Abstract
Inhibitors of the tyrosine kinase Zap70 are actively searched to improve treatments of lymphoid malignancies and autoimmune diseases associated with an abnormal T-cell response. The natural product withaferin A (WFA) has been characterized as a covalent inhibitor of Zap70 capable of blocking the migration of human T-cells. By analogy, we postulated that other withanolides equipped with a thiol-reactive, α,β-unsaturated ketone may form covalent complexes with Zap70. The hypothesis was tested using a molecular modeling approach with a panel of 12 withanolides docked onto the kinase domain of Zap70. Seven natural products revealed a capability to form stable complexes with Zap70 comparable to that of WFA, including withangulatin A, 4β-hydroxywithanolide E, withaperuvin, and ixocarpalactone A. Withangulatin A surpassed all the other withanolides for its ability to engage an interaction with Zap70 kinase and to form covalent complexes via bonding to the Cys346 residue close to the enzyme active site. The physicochemical and ADMET properties of withangulatin A were analyzed via Density Functional Theory calculations and an analysis of its Fukui function descriptors. The C3 position of the enone moiety was identified as the most reactive (nucleophilic) site of the molecule. Withangulatin A revealed a satisfactory ADMET profile with no major toxicity anticipated. It represents a potential hit to guide the design of Zap70 inhibitors.
1. Introduction
The protein Zap70 (zeta-chain-associated protein kinase 70 kDa) is a non-receptor tyrosine kinase which plays an essential role in T-cell receptor (TCR) signaling in thymocytes and peripheral T-cells. The kinase is recruited to the intracellular ζ-chains of the T-cell receptor to phosphorylate the TCR/CD3 multimeric protein complex, thus contributing to the diversification and amplification of TCR signaling. The correct functioning of the kinase is necessary to complete the development of thymocytes and T-cells in the thymus [1,2]. The protein is also expressed in NK cells and a subset of B cells. Aberrant expression of Zap70 has been reported in several inflammatory pathologies and its contribution to tumor immunity has been highlighted. Zap70 plays a role in several malignancies, notably in chronic lymphocytic leukemia (CLL) [3]. The protein is considered as a strong prognostic biomarker for patients with CLL [4]. Activated Zap70 represents a drug target to combat certain solid tumors due to its role as a driver of proliferation and tumor transformation and its implication in resistance to PI3K inhibitors in cancer cells [5]. Zap70 is viewed as an immunotherapeutic target to treat laryngeal cancer [6], lymphoid malignancies, and various autoimmune diseases associated with an abnormal T-cell response, including psoriasis and lupus [7,8].
These considerations have stimulated the search for different types of inhibitors of Zap70, acting either as direct blockers of the kinase domain or as disruptors of the interaction with the T-cell antigen receptor [8,9]. Zap70 inhibitors include natural and semi-synthetic products and diverse, rationally designed small molecules with a heterocyclic scaffold. In recent years, several types of inhibitors have been reported including arglabin derivatives, pyridopyrimidinones, and imidazopyrimidine-carboxamide derivatives to mention a few potent compounds [10,11,12]. Drug design approaches can benefit from a solid structural basis for the inhibition of the enzyme, with the crystallographic structure of the tyrosine kinase domain [13,14] and the identification of a druggable pocket close to the activation loop of the kinase [15].
Recently, the drug discovery strategy has been oriented toward the design and development of covalent inhibitors targeting cysteine residues that are essential for the correct functioning of the kinase. Thiol-reactive small molecules interfering with protein activity have been discovered [16]. There are several important cysteine residues in the kinase domain and the surrounding areas. In particular, C564 residue located in the kinase domain of Zap70 corresponds to a palmitoylation site, and its acylation contributes to the regulation of T-cell-mediated immunity [17,18]. The imidazopyrimidine-carboxamide derivative RDN009, bearing a chloroacetamide reactive group (IC50 = 44.8 nM), is one of the most potent covalent inhibitors of Zap70 targeting C346, which also represents a critical site (Figure 1) [10]. Recently, this compound has been optimized to enhance its activity, leading to the derivative RDN2150 potently active against the kinase (IC50 = 14 nM) and showing promising inhibitory effects on T-cell activation and inflammatory cytokine production [19]. The cysteine reactivity of RDN2150 is due to an acrylamide moiety, which is a mildly reactive warhead commonly found in approved covalent kinase drugs, such as afatinib and dacomitinib [20]. The α,β-unsaturated amide moiety of these compounds serves as a warhead to react with cysteine thiols. A similar reactive group with an α,β-unsaturated keto structure is found in the natural product β-epoxyarglabin and related sesquiterpene lactones such as grosheimin, which have been shown to react covalently with the C39 of Zap70 [12].
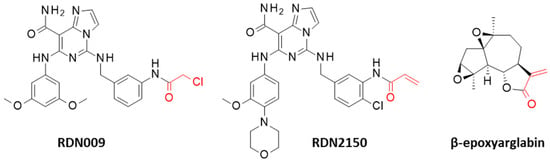
Figure 1.
Structures of three covalent inhibitors of Zap70. The thiol-reactive moiety is shown in red.
Covalent binding to Zap70 has also been demonstrated with the withanolide-type steroid called withaferin A (WFA), which is essentially found in the medicinal plant Withania somnifera (L.) Dunal [21]. This compound presents a C-28 steroidal lactone based on an ergostane skeleton (Figure 2). WFA shows marked antitumor and anti-inflammatory effects [22]. The compound belongs to a large family of more than 1200 withanolides, which includes many antitumor compounds such as withanolide A, withangulatin A, withanone, physapruin A, and others [23]. WFA stands as a leading compound in the family, owing to its multiple pharmacological properties and, in particular, its potent antitumor efficacy [24,25,26]. It is a reactive molecule capable of covalently binding to the exposed cysteine residues of certain proteins, such as vimentin, Hsp90, Ikkβ, Nrf2, annexin II, and a few others [27].
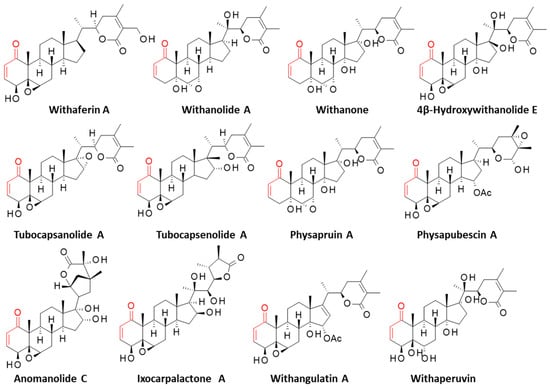
Figure 2.
Structures of selected withanolides bearing a thiol-reactive enone moiety (in red).
WFA has been shown to inhibit Zap70 by forming covalent complexes with cysteine residues. Covalent binding of the product to the kinase has been demonstrated experimentally, and a molecular modeling analysis has predicted that two cysteines C560 and/or C564 could serve as potential thiol donors for the covalent drug attachment [28]. But at present, the exact cysteine site in Zap70 has not been evidenced experimentally. WFA reacts with cysteine thiol via its α,β-unsaturated ketone (enone) moiety which functions as a Michael acceptor (electrophile) susceptible to covalently modifying cysteine residues in proteins or thiol-containing compounds such as glutathione (GSH) and homocysteine [29,30]. Occasionally, an adduct can form from the thiol addition to the epoxide at the C-6 position, as observed upon the bioconversion of an extract of Withania somnifera by the fungus Beauveria bassiana in the presence of glutathione [31]. But in most cases, it is the α,β-unsaturated ketone moiety that reacts with thiols to form conjugates and cysteine adducts.
