Abstract
In the search to cover the urgent need to combat infectious diseases, natural products have gained attention in recent years. The caespitate molecule, isolated from the plant Helichrysum caespititium of the Asteraceae family, is used in traditional African medicine. Caespitate is an acylphloroglucinol with biological activity. Acylphloroglucinols have attracted attention for treating tuberculosis due to their structural characteristics, highlighting the stabilizing effect of their intramolecular hydrogen bonds (IHBs). In this work, a conformational search for the caespitate was performed using the MM method. Posteriorly, DFT calculations with the APFD functional were used for full optimization and vibrational frequencies, obtaining stable structures. A population analysis was performed to predict the distribution of the most probable conformers. The calculations were performed in the gas phase and solution using the implicit SMD model for water, chloroform, acetonitrile, and DMSO solvents. Additionally, the multiscale ONIOM QM1/QM2 model was used to simulate the explicit solvent. The implicit and explicit solvent effects were evaluated on the global reactivity indexes using the conceptual-DFT approach. In addition, the QTAIM approach was applied to analyze the properties of the IHBs of the most energetically and populated conformers. The obtained results indicated that the most stable and populated conformer is in the gas phase, and chloroform has an extended conformation. However, water, acetonitrile, and DMSO have a hairpin shape. The optimized structures are well preserved in explicit solvent and the interaction energies for the IHBs were lower in explicit than implicit solvents due to non-covalent interactions formed between the solvent molecules. Finally, both methodologies, with implicit and explicit solvents, were validated with 1H and 13C NMR experimental data. In both cases, the results agreed with the experimental data reported in the CDCl3 solvent.
1. Introduction
Acylphloroglucinols (ACPLs) are phytochemical compounds with pharmacological properties. One relevant chemical characteristic of ACPLs is the formation of intramolecular non-covalent interactions in their structures, such as intramolecular hydrogen bonds (IHBs). These IHBs could be responsible for the chemical reactivity and biological activity observed in these compounds. On the other hand, it is important to simulate the solvent effect on the molecules. The effect of the solvent can be modeled with implicit models, which assume the solute is put into a cavity within a continuous dielectric medium simulating the solvent. Some more commonly used implicit models are the polarizable continuum model (PCM), or the universal solvation model based on density (SMD). Also, molecules of solvent can be explicitly incorporated into the system surrounding the solute to model the solute-solvent interactions. One explicit model widely used is our own N-layer integrated molecular orbital molecular mechanics (ONIOM), which uses different levels of theory to calculate different parts of the system. Generally, the solute is treated with a high level of theory calculation, while molecules of the solvent are treated at a lower level of theory. The chemical reactivity of ACPLs can be studied using global reactivity parameters based on the conceptual density functional theory (c-DFT) approach. c-DFT is based on describing chemical concepts from the quantitative prediction of the Koopmans theorem and the Kohn–Sham formalism, i.e., the energy of HOMO related to vertical ionization potential (IP) and the energy of LUMO related to vertical electron affinity (EA). The quantum theory of atoms in molecules (QTAIM) is used to characterize the intramolecular hydrogen bonds (IHBs) through parameters obtained from the electron density. As part of the validation of the theoretical methodology used, experimental spectroscopic data are very useful to compare the calculated values obtained. For example, experimental 1H and 13C NMR spectra of ACPLs have been reported, and our calculations of 1H and 13C NMR spectra are in agreement with them in both implicit and explicit calculated systems.
The Asteraceae, or Compositae, family is breathtakingly successful in human health. Many of its members have traditional therapeutic uses and have been cultivated for more than 3000 years [1]. Several species of this family have shown a wide range of anti-inflammatory, antimicrobial, antioxidant, and hepatoprotective activities [2]. Numerous compounds have been isolated from extracts of this family, such as caespitate [3] and caespitin [4], which are isolated from Helichrysum caespititium, a plant native to Lesotho, South Africa, and Zimbabwe [5,6], where this plant is used in traditional medicine to treat tuberculosis (TB), a disease with the world’s highest incidence caused by a single infectious agent, below COVID-19 and above HIV [7]. Therefore, there is an urgency to develop new possibilities for the treatment of TB based on phytochemical compounds.
Here, we are interested in the study of the anti-tuberculosis properties of caespitate. Caespitate (2-methyl-4-[2′,4′,6′-trihydroxy-3′-(2-methylpropanoyl)-phenyl]but-2-enyl acetate) is an acylphloroglucinol with an acyl group and three hydroxyl substituents on the benzene ring in 2, 4, and 6-positions, while in the meta position, it has a prenyl chain ending with an ester group, giving rise to two geometric isomers, Z and E. It has been demonstrated that the Z isomer is biologically active [8].
The use of ACPLs to treat TB has attracted the attention of several research groups due to the structural characteristics and stabilizing effects of the IHBs of several ACPLS compounds. The formation of IHBs depends on the type and position of the OH groups; some of them are available to interact with other solvent molecules, influencing the conformational preference and physicochemical properties of the molecule. The influence of the IHBs of type O–H···O on the conformational preferences and population analysis of caespitate was early studied using semiempirical methods [9]. Relevant geometry changes and increases in energy were observed in ACPL compounds and compared with caespitate, with the removal of IHB using MP2 and DFT/B3LYP calculations [10]. The implicit PCM solvent effect on the IHBs of a series of ACPLs, including caespitate, was analyzed using chloroform, acetonitrile, and water solvents with HF, MP2, and DFT/B3LYP methods [11,12]. The stabilization energy of the IHBs in polar solvents, acetonitrile and water, was higher than in chloroform, showing that the stabilization is increased with the solvent polarity. The IHB length was not significantly weakened in solution, even when evaluating the effect of the explicit solvent of adducts with water molecules [12,13]. The contribution of other weaker IHBs, such as C–H–O and O–H–π, to the conformational stabilization was also analyzed with the PCM implicit solvent model with HF, MP2, and DFT/B3LYP methods [14]. An extensive conformational search study of Z and E isomers of caespitate was carried out using the PCM model with HF, MP2, and DFT/B3LYP calculations, with a greater number of low-energy conformers for Z than the E isomer. Also, it was found that the ability to form IHBs is greater for the Z isomer than the E isomer, and the corresponding IHB has a greater stabilizing effect [15]. Z isomers can form two types of IHBs. The first is formed via the sp2 oxygen atom of the acyl group with the H atoms of the hydroxyl groups in the ortho position. The second IHB is formed with either of the two oxygen atoms (sp3 or sp2) of the prenyl chain and the H atoms of the hydroxyl group. Considering adducts with explicit water molecules approximating the first solvation layer, no significant differences in the patterns of IHB formation were found [15].
In this work, a conformational search on Z-caespitate was carried out using the MM method. Posteriorly, DFT calculations were used at two levels of theory, APFD/6-31+G(d) and APFD/6-311+G(2d,p), to obtain the most stable structures. Also, a population analysis was performed to predict the distribution of the most probable conformers. The calculations were carried out in the gas phase and solution phase with water, chloroform, acetonitrile, and DMSO as solvents, using the implicit SMD model. Additionally, a multiscale ONIOM (QM:XTB2) model was used to take into account the explicit solvent on caespitate conformers. On the preferred conformers of caespitate in each solvent, the implicit and explicit solvent effects on the global reactivity indices were analyzed using the conceptual DFT approach. Finally, the intramolecular hydrogen bonds (IHB) were characterized using the QTAIM approach.
