Abstract
Background: The edible gonads of the sea urchin Paracentrotus lividus are highly valued, yet sex cannot be determined externally, limiting selective harvest and quality control. Objective: We aimed to test whether headspace solid-phase microextraction gas chromatography–mass spectrometry combined with chemometrics can discriminate sex from gonadal volatilomes. Methods: Gonads from 29 individuals (21 females, 8 males) were profiled by this technique. Spectral data were modeled with Partial Least Squares–Linear Discriminant Analysis (PLS–LDA), Variable Importance in Projection scores highlighted key features, and Mann–Whitney tests assessed univariate differences. Tentative identifications were assigned by library match and curated for potential environmental artifacts. Results: Chemometric modeling yielded a clear female–male separation. Female gonads were enriched in low-odor-threshold oxygenates—aldehydes (hexanal, heptanal) and alcohols (1-penten-3-ol, 1-octen-3-ol)—together with diet-linked monoterpenes (e.g., D-limonene), consistent with PUFA LOX/HPL pathways and macroalgal inputs. Male gonads were dominated by saturated/branched hydrocarbons and long-chain alcohols with limited direct odor impact. Minor aromatic hydrocarbons (e.g., styrene; 1,3-bis(1,1-dimethylethyl)-benzene) were retained as environmental/artifact markers and excluded from biological interpretation. Conclusions: HS-SPME gas chromatography–mass spectrometry volatilomics coupled with PLS–LDA effectively distinguishes the sex of P. lividus gonads and rationalizes reported sensory differences. The marker set offers a basis for future non-destructive sexing workflows, pending confirmation with retention indices, authentic standards, and GC-olfactometry.
1. Introduction
Sea urchins are marine invertebrates that have gained increasing attention as high-value seafood, particularly in the Mediterranean and East Asian market [,]. Among the various species, Paracentrotus lividus is of special gastronomic interest due to the consumption of its gonads, often marketed as “sea urchin roe” or “uni” []. The gonads, which represent the reproductive and storage organs, exhibit significant variation in size, color, texture, and flavor depending on biological sex, reproductive stage, habitat, and feeding regime [,].
However, the growing commercial demand for edible gonads has led to intensive harvesting of wild populations, raising concerns about the ecological balance of coastal ecosystems [,,]. Overexploitation of sea urchin stocks can disrupt trophic dynamics and benthic community structure, contributing to the decline of macroalgal forests and the proliferation of barren grounds [,,]. Such imbalances reduce habitat complexity and biodiversity, with cascading effects on associated invertebrate and fish assemblages [,,]. Sustainable management of sea urchin fisheries and the development of aquaculture alternatives are therefore essential to mitigate ecological impacts while maintaining the economic and gastronomic value of this resource [].
In Mediterranean gastronomy, female gonads are often perceived as superior in taste and aroma and thus fetch higher market prices; nonetheless, controlled studies in echinoids indicate that sex and season jointly modulate sensory profiles, with gender effects not always unidirectional across contexts [,]. Despite this practical importance, the sex of sea urchins cannot be determined externally, especially before spawning [,]. Traditionally, sex identification requires dissection, which is destructive and incompatible with live handling or product quality preservation []. From the perspective of aquaculture, traceability, and food authentication, the development of non-invasive or minimally invasive tools for sex determination is a pressing need, enabling early selection for breeding or market purposes while enhancing product consistency.
In recent years, omics-based platforms have been applied to food authentication and quality assessment [,,]. Volatilomics, the study of volatile organic compounds (VOCs), is gaining ground as a non-destructive and sensitive strategy for detecting subtle biological differences in complex matrices. VOCs are produced as metabolic end-products of enzymatic pathways and reflect an organism’s physiological and genetic state. In sea urchins, volatiles may arise from the interaction between gonadal tissue enzymes, lipids, amino acids, and microbial flora, making them a promising source of sex-linked biochemical signals. Headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME–GC–MS) has become a powerful analytical technique for volatile profiling in foods and biological samples, offering high sensitivity, minimal sample preparation, and broad chemical coverage (aldehydes, alcohols, ketones, esters, hydrocarbons, terpenes) []. Furthermore, combining GC–MS data with chemometric analysis (e.g., PCA, PLS-DA) enables effective classification of samples based on multivariate VOC signatures [].