In the present study, we have analyzed the protein binding and compared the reactivity of a series of withanolides towards Zap70. The three-dimensional structure of the active kinase domain of Zap70 non-covalently bound to staurosporine was used as a structural model (PDB: 1U59) [14]. Twelve withanolides, all bearing a reactive α,β-unsaturated ketone moiety, were selected (Figure 2). Some of them are known to react covalently with cysteines in certain proteins. This is the case for WFA and tubocapsenolide A, which can form cysteine adducts with the chaperone protein Hsp90 [32,33], and for 4β-hydroxywithanolide E (4β-HWNE), which can bind to a cysteine residue of the transcription factor Keap1, so as to interrupt its interaction with Nrf2 [34]. Withangulatin A has been shown to form cysteine-bound covalent complexes with PHGDH (3-phosphoglycerate dehydrogenase), Prdx-6 (peroxiredoxin 6), and SERCA-2 (sarco/endoplasmic reticulum calcium-ATPase 2) [35,36,37]. The other withanolides have not been shown to form covalent complexes, but they carry the same reactive enone moiety. Some of them show prominent anticancer properties, such as physapruin A (from Physalis peruviana), which induces oxidative stress and DNA damages in cancer cells [38,39,40,41], and anomanolide C (from Tubocapsicum anomalum), which can suppress the progression of triple-negative breast tumors in mice [42]. By analogy to WFA, we hypothesized that some of these reactive compounds could also covalently bind to Zap70 and form covalent cysteine adducts. The results of our molecular docking study are presented here.
2. Materials and Methods
2.1. Molecular Structures and Software
The three-dimensional structure Zap70 non-covalently bound with staurosporine was retrieved from the protein data bank (PDB: 1U59) and used as a model for the docking analysis. It is a high-resolution structure (2.30 Å) obtained by X-ray diffraction [14]. The molecular docking analysis was performed with the GOLD software (version 5.3, Cambridge Crystallographic Data Centre, Cambridge, UK). Prior to the docking operations, the structure of each ligand was optimized using a classical Monte Carlo conformational searching procedure via the BOSS software v4.9 [43]. Molecular graphics and analysis were performed using Discovery Studio Visualizer, Biovia 2020 (Dassault Systèmes BIOVIA Discovery Studio Visualizer 2020, San Diego, Dassault Systèmes, 2020). The web server Computed Atlas of Surface Topography of proteins (CASTp) 3.0 was used to identify potential ligand-binding sites on the tubulin dimer. The molecular modeling software Chimera 1.15 was used for visualization [44].
2.2. In Silico Molecular Docking Procedure
The staurosporine binding area within the kinase domain of Zap70 was considered as the potential binding site for the studied withanolides. During the process, the side chains of the following amino acids within the binding site were rendered fully flexible: Leu344, Cys346, Phe349, Val352, Lys369, Glu386, Met390, Lys424, Asp479, and Phe480. A docking grid, centered in the volume defined by the central amino acid, has been defined based on shape complementarity and geometry considerations. In general, up to 100 poses considered as energetically reasonable are selected during the search for the correct binding mode of the ligand. The decision to select a trial pose is based on ranked poses, using the fitness scoring function (PLP score incorporated in GOLD v5.3) [45]. The same procedure was used to establish molecular models for all studied natural products.
In general, 6 poses are selected per analysis. The ranking leads to the evaluation of the empirical potential energy of the interaction (ΔE), defined using the expression ΔE(interaction) = E(complex) − [E(protein) + E(ligand)]. The SPASIBA spectroscopic force field is used to calculate the final energy. The required parameters are derived from vibrational wavenumbers obtained in the infrared and Raman spectra of a large series of compounds of diverse chemical nature (organic molecules, amino acids, saccharides, nucleic acids, and lipids). The last step corresponds to validation using the SPASIBA force field, an essential step to determine the best protein–ligand structure. This force field has been specifically developed to provide refined empirical MM force field parameters [46]. SPASIBA (integrated into CHARMM) empirical energies of interaction are calculated. It is an excellent system for reproducing crystal-phase infrared data. SPASIBA has been specifically developed to provide refined empirical molecular mechanics force field parameters [47]. The Boss program and the Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) procedure were used to evaluate the free energies of hydration (ΔG) in relation to aqueous solubility [48].
The docking analysis was performed with the different withanolides interacting with the kinase domain of Zap70 in a non-covalent process. The potential formation of a covalent C-S linkage between the α,β-unsaturated ketone unit of the natural products and the C346 thiol group was inferred from the calculated distance between the reactive groups, as described previously for other covalent drug–protein complexes [49,50].
2.3. DFT Calculations and Predicted Physicochemical and ADMET Properties
All Density Functional Theory (DFT) calculations were performed with the ORCA v5.0.4 software package, using the B3LYP-D3/def2-SVP computational level and the water implicit solvation CPCM [51], from the 3D coordinates of ligands previously generated using Auto3D [52]. The DFT calculations provided a better understanding of the reactivity and stability of our compounds, in particular, by obtaining the energies of the HOMO (Highest Occupied Molecular Orbital, serving as an electron donor) and the LUMO (Lowest Unoccupied Molecular Orbital, acting as an electron acceptor) orbitals, for the calculation of the HOMO-LUMO gaps. The reactivity of the molecules was further analyzed using Fukui functions and dual descriptors to determine the nucleophilicity (f−), electrophilicity (f+), and radical attack susceptibility (∆f(r)) of the heavy atoms.
Several machine learning-based approaches were used to predict a wide range of critical parameters, starting from the SMILES string of the studied compounds. SolTranNet [53] was used to predict the aqueous solubility with the calculation of logS values, PredPS [54] to predict the stability of compounds in human plasma using an attention-based graph neural network, and PredMS [55] to estimate the metabolic stability of the compounds in human liver microsomes from a random forest model. FP-ADMET [56], a toolbox of prediction models using mostly random forest models, allowed for the prediction of a wide range of physicochemical, ADMET, and ADMET-related parameters.
3. Results
3.1. Protein Structure and Binding Site Analysis
The structure of the active kinase domain of Zap70 bound to staurosporine (1U59) was used to locate the position of the different cysteine residues. The kinase domain comprises seven cysteine residues at C346, C405, C510, C560, C564, C575, and C596, but only one, C346, is positioned at the entrance of the ATP binding site where the crystallographic ligand (staurosporine) is bound, as represented in Figure 3. The other residues, notably C560-C564, are located on the external surface of the protein. A binding site analysis was performed using the web server CASTp 3.0, which is a convenient tool to analyze the topography of proteins and to locate potential drug-binding sites [57]. The analysis revealed the position of only one large binding area, corresponding to the ATP and staurosporine binding pocket (Figure 4). The cavity is quite large, sufficient to accommodate a polycyclic molecule such as WFA. Six other small cavities were identified with CAST, but they are very narrow (<50 Å3), probably too small to serve as potential binding sites. The estimated volume of the central cavity is 774.7 Å3 according to the method of static accessibility [58] and 2134.5 Å3 when measuring the solvent-accessible surface [57]. The same calculation method applied to WFA gave a value of 1374.5 Å3. It is thus clear that the molecule can fit only in the large cavity, not the small hollows around the protein. For this reason, we focused our analysis on the main kinase activity site and performed a docking analysis to compare the binding of WFA and its analogs to this site, and the possible formation of a covalent interaction with cysteine 346 located at the gate of the active site.
