Theoretical Insight into Non-Covalent Complexes of Closo-Borate Anions [BnHn−1X]y− with Glycine
Abstract
1. Introduction
2. Computational Details
3. Results
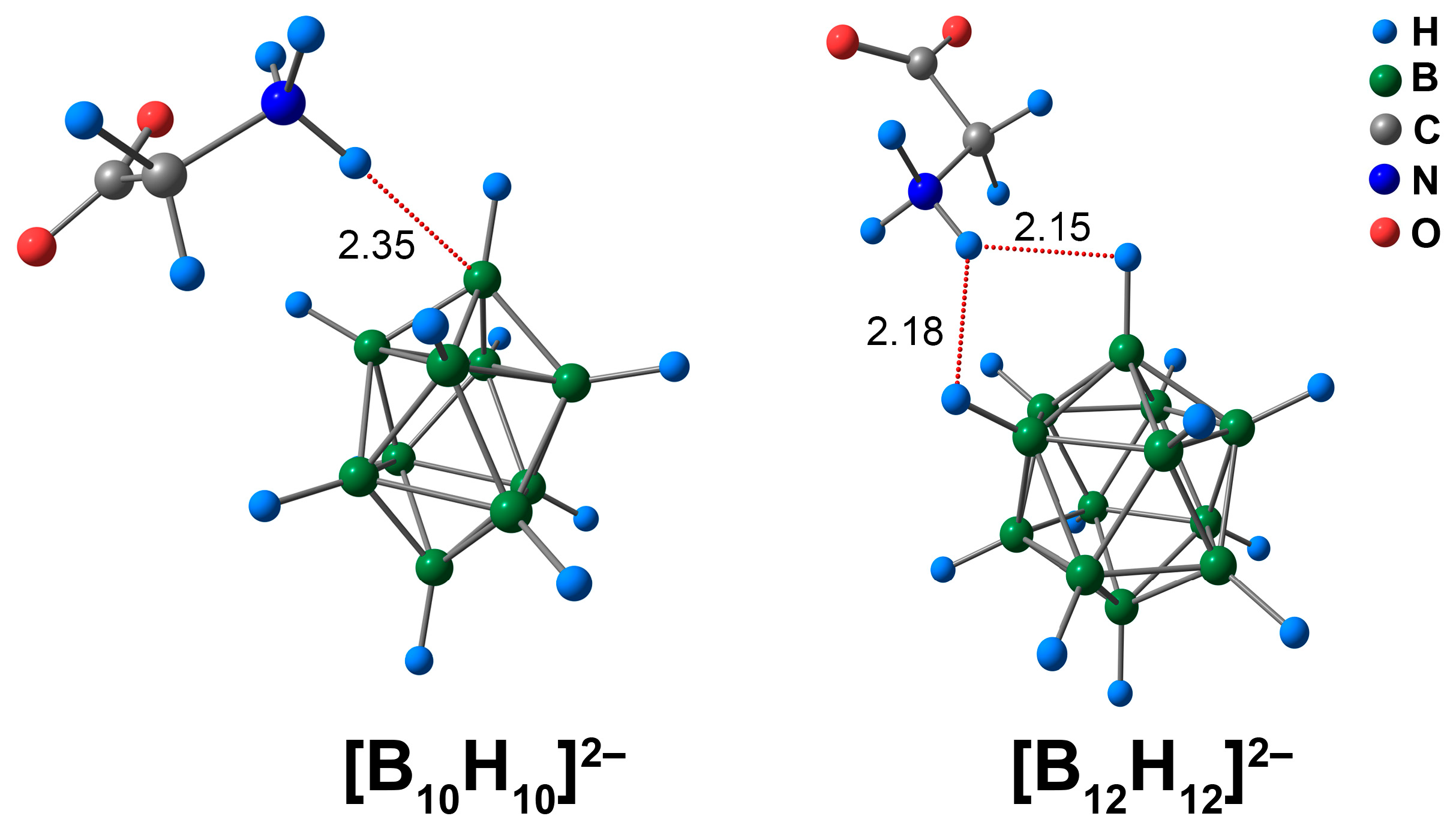
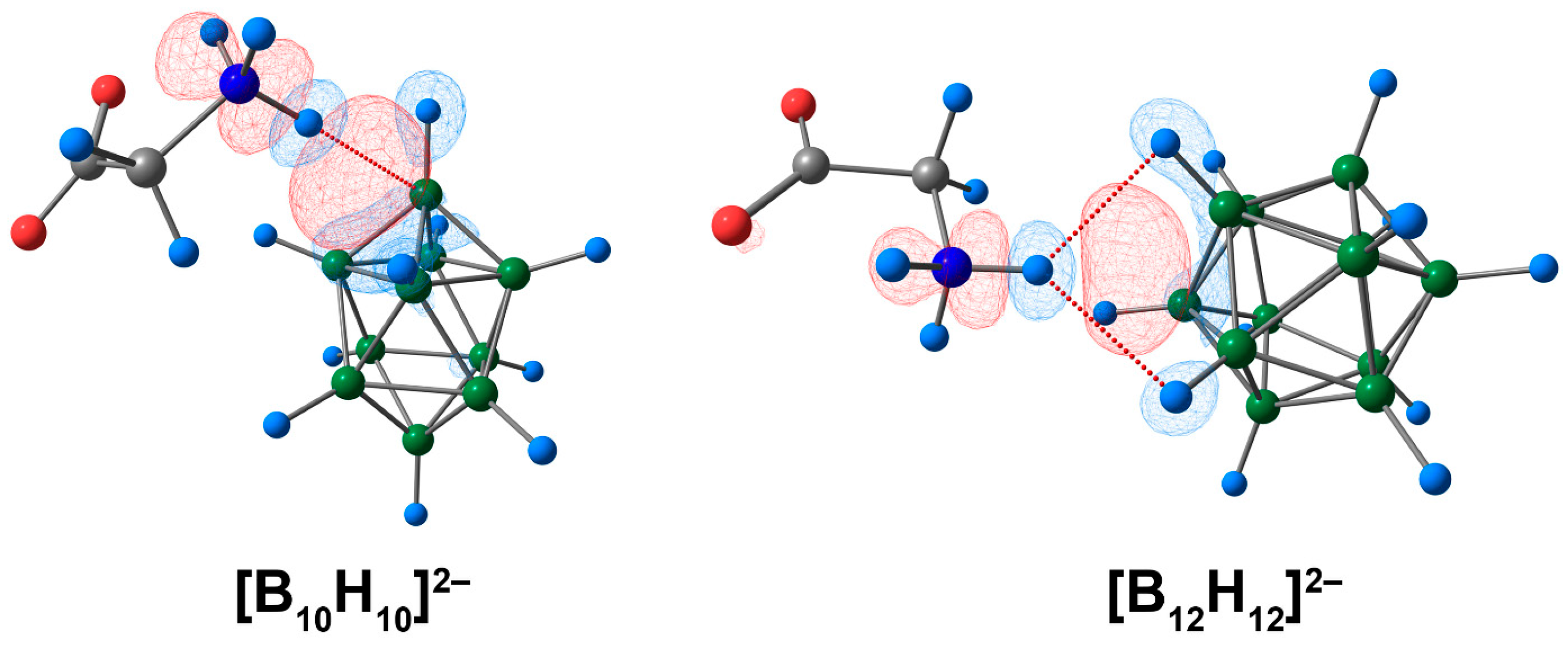
3.1. Complex of [BnHn]2− Clusters with Glycine
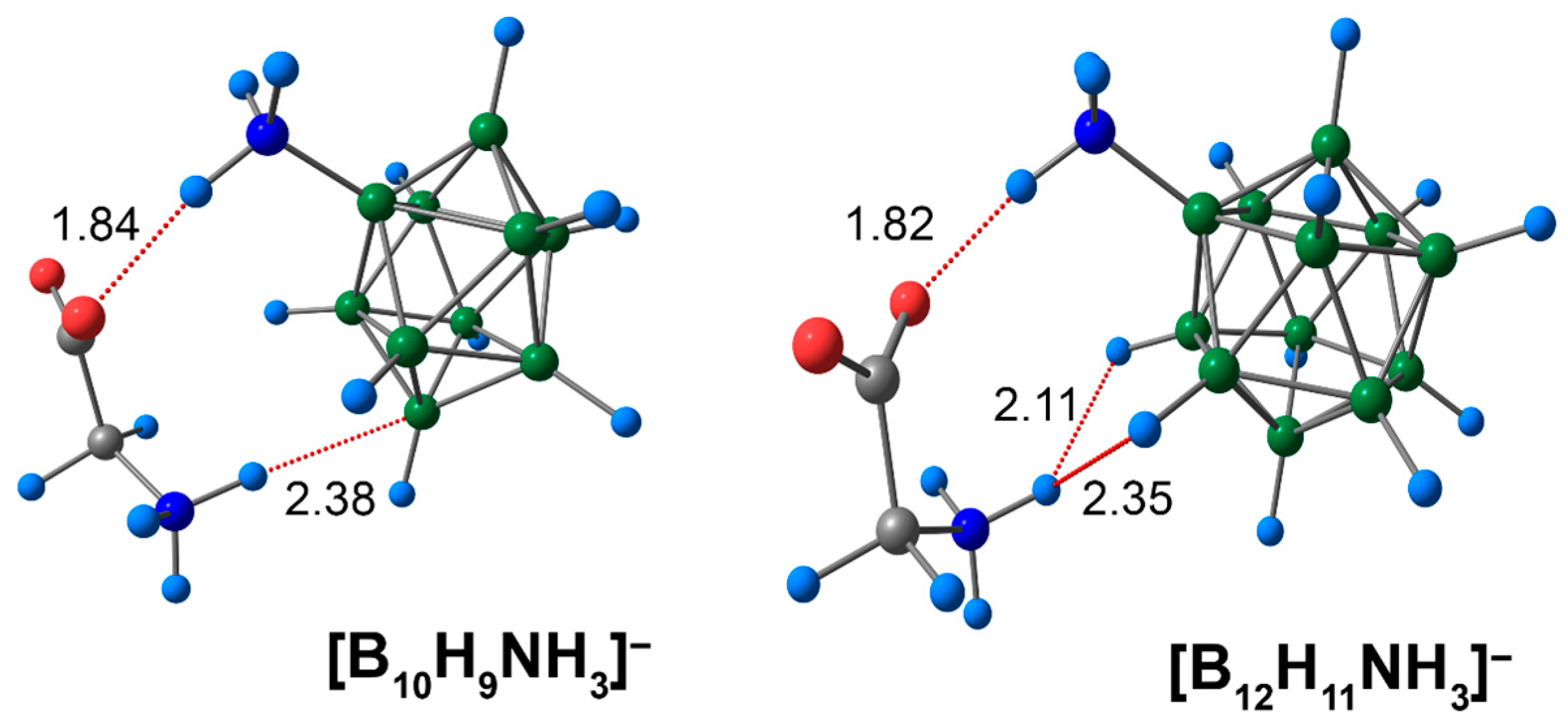
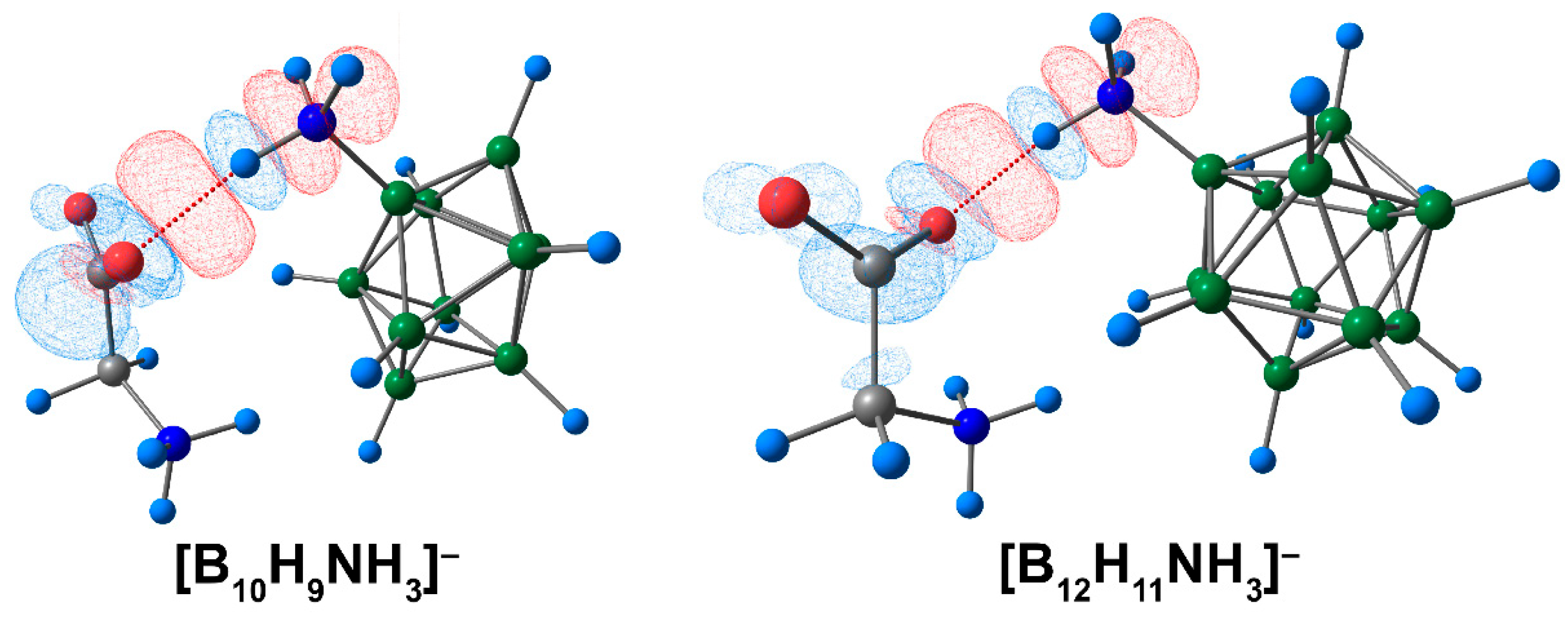
3.2. Complex of [BnHn−1NH3]− Clusters with Glycine
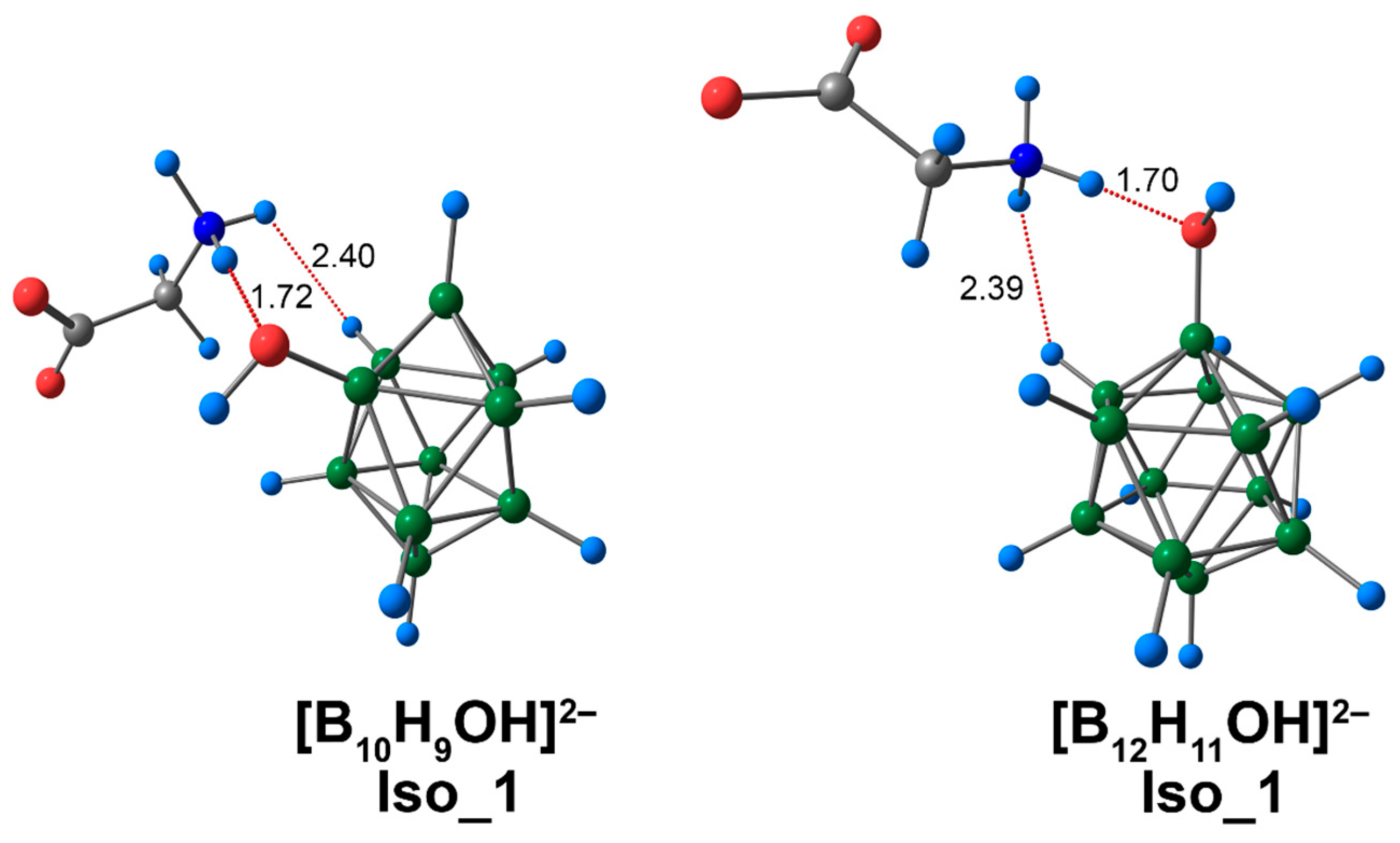
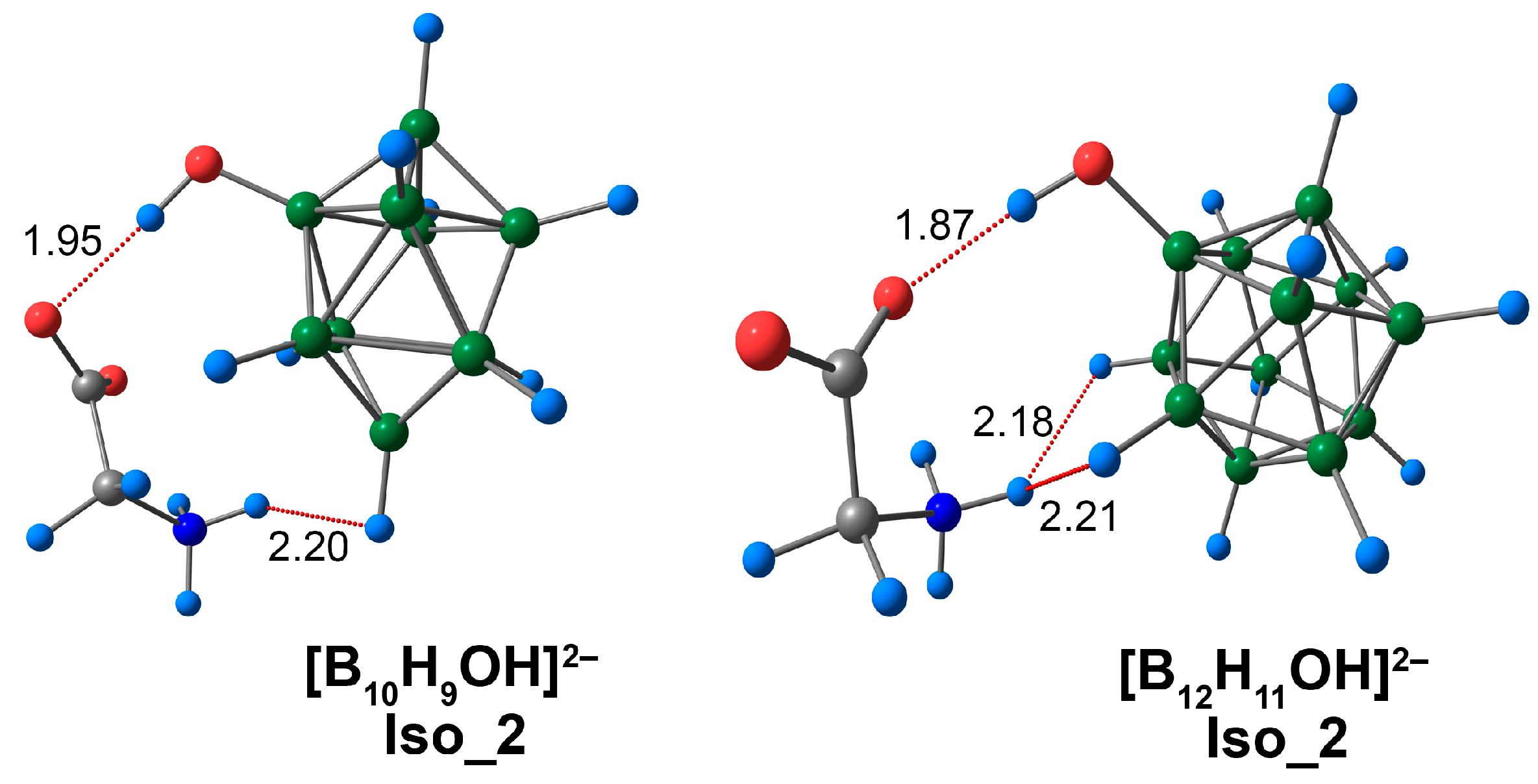
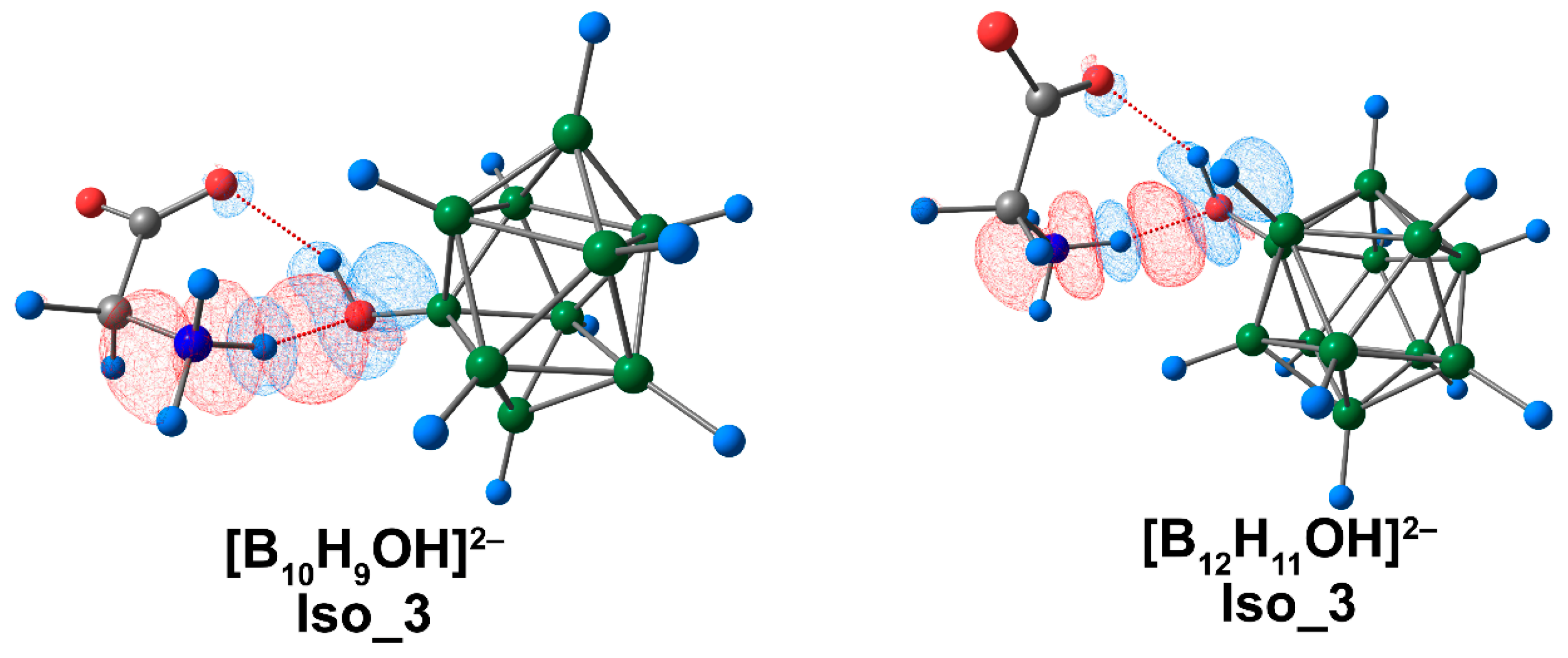
3.3. Complex of [BnHn−1OH]2− Clusters with Glycine
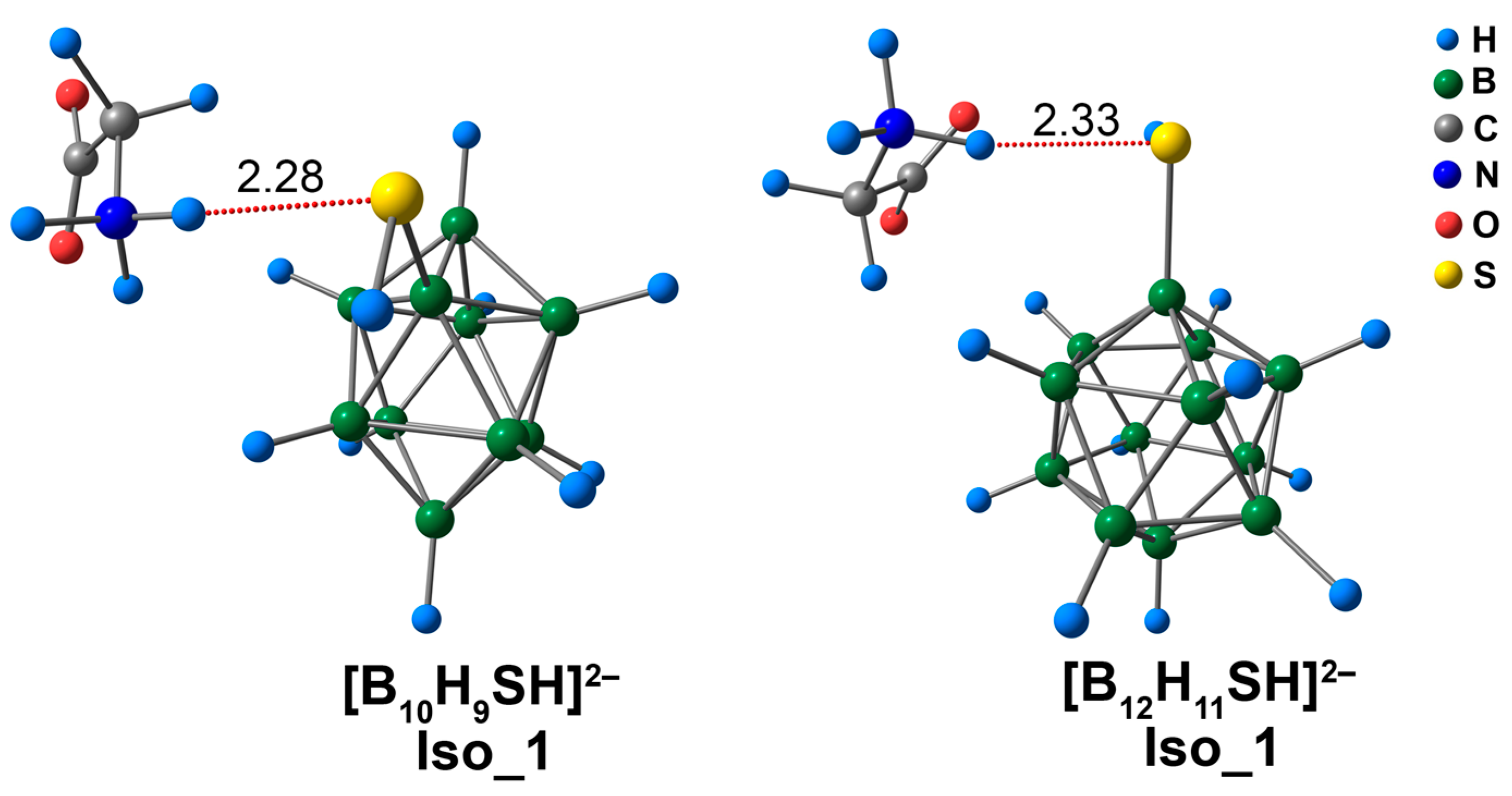
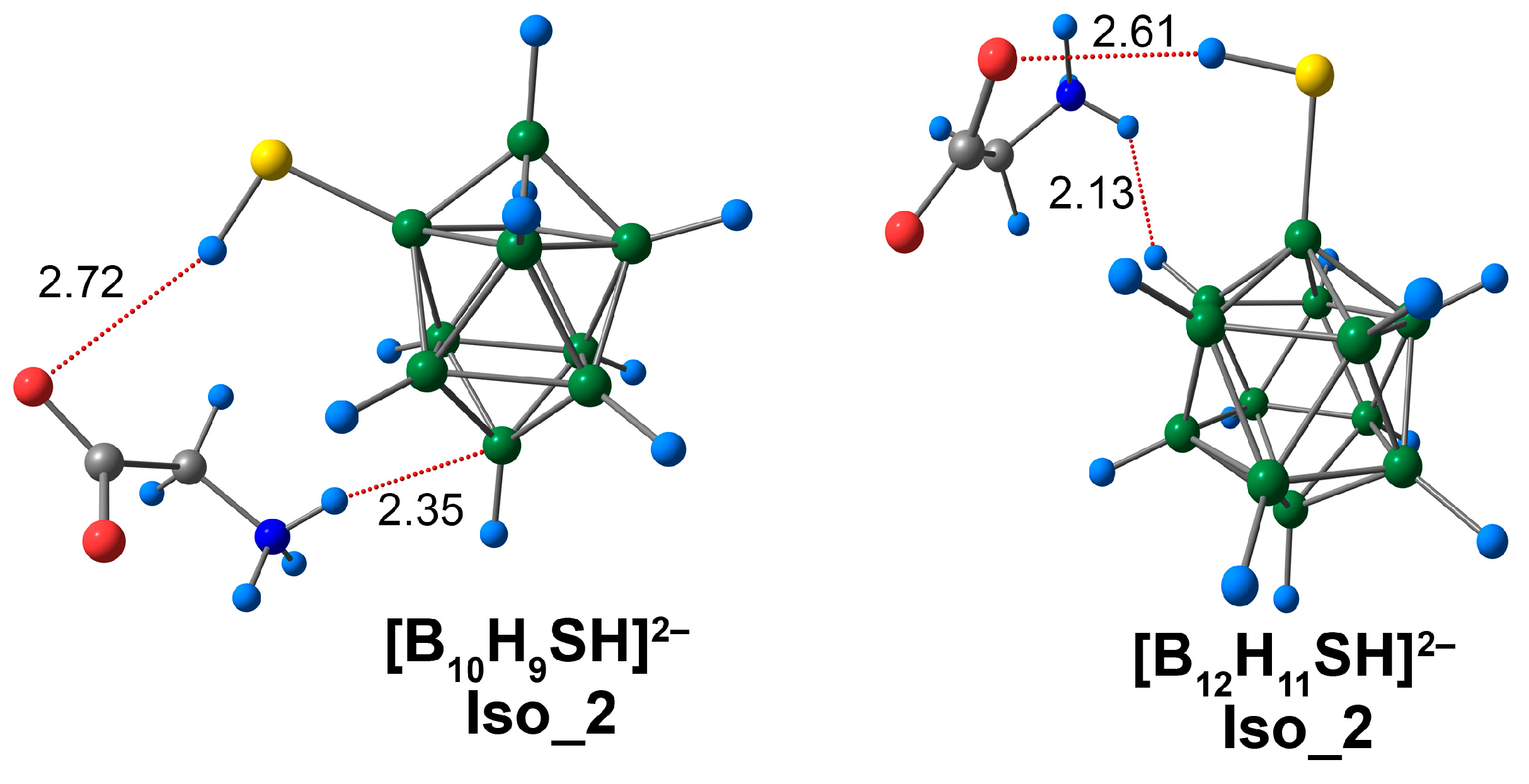
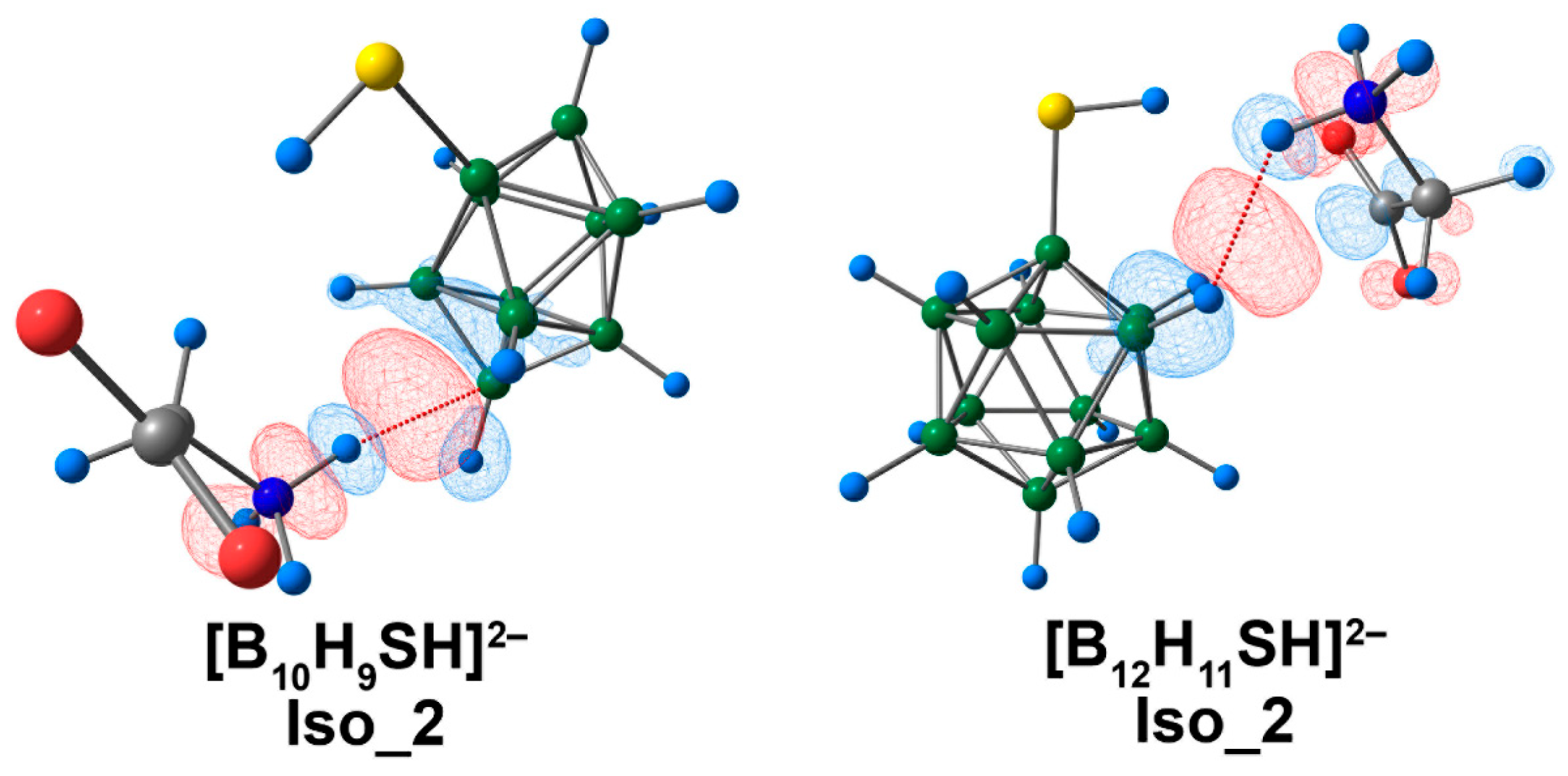
3.4. Complex of [BnHn−1SH]2− Clusters with Glycine
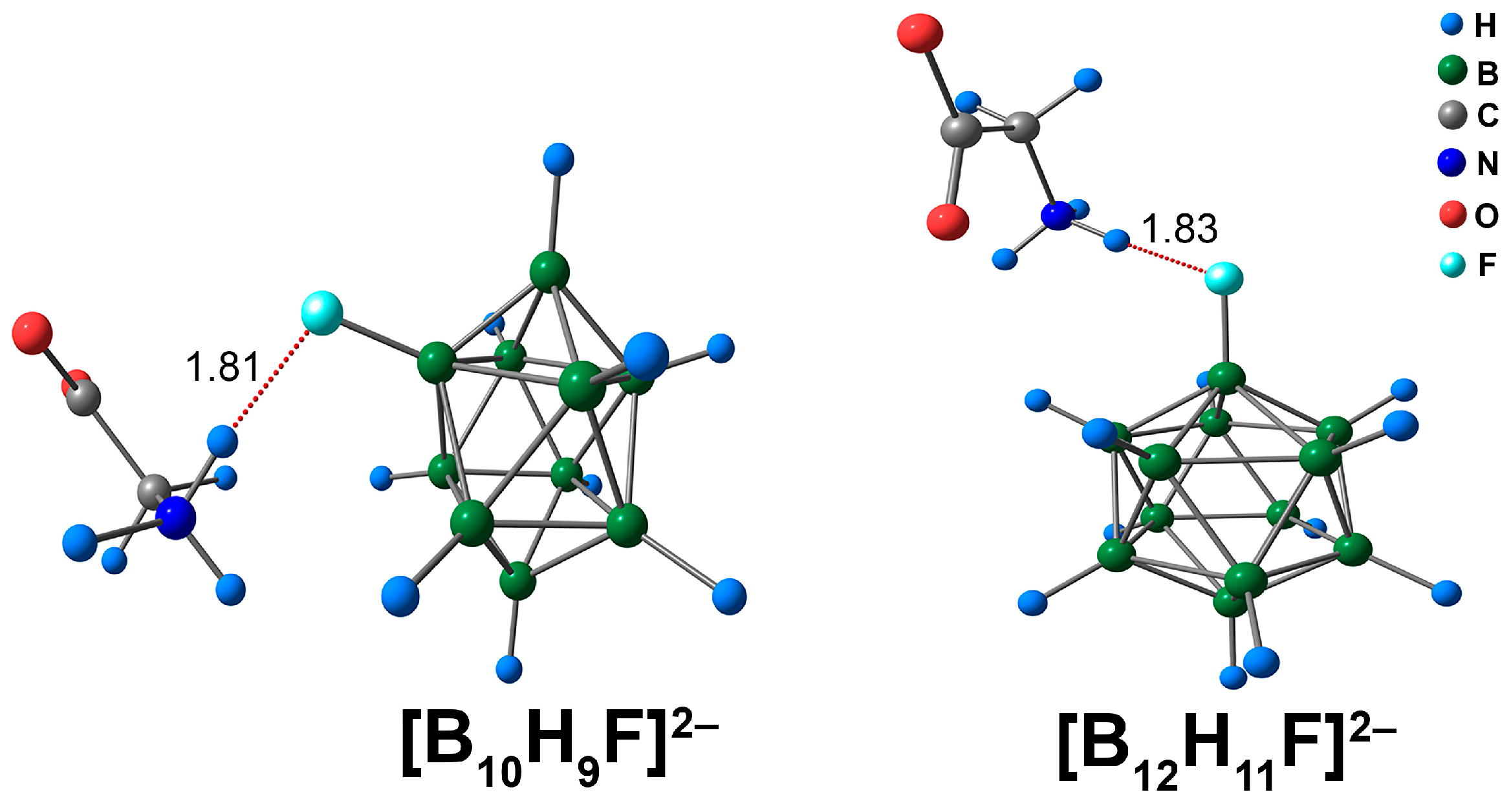
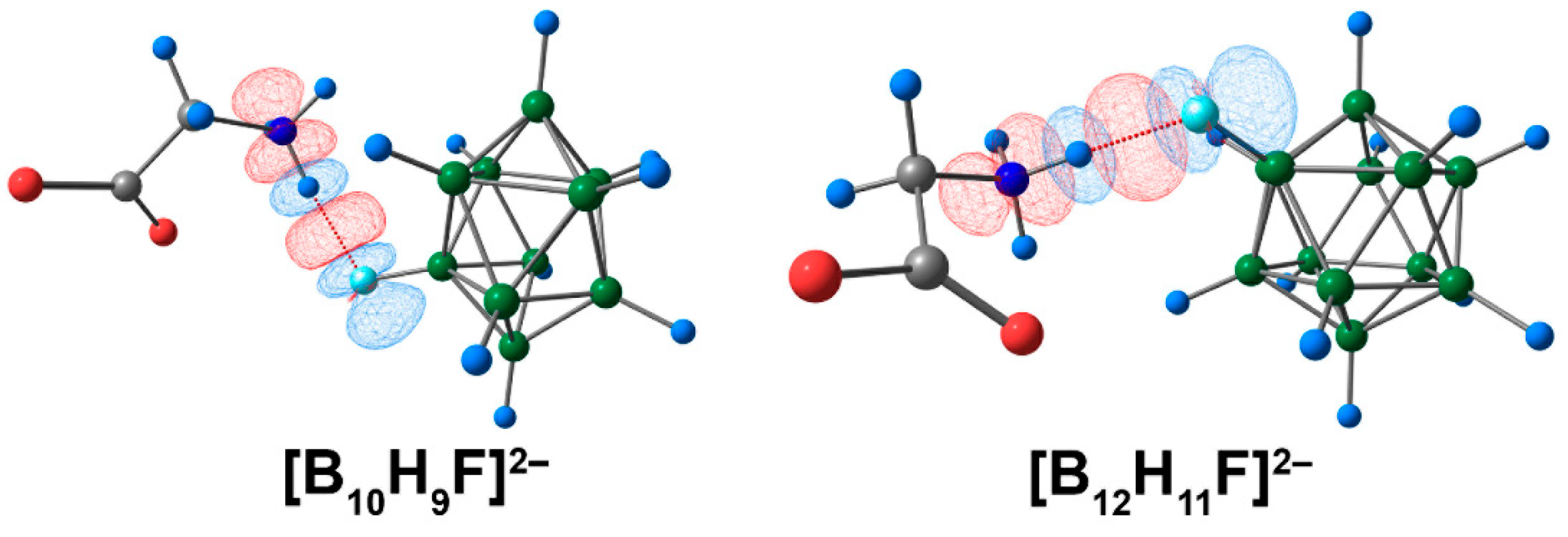
3.5. Complex with [BnHn−1F]2− Clusters with Glycine
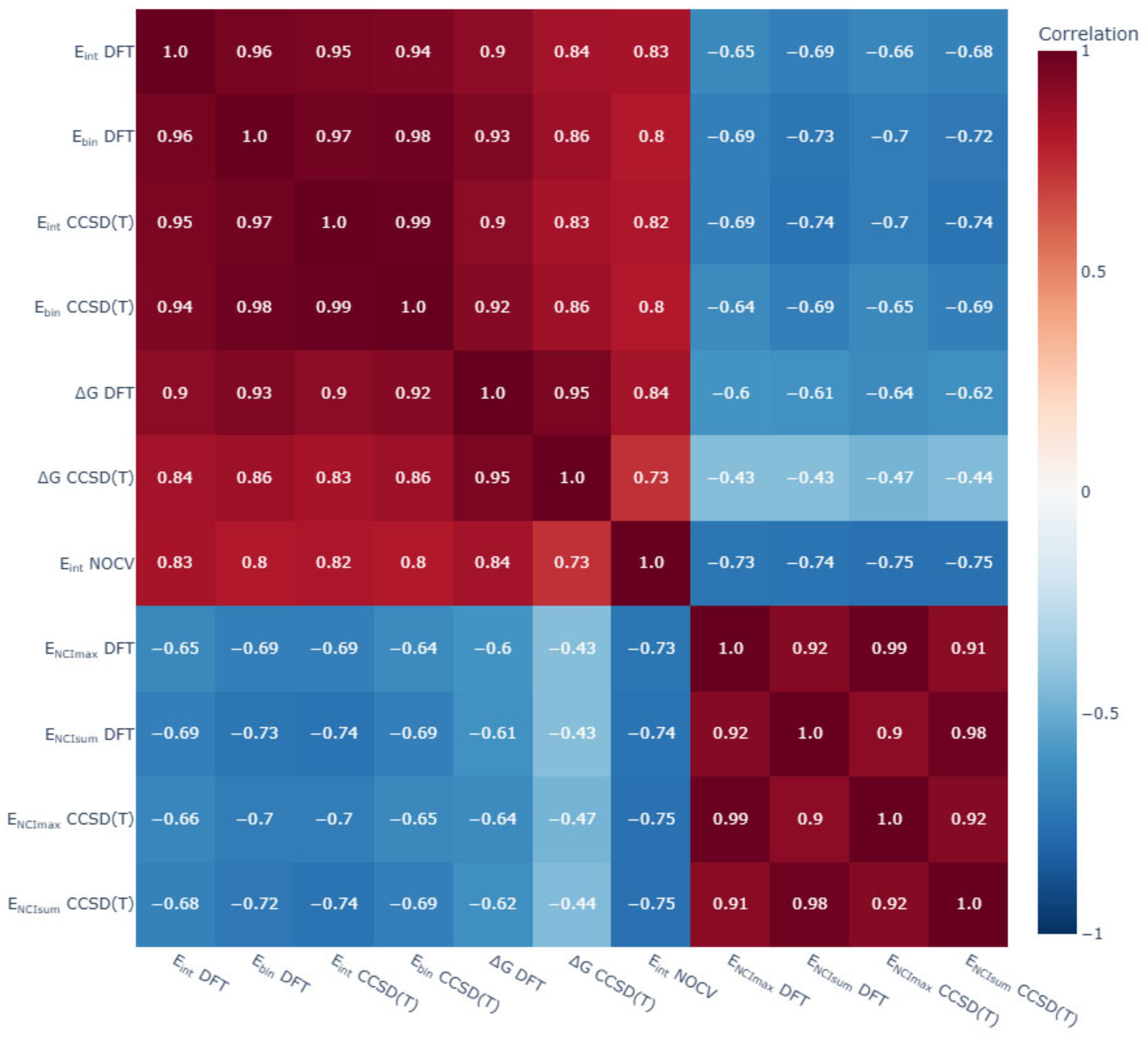
3.6. General Trends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Müller-Dethlefs, K.; Hobza, P. Noncovalent Interactions: A Challenge for Experiment and Theory. Chem. Rev. 2000, 100, 143–168. [Google Scholar] [CrossRef]
- Kollman, P.A. Noncovalent Interactions. Acc. Chem. Res. 1977, 10, 365–371. [Google Scholar] [CrossRef]
- Storer, M.C.; Hunter, C.A. The Surface Site Interaction Point Approach to Non-Covalent Interactions. Chem. Soc. Rev. 2022, 51, 10064–10082. [Google Scholar] [CrossRef]
- Tretiakov, S.; Nigam, A.; Pollice, R. Studying Noncovalent Interactions in Molecular Systems with Machine Learning. Chem. Rev. 2025, 125, 5776–5829. [Google Scholar] [CrossRef] [PubMed]
- Vladilo, G.; Hassanali, A. Hydrogen Bonds and Life in the Universe. Life 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Mukherjee, S.; Andreo, J.; Sinelshchikova, A.; Peccati, F.; Jiménez-Osés, G.; Wuttke, S.; Boxer, S.G. Electrostatic Atlas of Non-Covalent Interactions Built into Metal–Organic Frameworks. Nat. Chem. 2025, 17, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H. Binding Mechanisms in Supramolecular Complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef]
- Biedermann, F.; Schneider, H.-J. Experimental Binding Energies in Supramolecular Complexes. Chem. Rev. 2016, 116, 5216–5300. [Google Scholar] [CrossRef]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef]
- Robertazzi, A.; Krull, F.; Knapp, E.-W.; Gamez, P. Recent Advances in Anion–π Interactions. CrystEngComm 2011, 13, 3293. [Google Scholar] [CrossRef]