2. Computational Methodology
The conformational search of caespitate was initially performed using the protocol GMMX [16] implemented in the GaussView 6.0 program [17]. The MM method with the force field MMFF94 [18] was used to obtain 86 conformers with relative energies ≤ 6 kcal mol−1. Posteriorly, full optimization for all conformers was carried out using DFT calculations with APFD functional [19] and the basis set 6-31+G(d) [20], obtaining stable structures with energies of ≤4 kcal mol−1. Then, the APFD with the basis set 6-311+G(2d,p) [21] was used for the full optimization and vibrational frequency calculations on the stable structures with energies ≤ 2 kcal mol−1. Vibrational frequency calculations were evaluated to obtain the thermochemistry parameters and to assure local minimum structures with no imaginary frequencies for each conformer. The calculations were performed in gas phase and solution using the implicit SMD model [22] for solvents with different polarities: water (ε = 78.355), chloroform (ε = 4.711), acetonitrile (ε = 35.688), and DMSO (ε = 46.826) [23]. The population analysis was performed based on the Boltzmann distribution in the thermodynamic equilibrium, involving the relative free energies, ΔG, using the equation: , where is the probability to find the conformer i from the total number of conformers , T is the temperature of the system at 298.5 K, R is the universal gas constant, and M is the number of all accessible conformers [24]. Additionally, calculations using the multiscale model ONIOM (QM:XTB2) [25] were carried out to include the explicit solvent effect on the caespitate conformers. The first level QM was the ωB97X-D3/Def2-TZVP [26,27], adding auxiliar basis sets Def2/J [28] and Def2-TZVP/C [29] for caespitate conformers, while the second layer, a semiempirical with dispersion method XTB2 [30,31], was used for solvent. A cubic box of 30 × 30 × 30 Å containing 31 molecules of each solvent in order to surround the caespitate molecule was built using the AutoSolvate program [32]. The geometric differences between conformers obtained in implicit and explicit solvents were analyzed using the RMSD values. On the most stable and populated caespitate conformers in each phase, the global reactivity and the density topological parameters were analyzed. Frontier molecular orbitals (FMO) and molecular electrostatic potential (MEP) maps were analyzed. The FMO isosurfaces were graphed using an isovalue of 2.0 × 10−2 a.u. The MEP maps were graphed using an isovalue of 4.0 × 10−4 a.u. in a range from −3.0 × 10−2 e a.u.−3 to 3.0 × 10−2 e a.u.−3. The global reactivity indices were calculated using the conceptual-DFT approach [33] from the energies of HOMO and LUMO frontier orbitals through the equations for chemical potential , electronegativity , hardness , softness , and electrophilicity index . The intramolecular hydrogen bonds (IHBs) were characterized using the QTAIM approach [34], through the topological parameters as electronic density, , the Laplacian, , the Lagrangian kinetic energy, G, the Hamiltonian kinetic energy, H, the potential energy density, V, the interaction energy, EH…Y, the interatomic distance Dinter, the valence angle Ainter, and the delocalization indices, DI, where , and . Finally, the solvent models were validated using 1H and 13C NMR spectroscopy. NMR calculations were performed using the GIAO method [35]. TMS, the molecule used as a reference, was optimized for the same implicit and explicit models. All optimization, vibrational frequencies, NMR spectroscopy, and wavefunction calculations were performed using the Gaussian 16 program [36]. The ONIOM (QM:XTB2) calculations were performed in the ORCA 5.0.3 program [37]. RMSD values were obtained with the VMD program [38]. The QTAIM analysis was performed with the AIMAll 17.11.14 program [39]. The visualization of results was carried out using GaussView 6.0 [17] and ChemCraft 1.8 [40] programs.
3. Results and Discussion
3.1. Conformational and Population Analyses
After the full optimization, as described in the Computational methodology section, 24 conformers in the gas phase, 10 in water, 26 in chloroform, 33 in acetonitrile, and 34 in DMSO with free energies of ≤2 kcal mol−1 were obtained at the level of theory APFD/6-311+G(2d,p), as shown at Table S1. The relative free energies, ΔG, and population percentages of the five most stable conformers of caespitate in gas and solution phases are shown in Table 1.

Table 1.
Relative free energy (ΔG, kcal mol−1), population (%), and formation of IHBs of the most stable conformers of caespitate calculated at the APFD/6-311+G(2d,p) level of theory in gas and solution phases with the SMD implicit model.
In the gas phase, the absolute energy for the lowest-energy conformer G1 is −1111.892 a.u. in comparison with −1105.803 a.u. previously obtained at the HF/6-31G(d,p) level of theory in the gas phase [15], clearly showing the influence of including dispersion for using the APFD functional. In previous works, the importance of including the electronic correlation effect and diffuse functions to adequately describe the IHBs using the B3LYP and MP2 methods has been reported. The results obtained with the HF, B3LYP, and MP2 methods showed similar geometric patterns [10,11,12,13,14,15].
Figure 1 shows the optimized molecular structures of the most stable conformers of caespitate obtained in gas (G1), water (W1), chloroform (C1), acetonitrile (A1), and DMSO (D1) phases. Atom labels are shown. In gas (G1) and chloroform (C1), the conformers show extended conformations, while in water (W1), acetonitrile (A1) and DMSO (D1) have hairpin conformations. Two types of intramolecular hydrogen bonds (IHBs) are observed in the structures. The first IHB is formed via the sp2 oxygen atom of the acyl group with the H atoms of the ortho hydroxyl OH groups, in this case, between O12–H13···O15 for G1, W1, C1, and A1, or O10–H11···O15 for D1. The second IHB is formed with the sp2 oxygen atom of the prenyl chain and the hydrogen atom of the hydroxyl OH group, in this case, O8–H9···O41 for G1 and C1, and O12–H13···O41 for D1; see Figure 1. It is observed that extended conformations G1 and C1 and hairpin conformation D1 preserve both IHBs, while W1 and A1 only maintain one IHB. These conformations are in agreement with the most stable geometries previously reported at HF, B3LYP, and MP2 calculations in gas and water, chloroform, and acetonitrile solution phases [15]. Figure S1 shows the five lowest-energy conformers optimized in each phase. The five lowest-energy conformers optimized in the gas phase (G1–G5) maintain both IHBs. The same behavior occurs for the conformers in chloroform (C1–C5) and DMSO (D1–D5). For conformers in the acetonitrile phase, only four out of five (A2–A5) retain both IHBs, and for conformers in the water phase, only one conformer out of five (W2) retains both IHBs. It is observed that the conformers with extended conformation retain both IHBs in gas and solution implicit phases, while the conformers with hairpin conformation retain both IHBs in gas, chloroform, and DMSO, and hairpin conformations with only IHB are found in water and acetonitrile. In the gas phase, the extended conformation is dominant, while in the solution phase, the hairpin conformation is dominant. These results do not follow exactly the same trend previously reported [15], where it is mentioned that the number of lowest-energy conformers having both IHB decreases as the polarity of the medium increases, i.e., in chloroform and acetonitrile phases, there are more stable conformations with two IHB than in water solutions, which only contain one IHB.
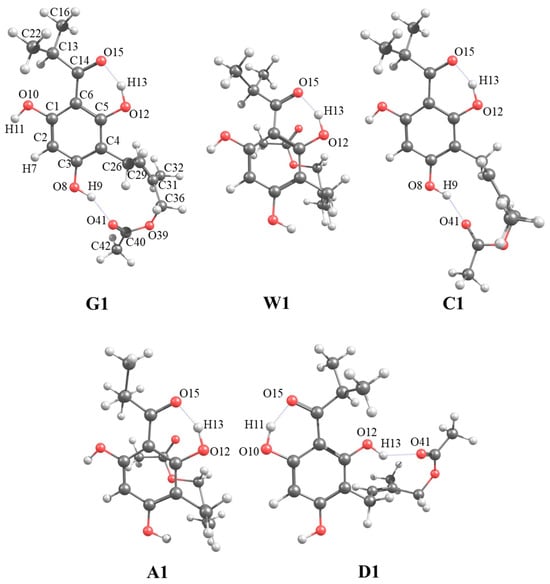
Figure 1.