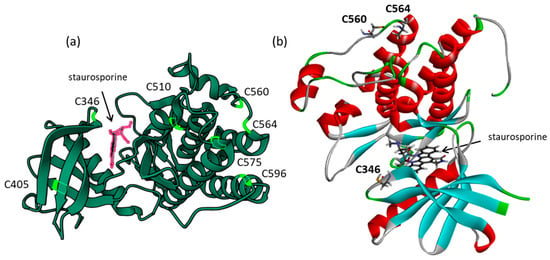
Figure 3.
Structure of the active kinase domain of Zap70 bound to staurosporine (from Lys328 to His612, from PDB 1U59) [14]. (a) A global view of the active kinase domain with the different cysteine residues. (b) A view of the staurosporine-bound protein with the α-helices (in red) and β-sheets (in cyan). The position of three cysteine residues is indicated, including C346 in the kinase active site and C560-C564 exposed on the protein surface.
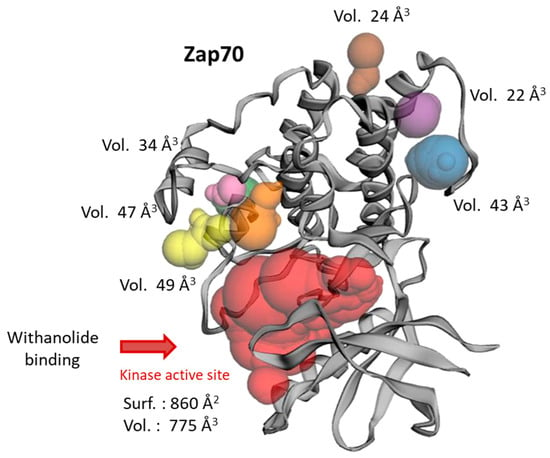
Figure 4.
The binding site analysis of Zap70 (using web server CASTp 3.0) primarily reveals the position of the ATP-binding site in the center of the protein (in red) and a few minor areas around the protein, with the indicated volumes.
3.2. Covalent Binding of WFA to Zap70
WFA was docked into the active kinase domain of Zap70, and the empirical energy of interaction (ΔE) and the free energy of hydration (ΔG) of the interaction were calculated (Table 1). WFA can fit easily into the binding cavity, as represented in Figure 5. The compound remains significantly exposed, because the cavity is large, with an extended solvent-accessible area. However, the extended molecule bridges the two lips of the cavity and anchors itself relatively deeply into the protein site. The fit is satisfactory (ΔE = −69.30 kcal/mol), controlled by the diversity of molecular contacts between the ligand and the protein. Notably, four hydrogen bonds contribute to the stability of the complex, between the withanolide and residues Asn348, Phe349, Arg460, and Lys484, in addition to various van der Waals contacts (Figure 6). Interestingly, the small molecule sits at a short distance from Cys346 to position the enone moiety close to the thiol group of the cysteine residue. Under these conditions, covalent binding can be easily established, as represented in Figure 7. The molecule adopts a configuration which places its enone moiety close to the SH group of C346, thus favoring the subsequent formation of the thioether linkage, while the opposite lactone part of the molecule is interacting with the protein via residues Arg460 and Lys484. The covalent binding of the natural product to the C346 residue of Zap70 deserves further study using a more appropriate methodology (e.g., quantum mechanical) for describing the covalent bond formation with greater precision.

Table 1.
Calculated potential energy of interaction (ΔE) and free energy of hydration (ΔG) for the interaction of withanolides with Zap70 (1U59).
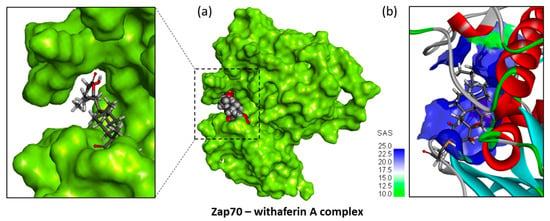
Figure 5.
Molecular model of compound WFA bound to Zap70. (a) A surface model of the protein with the compound bound to the kinase site and a close-up view of the binding area. (b) A ribbon model of Zap70 with the WFA bound to the active site. The α-helices (in red) are shown together with the solvent-accessible surface (SAS) area surrounding the drug-binding zone (color code indicated).
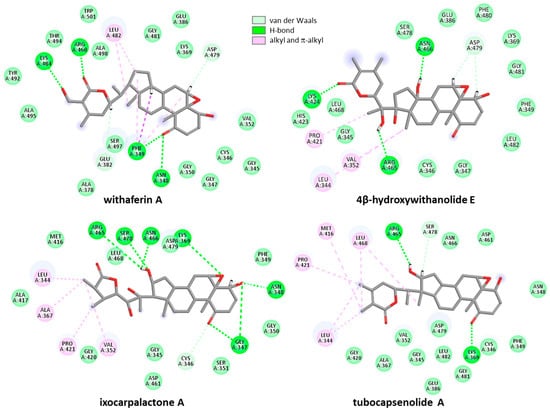
Figure 6.
Binding map contacts for four withanolides bound to Zap70 (color code indicated). The compounds rank in order in terms of potential binding to Zap70: 4β-hydroxywithanolide E = ixocarpalactone A > withaferin A > tubocapsenolide A (Table 1).
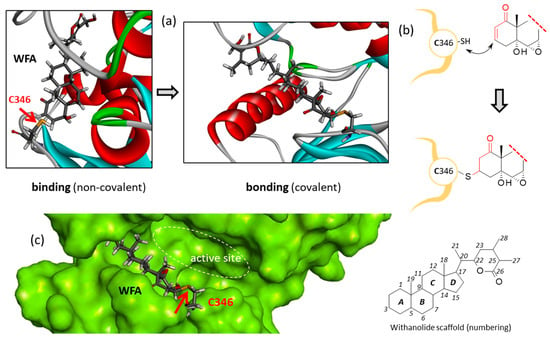
Figure 7.
Covalent binding of WFA to Zap70 via Cys346. (a) The binding to bonding process permitted by the close proximity between the enone moiety of WFA and thiol group of C346. (b) The reaction scheme and (c) a detailed view of WFA covalently bound to C346 at the gate of the kinase active site.
3.3. Comparative Molecular Docking of Withanolides to Zap70
The docking analysis was repeated with each of the 12 selected withanolides to calculate the corresponding binding energies (Table 1). Although all molecules present the same framework, with a common ergostane scaffold and the key reactive enone group, significant variations were observed between the molecules in terms of binding to Zap70. As expected, the free energy of hydration (ΔG) is relatively similar, whereas the potential energy of interaction (ΔE) varies importantly from one molecule to the other. Arbitrarily, we can separate the compounds in three groups: (1) the weak binder including withanone, withanolide A, physapruin A, anomanolide C, and tubocapsanolide A (−55.3 kcal/mol < ΔE < −63.4 kcal/mol); (2) the good binder including 4β-hydroxywithanolide E, ixocarpalactone A, physapubescin, tubocapsenolide A, WFA, and withaperuvin (−69.3 kcal/mol < ΔE < −75.5 kcal/mol); and (3) a single compound with a very favorable binding energy, withangulatin A (Table 1).
The compounds of the second group showed a behavior more or less comparable to that of WFA. They all can fit into the protein active site at a relative proximity to the C346 residue. The binding configuration varies slightly from one compound to another, leading to variable molecular contacts. Typical examples are represented in Figure 6. The case of ixocarpalactone A is interesting to note because its C16-OH group contributes importantly to the protein interaction with an implication in up to three H-bonds with residues Arg465, Asn466, and Ser478. But the molecular arrangement moves away the C346-SH group, which then becomes less accessible for bonding. The same observation was made with the weakest ligand, withanone. In this case, the compound is positioned close to the upper lip of the protein, with a short H-bond distance between Arg465 and the C1-carbonyl and C5-OH (2.6 Å), thus placing the C3 position away from the C346-SH group. On the opposite side, the same H-bond distances are longer with withangulatin A (3.0–3.3 Å), positioning the ligand closer to the C346-SH group, and thus favoring the realization of the covalent link. The spatial distance between the C346-SH group and the C3 position of the different withanolides tested here varied from 3.0 Å to >5.0 Å. The shortest distance was observed with withangulatin A (Figure 8).