- Karas, L.J.; Wu, C.; Das, R.; Wu, J.I. Hydrogen Bond Design Principles. WIREs Comput. Mol. Sci. 2020, 10, e1477. [Google Scholar] [CrossRef] [PubMed]
- van der Lubbe, S.C.C.; Fonseca Guerra, C. The Nature of Hydrogen Bonds: A Delineation of the Role of Different Energy Components on Hydrogen Bond Strengths and Lengths. Chem. Asian J. 2019, 14, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Wager, J.F. New Perspective on Hydrogen Bonding. ACS Omega 2023, 8, 41674–41679. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the Hydrogen Bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Herschlag, D.; Pinney, M.M. Hydrogen Bonds: Simple after All? Biochemistry 2018, 57, 3338–3352. [Google Scholar] [CrossRef]
- Martínez, R.F.; Matamoros, E.; Cintas, P.; Palacios, J.C. Imine or Enamine? Insights and Predictive Guidelines from the Electronic Effect of Substituents in H-Bonded Salicylimines. J. Org. Chem. 2020, 85, 5838–5862. [Google Scholar] [CrossRef]
- Wenthold, P.G.; Squires, R.R. Bond Dissociation Energies of F2– and HF2–. A Gas-Phase Experimental and G2 Theoretical Study. J. Phys. Chem. 1995, 99, 2002–2005. [Google Scholar] [CrossRef]
- Howard, J.A.K.; Hoy, V.J.; O’Hagan, D.; Smith, G.T. How Good Is Fluorine as a Hydrogen Bond Acceptor? Tetrahedron 1996, 52, 12613–12622. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry; Cornell University Press: Ithaca, NY, USA, 1960; ISBN 0801403332. [Google Scholar]
- Shan, S.; Loh, S.; Herschlag, D. The Energetics of Hydrogen Bonds in Model Systems: Implications for Enzymatic Catalysis. Science 1996, 272, 97–101. [Google Scholar] [CrossRef]
- Matienko, L.I.; Mil, E.M.; Albantova, A.A.; Goloshchapov, A.N. The Role H-Bonding and Supramolecular Structures in Homogeneous and Enzymatic Catalysis. Int. J. Mol. Sci. 2023, 24, 16874. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Hydrogen-Bonds in Molecular Solids—From Biological Systems to Organic Electronics. J. Mater. Chem. B 2013, 1, 3742. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.V.; Stogniy, M.Y.; Anufriev, S.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Intramolecular Proton-Hydride Activation Using the Example of Cobalt Bis(Dicarbollide) Derivative [8-Et(HO)C HN-3,3′-Co(1,2-C2B9H10)(1′,2′-C2B9H11)]. Inorganica Chim. Acta 2025, 578, 122529. [Google Scholar] [CrossRef]