Molecular structures of the most stable conformers of caespitate calculated at the APFD/6-311+G(2d,p) level of theory in gas and solution phases with the SMD implicit model in gas (G1), water (W1), chloroform (C1), acetonitrile (A1), and DMSO (D1) phases.
In general, the conformations generated in this work with hybrid MM and DFT methods (86 conformers with energies ≤ 6 kcal mol−1) are in agreement with those obtained in gas and water, chloroform, and acetonitrile with the PCM model [15], calculated at the HF/6-31G(d,p) level of theory with energies ≤ 10 kcal mol−1.
The results of the population analysis show that in the gas phase, the G1 conformer is the most populated with 24.60%, but G2 and G3 are significantly populated with 23.65% and 19.88%, respectively; therefore, the three lowest-energy conformers represent 68.10% of the total population. This population is lower than that reported previously of 76.60% for the most stable conformer in gas, and the following conformers are 18.30% and 1.30% [15]. According to the solution results, in water, W1 is the most populated with 26.50%, and the following conformers have populations between 10.72 and 17.19%. In chloroform, C1 is the most populated conformer with 49.10%, C2 has a significant population of 22.76%, and C3–C5 decrease considerably with values in the range of 5.58–6.37%. In acetonitrile, the conformer A1 is the most populated with 20.25%, and the following conformers A2–A5 have similar populations in the range of 11.96–15.54%. Finally, for DMSO, D1 is the most populated with 23.62%. D2 is similarly populated as D1, with 21.66%. The following conformers (D3–D5) decrease in population with values between 7.09 and 13.10%. In general, with these results, it is possible to observe that the extended conformation, which preserves both IHBs, is the dominant conformation in the gas phase (G1) and chloroform solution phase (C1); see Table 1.
3.2. ONIOM DFT
The total energies obtained with the ONIOM (ωB97X-D3/Def2-TZVP:XTB2) method of the explicit solute-solvent systems were obtained from more stable structures optimized in gas (see Figure S2) and solution implicit phases (see Figure 2). In Table 2, the total energies of the different systems with 31 molecules of each explicit solvent are compared. The optimized geometries of the explicit solute-solvent systems were characterized as energetic minimal structures with a vibrational frequency analysis ().
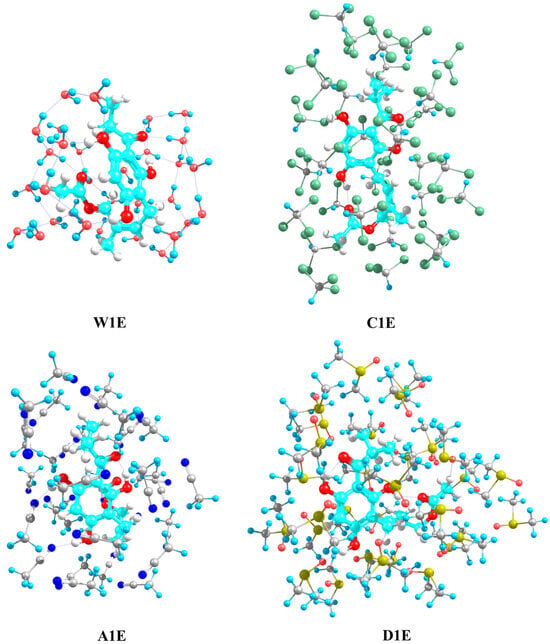
Figure 2.
Optimized geometries of the caespitate—explicit solvent systems calculated with the ONIOM (ωB97X-D3/Def2-TZVP:XTB2) method obtained from the most stable conformers in implicit solution in water (W1E), chloroform (C1E), acetonitrile (A1E), and DMSO (D1E).

Table 2.
Total (a.u.) and relative (kcal mol−1) energies of the most stable conformers of caespitate calculated with the ONIOM (ωB97X-D3/Def2-TZVP:XTB2) method in explicit solution, including explicit water (WG1E/W1E), explicit chloroform (CG1E/C1E), explicit acetonitrile (AG1E/A1E), and explicit DMSO (DG1E/D1E) phases.
It is observed that the energy is stabilized when the gas phase conformation G1 turns into solvent phase conformations (from the W1, C1, and A1 conformers), except when surrounded by 31 molecules of DMSO (from the D1 conformer). For the most stable conformer in chloroform, C1E, the stabilization energy (the most negative value) is −4.97 kcal mol−1, while for the W1E, A1E, and D1E conformers, the stabilization energy is −0.33, −2.03, and 1.41 kcal mol−1, respectively.
The results show that the 31 molecules of chloroform provide higher stability to caespitate, which has extended form in implicit solvent (see C1 in Figure 1) as well as in explicit solvent (see CG1E and C1E in Figure 2 and Figure S2, respectively). On the other hand, the 31 molecules of water and acetonitrile favor the hairpin form (see W1E and A1E in Figure 2). Contrary to this, in the DMSO conformer, the stability energy was the highest (1.41 kcal mol−1), indicating the extended form (see DG1E in Figure S2) is preferentially favored over the hairpin form (see D1E in Figure 2) and therefore suggesting that the caespitate conformers in DMSO could preferentially exist in extended form surrounded solvent molecules. Additionally, the energetic stability, considering the gas phase structure (G1) as the implicit solvent phase structures (W1, C1, A1, and D1) as the initial guess, presents the same trend in stability in each solvent in the explicit model: ECG1E/EC1E > EDG1E/ED1E > EAG1E/EA1E > EWG1E/EW1E.
The selected distances and angles for both implicit and explicit solvents are shown in Table 3. In general, the conformers surrounded by explicit solvent molecules of caespitate maintain their conformation like that obtained in an implicit solvent. It can be observed that there is a slight increment in the bond lengths of the hydrogen bonds and a slight decrease in the valence angles in the explicit solvent. Also, in dihedral angles, there is a variation in the angle O15–C14–C6–C5, indicating that the O15 atom is found in the plane of the aromatic ring of phloroglucinol in conformers in explicit solvent rather than in implicit solvent. In general, the prenylated chain maintains its position, given the angle, except for the conformer in chloroform, C1E, with the largest variation of approximately 20°.

Table 3.
Selected parameters (bond lengths in Å, angles in degrees) of the most stable conformers of caespitate calculated at the APFD/6-311+G(2d,p) level of theory and ONIOM (ωB97X-D3/Def2–TZVP:XTB2) in solution phases.
The variation between conformers in implicit and explicit solvents was also analyzed by RMSD value, where it was observed that conformer G1, surrounded by 31 molecules of each solvent, WG1E, CG1E, AG1E, and DG1E, maintains the extended G1 conformation with RMSD values of 0.19 to 0.79 Å (see Figure S3), with the largest variation in DMSO (see DG1E in Figure S3). The conformers WG1E, CG1E, and AG1E maintain both IHBs similar to conformer G1, while DG1E only maintains the second IHB (see Figure S3). On the other hand, the variation of the conformers W1, C1, A1, and D1 surrounded by their corresponding explicit solvent has RMSD values of 0.22 to 0.73 Å (see Figure 3), with the largest variation using water as explicit solvent (see W1E in Figure 3). The conformers W1E and A1E have hairpin conformation, maintaining the first IHB, while D1E, also in hairpin conformation, maintains both IHBs, similarly as in the implicit solvent systems (see W1, A1, and D1 in Figure 1). C1E maintains the extended conformation and both IHBs as in the implicit solvent (see C1, Figure 1).