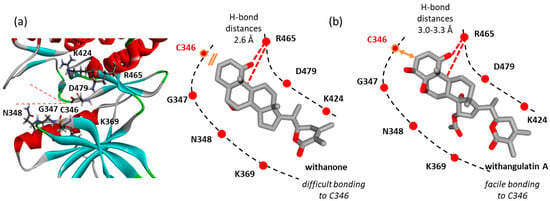
Figure 8.
Withangulatin A binding to the kinase site. (a) A view of the binding area with the two lips of the site. A hinge region separates two zones with residues K369, C346, G347, and N348 on one side and K424, R465, and D479 on the other side. (b) Withanone becomes close to R465 but distant from C346, whereas withangulatin A remains close to C346, at a distance suitable for covalent binding to C346.
Withangulatin A was found to be the best ligand in the series, with a strong capability to form stable complexes with the kinase. It surpassed all the other withanolide derivatives with a free energy of interaction largely more negative (about 35%) compared to WFA and the other compounds (Table 1). The compound is ideally shaped to interact with the enzyme. The binding (non-covalent) step places the ligand in the kinase domain through the H-bond interaction with two key lysine residues, Lys369 and Lys424. The formation of the covalent linkage slightly modifies the positioning of the drug in its site, but the H-bond between the lactone unit and Lys424 is maintained (Figure 9).
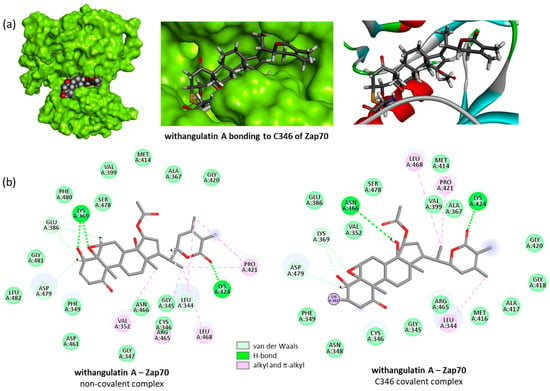
Figure 9.
Binding and bonding of withangulatin A to Zap70-active kinase domain. (a) Molecular model of the drug–protein complex, with a close-up view of the ligand covalently bound to C346. (b) Binding map contacts for withangulatin A non-covalently and covalently bound to Zap70 (color code indicated).
To sum it up, the docking analysis suggested that WFA can form covalent complexes with the Cys346 of Zap70 kinase, and similar stable complexes can form with a few other withanolides including ixocarpalactone A, physapubescin, tubocapsenolide A, and withaperuvin. But one product stands out with its high capability for binding to Zap70 kinase: withangulatin A. This antitumor natural product exhibits a remarkable ability for bonding to the Cys346 thiol residue of Zap70, surpassing all other withanolides in terms of protein-binding capability.
3.4. Comparative Reactivity and Druggability of Withangulatin A and WFA
The discovery of withangulatin A as a potential top binder to Zap70 prompted us to further analyze this natural product as a drug candidate. We used additional in silico methods, notably the Density Functional Theory (DFT) and machine learning approaches, to evaluate the relative chemical stability and reactivity of this compound compared to WFA. The DFT analysis, performed at the B3LYP-D3/def2-SVP computational level of theory, allowed us to compare their electronic behavior. The calculation showed that the two compounds exhibit similar HOMO and LUMO energies. The HOMO-LUMO gap energy (about 3 eV) is comparable for the two compounds, thus suggesting an identical chemical stability (Figure 10). However, the two compounds have different charge density locations in the HOMO and LUMO. For instance, the LUMO and HOMO in WFA are mainly located on the A-ring around the enone moiety, whereas in withangulatin A, the LUMO has the charge density on the enone part of the ring A, but the HOMO has electron density on the D-ring (Figure 10b). For WFA, both the HOMO and LUMO are located on the same side of the molecular plane near the reactive position (C23/C31). For withangulatin A, the orbital interactions are different, with more distance between the HOMO and LUMO sites. It seems that the presence of the acetyl group (at C5) of withangulatin A can affect the rate at which an electronic transition can occur between orbitals. The DFT analysis predicts that the two natural products are equally stable, but the enone moiety of WFA may be slightly more reactive than that of withangulatin A.
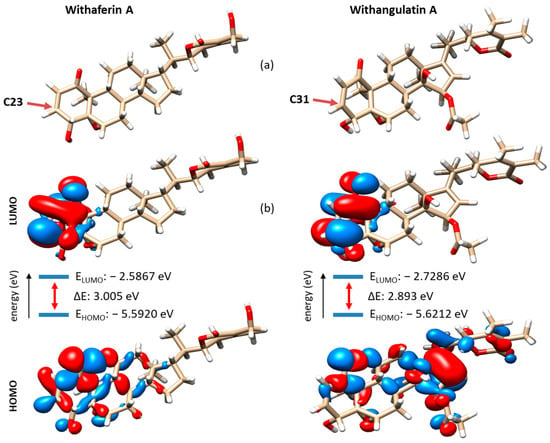
Figure 10.
(a) Optimized structures of withaferin A and withangulatin A. The arrow points to the most nucleophilic site, as detailed in Figure S1. (b) Electron density distribution of HOMO and LUMO. The energy band gap is indicated with the respective frontier molecular orbital energies (E = ELUMO − EHOMO).
In parallel, we used Fukui functions and the dual descriptor to obtain quantitative measures of the nucleophilicity and electrophilicity of all atoms in the two compounds. Data for the carbon and oxygen atoms are shown in Figure S1 (H atoms are not shown for clarity, but all atomic positions were analyzed). The analysis clearly shows that the most reactive C-site of the molecule is the expected C3 enone position (labeled C23 and C31 for WFA and withangulatin A, respectively, in Figure S1). This C3 position is significantly more reactive than the two carbons of the epoxide and also corresponds to the most sterically accessible position, as shown out in Figure 10a. It is also interesting to note that the nucleophilicity index measured for WFA at position C5 (position C23 in Figure S1: nucleophilicity 0.07713) is lower than that measured for withangulatin A at the same position (position C31 in Figure S1: nucleophilicity 0.08533). Therefore, this specific position in withangulatin A may be more reactive than initially expected based on the DFT calculations mentioned above.
The expression of Fukui functions (interpreted as a variation in nucleophilicity or electrophilicity) points out to a locus in the chemical reactivity of the withanolides. To our knowledge, such an analysis has never been performed before. The only related work refers to a quantum mechanical study, also based on DFT calculations, with two different withanolides: withacoagulin J and withanolide H isolated from W. coagulans [59]. The band gap was a little larger with these two compounds (4.8 eV). Withanolide H, which has a reactive enone in the A-ring unlike withacoagulin J, showed a HOMO/LUMO pattern comparable to that of WFA. This type of analysis is useful to predict the chemical reactivity of the molecules.