- Zimmermann, L.W.; Aghaei Hakkak, R.; Ranjbar, M.; Schleid, T. Crystal Structures and Thermal Analyses of Three New High-Energy Hydrazinium Hydro-Closo-Borates. Int. J. Hydrogen Energy 2024, 49, 1469–1477. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Vologzhanina, A.V.; Malinina, E.A.; Kuznetsov, N.T. Dihydrogen Bonds in Salts of Boron Cluster Anions [BnHn]2− with Protonated Heterocyclic Organic Bases. Crystals 2019, 9, 330. [Google Scholar] [CrossRef]
- Li, J.; Kim, J.S.; Fan, J.; Peng, X.; Matějíček, P. Boron Cluster Leveraged Polymeric Building Blocks. Chem. Soc. Rev. 2025, 54, 4104–4134. [Google Scholar] [CrossRef]
- Tu, D.; Li, J.; Sun, F.; Yan, H.; Poater, J.; Solà, M. Cage– ···Cage– Interaction: Boron Cluster-Based Noncovalent Bond and Its Applications in Solid-State Materials. JACS Au 2021, 1, 2047–2057. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Pecina, A.; Řezáč, J.; Lepšík, M.; Sárosi, M.B.; Hnyk, D.; Hobza, P. Benchmark Data Sets of Boron Cluster Dihydrogen Bonding for the Validation of Approximate Computational Methods. ChemPhysChem 2020, 21, 2599–2604. [Google Scholar] [CrossRef]
- Shubina, E.S.; Bakhmutova, E.V.; Filin, A.M.; Sivaev, I.B.; Teplitskaya, L.N.; Chistyakov, A.L.; Stankevich, I.V.; Bakhmutov, V.I.; Bregadze, V.I.; Epstein, L.M. Dihydrogen Bonding of Decahydro-Closo-Decaborate(2-) and Dodecahydro-Closo-Dodecaborate(2-) Anions with Proton Donors: Experimental and Theoretical Investigation. J. Organomet. Chem. 2002, 657, 155–162. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Lepšík, M.; Horinek, D.; Havlas, Z.; Hobza, P. Interaction of Carboranes with Biomolecules: Formation of Dihydrogen Bonds. ChemPhysChem 2006, 7, 1100–1105. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Hnyk, D.; Lepšík, M.; Hobza, P. Interaction of Heteroboranes with Biomolecules: Part 2. The Effect of Various Metal Vertices and Exo-Substitutions. Phys. Chem. Chem. Phys. 2007, 9, 2085–2093. [Google Scholar] [CrossRef]
- Ali, F.; S Hosmane, N.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [PubMed]
- Sivaev, I.B.; Bregadze, V.V. Polyhedral Boranes for Medical Applications: Current Status and Perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. [Google Scholar] [CrossRef]