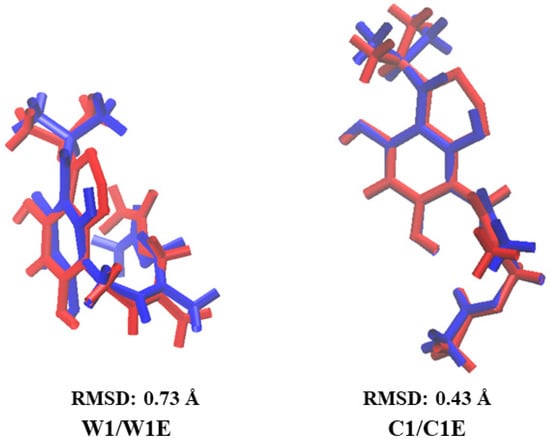
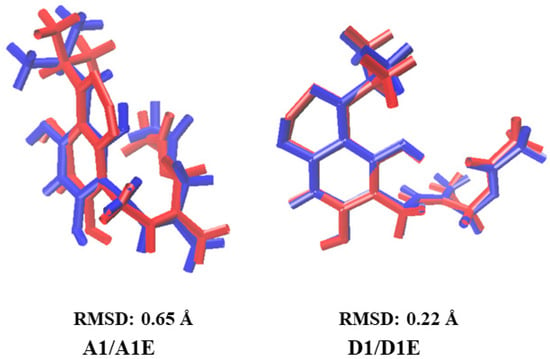
Figure 3.
Comparison of the optimized structures of the most stable conformer in implicit solvent, water (W1), chloroform (C1), acetonitrile (A1), and DMSO (D1) (in red color), with the corresponding structure in explicit solvent, in water (W1E), chloroform (C1E), acetonitrile (A1E), and DMSO (D1E) (in blue color).
3.3. Global Reactivity Indices
The conceptual DFT approach [33] was used to analyze the global reactivity descriptors: chemical potential (μ), electronegativity (χ), hardness (η), softness (S), and the electrophilicity index (ω) from the FMO energies, EHOMO and ELUMO, for the most stable conformers of caespitate in gas and implicit and explicit solution phases. Table 4 presents the results.

Table 4.
HOMO and LUMO energies and global reactivity descriptors (eV) of the most stable conformers of caespitate calculated at the APFD/6-311+G(2d,p) level of theory in gas and implicit and explicit solution phases.
The HOMO energy value is −6.12 in the gas phase for G1, while in the implicit and explicit solution they are in a similar range of −6.26 to −6.15 eV. The C1 and D1 conformers have higher HOMO energy values in implicit solvent, while in explicit solvent, the highest HOMO value corresponds to the acetonitrile conformer A1E. These values are related to the electron donor’s ability. A higher HOMO value indicates that these conformers have a higher electron donor behavior compared to the others. C1 has an extended form with two IHBs, while D1 and A1E have a hairpin conformation retaining two IHBs and one IHB, respectively; see Figure 3. On the other hand, the LUMO energy value is −1.28 eV in the gas phase for G1, while the implicit solvent and explicit solvent have values in the range of −1.61 to −1.39 eV and −1.50 to −1.38 eV, respectively. The lowest LUMO energy values correspond to the conformers in water (W1 and W1E). Both conformers in water have a hairpin conformation with the first IHB; see Figure 3. Small LUMO energy values indicate higher electron affinity or electron withdrawing ability.
Figure S4 shows the distribution of the FOM of the most stable conformers in gas and implicit solution phases. A similar distribution of the HOMO orbitals is observed in the conformers G1 and C1, with higher contributions of the OH groups in ortho position and of the phenyl ring, both with extended conformation, while the HOMO orbitals of the conformers W1, A1, and D1 show a similar distribution with higher contributions of the three OH groups of the phenyl ring, the carbonyl group, and the phenyl ring. In this case, all of them have a hairpin conformation. LUMO orbitals have a similar distribution in G1, C1, and D1 conformers, with major contributions attributed to the OH, carbonyl, and aromatic ring groups. No contribution from the prenylated chain is observed. In conformers W1 and A1, higher contributions from the OH groups, the acyl group, and the aromatic ring are observed. In Figure S5, for the distribution of the FOM of caespitate conformers obtained from systems with explicit solvent, a similar distribution of the HOMO orbitals for conformers C1E and A1E is observed, with a higher contribution of the aromatic ring and the OH groups, while the HOMO orbitals of conformers W1E and D1E show additional contributions of the prenylated chain. For the distribution of the LUMO orbitals, in W1E, C1E, and D1E, they are similar, with higher contributions of carbonyl, OH, and aromatic rings, and the contribution of the prenylated chain is not observed, while in A1E a smaller contribution of the methyl groups linked to the acyl group is observed. In general, the distributions of the HOMO and LUMO orbitals are similar in implicit solvent as well as explicit solvent.
With respect to the values of the global reactivity descriptors, the highest value of the chemical potential, μ, corresponds to the conformer G1 (−3.70 eV) in the gas phase, while in the implicit solution, the conformers in chloroform and DMSO, C1 and D1 (−3.78 eV), have slightly higher values than the rest of them. Conformers C1E, A1E, and D1E have a slightly higher value (−3.80 eV) than W1E in explicit solvent. These values represent higher electron density exchange with the surroundings. Conversely, these conformers have the lowest electronegativity values, χ, i.e., they present the lowest resistance to electron density loss. The conformers with higher values of hardness, η, are G1 (4.83 eV) in the gas phase, C1 and D1 (4.73–4.76 eV) in the implicit solvent, and C1E (4.84 eV) in the explicit solvent, indicating that these conformers present more resistance to change their electronic distribution. Contrary, W1/W1E and A1/A1E (0.21 eV) in implicit/explicit solvents have slightly higher softness values, s, than in the other solvents and in the gas phase (0.20 eV), indicating that these conformers can easily modify their electron density. On the other hand, the conformers with the highest electrophilicity index, ω, are W1/W1E (1.66 and 1.59 eV, respectively) in implicit/explicit solvents, which indicates better electrophilic behavior. These values are higher by approximately two eV with respect to the value of G1 in the gas phase (1.42 eV).
The use of global reactivity descriptors is common in predicting the activity of antimicrobial compounds [41,42,43,44]. For example, the electrophilicity index, ω, has been used in the evaluation of the toxicity of molecules with anti-microbial activity, allowing the quantification of the drug-receptor biological interaction [45]. A low value of the electrophilicity index, ω, is related to low toxicity [43,44]. In our case, the caespitate conformers, both in implicit and explicit chloroform solvents, C1 and C1E, show a lower value of ω, suggesting a lower toxicity with respect to the other conformers.