Next, we used machine learning models to predict the physicochemical and metabolic properties of the two compounds. As expected, the machine learning model SolTranNet predicted a very low aqueous solubility for the two compounds, with logS values of −4.87 and −5.26 for WFA and withangulatin A, respectively (a soluble compound is defined as a compound with logS > −4 [53]). Such compounds require the development of a solvent-based formulation or liposomal drug delivery system, as proposed for WFA [60,61]. The attention-based graph neural network PredPS [52] predicted that the two molecules are relatively unstable in human plasma (with probability scores of 0.83 and 0.98 for WFA and withangulatin A, respectively). We also tested the random forest model PredMS [62] to predict their metabolic stability, but in this case, the calculated probability scores were considered unreliable (0.47 and 0.54 for WFA and withangulatin A, respectively). Nevertheless, WFA was predicted to be more stable than withangulatin A.
The predictive models of FP-ADMET [63] were used to compare the ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties of the two compounds. The system is based on a repository of molecular fingerprints, which essentially correspond to drug-like molecules. In our case, the predictions have a relatively low confidence and credibility because this type of complex molecule is underrepresented in the training set. Nevertheless, the machine learning model pointed out three noteworthy aspects: (i) The two compounds seem to be phototoxic in vitro (parameter credibility 0.62–0.75) but not phototoxic to humans; (ii) the predicted hERG cardiotoxicity was negative for the two compounds, and, similarly, they do not present myotoxicity; and (iii) the predicted urinary toxicity was negative for WFA but positive for withangulatin A. However, these machine learning results should be treated with caution, as the training sets contain few examples of such complex compounds. A larger training set would be required to refine the predictions of their ADMET properties in addition to experimental measurements.
4. Discussion
The medicinal use of the plant Withania somnifera (L.) Dunal is well recognized, notably in the traditional Indian medical system to treat inflammatory diseases, microbial infections, hepatic dysfunctions, and other ailments. The plant is also used to treat cancer and in the prevention of neurodegenerative diseases [64,65,66]. W. somnifera is a rich source of bioactive molecules, in particular withanolides endowed with anticancer properties [21,23]. The lead compound is withaferin A (WFA), which has revealed a large panel of pharmacological activities useful to combat inflammation, cancer, and neuropathological disorders [25]. It is a highly potent withanolide capable of binding to many proteins and triggering covalent reactions with cysteine residues of various proteins including NFκB, SERCA-2, β-tubulin, vimentin, and a few enzymes such as Pin1 and Zap70 [27]. Up to now, WFA is the only withanolide known to bind to Zap70, which is a key tyrosine kinase implicated in T-cell immunity. The covalent binding of WFA to the exposed cysteines of Zap70 has been evidenced recently [28]. This study paved the way for the discovery of additional binders with a withanolide scaffold similar to WFA. In this frame, we selected 12 withanolides equipped with a reactive α,β-unsaturated ketone moiety to compare their ability to form covalent complexes with the kinase. The selected withanolides include compounds previously characterized as potent anticancer agents, such as physapruin A, 4β-hydroxywithanolide E, and withanone [41,67,68]. Our molecular docking approach is comparable to that used recently to study other anticancer agents [69,70].
The molecular docking analysis points to seven compounds susceptible to form stable complexes with Zap70: 4β-hydroxywithanolide E, ixocarpalactone A, physapubescin, tubocapsenolide A, WFA, withaperuvin, and withangulatin A. The first six compounds exhibit a roughly equal capability to engage molecular interactions in the active site of Zap70. In particular, 4β-hydroxywithanolide E, withaperuvin, and ixocarpalactone A display the same high ability to form stable complexes with Zap70. The analysis of the structure-binding relationships in the series is difficult due to the complexity of the molecules. However, the following four criteria can be underlined: (i) The C5-C6 epoxide moiety of WFA is not absolutely required for kinase interaction (not present in whitaperuvin A), (ii) a hydroxyl group at C16 contributes favorably to the kinase interaction (as observed with ixocarpalactone A and tubocapsenolide A), (iii) the presence of a hydroxyl group at C20 reinforces the protein interaction, and (iv) in all cases, the lactone E-ring plays an important role in the protein interaction. Thus, the docking analysis suggests clearly that WFA is not the only withanolide susceptible to forming covalent complexes with Zap70.
One compound emerges from the study as a promising Zap70 binder: withangulatin A, which is a well-known anti-inflammatory, immuno-suppressive, and antifibrotic agent [71,72,73]. This withanolide, essentially isolated from Physalis angulata L. [55,56], has been characterized previously as a covalent protein binder. Withangulatin A has been shown to form cysteine-mediated covalent complexes with the enzymes 3-phosphoglycerate dehydrogenase (PHGDH) [35], peroxiredoxin 6 (Prdx-6) [36], and sarco/endoplasmic reticulum calcium-ATPase 2 (SERCA-2) [37]. It is also an inhibitor of glutaminase 1 (IC50 = 18.2 μM) [74] and a regulator of the expression of cyclooxygenase 2 [75] and ADP-ribosylation factor 6 (ARF6) [76]. This multitargeted reactive molecule can interfere with different processes in cells. Here, we provide in silico evidence that it is also a potential strong binder to the kinase Zap70.
Withangulatin A surpassed all the other tested withanolides in terms of complex formation and reactivity with the Cys346 position of Zap70. This cysteine residue located at the entrance of the active site represents a more attractive site than those located on the external surface of the protein, such as C560-C564. The product fits well into the active site of the kinase, placing its reactive enone moiety close to the thiol group of Cys346. The model suggests that a covalent reaction can occur easily to generate a stable protein–drug adduct, as described experimentally with WFA. A detailed analysis of the reactivity of the molecule, using DFT calculations, indicates that the enone moiety of withangulatin A exhibits a high reactivity, with the C3 position (labeled C31 in Figure 10) as the main reactive site. The docking analysis was performed with a catalytic subunit of Zap70 corresponding to the active kinase domain of the protein (open conformation). It would be useful to determine if the covalent binding of WFA can also occur when the protein is in a closed, autoinhibited conformation, so as to prevent the opening of its conformation [77].
The ADMET profile of withangulatin A suggests that it is a druggable molecule. The only point to watch out for is potential urinary toxicity, but apart from that, the molecule does not present major toxic aspects or unacceptable properties. This natural product exhibits a low oral bioavailability, and it can easily hydrolyze in human plasma [78]. Withangulatin A has already been exploited in drug design strategies. Recently, Zhou and coworkers have synthesized two large series of withangulatin derivatives and identified potent antiproliferative agents, including a C-4 ester derivative 70 times more potent than the parent compound, withangulatin A [74,79]. In the same vein, semisynthetic analogs of withangulatin A targeting thioredoxin reductase have been designed, and potent cysteine-reactive analogs bearing an α,β-unsaturated ketone have been identified [80]. Other derivatives targeting the allosteric site of glutaminase C have been proposed recently [81]. According to our calculations, withangulatin A is a potential hit compound, which can be used to guide the design of drug candidates. Zap70 shall be considered as a potential target for these compounds.
5. Conclusions
The computational study has identified withangulatin A as a potential inhibitor of the kinase Zap70 via covalent binding to the Cys346 residue in the enzyme active site. This compound emerged as the best ligand among a series of 12 withanolides equipped with a thiol-reactive, α,β-unsaturated ketone. The study paves the way for the design of novel Zap70 inhibitors, which are needed to improve the treatment of lymphoid malignancies and autoimmune diseases associated with an abnormal T-cell response.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/computation13090207/s1, Figure S1: Condensed Fukui functions and dual descriptors for the heavy atoms of withaferin A and withangulatin A.
