 2020, 10, 6370. [Google Scholar] [CrossRef]
- Moghimi, N.; Hosseini, S.A.; Dalan, A.B.; Mohammadrezaei, D.; Goldman, A.; Kohandel, M. Controlled tumor heterogeneity in a co-culture system by 3D bio-printed tumor-on-chip model. Sci. Rep. 2023, 13, 13648. [Google Scholar] [CrossRef]
- Larracuente, A.M.; Ferree, P.M. Simple method for fluorescence DNA in situ hybridization to squashed chromosomes. J. Vis. Exp. 2015, 95, 52288. [Google Scholar] [CrossRef]
- Saji Joseph, J.; Tebogo Malindisa, S.; Ntwasa, M. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culturing in Drug Discovery. In Cell Culture; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part. B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Rana, D.; Arulkumar, S.; Vishwakarma, A.; Ramalingam, M. Considerations on Designing Scaffold for Tissue Engineering. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Sultana, N. Mechanical and biological properties of scaffold materials. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- González-Díaz, E.C.; Varghese, S. Hydrogels as Extracellular Matrix Analogs. Gels 2016, 2, 20. [Google Scholar] [CrossRef]
- Padrão, J.; Melro, L.; Fernandes, M.; Fernandes, R.D.V.; Ribeiro, A.I.; Song, X.; Yu, L.; Zille, A. Biocompatibility of Nanocellulose. In Handbook of Biopolymers; Thomas, S., Ar, A., Chirayil, C.J., Thomas, B., Eds.; Springer: Singapore, 2023; Volume 1, pp. 975–1006. [Google Scholar]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Bown, A.J. The Chemical Action of Pure Cultivation of Bacterium Aceti. J. Chem. Soc. Trans. 1886, 49, 172–187. [Google Scholar]
- Ryngajłło, M.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Jacek, P.; Bielecki, S. Comparative genomics of the Komagataeibacter strains—Efficient bionanocellulose producers. Microbiologyopen 2019, 8, e00731. [Google Scholar] [CrossRef] [PubMed]
- Čolić, M.; Tomić, S.; Bekić, M. Immunological aspects of nanocellulose. Immunol. Lett. 2020, 222, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Archer, A.J.; Chen, X.; Liu, C.; Yang, G.; Liu, Y. Dehydration of bacterial cellulose and the water content effects on its viscoelastic and electrochemical properties. Sci. Technol. Adv. Mater. 2018, 19, 203–211. [Google Scholar] [CrossRef]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface Modification of Bacterial Cellulose for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef]
- Reis, E.M.; Berti, F.V.; Colla, G.; Porto, L.M. Bacterial nanocellulose-IKVAV hydrogel matrix modulates melanoma tumor cell adhesion and proliferation and induces vasculogenic mimicry in vitro. J. Biomed. Mater. Res. Part B 2017, 106, 2741–2749. [Google Scholar] [CrossRef]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and differentiation of SH-SY5Y neuroblastoma cells on bacterial nanocellulose scaffolds. Artif. Cells Nanomed. Biotechnol. 2013, 42, 302–308. [Google Scholar] [CrossRef]
- Martínez Ávila, H.; Feldmann, E.M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Müller, R.; Hendriks, J.; Rotter, N.; et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 2015, 44, 122–133. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Thomas, D.; Madhavan, A.; Sindhu, R.; Binod, P.; Varjani, S.; Awasthi, M.K.; Pandley, A. Bacterial nanocellulose: Engineering, production, and applications. Bioengineered 2021, 12, 11463–11483. [Google Scholar] [CrossRef]