- Viñas I Teixidor, C. The Uniqueness of Boron as a Novel Challenging Element for Drugs in Pharmacology, Medicine and for Smart Biomaterials. Future Med. Chem. 2013, 5, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.Y.; Chiu, Y.-L.; Huang, S.-C.; Huang, W.-Y.; Hsu, F.-T.; Lee, H.Y.; Wang, T.-W.; Keng, P.Y. Boron Neutron Capture Therapy Enhanced by Boronate Ester Polymer Micelles: Synthesis, Stability, and Tumor Inhibition Studies. Biomacromolecules 2024, 25, 4215–4232. [Google Scholar] [CrossRef]
- Futamura, G.; Kawabata, S.; Siba, H.; Kuroiwa, T.; Suzuki, M.; Kondo, N.; Ono, K.; Sakurai, Y.; Tanaka, M.; Todo, T.; et al. A Case of Radiation-Induced Osteosarcoma Treated Effectively by Boron Neutron Capture Therapy. Radiat. Oncol. 2014, 9, 237. [Google Scholar] [CrossRef]
- Gona, K.B.; Gómez-Vallejo, V.; Padro, D.; Llop, J. [18F]Fluorination of o-Carborane via Nucleophilic Substitution: Towards a Versatile Platform for the Preparation Of 18F-Labelled BNCT Drug Candidates. Chem. Commun. 2013, 49, 11491–11493. [Google Scholar] [CrossRef]
- Pitto-Barry, A. Polymers and Boron Neutron Capture Therapy (BNCT): A Potent Combination. Polym. Chem. 2021, 12, 2035–2044. [Google Scholar] [CrossRef]
- Coghi, P.; Li, J.; Hosmane, N.S.; Zhu, Y. Next Generation of Boron Neutron Capture Therapy (BNCT) Agents for Cancer Treatment. Med. Res. Rev. 2023, 43, 1809–1830. [Google Scholar] [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. Int. J. Part. Ther. 2022, 9, 71–82. [Google Scholar] [CrossRef]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron Neutron Capture Therapy: Current Status and Future Perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Kikuoka, Y.; Aihara, T.; Higashino, M.; Kurisu, Y.; Haginomori, S.; Nihei, K.; Ono, K. Expression Rate of LAT1 in High-Grade Parotid Gland Carcinoma and Potential of BNCT as a Treatment Option for Recurrent Parotid Gland Carcinoma. Appl. Radiat. Isot. 2025, 218, 111657. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sanada, Y.; Hattori, Y.; Suzuki, M. Correlation between the Expression of LAT1 in Cancer Cells and the Potential Efficacy of Boron Neutron Capture Therapy. J. Radiat. Res. 2023, 64, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Hosmane, N.S.; Zhu, Y. In Silico Assessments of the Small Molecular Boron Agents to Pave the Way for Artificial Intelligence-Based Boron Neutron Capture Therapy. Eur. J. Med. Chem. 2024, 279, 116841. [Google Scholar] [CrossRef]