Figure S6 shows the molecular electrostatic potential (MEP) map in the gas and implicit solution phases. It is observed that the highest electron density (red regions) is concentrated on the O atom of the –OH group in the para position of the aromatic ring and slightly on the O atom of the acyl group in the conformers W1 and A1, while in the conformers G1, C1, and D1, the highest electron density is concentrated on the O atom of the acyl group and the O atoms of the three –OH groups of the aromatic ring. The electron density-deficient zones (blue regions) are around the H atom of the –OH group in the ortho position in the conformers G1, W1, C1, and A1, while in D1 it is around the H atom of the –OH group in the para position. In both cases, this H atom does not form a hydrogen bond. Another area of low electron density is on the methyl ester group of the prenylated chain in all conformers. Figure S7 shows the MEP map from explicit solution systems. In general, the highest electron density is concentrated on the aromatic ring, the O atom of the –OH groups in the ortho and para positions of the aromatic ring, the O atom of the acyl group, and the O atom of the ester group of the prenylated chain of the conformers C1E and D1E, while in the conformers W1E and A1E, the highest electron density is concentrated on the O atom of the carbonyl group and the O atom of the ester group of the prenylated chain. The electron density-deficient zones are observed in the prenylated chain in conformers C1E and D1E, while for W1E and A1E, these regions are observed on the methyl groups and some protons of the aromatic ring.
3.4. Density Topological Parameters
Intramolecular hydrogen bonds (IHBs) are particularly relevant for biologically active molecules due to their roles in molecular recognition, anticancer activity, chemical selectivity, and interactions between the molecule and its biological target [46,47,48,49]. The interesting ability of the Z isomer of caespitate to form two IHBs has been studied in previous works [9,10,11,12,14,15,49], in comparison with its E isomer, which forms only one IHB. Specifically, it has been shown that the formation of the second IHB dominates the conformational preferences in the gas and solvent phases [15]. It could explain the bioactivity associated with the Z isomer. The first IHB is formed by O15⋯H13 in G1, W1/W1E, C1/C1E, and A1/A1E, or O15⋯H11 in D1/D1E conformers. The second IHB is formed by O41⋯H9 in G1 and C1/C1E, or O41⋯H13 in D1/D1E; see Figure 4 and Figure 5. Figure 4 and Figure 5 show the molecular graphs of the most stable conformers in gas and the implicit and explicit solution phases. Bond critical points (BCPs) are indicated in green dots, and ring critical points (RCPs) are indicated in purple dots. Table 5 shows the values of the electron density, , the Laplacian of the electron density, , the Lagrangian kinetic energy, , the potential energy density, , the Hamiltonian kinetic energy, , the interaction energy, , the interatomic distance, Dinter, the valence angle, Ainter, and the delocalization indices, DI, of the BPCs corresponding to both IHBs. The electron density values, , of the bond critical points (BCP) and of the ring critical points (RCP) show the formation of non-covalent O⋯H bonds and the formation of stable rings in all conformers. The first IHB corresponds to BCP O15⋯H13 for G1, W1, C1, and A1, and BCP O15⋯H11 for D1 with values in the range 0.079 to 0.083 a.u., while the second IHB corresponds to BCP O41⋯H9 for conformers G1 and C1, and BCP O41⋯H13 for D1 with values from 0.026 to 0.030 a.u. in explicit solvent, to BCP O15⋯H13 for A1E, C1E, and Ac1E, and O15⋯H11 for D1E the values in the range 0.038 to 0.076 a.u., while BCP O41⋯H9 of C1E and BCP O41⋯H13 for D1 have values of 0.022 to 0.029 a.u. In both cases, for implicit and explicit models, it indicates that the first IHB is stronger than the second one. Also, positive values of on BCPs confirm the existence of weak or medium-strength non-covalent hydrogen bonds, exhibiting a local depletion of density ( [50]. In addition, the positive sign of the parameter indicates that the accumulation of charge density on the hydrogen bond has a destabilizing effect ( > 0) [51]. is interpreted as a consequence of the charge density accumulation at the interaction BCP [52]. Weak or medium-strength hydrogen bonds show positive values of and [53]. The interaction energy values, , for the first IHB, is in the range of 28.43 to 30.47 kcal mol−1, with conformer A1 having the highest magnitude for implicit solvent. For explicit solvent, the values are in the range of 10.61 to 27.29 kcal mol−1, with A1E having the highest value. For the second IHB of conformers G1, C1, and D1, the values are in the range of 6.62 to 7.72 kcal mol−1, with C1 being the conformer with the highest value. For the explicit solvent, the values are in the range of 5.21 to 7.65 kcal mol−1, with C1E with the highest value. For both implicit and explicit models, the values of the first IHB are clearly higher than those of the second IHB, indicating that the first IHB has a greater stabilizing effect compared to the second IHB. The hydrogen bond strength classification indicates that strong hydrogen bonds have an energy of 15–40 kcal mol−1 and medium-strength hydrogen bonds have an energy of 4–15 kcal mol−1 [54]. According to this classification, the first IHB is a strong hydrogen bond, and the second is a medium-strength hydrogen bond. On the other hand, the delocalization indices, DI, have values <1.0, corresponding to non-covalent interactions for both IHBs. The DI for the BCP of the covalent bonds, O12–H13 and O10–H11, have values of ≅1.0 a.u., indicating that these bonds have the strength of a single bond, and the H13 and H11 atoms do not contribute to a great extent to the formation of the hydrogen bond with the O15 atom. The bond distances of both hydrogen bonds O⋯H, Dinter ≅ 1.50 and 1.80 Å, for the implicit model, and Dinter ≅ 1.52–1.56 and 1.81-1.93 Å for the explicit model, indicate that they could be strong bonds; however, the valence angles H–O⋯H are not optimal (Ainter ≅ 142.5–156.0° for the implicit model, and 146.6–156.1° for the explicit model) for the formation of strong hydrogen bonds, which should have a value close to 180° [55]. With respect to in the RCPs, the values of the first IHB of all conformers are similar, in the range of 0.0239–0.0243 a.u. for the implicit solvent and 0.0198–0.0238 a.u. for the explicit solvent; the values of in the RCP of the second IHB are 0.0079–0.0091 a.u. for the implicit solvent, and 0.0085–0.0098 a.u. for the explicit solvent, showing that the ring structures formed by the hydrogen bond O15⋯H13 or O15⋯H11 stabilize the structure in the same order at all implicit and explicit conformers, while the ring in conformers G1 and C1/C1E with the formation of O41⋯H9, and D1/D1E with the formation of O41⋯H13 do not contribute significantly to the stabilization of the system. Other non-covalent interactions of electrostatic type are also observed, including the interaction formed between the hydrogen atoms of the methyl group of the acyl group and the oxygen atoms of the OH groups in the ortho position, which does not form the first IHB, and some other interactions in the prenylated chain; see Figure 4 and Figure 5. In general, the interaction energies, , of the explicit solvent systems are smaller (10.60–27.30 kcal mol−1 for the first IHB and 5.20–6.55 kcal mol−1 for the second IHB) with respect to those obtained with the implicit solvent model (28.43–30.37 kcal mol−1 for the first IHB and 6.62–7.72 kcal mol−1 for the second IHB), which can be explained due to the non-covalent interactions between solvent molecules that surround the caespitate molecule in the systems W1E, C1E, A1E, and D1E.
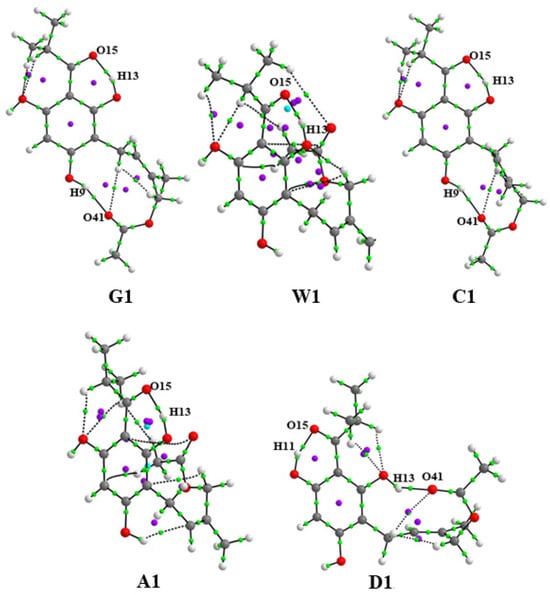
Figure 4.