- Gama, F.M.; Gatenholm, P.; Klemm, D. Bacterial NanoCellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Berti, F.V.; Rambo, C.R.; Dias, P.F.; Porto, L.M. Nanofiber density determines endothelial cell behavior on hydrogel matrix. Mater. Sci. Eng. C 2013, 33, 4684–4691. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.D.; Carvalho, J.L.; Novikoff, S.; Berti, F.V.; Porto, L.M.; Assis, D.; de Goes, A.M. Bacterial Cellulose Membranes Constitute Biocompatible Biomaterials for Mesenchymal and Induced Pluripotent Stem Cell Culture and Tissue Engineering. J. Tissue Sci. Eng. 2012, 11, 5. [Google Scholar] [CrossRef]
- Hu, Y.; Catchmark, J.M.; Zhu, Y.; Abidi, N.; Zhou, X.; Wang, J.; Liang, N. Engineering of porous bacterial cellulose toward human fi broblasts ingrowth for tissue engineering. J. Mater. Res. 2014, 29, 2682–2693. [Google Scholar] [CrossRef]
- Mastrodimos, M.; Jain, S.; Badv, M.; Shen, J.; Montazerian, H.; Meyer, C.E.; Annabi, N.; Weiss, P.S. Human Skeletal Muscle Myoblast Culture in Aligned Bacterial Nanocellulose and Commercial Matrices. ACS Appl. Mater. Interfaces 2024, 16, 47150–47162. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Multicompartment Hydrogels. Macromol. Rapid Commun. 2022, 43, 2100895. [Google Scholar] [CrossRef]
- Meng, Y.; Giannini Beillon, G.; Lauby, M.; Elharar, I.; Schoefs, B.; Marchand, J.; Nicol, E. Triphasic hydrogel for cell co-culture in compartmentalized all-liquid micro-bioreactor. Algal Res. 2024, 84, 103803. [Google Scholar] [CrossRef]
- Jedrusik, N.; Meyen, C.; Finkenzeller, G.; Stark, G.B.; Meskath, S.; Schulz, S.D.; Steinberg, T.; Eberwein, P.; Strassburg, S.; Tomakidi, P. Nanofibered Gelatin-Based Nonwoven Elasticity Promotes Epithelial Histogenesis. Adv. Healthc. Mater. 2018, 7, 1700895. [Google Scholar] [CrossRef]
- Taymour, R.; Chicaiza-Cabezas, N.A.; Gelinsky, M.; Lode, A. Core-shell bioprinting of vascularized in vitro liver sinusoid models. Biofabrication 2022, 14, 045019. [Google Scholar] [CrossRef]
- Vinson, B.T.; Phamduy, T.B.; Shipman, J.; Riggs, B.; Strong, A.L.; Sklare, S.C.; Murfee, W.L.; Burow, M.E.; Bunnell, B.A.; Huang, Y.; et al. Laser direct-write based fabrication of a spatially-defined, biomimetic construct as a potential model for breast cancer cell invasion into adipose tissue. Biofabrication 2017, 9, 025013. [Google Scholar] [CrossRef]
- Souza, S.S.d.; Berti, F.V.; Oliveira, K.P.V.d.; Pittella, C.Q.P.; Castro, J.V.d.; Pelissari, C.; Rambo, C.R.; Porto, L.M. Nanocellulose biosynthesis by Komagataeibacter hansenii in a defined minimal culture medium. Cellulose 2018, 4, 1641–1655. [Google Scholar] [CrossRef]
- Saito, H.; Sakurai, A.; Sakakibara, M.; Saga, H. Preparation and properties of transparent cellulose hydrogels. J. Appl. Polym. Sci. 2003, 90, 3020–3025. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Vogelaar, L.; Bolhuis-Versteeg, L.A.M.; Lammertink, R.G.H.; Stamatialis, D.; Wessling, M. One-step fabrication of porous micropatterned scaffolds to control cell behavior. Biomaterials 2007, 28, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef]
- Liu, T.; Lin, B.; Qin, J. Carcinoma-associated fibroblasts promoted tumor spheroid invasion on a microfluidic 3D co-culture device. Lab. Chip 2010, 10, 1671–1677. [Google Scholar] [CrossRef]
- Eiro, N.; González, L.; Martínez-Ordoñez, A.; Fernandez-Garcia, B.; González, L.O.; Cid, S.; Dominguez, F.; Perez-Fernandez, R.; Vizoso, F.J. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell. Oncol. 2018, 41, 369–378. [Google Scholar] [CrossRef]