Several studies have demonstrated the capacity of GC–MS-based VOC fingerprinting to distinguish between products with subtle differences. For example, honey varieties and bee species have been differentiated by HS-SPME–GC–MS volatile fingerprints []. In meat systems, this technique and workflow has identified quantitative markers of shelf-life and freshness across multiple product types under controlled storage []. Comparable strategies have been applied to fungal matrices such as truffles, where comprehensive reviews and recent studies align sensory notes with specific volatile families and analytical workflows [,,]. In seafood quality/shelf-life, VOC profiling likewise underpins objective discrimination and spoilage assessment. Together, these examples illustrate how standardized HS-SPME–GC–MS pipelines, interpreted through chemometrics, can robustly classify complex food matrices.
However, no study has yet tested VOC fingerprinting for sex determination in echinoderms, and particularly in Paracentrotus Lividus (Lamarck, 1816). Nevertheless, echinoid work indicates that diet and physiology strongly shape odor-active volatiles: kelp-feeding in Mesocentrotus nudus (A.Agassiz, 1964) modulates odor-active compounds and reduces off-notes, while early P. lividus GC-MS data documented matrix-dependent volatile patterns (fresh vs. processed) [,]. Building on this rationale, we propose a novel methodological approach to distinguish male and female P. lividus gonads based on HS-SPME–GC–MS VOC fingerprinting coupled with multivariate analysis. Our goal is to identify sex-specific VOC signatures that may serve not only for biological classification but also as predictors of organoleptic quality in gourmet markets. Our dataset includes samples from 29 individuals (21 females and 8 males), with volatile profiles extracted under standardized conditions and subjected to chemometric evaluation.
2. Materials and Methods
2.1. Sample Collection and Preparation
A total of 29 adult specimens of Paracentrotus lividus were collected manually in May 2024, during the peak reproductive season, from the Mediterranean coast of Alicante (Spain) (Figure 1). Individuals were transported live to the Marine Science laboratory of the University of Alicante in aerated seawater and sacrificed immediately under ethical guidelines approved for marine invertebrate studies. Collection complied with environmental regulations limiting the harvest of P. lividus.
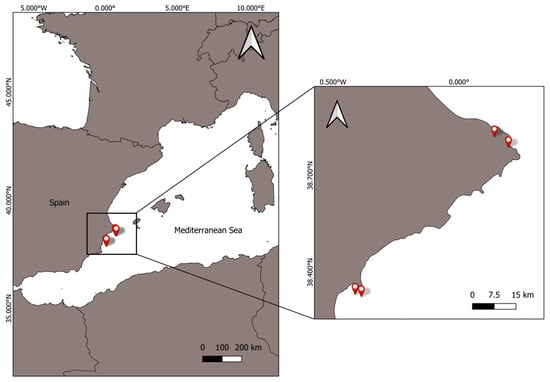
Figure 1.
Location of the study area in Alicante coast (Western Mediterranean), indicating sampling sites of Paracentrotus lividus.
Sex identification was performed through visual inspection of gonads after dissection, based on secondary-sexual characteristics, resulting in 21 females and 8 males []. Gonads were excised, homogenized, and initially stored at −20 °C. All samples were subsequently deep-frozen at −80 °C to prevent volatile degradation and then lyophilized under vacuum. Approximately 100 mg of the freeze-dried gonadal tissue from each individual was weighed and transferred into 20 mL glass vials sealed with PTFE/silicone septa for headspace analysis. The vials were stored at −20 °C until gas chromatography–mass spectrometry (GC–MS) analysis.