Molecular graphs of the most stable conformers of caespitate calculated at the APFD/6-311+G(2d,p) level of theory in gas and solution phases. Gas (G1), water (W1), chloroform (C1), acetonitrile (A1), and DMSO (D1) phases.
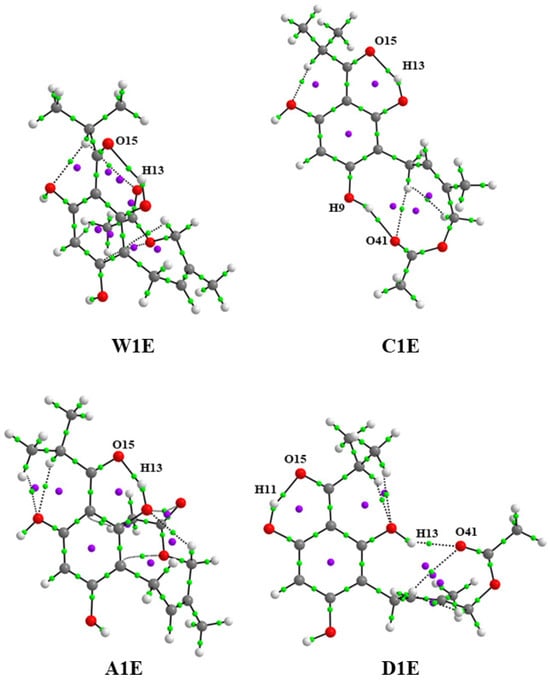
Figure 5.
Molecular graphs of the caespitate conformers calculated with the ONIOM (ωB97X-D3/Def2—TZVP:XTB2) method with explicit solvent obtained from the most stable conformers in implicit solution in water (W1E), chloroform (C1E), acetonitrile (A1E), and DMSO (D1E).

Table 5.
Topological parameters (a.u.), EH…Y (kcal mol−1), interatomic distances (Dinter, Å), and valence angles (Ainter, °) of the most stable conformers of caespitate calculated in implicit solvent at the APFD/6-311+G(2d,p) level of theory and in explicit solvent at the APFD/6-311+G(2d,p)//ONIOM (ωB97X-D3/Def2-TZVP:XTB2) level of theory in the gas phase (G1); in the implicit solvent: water (W1), chloroform (C1), acetonitrile (A1), and DMSO (D1) phases; and in the explicit solvent: water (W1E), chloroform (C1E), acetonitrile (A1E), and DMSO (D1E) phases.
3.5. Validation of the Solvent Models by NMR Spectroscopy
To validate our results regarding the structure of the most stable conformers of caespitate considering the implicit and explicit solvent, calculations of the 1H and 13C NMR spectra were performed and compared with the experimental spectra at chloroform solvent [3]. Chemical shifts (δ) of 1H and 13C are reported in ppm using tetramethylsilane (TMS) as a reference. A correlation of the calculated values with the experimental data is carried out in order to validate the level of theory used and confirm that the chemical shifts correspond to the extended conformation of caespitate in the gas phase (G1) and chloroform solvent using implicit and explicit models (C1 and C1E). Table 6 and Table 7 show the experimental (δExp), calculated (δCalc), and recalculated (δRecalc) chemical shift values of the 1H and 13C NMR spectra, respectively. The δrecalc values were estimated through a simple linear regression equation, δRecalc = (δCalc−b)/m, with slope m and the intercept b [56]. Calculated chemical shifts using the APFD/6-311+G(2d,p) level of theory at the gas phase and implicit and explicit solvent models in chloroform were used to perform the fundamental assignments of caespitate according to Figure 6.

Table 6.
Experimental, calculated, and recalculated 1H-NMR chemical shifts δ (ppm) with respect to TMS of the most stable conformer of caespitate calculated at the APFD/6-311+G(2d,p) level of theory in gas and implicit and explicit solution phases.

Table 7.
Experimental, calculated, and recalculated 13C-NMR chemical shifts δ (ppm) with respect to TMS of the most stable conformer of caespitate calculated at the APFD/6-311+G (2d,p) level of theory in gas and implicit and explicit solution phases.
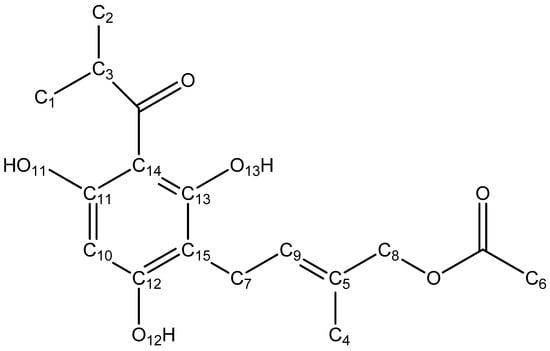
Figure 6.
Structure of caespitate for 1H and 13C NMR chemical shifts δ assignation.
The chemical shift (δ) calculations for the extended conformer of caespitate (G1, C1, and C1E) were carried out to validate the methodology used in this work and verify that the extended conformation of caespitate was the most stable reported experimentally [3]. The values in Table 6 and Table 7 show a clear correlation between the experimental, calculated, and recalculated values. Using the parameters in the simple linear regression equation, it was possible to estimate values of the correlation coefficients R2 in the range of 0.9753 to 0.9995 for 1H and of 0.9986 to 0.9991 for 13C-NMR chemical shifts. In Table 6, for 1H-NMR δ, some values were overestimated with respect to the experimental data; however, considering the implicit solvent, several values were improved, and finally, taking into account the explicit solvent, most of the values were in good agreement with the experimental data. In Table 7, a similar trend is observed for 13C-NMR δ. In the gas phase, some values were overestimated with respect to the experimental data, but considering the implicit solvent, the results were improved, and finally, the best concordance was found for explicit solvent results.