- Retini, M.; Järvinen, J.; Bahrami, K.; Tampio, J.; Bartoccini, F.; Riihelä, P.; Pehkonen, H.; Värä, A.; Laitinen, T.; Huttunen, K.M.; et al. Asymmetric Synthesis and Biological Evaluation of Both Enantiomers of 5- and 6-Boronotryptophan as Potential Boron Delivery Agents for Boron Neutron Capture Therapy. ACS Med. Chem. Lett. 2024, 15, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Cígler, P.; Kožíšek, M.; Řezáčová, P.; Brynda, J.; Otwinowski, Z.; Pokorná, J.; Plešek, J.; Grüner, B.; Dolečková-Marešová, L.; Máša, M.; et al. From Nonpeptide toward Noncarbon Protease Inhibitors: Metallacarboranes as Specific and Potent Inhibitors of HIV Protease. Proc. Natl. Acad. Sci. USA 2005, 102, 15394–15399. [Google Scholar] [CrossRef] [PubMed]
- Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáčová, P.; Brynda, J.; Pokorná, J.; Plešek, J.; Grüner, B.; Grantz Šašková, K.; et al. Inorganic Polyhedral Metallacarborane Inhibitors of HIV Protease: A New Approach to Overcoming Antiviral Resistance. J. Med. Chem. 2008, 51, 4839–4843. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Brynda, J.; Řezáč, J.; Hobza, P.; Lepšík, M. Interpretation of Protein/Ligand Crystal Structure Using QM/MM Calculations: Case of HIV-1 Protease/Metallacarborane Complex. J. Phys. Chem. B 2008, 112, 15094–15102. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Brynda, J.; Kugler, M.; Lepšík, M.; Pospíšilová, K.; Holub, J.; Hnyk, D.; Nekvinda, J.; Grüner, B.; Řezáčová, P. B–H⋯π and C–H⋯π Interactions in Protein–Ligand Complexes: Carbonic Anhydrase II Inhibition by Carborane Sulfonamides. Phys. Chem. Chem. Phys. 2023, 25, 1728–1733. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzá, A. Closo -Carboranes as Dual CH⋯π and BH⋯π Donors: Theoretical Study and Biological Significance. Phys. Chem. Chem. Phys. 2019, 21, 19944–19950. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Friede, M.; Ehlert, S.; Grimme, S.; Mewes, J.-M. Do Optimally Tuned Range-Separated Hybrid Functionals Require a Reparametrization of the Dispersion Correction? It Depends. J. Chem. Theory Comput. 2023, 19, 8097–8107. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized Accurate Auxiliary Basis Sets for RI-MP2 and RI-CC2 Calculations for the Atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Martynova, S.; Krisyuk, V.; Sukhikh, A.; Benassi, E. β-Diketonate Coordination: Vibrational Properties, Electronic Structure, Molecular Topology, and Intramolecular Interactions. Beryllium(II), Copper(II), and Lead(II) as Study Cases. J. Phys. Chem. A 2025, 129, 924–945. [Google Scholar] [CrossRef] [PubMed]