- Wei, L.; Ye, H.; Li, G.; Lu, Y.; Zhou, Q.; Zheng, S.; Lin, Q.; Liu, Y.; Li, Z.; Chen, R. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2018, 9, 1065. [Google Scholar] [CrossRef]
- Yavuz, B.G.; Gunaydin, G.; Gedik, M.E.; Kosemehmetoglu, K.; Karakoc, D.; Ozgur, F.; Guc, D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1 + TAMs. Sci. Rep. 2019, 9, 1–15. [Google Scholar]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Ireland, L.V.; Mielgo, A. Macrophages and fibroblasts, key players in cancer chemoresistance. Front. Cell Dev. Biol. 2018, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Liu, F.; Chen, Y.; Yang, Q. Crosstalk between cancer-associated fibroblasts and immune cells in cancer. J. Cell Mol. Med. 2020, 24, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, F.; Engler, A.J.; Eckhardt, A.; Ahmed, F.; Discher, D.E. Cell responses to the mechanochemical microenvironment-Implications for regenerative medicine and drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 1329–1339. [Google Scholar] [CrossRef]
- Peter, M.; Singh, A.; Mohankumar, K.; Jeenger, R.; Joge, P.A.; Gatne, M.M.; Tayalia, P. Gelatin-Based Matrices as a Tunable Platform to Study in Vitro and in Vivo 3D Cell Invasion. ACS Appl. Bio Mater. 2019, 2, 916–929. [Google Scholar] [CrossRef]
- Wu, P.H.; Gilkes, D.M.; Phillip, J.M.; Narkar, A.; Cheng, T.W.T.; Marchand, J.; Lee, M.H.; Li, R.; Wirtz, D. Single-cell morphology encodes metastatic potential. Sci. Adv. 2020, 6, eaaw6938. [Google Scholar] [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes. Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part. A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Whang, K.; Ph, D.; Healy, K.E.; Ph, D.; Elenz, D.R.; Nam, E.K. Engineering Bone Regeneration with Bioabsorbable Scaffolds with Novel Microarchitecture. Tissue Eng. 1999, 5, 35–51. [Google Scholar] [CrossRef]
- Lien, S.; Ko, L.; Huang, T. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Tien, J.; Ghani, U.; Dance, Y.W.; Seibel, A.J.; Karakan, M.Ç.; Ekinci, K.L.; Nelson, C.M. Matrix Pore Size Governs Escape of Human Breast Cancer Cells from a Microtumor to an Empty Cavity. iScience 2020, 23, 101673. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Rev. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, K.M.; Pettee, K.M.; Rubinic-Minotti, K.; Su, R.; Nestor-Kalinoski, A.; Eisenmann, K.M. Carcinoma associated fibroblasts (CAFs) promote breast cancer motility by suppressing mammalian Diaphanous-related formin-2 (mDia2). PLoS ONE 2018, 13, e0195278. [Google Scholar] [CrossRef] [PubMed]
- Duda, D.G.; Duyverman, A.M.M.J.; Kohno, M.; Snuderl, M.; Steller, E.J.A.; Fukumura, D.; Jain, R.K. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl. Acad. Sci. USA 2010, 107, 21677–21682. [Google Scholar] [CrossRef]
- McCarthy, J.B.; El-Ashry, D.; Turley, E.A. Hyaluronan, cancer-associated fibroblasts and the tumor microenvironment in malignant progression. Front. Cell Dev. Biol. 2018, 6, 48. [Google Scholar]
- Cui, K.; Ardell, C.L.; Podolnikova, N.P.; Yakubenko, V.P. Distinct migratory properties of M1, M2, and resident macrophages are regulated by αdβ2and αmβ2integrin-mediated adhesion. Front. Immunol. 2018, 9, 2650. [Google Scholar] [CrossRef]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus biological significance: Are PMA-differentiated THP-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front. Pharmacol. 2018, 9, 200747. [Google Scholar] [CrossRef]