4. Conclusions
In this work, 86 caespitate conformers were generated using the MM with relative energy values ΔE ≤ 6 kcal mol−1. Posteriorly, DFT optimization calculations at the APFD/6-31+G(d) level of theory were used to obtain the structures with energies ΔE ≤ 4 kcal mol−1. Subsequently, a higher level of theory, APFD/6-311+G(2d,p), was employed as a second filter, where the optimization and calculation of vibrational frequencies were performed to obtain stable structures with energies ΔE ≤ 2 kcal mol−1. Calculations were carried out both in the gas phase and implicit solution phases with different solvents, including water, chloroform, acetonitrile, and DMSO. In the gas phase (G), a total of 24 conformers were obtained, while in the water phase (W), 10 conformers, 26 conformers in chloroform (C), 33 conformers in acetonitrile (A), and 34 conformers in DMSO (D) were identified. The most stable structures in each phase presented extended conformation in the gas phase (G1) and in chloroform (C1), while in water (W1), acetonitrile (A1) and DMSO (D1) had a hairpin conformation. The extended forms G1 and C1 showed two intramolecular hydrogen bonds (IHBs), whereas in the hairpin conformations, only D1 showed both hydrogen bonds, while W1 and A1 showed only one IHB. Population analysis based on the Boltzmann distribution revealed the prevalence of the extended conformation, which preserved both IHBs. The extended conformation was predominant in the gas phase (G1) with 24.60% and in the implicit chloroform solution (C1) with 49.10%. To assess the explicit solvent influence in caespitate conformers, systems surrounded by 31 molecules for each solvent were constructed using the ONIOM methodology (ωB97X-D3/Def2-TZVP:XTB2). Optimized structures were obtained from the caespitate in the gas phase (WG1E, CG1E, AG1E, and DG1E), as well as from the implicit solvent structures (W1E, C1E, A1E, and D1E). The results showed a greater stabilization energy of –4.97 kcal mol−1 for caespitate when surrounded by chloroform compared to the other solvents. Additionally, the stability order of both considering the conformer in the gas phase structure (G1) and the most stable implicit solvent structures (W1, C1, A1, and D1) followed the same trend: ECG1E/EC1E > EDG1E/ED1E > EAG1E/EA1E > EWG1E/EW1E. In general, the caespitate conformers maintained a conformation similar to that obtained in the implicit solvent when surrounded by each of the solvents explicitly. It was confirmed through the assessment of RMSD values between each pair of conformers in implicit and explicit solvents. Global reactivity descriptors were analyzed using the conceptual DFT approach from the FOM HOMO and LUMO energies. In general, the global reactivity indices exhibited slightly higher values in explicit solvent compared to those in implicit solvent. On the other hand, the QTAIM analysis was employed for characterizing the IHBs in the caespitate structures. The EH...Y interaction energies were lower in the systems with explicit solvent compared to those obtained with implicit solvent. This can be attributed to the non-covalent interactions between the solvent molecules surrounding the caespitate molecule in the W1E, C1E, A1E, and D1E systems. Finally, NMR spectroscopy was used to validate the methods. In both 1H and 13C-NMR δ, in the gas phase, some values overestimated the experimental data; however, considering both implicit and explicit solvents, the results were better fitted to experimental values. The correlation coefficients confirmed the good concordance between calculated and experimental data, validating the methodology used in this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/computation12010005/s1, Table S1. Relative free energy (ΔG) and population (%) of conformers of caespitate with free energies ≤ 2 kcal mol−1 calculated at the APFD/6-311+G(2d,p) level of theory in gas and solution phases; Figure S1. Molecular structures of the five most stable conformers of caespitate calculated at the APFD/6-311+G(2d,p) level of theory in gas and solution phases in gas (G1–G5), water (W1–W5), chloroform (C1–C5), acetonitrile (A1–A5), and DMSO (D1–D5) phases. Figure S2. Optimized geometries of the caespitate—explicit solvent systems calculated with the ONIOM (ωB97X-D3/Def2-TZVP:XTB2) method obtained from the most stable conformer in the gas phase (G1) surrounded by water (WG1E), chloroform (CG1E), acetonitrile (AG1E), and DMSO (DG1E) molecules; Figure S3. Comparison of the optimized structures of the most stable conformer in the gas phase (G1) (in red color) surrounded by explicit solvent in water (WG1E), chloroform (CG1E), acetonitrile (AcG1E), and DMSO (DG1E) (in blue color); Figure S4. Isosurfaces of the frontier molecular orbitals HOMO and LUMO of the most stable caespitate conformers calculated at the APFD/6-311+G(2d,p) level of theory in gas and solution phases in gas (G1), water (W1), chloroform (C1), acetonitrile (A1), and DMSO (D1) phases; Figure S5. Isosurfaces of the frontier molecular orbitals HOMO and LUMO of the caespitate conformers calculated at the ONIOM (ωB97X-D3/Def2—TZVP:XTB2) method with explicit solvent obtained from the most stable conformers in implicit solution in water (W1E), chloroform (C1E), acetonitrile (A1E), and DMSO (D1E); Figure S6. Molecular electrostatic potential (MEP) of the most stable caespitate conformers calculated at the APFD/6-311+G(2d,p) level of theory in gas and solution phases in gas (G1), water (W1), chloroform (C1), acetonitrile (A1), and DMSO (D1) phases; Figure S7. Molecular Electrostatic Potential (MEP) of the caespitate conformers calculated at the ONIOM (ωB97X-D3/Def2-TZVP:XTB2) method with explicit solvent obtained from the most stable conformers in implicit solution in water (W1E), chloroform (C1E), acetonitrile (A1E), and DMSO (D1E).
Author Contributions
Conceptualization, M.E.C. and F.J.M.; methodology, A.M.-C., M.E.C., F.J.M. and L.M.; software, M.E.C., L.M., N.A.C. and F.J.M.; validation, A.M.-C. and L.M.; writing—original draft preparation, A.M.-C., M.E.C. and F.J.M.; writing—review and editing, L.M. and N.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
Vicerrectoría de Investigación y Estudios de Posgrado (VIEP-BUAP, Mexico) (100517029-VIEP2023 project), and the PRODEP Academic Group (SEP, Mexico) (BUAP-CA-263).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
AMC thanks CONACYT-México for financial support (fellowship No. 769481). The authors thank the Laboratorio Nacional de Supercómputo del Sureste de México (LNS-BUAP) of the CONACYT network of national laboratories for the computer resources and support provided and the PRODEP Academic Group BUAP-CA-263 (SEP, Mexico).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 63009. [Google Scholar] [CrossRef] [PubMed]
- Achika, J.; Arthur, D.; Gerald, I.; Adedayo, A. A Review on the Phytoconstituents and Related Medicinal Properties of Plants in the Asteraceae Family. IOSR J. Appl. Chem. 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Mathekga, A.D.M.; Meyer, J.J.M.; Horn, M.M.; Drewes, S.E. An acylated phloroglucinol with antimicrobial properties from Helichrysum caespititium. Phytochemistry 2000, 53, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.G.; Fourie, T.G.; Snyckers, F.O.; van der Schyf, C.J. Studies of South African medicinal plants. Part 2. Caespitin, a new phloroglucinol derivative with antimicrobial properties from Helichrysum caespititium. S. Afr. J. Chem. 1983, 36, 114–116. Available online: https://journals.co.za/doi/pdf/10.10520/AJA03794350_1036 (accessed on 19 October 2023).
- Mapaura, A.; Timberlake, J. A Checklist of Zimbabwean Vascular Plants Southern African Botanical Diversity Network Report No. 33; SABONET Publications: Pretoria, South Africa, 2004; p. 26. [Google Scholar]
- Pooley, E. A Field Guide to the Wild Flowers of KwaZulu-Natal and the Eastern Region; Natal Flora Publications Trust: Durban, South African, 1998; pp. 442–443. [Google Scholar]
- WHO. Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 20 October 2023).
- Mathekga, A.D.M. Antimicrobial Activity of Helichrysum Species and the Isolation of a New Phloroglucinol from Helichrysum Caespititium. Doctoral Thesis, University of Pretoria, UPSpace International Repository, Pretoria, South Africa, 2001. Available online: https://repository.up.ac.za/handle/2263/23672 (accessed on 20 October 2023).
- Mammino, L.; Kabanda, M.M. Model structures for the study of acylated phloroglucinols and computational study of the caespitate molecule. J. Mol. Struct. THEOCHEM 2007, 805, 39–52. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. A study of the intramolecular hydrogen bond in acylphloroglucinols. J. Mol. Struct. THEOCHEM 2009, 901, 210–219. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. The conformational preferences of acylphloroglucinols—A promising class of biologically active compounds. Int. J. Comput. Chem. 2012, 112, 3691–3702. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. A Computational Study of the Effects of Different Solvents on the Characteristics of the Intramolecular Hydrogen Bond in Acylphloroglucinols. J. Phys. Chem. A 2009, 113, 15064–15077. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. The role of additional O–H···O intramolecular hydrogen bonds for acylphloroglucinols’ conformational preferences in vacuo and in solution. Mol. Simul. 2013, 39, 1–13. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. Computational study of the patterns of weaker intramolecular hydrogen bonds stabilizing acylphloroglucinols. Int. J. Quantum Chem. 2012, 112, 2650–2658. [Google Scholar] [CrossRef]
- Mammino, L.; Kabanda, M.M. The Geometric Isomers of Caespitate: A Computational Study in Vacuo and in Solution. Int. J. Biol. Biomed. Eng. 2012, 6, 114–133. Available online: http://ijdri.com/ijbbe/2012/17-931.pdf (accessed on 19 October 2023).