- Najibi, A.; Casanova-Páez, M.; Goerigk, L. Analysis of Recent BLYP- and PBE-Based Range-Separated Double-Hybrid Density Functional Approximations for Main-Group Thermochemistry, Kinetics, and Noncovalent Interactions. J. Phys. Chem. A 2021, 125, 4026–4035. [Google Scholar] [CrossRef]
- Ahmad, S.; Eng, J.; Penfold, T.J. Conformational Control of Donor–Acceptor Molecules Using Non-Covalent Interactions. J. Phys. Chem. A 2024, 128, 8035–8044. [Google Scholar] [CrossRef]
- Neese, F. An Improvement of the Resolution of the Identity Approximation for the Formation of the Coulomb Matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Mahfouz, N.; Ghaida, F.A.; El Hajj, Z.; Diab, M.; Floquet, S.; Mehdi, A.; Naoufal, D. Recent Achievements on Functionalization within Closo-Decahydrodecaborate [B10H10]2− Clusters. ChemistrySelect 2022, 7, e202200770. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Naoufal, D. Fifty Years of the Closo-Decaborate Anion Chemistry. Collect. Czechoslov. Chem. Commun. 2010, 75, 1149–1199. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. QTAIM Analysis of Mono-Hydroxy Derivatives of Closo-Borate Anions [BnHn–1OH]2– (n = 6, 10, 12). Russ. J. Inorg. Chem. 2019, 64, 1825–1828. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Vlasova, Y.S.; Novikov, A.S.; Zhdanov, A.P.; Hagemann, H.R.; Zhizhin, K.Y.; Kuznetsov, N.T. B-F Bonding and Reactivity Analysis of Mono- and Perfluoro-Substituted Derivatives of Closo-Borate Anions (6, 10, 12): A Computational Study. Polyhedron 2022, 211, 115559. [Google Scholar] [CrossRef]
- El Anwar, S.; Holub, J.; Tok, O.; Jelínek, T.; Růžičková, Z.; Fojt, L.; Šolínová, V.; Kašička, V.; Grüner, B. Synthesis and Selected Properties of Nonahalogenated 2-Ammonio-Decaborate Anions and Their Derivatives Substituted at N-Centre. J. Organomet. Chem. 2018, 865, 189–199. [Google Scholar] [CrossRef]
- Riplinger, C.; Neese, F. An Efficient and Near Linear Scaling Pair Natural Orbital Based Local Coupled Cluster Method. J. Chem. Phys. 2013, 138, 034106. [Google Scholar] [CrossRef] [PubMed]
- Riplinger, C.; Sandhoefer, B.; Hansen, A.; Neese, F. Natural Triple Excitations in Local Coupled Cluster Calculations with Pair Natural Orbitals. J. Chem. Phys. 2013, 139, 134101. [Google Scholar] [CrossRef]