Author Contributions
C.B. (Corentin Bedart), Methodology, Formal analysis, Software, Writing—original draft, and Writing—review and editing; G.V., Methodology, Software; C.B. (Christian Bailly), Conceptualization, Investigation, Supervision, Writing—original draft, and Writing—review and editing; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
Author Christian Bailly was employed by the company OncoWitan. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CLL | Chronic lymphocytic leukemia |
| DFT | Density functional theory |
| 4β-HWNE | 4β-hydroxywithanolide E |
| WFA | Withaferin A |
| Zap70 | Zeta-chain associated protein kinase 70 kDa |
References
- Ashouri, J.F.; Lo, W.L.; Nguyen, T.T.T.; Shen, L.; Weiss, A. ZAP70, too little, too much can lead to autoimmunity. Immunol. Rev. 2022, 307, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, B.B.; Shah, N.H.; Shen, L.; Weiss, A. ZAP-70 in Signaling, Biology, and Disease. Annu. Rev. Immunol. 2018, 36, 127–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Moore, A.; Ringshausen, I. ZAP-70 Shapes the Immune Microenvironment in B Cell Malignancies. Front. Oncol. 2020, 10, 595832. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Yang, J.; Bi, Y.; Wang, H. ZAP-70 in chronic lymphocytic leukemia: A meta-analysis. Clin. Chim. Acta 2018, 483, 82–88. [Google Scholar] [CrossRef]
- Demir, M.; Cizmecioglu, O. ZAP70 Activation Compensates for Loss of Class IA PI3K Isoforms Through Activation of the JAK-STAT3 Pathway. Cancer Diagn. Progn. 2022, 2, 391–404. [Google Scholar] [CrossRef]
- Ren, L.; Li, P.; Li, Z.; Chen, Q. AQP9 and ZAP70 as immune-related prognostic biomarkers suppress proliferation, migration and invasion of laryngeal cancer cells. BMC Cancer 2022, 22, 465. [Google Scholar] [CrossRef]
- Yang, M.L.; Lam, T.T.; Kanyo, J.; Kang, I.; Zhou, Z.S.; Clarke, S.G.; Mamula, M.J. Natural isoaspartyl protein modification of ZAP70 alters T cell responses in lupus. Autoimmunity 2023, 56, 2282945. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, M.; Silakari, O. Insight into the therapeutic aspects of ‘Zeta-Chain Associated Protein Kinase 70 kDa’ inhibitors: A review. Cell Signal. 2014, 26, 2481–2492. [Google Scholar] [CrossRef]
- Visperas, P.R.; Wilson, C.G.; Winger, J.A.; Yan, Q.; Lin, K.; Arkin, M.R.; Weiss, A.; Kuriyan, J. Identification of Inhibitors of the Association of ZAP-70 with the T Cell Receptor by High-Throughput Screen. SLAS Discov. 2017, 22, 324–331. [Google Scholar] [CrossRef]
- Rao, D.; Li, H.; Ren, X.; Sun, Y.; Wen, C.; Zheng, M.; Huang, H.; Tang, W.; Xu, S. Discovery of a potent, selective, and covalent ZAP-70 kinase inhibitor. Eur. J. Med. Chem. 2021, 219, 113393. [Google Scholar] [CrossRef]
- Masip, V.; Lirio, Á.; Sánchez-López, A.; Cuenca, A.B.; Puig de la Bellacasa, R.; Abrisqueta, P.; Teixidó, J.; Borrell, J.I.; Gibert, A.; Estrada-Tejedor, R. Expanding the Diversity at the C-4 Position of Pyrido[2,3-d]pyrimidin-7(8H)-ones to Achieve Biological Activity against ZAP-70. Pharmaceuticals 2021, 14, 1311. [Google Scholar] [CrossRef]
- Khlebnikov, A.I.; Schepetkin, I.A.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Kirpotina, L.N.; Quinn, M.T. Inhibition of T Cell Receptor Activation by Semi-Synthetic Sesquiterpene Lactone Derivatives and Molecular Modeling of Their Interaction with Glutathione and Tyrosine Kinase ZAP-70. Molecules 2019, 24, 350. [Google Scholar] [CrossRef] [PubMed]
- Deindl, S.; Kadlecek, T.A.; Brdicka, T.; Cao, X.; Weiss, A.; Kuriyan, J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell 2007, 129, 735–746. [Google Scholar] [CrossRef]
- Jin, L.; Pluskey, S.; Petrella, E.C.; Cantin, S.M.; Gorga, J.C.; Rynkiewicz, M.J.; Pandey, P.; Strickler, J.E.; Babine, R.E.; Weaver, D.T.; et al. The three-dimensional structure of the ZAP-70 kinase domain in complex with staurosporine: Implications for the design of selective inhibitors. J. Biol. Chem. 2004, 279, 42818–42825. [Google Scholar] [CrossRef]
- Huber, R.G.; Fan, H.; Bond, P.J. The Structural Basis for Activation and Inhibition of ZAP-70 Kinase Domain. PLoS Comput. Biol. 2015, 11, e1004560. [Google Scholar] [CrossRef]
- Visperas, P.R.; Winger, J.A.; Horton, T.M.; Shah, N.H.; Aum, D.J.; Tao, A.; Barros, T.; Yan, Q.; Wilson, C.G.; Arkin, M.R.; et al. Modification by covalent reaction or oxidation of cysteine residues in the tandem-SH2 domains of ZAP-70 and Syk can block phosphopeptide binding. Biochem. J. 2015, 465, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Schnurra, M.; El-Bizri, A.; Woessner, N.M.; Hartmann, S.; Hartig, R.; Minguet, S.; Schraven, B.; Simeoni, L. A Cysteine Residue within the Kinase Domain of Zap70 Regulates Lck Activity and Proximal TCR Signaling. Cells 2022, 11, 2723. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Shayahati, B.; Fan, Y.; Akimzhanov, A.M. T cell receptor-dependent S-acylation of ZAP-70 controls activation of T cells. J. Biol. Chem. 2021, 296, 100311. [Google Scholar] [CrossRef]
- Rao, D.; Yang, T.; Feng, H.; An, Q.; Zhang, S.; Yu, J.; Ren, X.; Diao, X.; Huang, H.; Tang, W.; et al. Discovery and Structural Optimization of Covalent ZAP-70 Kinase Inhibitors against Psoriasis. J. Med. Chem. 2023, 66, 12018–12032. [Google Scholar] [CrossRef]
- Boike, L.; Henning, N.J.; Nomura, D.K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 2022, 21, 881–898. [Google Scholar] [CrossRef]
- Yadav, N.; Tripathi, S.; Sangwan, N.S. Phyto-therapeutic potential of Withania somnifera: Molecular mechanism and health implications. Phytother. Res. 2024, 38, 1695–1714. [Google Scholar] [CrossRef]
- Xing, Z.; Su, A.; Mi, L.; Zhang, Y.; He, T.; Qiu, Y.; Wei, T.; Li, Z.; Zhu, J.; Wu, W. Withaferin A: A Dietary Supplement with Promising Potential as an Anti-Tumor Therapeutic for Cancer Treatment—Pharmacology and Mechanisms. Drug Des. Devel Ther. 2023, 17, 2909–2929. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cao, S.; Kang, N.; Qiu, F. Withanolides: Promising candidates for cancer therapy. Phytother. Res. 2024, 38, 1104–1158. [Google Scholar] [CrossRef]
- Kumar, S.; Mathew, S.O.; Aharwal, R.P.; Tulli, H.S.; Mohan, C.D.; Sethi, G.; Ahn, K.S.; Webber, K.; Sandhu, S.S.; Bishayee, A. Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal. Pharmaceuticals 2023, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Banik, S.P.; Goel, A.; Chakraborty, S.; Bagchi, M.; Bagchi, D. Revisiting the Multifaceted Therapeutic Potential of Withaferin A (WA), a Novel Steroidal Lactone, W-ferinAmax Ashwagandha, from Withania somnifera (L) Dunal. J. Am. Nutr. Assoc. 2024, 43, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Abeesh, P.; Guruvayoorappan, C. The Therapeutic Effects of Withaferin A against Cancer: Overview and Updates. Curr. Mol. Med. 2024, 24, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Covalent binding of withanolides to cysteines of protein targets. Biochem. Pharmacol. 2024, 226, 116405. [Google Scholar] [CrossRef]