- Tobiason, F.L.; Vergoten, G. Chapter: GMMX Conformation Searching and Prediction of NMR Proton-Proton Coupling Constants. In Biomolecular Structure and Dynamics; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1997; Volume 342, pp. 179–186. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView; Version 6.0; Semichem Inc.: Shawnee Mission, KS, USA, 2019; Available online: https://gaussian.com/gaussview6/ (accessed on 24 October 2023).
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A density functional with spherical atom dispersion terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.D. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639. [Google Scholar] [CrossRef]
- Binkley, J.S.; Pople, J.A.; Hehre, W.J. Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J. Am. Chem. Soc. 1980, 102, 939–947. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Solvents List SCRF. Gaussian. Available online: http://gaussian.com/scrf/?tabid=7 (accessed on 10 October 2023).
- Ott, J.B.; Boerio-Goates, J. Chemical Thermodynamics: Advanced Applications, 1st ed.; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Chung, L.W.; Sameera, W.M.C.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.; Liu, F.; et al. The ONIOM Method and Its Applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef]
- Pantazis, D.; Neese, F. All-electron scalar relativistic basis sets for the 6p elements. Theor. Chem. Acc. 2012, 131, 1292. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.E.; Rangel-Galván, M.; Morales Dávila, E.; Caballero, N.A.; Melendez, F.J. Theoretical NMR and IR spectroscopic analyses of the preferred conformers of the neurotransmitter anandamide. Int. J. Quantum Chem. 2023, 123, e27098. [Google Scholar] [CrossRef]
- Hruska, E.; Gale, A.; Huang, X.; Liu, F. AutoSolvate: A Toolkit for Automating Quantum Chemistry Design and Discovery of Solvated Molecules. J. Chem. Phys. 2022, 156, 124801. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224180. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Tod, A.K. TK Gristmill Software, AIMAll Version 17.11.14; Overland Park, KS, USA, 2017. Available online: https://aim.tkgristmill.com (accessed on 14 October 2023).
- Andrienko, G.A. Chemcraft—Graphical Program for Visualization of Quantum Chemistry Computations; Version 1.8; Chemcraft Authors: Ivanovo, Russia, 2016; Available online: http://www.chemcraftprog.com (accessed on 10 October 2023).
- Parthasarathi, R.; Padmanabhan, J.; Sarkar, U.; Maiti, B.; Subramanian, V.; Chattaraj, P.K. Toxicity Analysis of Benzidine Through Chemical Reactivity and Selectivity Profiles: A DFT Approach. Internet Electron. J. Mol. Des. 2003, 2, 798–813. Available online: http://www.biochempress.com/av02_0798.html (accessed on 19 December 2023).
- Pradeep Kumar, C.B.; Raghu, M.S.; Prasad, K.N.N.; Chandrasekhar, S.; Jayanna, B.K.; Alharthi, F.A.; Prashanth, M.K.; Yoghes Kumar, K. Expatiating biological excellence of 2,3-disubstituted quinazolin-4(1H)-ones against Mycobacterium tuberculosis and DNA using docking, spectroscopic and DFT studies. New J. Chem. 2020, 45, 403–414. [Google Scholar] [CrossRef]
- Mishra, V.R.; Ghanavatkar, C.W.; Mali, S.N.; Chaudhari, H.K.; Sekar, N. Schiff base clubbed benzothiazole: Synthesis, potent antimicrobial and MCF-7 anticancer activity, DNA cleavage and computational study. J. Biomol. Struct. Dyn. 2019, 38, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.N.; Pandey, A.; Thorat, B.R.; Lai, C. Multiple 3D- and 2D-quantitative structure–activity relationship models (QSAR), theoretical study and molecular modeling to identify structural requirements of imidazopyridine analogues as anti-infective agents against tuberculosis. Struct. Chem. 2022, 33, 679–694. [Google Scholar] [CrossRef]
- Eno, E.A.; Mbonu, J.I.; Louis, H.; Patrick-Inezi, F.S.; Gber, T.E.; Unimuke, T.O.; Okon, E.E.D.; Benjamin, I.; Offiong, O.E. Antimicrobial activities of 1-phenyl-3-methyl-4-trichloroacetyl-pyrazolone: Experimental, DFT studies, and molecular docking investigation. J. Indian Chem. Soc. 2022, 99, 100524. [Google Scholar] [CrossRef]
- Laurence, C.; Berthelot, M. Observations on the strength of hydrogen bonding. Perspect. Drug Discov. Des. 2000, 18, 39–60. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Lightner, D.A. Influence of Conformation and Intramolecular Hydrogen Bonding on the Acyl Glucuronidation and Biliary Excretion of Acetylenic Bis-Dipyrrinones Related to Bilirubin. J. Med. Chem. 2007, 50, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Millan, D.S.; Perez, M.; Wakenhut, F.; Whitlock, G.A. Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space. Med. Chem. Commun. 2011, 2, 669–674. [Google Scholar] [CrossRef]
- Meyer, J.J.M.; Lall, N.; Mathekga, A.D.M.; Jäger, A.K. In vitro inhibition of drug-resistant and drug-sensitive strains of Mycobacterium tuberculosis by Helichrysum caespititium. S. Afr. J. Bot. 2002, 68, 90–93. [Google Scholar] [CrossRef]
- Pinto, A.V.; Magalhães, A.L. Intramolecular Hydrogen Bonds in Tip-Functionalized Single-Walled Carbon Nanotubes as pH-Sensitive Gates. J. Phys. Chem. A 2020, 124, 9542–9551. [Google Scholar] [CrossRef]
- Grabowski, S.J. What Is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef]
- Duarte, D.J.R.; Angelina, E.L.; Peruchena, N.M. Physical meaning of the QTAIM topological parameters in hydrogen bonding. J. Mol. Model. 2014, 20, 2510. [Google Scholar] [CrossRef] [PubMed]
- Rozas, I.; Alkorta, I.; Elguero, J. Behavior of Ylides Containing N, O, and C Atoms as Hydrogen Bond Acceptors. J. Am. Chem. Soc. 2000, 122, 11154–11161. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V.; Sigalov, M.V. Estimating the energy of intramolecular hydrogen bonds from1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199. [Google Scholar] [CrossRef] [PubMed]
- Alareeqi, S.; Bahamon, D.; Nogueira, R.P.; Vega, L.F. Understanding the relationship between the structural properties of three corrosion inhibitors and their surface protectiveness ability in different environments. Appl. Surf. Sci. 2021, 542, 148600. [Google Scholar] [CrossRef]
- Castro Sánchez, M.E.; Noriega, L.; Perez-Aguilar, J.M.; Caballero-Concha, N.A.; Merino-Montiel, P.; Romero López, A.; Melendez Bustamante, F.J. Advances in Green and Sustainable Chemistry. In Green Chemistry and Computational Chemistry: Shared Lessons in Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 8; pp. 193–214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).