- Kashani, A.S.; Packirisamy, M. Cancer cells optimize elasticity for efficient migration: Migratory index. R. Soc. Open Sci. 2020, 7, 200747. [Google Scholar] [CrossRef]
- Gefen, A.; Dilmoney, B. Mechanics of the normal woman’s breast. Technol. Health Care 2007, 15, 259–271. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Zigon-Branc, S.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Dubruel, P.; Zerobin, E.; Baudis, S.; Ovsianikov, A. Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids Sara. Tissue Eng. Part A 2019, 25, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Sarrió, D.; Palacios, J.; Hergueta-Redondo, M.; Gómez-López, G.; Cano, A.; Moreno-Bueno, G. Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer 2009, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Eslami Amirabadi, H.; Tuerlings, M.; Hollestelle, A.; SahebAli, S.; Luttge, R.; van Donkelaar, C.C.; Martens, J.W.M.; den Toonder, J.M.J. Characterizing the invasion of different breast cancer cell lines with distinct E-cadherin status in 3D using a microfluidic system. Biomed. Microdevices 2019, 21, 101. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Dunstan, C.R.; Sasaki, A.; Williams, P.J.; Mundy, G.R.; Yoneda, T. E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res. 1996, 56, 4063–4070. [Google Scholar]
- Huang, Z.; Yu, P.; Tang, J. Characterization of triple-negative breast cancer MDA-MB-231 cell spheroid model. OncoTargets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Russo, G.C.; Karl, M.N.; Clark, D.; Cui, J.; Carney, R.; Su, B.; Starich, B.; Lih, T.S.; Zhang, Q.; Wu, P.H.; et al. E-cadherin promotes cell hyper-proliferation in breast cancer. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sundqvist, A.; Morikawa, M.; Ren, J.; Vasilaki, E.; Kawasaki, N.; Kobayashi, M.; Koinuma, D.; Aburatani, H.; Miyazono, K.; Heldin, C.-H.; et al. JUNB governs a feed-forward network of TGFβ signaling that aggravates breast cancer invasion. Nucleic Acids Res. 2018, 46, 1180–1195. [Google Scholar] [CrossRef]
- Pang, M.F.; Georgoudaki, A.-M.; Lambut, L.; Johansson, J.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016, 35, 748–760. [Google Scholar] [CrossRef]
- Piechaczyk, M.; Farràs, R. Regulation and function of JunB in cell proliferation. In Biochemical Society Transactions; Portland Press: South Portland, ME, USA, 2008; Volume 36, pp. 864–867. [Google Scholar]
- Kallergi, G.; Tsintari, V.; Sfakianakis, S.; Bei, E.; Lagoudaki, E.; Koutsopoulos, A.; Zacharopoulou, N.; Alkahtani, S.; Alarif, S.; Stournaras, C.; et al. The prognostic value of JUNB-positive CTCs in metastatic breast cancer: From bioinformatics to phenotypic characterization. Breast Cancer Res. 2019, 21, 86. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, P.; Long, X. Differential Expression Profiles of the Transcriptome in Breast Cancer Cell Lines Revealed by Next Generation Sequencing. Cell. Physiol. Biochem. 2017, 44, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, H.; Liu, S.; Yang, Z.; Li, L.; Yao, N.; Cheng, S.; Dong, X.; Liang, X.; Chen, C.; et al. The suppression of DUSP5 expression correlates with paclitaxel resistance and poor prognosis in basal-like breast cancer. Int. J. Med. Sci. 2018, 15, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, L.; Li, H.; Huang, L.; Yin, M.; Pan, C.; Qin, H.; Jin, Z. Dual Specificity Phosphatase 5 Is a Novel Prognostic Indicator for Patients with Advanced Colorectal Cancer. Am. J. Cancer Res. 2016, 6, 2323. [Google Scholar] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]