- Fazil, M.H.U.T.; Chirumamilla, C.S.; Perez-Novo, C.; Wong, B.H.S.; Kumar, S.; Sze, S.K.; Vanden Berghe, W.; Verma, N.K. The steroidal lactone withaferin A impedes T-cell motility by inhibiting the kinase ZAP70 and subsequent kinome signaling. J. Biol. Chem. 2021, 297, 101377. [Google Scholar] [CrossRef]
- Fuska, J.; Fusková, A.; Rosazza, J.P.; Nicholas, A.W. Novel cytotoxic and antitumor agents. IV. Withaferin A: Relation of its structure to the in vitro cytotoxic effects on P388 cells. Neoplasma 1984, 31, 31–36. [Google Scholar]
- Nicholas, A.W.; Rosazza, J.P. Reactions of withaferin-A with model biological nucleophiles. Bioorg Chem. 1976, 5, 367–372. [Google Scholar] [CrossRef]
- Rabhi, C.; Arcile, G.; Le Goff, G.; Da Costa Noble, C.; Ouazzani, J. Neuroprotective Effect of CR-777, a Glutathione Derivative of Withaferin A, Obtained through the Bioconversion of Withania somnifera (L.) Dunal Extract by the Fungus Beauveria bassiana. Molecules 2019, 24, 4599. [Google Scholar] [CrossRef]
- Goode, K.M.; Petrov, D.P.; Vickman, R.E.; Crist, S.A.; Pascuzzi, P.E.; Ratliff, T.L.; Davisson, V.J.; Hazbun, T.R. Targeting the Hsp90 C-terminal domain to induce allosteric inhibition and selective client downregulation. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1992–2006. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chang, F.R.; Huang, Z.Y.; Chen, J.H.; Wu, Y.C.; Wu, C.C. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J. Biol. Chem. 2008, 283, 17184–17193. [Google Scholar] [CrossRef]
- Yang, W.J.; Chen, X.M.; Wang, S.Q.; Hu, H.X.; Cheng, X.P.; Xu, L.T.; Ren, D.M.; Wang, X.N.; Zhao, B.B.; Lou, H.X.; et al. 4β-Hydroxywithanolide E from Goldenberry (Whole Fruits of Physalis peruviana L.) as a Promising Agent against Chronic Obstructive Pulmonary Disease. J. Nat. Prod. 2020, 83, 1217–1228. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, T.; Liu, X.; Zhu, D.; Zhang, Y.; Wu, S.; Han, C.; Zhang, H.; Luo, J.; Kong, L. Identification of a novel PHGDH covalent inhibitor by chemical proteomics and phenotypic profiling. Acta Pharm. Sin. B 2022, 12, 246–261. [Google Scholar] [CrossRef]
- Chen, C.; Gong, L.; Liu, X.; Zhu, T.; Zhou, W.; Kong, L.; Luo, J. Identification of peroxiredoxin 6 as a direct target of withangulatin A by quantitative chemical proteomics in non-small cell lung cancer. Redox Biol. 2021, 46, 102130. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, C.; Wang, S.; Zhang, Y.; Zhu, D.; Li, L.; Luo, J.; Kong, L. Cellular target identification of Withangulatin A using fluorescent analogues and subsequent chemical proteomics. Chem. Commun. 2019, 55, 8231–8234. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.J.; Cheng, Y.B.; Lin, L.C.; Tsai, Y.H.; Yao, B.Y.; Tang, J.Y.; Chang, F.R.; Yen, C.H.; Ou-Yang, F.; Chang, H.W. Physalis peruviana-Derived Physapruin A (PHA) Inhibits Breast Cancer Cell Proliferation and Induces Oxidative-Stress-Mediated Apoptosis and DNA Damage. Antioxidants 2021, 10, 393. [Google Scholar] [CrossRef]
- Yu, T.J.; Shiau, J.P.; Tang, J.Y.; Yen, C.H.; Hou, M.F.; Cheng, Y.B.; Shu, C.W.; Chang, H.W. Physapruin A Induces Reactive Oxygen Species to Trigger Cytoprotective Autophagy of Breast Cancer Cells. Antioxidants 2022, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.J.; Yen, C.Y.; Cheng, Y.B.; Yen, C.H.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Physapruin A Enhances DNA Damage and Inhibits DNA Repair to Suppress Oral Cancer Cell Proliferation. Int. J. Mol. Sci. 2022, 23, 8839. [Google Scholar] [CrossRef]
- Yu, T.J.; Shiau, J.P.; Tang, J.Y.; Farooqi, A.A.; Cheng, Y.B.; Hou, M.F.; Yen, C.H.; Chang, H.W. Physapruin A Exerts Endoplasmic Reticulum Stress to Trigger Breast Cancer Cell Apoptosis via Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 8853. [Google Scholar] [CrossRef]
- Chen, Y.M.; Xu, W.; Liu, Y.; Zhang, J.H.; Yang, Y.Y.; Wang, Z.W.; Sun, D.J.; Li, H.; Liu, B.; Chen, L.X. Anomanolide C suppresses tumor progression and metastasis by ubiquitinating GPX4-driven autophagy-dependent ferroptosis in triple negative breast cancer. Int. J. Biol. Sci. 2023, 19, 2531–2550. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. J. Comput. Chem. 2005, 26, 1689–1700. [Google Scholar] [CrossRef]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Meziane-Tani, M.; Lagant, P.; Semmoud, A.; Vergoten, G. The SPASIBA force field for chondroitin sulfate: Vibrational analysis of D-glucuronic and N-acetyl-D-galactosamine 4-sulfate sodium salts. J. Phys. Chem. A 2006, 110, 11359–11370. [Google Scholar] [CrossRef] [PubMed]
- Lagant, P.; Nolde, D.; Stote, R.; Vergoten, G.; Karplus, M. Increasing normal modes analysis accuracy: The SPASIBA spectroscopic force field introduced into the CHARMM program. J. Phys. Chem. A 2004, 108, 4019–4029. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Ulmschneider, J.P.; Tirado-Rives, J. Free energies of hydration from a generalized Born model and an ALL-atom force field. J. Phys. Chem. B 2004, 108, 16264–16270. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Molecular docking of cryptoconcatones to α-tubulin and related pironetin analogues. Plants 2023, 12, 296. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Interaction of microcolin cyanobacterial lipopeptides with phosphatidylinositol transfer protein (PITP)—Molecular docking analysis. Future Pharmacol. 2025, 5, 13. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Liu, Z.; Zubatiuk, T.; Roitberg, A.; Isayev, O. Auto3D: Automatic Generation of the Low-Energy 3D Structures with ANI Neural Network Potentials. J. Chem. Inf. Model. 2022, 62, 5373–5382. [Google Scholar] [CrossRef]
- Francoeur, P.G.; Koes, D.R. SolTranNet-A Machine Learning Tool for Fast Aqueous Solubility Prediction. J. Chem. Inf. Model. 2021, 61, 2530–2536, Erratum in J. Chem. Inf. Model. 2021, 61, 4120–4123. [Google Scholar] [CrossRef]
- Jang, W.D.; Jang, J.; Song, J.S.; Ahn, S.; Oh, K.S. PredPS: Attention-based graph neural network for predicting stability of compounds in human plasma. Comput. Struct. Biotechnol. J. 2023, 21, 3532–3539. [Google Scholar] [CrossRef]