- Riplinger, C.; Pinski, P.; Becker, U.; Valeev, E.F.; Neese, F. Sparse Maps—A Systematic Infrastructure for Reduced-Scaling Electronic Structure Methods. II. Linear Scaling Domain Based Pair Natural Orbital Coupled Cluster Theory. J. Chem. Phys. 2016, 144, 024109. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.; Legare, D. Properties of Atoms in Molecules: Structures and Reactivities of Boranes and Carboranes. Can. J. Chem. 1992, 70, 657–677. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A. Donor–Acceptor Properties of Ligands from the Natural Orbitals for Chemical Valence. Organometallics 2007, 26, 6576–6580. [Google Scholar] [CrossRef]
- Sabando, R.C.; Riplinger, C.; Wennmohs, F.; Neese, F.; Bistoni, G. Broadening the Scope of the ETS-NOCV Scheme: A Versatile Implementation in ORCA. J. Chem. Theory Comput. 2025, 21, 7920–7934. [Google Scholar] [CrossRef] [PubMed]
- Kukułka, M.; Żurowska, O.; Mitoraj, M.; Michalak, A. ETS-NOCV Description of Chemical Bonding: From Covalent Bonds to Non-Covalent Interactions. J. Mol. Model. 2025, 31, 6. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Version 1.8, Build 682. Available online: https://www.chemcraftprog.com (accessed on 20 November 2025).
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997; ISBN 9780195095494. [Google Scholar]
- Nowroozi, A.; Hajiabadi, H.; Akbari, F. OH···O and OH···S Intramolecular Interactions in Simple Resonance-Assisted Hydrogen Bond Systems: A Comparative Study of Various Models. Struct. Chem. 2014, 25, 251–258. [Google Scholar] [CrossRef]
- Grabowski, S. Intramolecular Hydrogen Bond Energy and Its Decomposition—O–H∙∙∙O Interactions. Crystals 2020, 11, 5. [Google Scholar] [CrossRef]
- Adhav, V.A.; Saikrishnan, K. The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function. ACS Omega 2023, 8, 22268–22284. [Google Scholar] [CrossRef]
- Pietruś, W.; Kafel, R.; Bojarski, A.J.; Kurczab, R. Hydrogen Bonds with Fluorine in Ligand–Protein Complexes-the PDB Analysis and Energy Calculations. Molecules 2022, 27, 1005. [Google Scholar] [CrossRef]
- Dalvit, C.; Invernizzi, C.; Vulpetti, A. Fluorine as a Hydrogen-Bond Acceptor: Experimental Evidence and Computational Calculations. Chem. A Eur. J. 2014, 20, 11058–11068. [Google Scholar] [CrossRef]
- Zhou, P.; Zou, J.; Tian, F.; Shang, Z. Fluorine Bonding—How Does It Work In Protein−Ligand Interactions? J. Chem. Inf. Model. 2009, 49, 2344–2355. [Google Scholar] [CrossRef]
- Kjaersgaard, A.; Lane, J.R.; Kjaergaard, H.G. Room Temperature Gibbs Energies of Hydrogen-Bonded Alcohol Dimethylselenide Complexes. J. Phys. Chem. A 2019, 123, 8427–8434. [Google Scholar] [CrossRef]