2.2. VOC Extraction and GC–MS Analysis
Volatile organic compounds were extracted using headspace solid-phase microextraction (HS-SPME). Each vial was incubated at 50 °C for 10 min with agitation. A 50/30 μm divinylbenzene/carboxen/polydimethylsiloxanefiber (Supelco, Bellefonte, PA, USA) was then inserted into the headspace and exposed for 30 min to allow VOC adsorption. GC–MS analysis was performed using an Agilent 7890A gas chromatograph coupled to a 5975C inert mass selective detector (Agilent Technologies, Santa Clara, CA, USA). The instrument was equipped with an HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness). The temperature program was as follows: initial 40 °C for 5 min, ramped at 5 °C/min to 250 °C, and held for 5 min. The injector was maintained at 250 °C in splitless mode. Helium was used as the carrier gas at a constant flow of 1.0 mL/min. Mass spectra were recorded in electron ionization (EI) mode at 70 eV with a scan range of m/z 35–400. Compound identification was achieved by spectral comparison with the NIST 11 Mass Spectral Library, selecting the compound with the highest matching quality score (Qual%). Only identifications with Qual% ≥ 70 were retained for analysis, as recommended for untargeted volatilomics studies [].
2.3. Data Processing and Statistical Analysis
Supervised models were built with Partial Least Squares–Linear Discriminant Analysis (PLS–LDA) using the plslda routine from libPLS v1.95 in MATLAB version 2024 (MathWorks, Natick, MA, USA). In this approach, PLS extracts latent variables (LVs) that maximize covariance between X and y, and LDA is then fitted on the PLS score space to obtain a linear decision boundary for class prediction. The libPLS toolbox provides integrated routines for pretreatment, cross-validation and PLS-LDA modeling; it is openly available at the project website (https://www.libpls.net/, accessed on 12 November 2025) [,,].
Variable Importance in Projection (VIP) scores were computed from the final PLS model to rank VOC contributions. Complementary Mann–Whitney U tests compared autoscaled peak areas by sex; resulting p-values were adjusted by Benjamini–Hochberg FDR, and Cliff’s δ quantified effect size.
3. Results
The gonadal samples of Paracentrotus lividus were analyzed using Gas Chromatography–Mass Spectrometry (GC-MS), yielding their corresponding chromatographic profiles (Figure 2). Although the figure displays only two representative spectra—one from a female and one from a male specimen—clear differences can be observed between them (Figure 2).
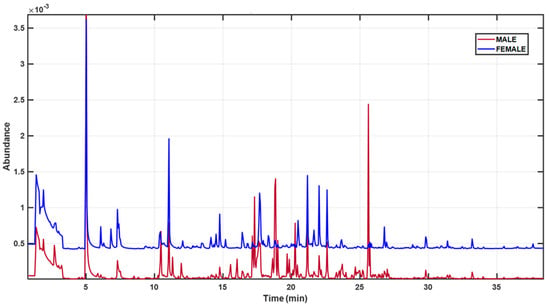
Figure 2.
Representative HS-SPME-GC-MS TICs of P. lividus gonads from a female (blue) and a male (red) specimen.
To assess the consistency of GC–MS profiles within each sex, the variability of the 35 selected peak areas was evaluated after total ion current (TIC) normalization. Overall, chromatograms within each sex exhibited a high degree of reproducibility, although female samples showed slightly greater dispersion. The median within-group coefficients of variation (CV) were 53.5% for females and 28.1% for males (robust CV = 47.7% and 24.8%, respectively), indicating a more heterogeneous volatilome among females. Principal component analyses (PCA) performed separately for each sex confirmed this trend: PC1–PC2 accounted for 60.9% of the total variance in females and 79.7% in males, with compact score distributions in both cases. These results demonstrate that the sex-specific volatilome differences are robust and reproducible, and that the slightly higher variability among females likely reflects greater biochemical and dietary heterogeneity during the reproductive phase.