| Analysis | SLBNC | 2LBNC | 3LBNC | Individual Layers | Type of Cell: Number | |
|---|---|---|---|---|---|---|
| Single-Layer (Control) (7 Days Incubation) | Double-Layer (14 Days Incubation) | Triple-Layer (21 Days Incubation) | When Applicable | |||
| Physical Characterization | Thickness | √ | n/a | √ | √ | n/a |
| Transparency | √ | √ | √ | n/a | n/a | |
| Pore size | √ | n/a | √ | √ | n/a | |
| Nutrient transport | √ | √ | n/a | n/a | n/a | |
| Rheology | n/a | √ | √ | n/a | n/a | |
| Biological Characterization | Cell viability | n/a | √ | √ | n/a | EOMA: 104 and 105 |
| Cell metabolic activity | n/a | √ | n/a | n/a | MDA-MB-231: 2 × 106 | |
| Cell migration | n/a | n/a | √ | n/a | EA.hy926: 106 | |
| Confocal microscopy | n/a | n/a | √ | n/a | BC-CAFs: 9 × 104 MDA-MB-231: 2 × 106 M2: 4 × 105 | |
| Gene expression | n/a | n/a | √ | n/a | BC-CAFs: 9 × 104 MDA-MB-231: 2 × 106 M2: 4 × 104 |
| NCBI ID | Gene | Forward/ Reverse | Sequence (5′ → 3′) | Start | End |
|---|---|---|---|---|---|
| NM_002046.7 | GAPDH | F | CACCCACTCCTCCACCTTTG | 943 | 963 |
| R | CCACCACCCTGTTGCTGTAG | 1052 | 1032 | ||
| NM_002229.3 | JUNB | F | TTCAAGGAGGAACCGCAGAC | 1001 | 1021 |
| R | TGAGCGTCTTCACCTTGTCC | 1196 | 1176 | ||
| NM_004419.4 | DUSP5 | F | CCAACTTTGGCTTCATGGGC | 1120 | 1140 |
| R | GCTCAGTGTCTGCAAATGGC | 1253 | 1233 | ||
| Z13009.1 | CDH1 * | F | GGTCTCTCTCACCACCTCCA | 1483 | 1503 |
| R | GGATGTGATTTCCTGGCCCA | 1615 | 1595 |
| 1st Layer | 2nd Layer | 3rd Layer | 2LBNC | 3LBNC | |
|---|---|---|---|---|---|
| Young’s Module—E (Pa) | 569.67 ± 515.39 | 869.34 ± 366.51 | 927.44 ± 102.29 | 183.61 ± 85.22 | 723.68 ± 306.53 |
| Storage Modulus—G’ (Pa) | 712.36 ± 0.00 a | 1756.44 ± 0.00 a | 382.66 ± 0.00 a | 2067.95 ± 237.98 | 3257.93 ± 450.07 |
| Loss Modulus—G” (Pa) | 125.74 ± 0.00 a | 219.17 ± 0.00 a | 53.64 ± 0.00 a | 309.21 ± 6.93 | 540.28 ± 66.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, K.P.V.; Yitayew, M.Y.; Bastos, A.P.A.; Mandrik, S.C.N.; Porto, L.M.; Tabrizian, M. Transparent 3-Layered Bacterial Nanocellulose as a Multicompartment and Biomimetic Scaffold for Co-Culturing Cells. J. Funct. Biomater. 2025, 16, 208. https://doi.org/10.3390/jfb16060208
de Oliveira KPV, Yitayew MY, Bastos APA, Mandrik SCN, Porto LM, Tabrizian M. Transparent 3-Layered Bacterial Nanocellulose as a Multicompartment and Biomimetic Scaffold for Co-Culturing Cells. Journal of Functional Biomaterials. 2025; 16(6):208. https://doi.org/10.3390/jfb16060208
Chicago/Turabian Stylede Oliveira, Karla Pollyanna Vieira, Michael Yilma Yitayew, Ana Paula Almeida Bastos, Stefanie Cristine Nied Mandrik, Luismar Marques Porto, and Maryam Tabrizian. 2025. "Transparent 3-Layered Bacterial Nanocellulose as a Multicompartment and Biomimetic Scaffold for Co-Culturing Cells" Journal of Functional Biomaterials 16, no. 6: 208. https://doi.org/10.3390/jfb16060208
APA Stylede Oliveira, K. P. V., Yitayew, M. Y., Bastos, A. P. A., Mandrik, S. C. N., Porto, L. M., & Tabrizian, M. (2025). Transparent 3-Layered Bacterial Nanocellulose as a Multicompartment and Biomimetic Scaffold for Co-Culturing Cells. Journal of Functional Biomaterials, 16(6), 208. https://doi.org/10.3390/jfb16060208