- Ruscic, B. Active Thermochemical Tables: Water and Water Dimer. J. Phys. Chem. A 2013, 117, 11940–11953. [Google Scholar] [CrossRef]
- Isayev, O.; Gorb, L.; Leszczynski, J. Theoretical Calculations: Can Gibbs Free Energy for Intermolecular Complexes Be Predicted Efficiently and Accurately? J. Comput. Chem. 2007, 28, 1598–1609. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, C.S.; Mackeprang, K.; Du, L.; Jørgensen, S.; Kjaergaard, H.G. Similar Strength of the NH···O and NH···S Hydrogen Bonds in Binary Complexes. J. Phys. Chem. A 2014, 118, 11074–11082. [Google Scholar] [CrossRef]
- Sokolov, A.A.; Khisamiev, M.B.; Solomonov, B.N.; Yagofarov, M.I. Compensation Relationship in Thermodynamics of Gas-Phase Molecular Complexation. Phys. Chem. Chem. Phys. 2025. Preprint. [Google Scholar] [CrossRef]
- Kjaersgaard, A.; Pal, D.; Vogt, E.; Skovbo, T.E.; Kjaergaard, H.G. Room Temperature Gas-Phase Detection and Formation Gibbs Energy of the Water Dimethyl Ether Bimolecular Complex. J. Phys. Chem. A 2025, 129, 4438–4446. [Google Scholar] [CrossRef] [PubMed]
- Senthilnithy, R.; Weerasingha, M.S.S.; Dissanayake, D.P. Interaction of Caffeine Dimers with Water Molecules. Comput. Theor. Chem. 2014, 1028, 60–64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyukin, I.N.; Kolbunova, A.V.; Novikov, A.S.; Klyukina, A.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Theoretical Insight into Non-Covalent Complexes of Closo-Borate Anions [BnHn−1X]y− with Glycine. Computation 2025, 13, 285. https://doi.org/10.3390/computation13120285
Klyukin IN, Kolbunova AV, Novikov AS, Klyukina AA, Zhizhin KY, Kuznetsov NT. Theoretical Insight into Non-Covalent Complexes of Closo-Borate Anions [BnHn−1X]y− with Glycine. Computation. 2025; 13(12):285. https://doi.org/10.3390/computation13120285
Chicago/Turabian StyleKlyukin, Ilya N., Anastasia V. Kolbunova, Alexander S. Novikov, Alexandra A. Klyukina, Konstantin Y. Zhizhin, and Nikolay T. Kuznetsov. 2025. "Theoretical Insight into Non-Covalent Complexes of Closo-Borate Anions [BnHn−1X]y− with Glycine" Computation 13, no. 12: 285. https://doi.org/10.3390/computation13120285
APA StyleKlyukin, I. N., Kolbunova, A. V., Novikov, A. S., Klyukina, A. A., Zhizhin, K. Y., & Kuznetsov, N. T. (2025). Theoretical Insight into Non-Covalent Complexes of Closo-Borate Anions [BnHn−1X]y− with Glycine. Computation, 13(12), 285. https://doi.org/10.3390/computation13120285