The GC-MS spectra of all samples were subjected to chemometric analysis, specifically employing Partial Least Squares–Linear Discriminant Analysis (PLS-LDA) (Figure 3) [,,]. The resulting score plot (Figure 3B) revealed a clear separation between male and female samples, indicating distinct VOC profiles associated with sex (Table 1) [,]. Furthermore, the Variable Importance in Projection (VIP) scores derived from the PLS-LDA model identified the chromatographic signals contributing most significantly to this separation (Figure 2).
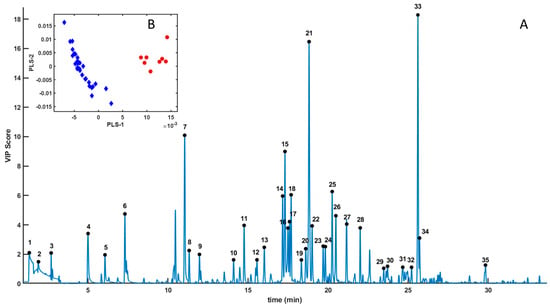
Figure 3.
Sex discrimination based on GC-MS volatilome. (A) VIP scores derived from the PLS-LDA model applied to GC-MS spectral data of P. lividus gonads. The most significant VIP peaks were annotated numerically (black circles). The inset (B) shows the PLS-LDA score plot showing male (red circles) and female (blue diamonds).

Table 1.
Identifications of the peaks by retention time (RT).
In the VIP profile, the most significant peaks contributing to the discrimination between sexes were annotated numerically (Table 1) [,,]. The next step in our workflow involved extracting the normalized peak areas corresponding to these signals from the raw GC-MS data. Peak identification was performed using the GC-MS instrument software (NIST 11 Mass Spectral Library), which also provided identification quality scores, acknowledging that not all identifications are equally reliable or unambiguous.
Once the normalized peak areas were extracted for all samples, we employed supervised chemometric modeling (PLS_LDA) to determine which compounds were most influential in differentiating between male and female gonadal profiles. Again, a PLS-LDA approach was applied, this time using only the 35 selected peak areas (Table 1) as input variables. The resulting score plot (Figure 3B) displayed an excellent separation between male and female groups, reinforcing the discriminative power of these selected VOCs.
Additionally, the VIP scores and corresponding loadings plot (Figure 4) highlighted the key compounds driving the separation observed in the PLS-LDA model. This refinement of the dataset allowed us to focus on a subset of VOCs with both statistical and sensory relevance in the differentiation of sexes.
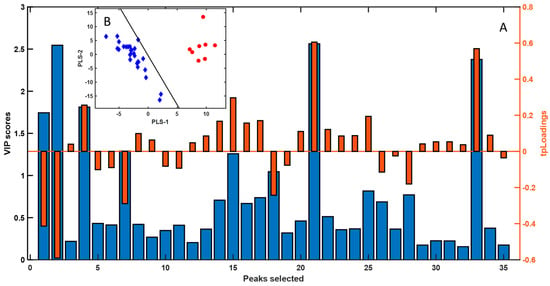
Figure 4.
(A) VIP scores and corresponding loadings plot from the PLS-LDA model, constructed using the normalized areas of the selected GC-MS peaks (Table 1). The inset (B) shows the PLS-LDA score plot depicting sample classification based on sex. Male (red circles) and female (blue diamonds).
The Mann–Whitney U test identified statistically significant differences in the relative abundance of multiple volatile compounds between male and female Paracentrotus lividus gonads. The analysis revealed that 30 out of 35 compounds exhibited statistically significant differences (p < 0.05), with normalized U statistics (U_stat) ranging from –0.67 to +1.32. These values indicate both the magnitude and direction of enrichment: positive U_stat values reflect compounds more abundant in males, while negative values indicate female enrichment. To visualize these differences, histograms were generated for each compound, displaying the distribution of normalized peak areas across both sexes (Figure 5). These histograms clearly illustrate the distinct abundance patterns for key volatiles, with several compounds showing markedly higher concentrations in female gonads (e.g., hexanal, 1-octen-3-ol, or D-limonene) [,], while others, such as methylamine derivatives, were more prevalent in male samples [,,].