- Lee, S.W.; Pan, M.H.; Chen, C.M.; Chen, Z.T. Withangulatin I, a new cytotoxic withanolide from Physalis angulata. Chem. Pharm. Bull. 2008, 56, 234–236. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, Z.T.; Hsieh, C.H.; Li, W.S.; Wen, S.Y. Withangulatin A, a new withanolide from Physalis angulata. Heterocycles 1990, 31, 1371–1375. [Google Scholar] [CrossRef]
- Connolly, M.L. Solvent-accessible surfaces of proteins and nucleic acids. Science 1983, 221, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Richards, F.M. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 1971, 55, 379–400. [Google Scholar] [CrossRef]
- Khan, S.A.; Adhikari, A.; Ayub, K.; Farooq, A.; Mahar, S.; Qureshi, M.N.; Rauf, A.; Khan, S.B.; Ludwig, R.; Mahmood, T. Isolation, characterization and DFT studies of epoxy ring containing new withanolides from Withania coagulans Dunal. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 217, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Abeesh, P.; Vishnu, W.K.; Guruvayoorappan, C. Preparation and characterization of withaferin A loaded pegylated nanoliposomal formulation with high loading efficacy: In vitro and in vivo anti-tumour study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112335. [Google Scholar] [CrossRef]
- Abeesh, P.; Guruvayoorappan, C. Withaferin A-Encapsulated PEGylated Nanoliposomes Induce Apoptosis in B16F10 Melanoma Cells by Regulating Bcl2 and Bcl xl Genes and Mitigates Murine Solid Tumor Development. J. Environ. Pathol. Toxicol. Oncol. 2024, 43, 29–42. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Lee, J.H.; Lee, B.H.; Song, J.S.; Ahn, S.; Oh, K.S. PredMS: A random forest model for predicting metabolic stability of drug candidates in human liver microsomes. Bioinformatics 2022, 38, 364–368. [Google Scholar] [CrossRef]
- Venkatraman, V. FP-ADMET: A compendium of fingerprint-based ADMET prediction models. J. Cheminform. 2021, 13, 75. [Google Scholar] [CrossRef]
- Philips, C.A.; Theruvath, A.H. A comprehensive review on the hepatotoxicity of herbs used in the Indian (Ayush) systems of alternative medicine. Medicine 2024, 103, e37903. [Google Scholar] [CrossRef]
- Lerose, V.; Ponticelli, M.; Benedetto, N.; Carlucci, V.; Lela, L.; Tzvetkov, N.T.; Milella, L. Withania somnifera (L.) Dunal, a Potential Source of Phytochemicals for Treating Neurodegenerative Diseases: A Systematic Review. Plants 2024, 13, 771. [Google Scholar] [CrossRef]
- Kumar, P.; Banik, S.P.; Goel, A.; Chakraborty, S.; Bagchi, M.; Bagchi, D. A critical assessment of the whole plant-based phytotherapeutics from Withania somnifera (L.) Dunal with respect to safety and efficacy vis-a-vis leaf or root extract-based formulation. Toxicol. Mech. Methods 2023, 33, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.; Tune, B.X.J.; Wu, Y.S.; Batumalaie, K.; Sekar, M.; Sarker, M.M.R.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Gopinath, S.C.B. Withanone as an Emerging Anticancer Agent and Understanding Its Molecular Mechanisms: Experimental and Computational Evidence. Curr. Cancer Drug Targets 2025, 25, 574–585. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, L.; Chen, M.; Zhong, R.; Liu, J. Amelioration of systemic lupus erythematosus by Withangulatin A in MRL/lpr mice. J. Cell Biochem. 2011, 112, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Mosallam, A.M.; Ibrahim, A.O.A.; Badr, M.; Abdelmonsef, A.H. Novel 3-phenylquinazolin-2,4(1H,3H)-diones as dual VEGFR-2/c-Met-TK inhibitors: Design, synthesis, and biological evaluation. Sci. Rep. 2023, 13, 18567. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelhady, H.A.; Hassain, D.Z.H.; Abdelmonsef, A.H.; El-Naggar, M.; Elaasser, M.M.; Mahmoud, H.K. Thiazole-Based Thiosemicarbazones: Synthesis, Cytotoxicity Evaluation and Molecular Docking Study. Drug Des. Devel Ther. 2021, 15, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Hu, H.H.; Chang, F.R.; Tsai, J.Y.; Kuo, C.Y.; Wu, Y.C.; Wu, C.C. Different effects of 4beta-hydroxywithanolide E and withaferin A, two withanolides from Solanaceae plants, on the Akt signaling pathway in human breast cancer cells. Phytomedicine 2019, 53, 213–222. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.W.; Liu, P.; Yu, Y.J.; Ma, L.; Hu, L.H. Immunosuppression effect of Withangulatin A from Physalis angulata via heme oxygenase 1-dependent pathways. Process Biochem. 2011, 46, 482–488. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Wang, X.; Yu, L.; Hu, L.H.; Shen, X. Withagulatin A inhibits hepatic stellate cell viability and procollagen I production through Akt and Smad signaling pathways. Acta Pharmacol. Sin. 2010, 31, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.X.; Chen, C.; Liu, X.Q.; Li, Y.; Lin, Y.L.; Wu, X.T.; Kong, L.Y.; Luo, J.G. Discovery and optimization of withangulatin A derivatives as novel glutaminase 1 inhibitors for the treatment of triple-negative breast cancer. Eur. J. Med. Chem. 2021, 210, 112980. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, J.; Cui, D.; Li, J.; Yu, Y.; Ma, L.; Hu, L. Anti-inflammatory function of Withangulatin A by targeted inhibiting COX-2 expression via MAPK and NF-kappaB pathways. J. Cell Biochem. 2010, 109, 532–541. [Google Scholar] [CrossRef]
- Sun, D.J.; Yang, Y.Y.; Liu, Y.; Ma, X.X.; Li, H.; Chen, L.X. Identification of ADP-ribosylation factor 6 as the cellular target of withangulatin A against TNBC cells by ferroptosis. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Klammt, C.; Novotná, L.; Li, D.T.; Wolf, M.; Blount, A.; Zhang, K.; Fitchett, J.R.; Lillemeier, B.F. T cell receptor dwell times control the kinase activity of Zap70. Nat. Immunol. 2015, 16, 961–969. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; Li, N.; Meng, H.; Li, Z.; Luo, J.; Qiu, Z. Hydrolytic Metabolism of Withangulatin A Mediated by Serum Albumin Instead of Common Esterases in Plasma. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.X.; Chen, C.; Liu, X.Q.; Li, Y.; Kong, L.Y.; Luo, J.G. Synthesis and biological evaluation of novel withangulatin A derivatives as potential anticancer agents. Bioorg. Chem. 2021, 108, 104690. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Zhao, J.; Yang, H.; Yin, F.; Ding, M.; Luo, J.; Wang, X.; Kong, L. Design and SAR of Withangulatin A Analogues that Act as Covalent TrxR Inhibitors through the Michael Addition Reaction Showing Potential in Cancer Treatment. J. Med. Chem. 2020, 63, 11195–11214. [Google Scholar] [CrossRef]
- Saghiri, K.; Daoud, I.; Melkemi, N.; Mesli, F. Molecular docking/dynamics simulations, MEP analysis, and pharmacokinetics prediction of some withangulatin A derivatives as allosteric glutaminase C inhibitors in breast cancer. Chem. Data Collect. 2023, 46, 101044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).