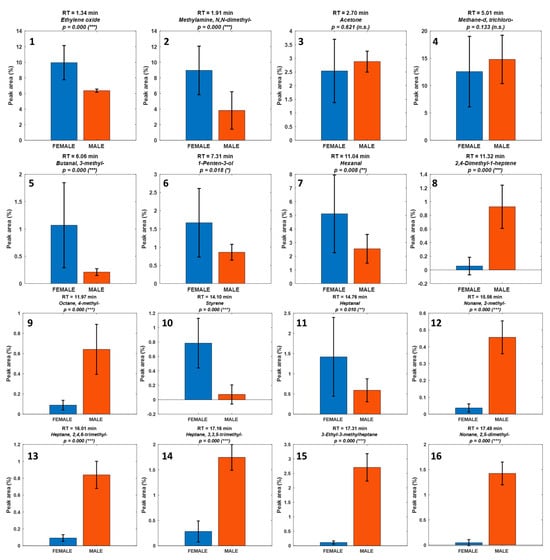
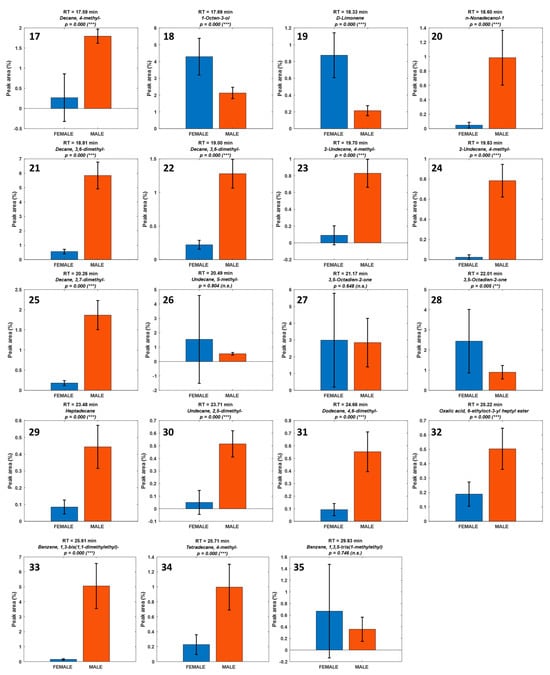
Figure 5.
Histograms representing the normalized peak area distribution of the identified volatile compounds in male (orange) and female (blue) Paracentrotus lividus gonads. Each subplot corresponds to a specific compound (Table 1). Statistical significance was assessed using the Mann–Whitney U test, with p-values indicated for each compound. Compounds significant are marked (* p < 0.05, ** p < 0.01, *** p < 0.001), indicating significant sex-based differences in abundance.
Compounds with the highest U_stat values—such as 2,4-Dimethyl-1-heptene, 4-Methyl-octane, 2-Methyl-nonane, and 2,4,6-Trimethyl-heptane (U_stat = 1.321, p ≤ 2 × 10−5)— were consistently more abundant in male samples. In contrast, compounds such as N,N-Dimethyl-methylamine (U_stat = –0.554, p = 2.1 × 10−4), Ethylene oxide (U_stat = –0.536, p = 2.8 × 10−4), and D-Limonene (U_stat = –0.67, p = 3 × 10−5), with negative U-statistics, were significantly enriched in females.
The combined use of univariate (Mann–Whitney U test) and visual (histogram) analyses (Figure 4) provided robust evidence supporting the hypothesis that the volatile profile of P. lividus gonads is influenced by sex. This statistical approach not only confirmed the trends observed in the chemometric models but also highlighted specific compounds with the greatest discriminatory power.
The family-level grouping in Table 2 delineates two distinct volatilome signatures. Female gonads are enriched in low-threshold oxygenated volatiles [,]—notably aldehydes (hexanal, heptanal) and alcohols (1-penten-3-ol, 1-octen-3-ol)—together with diet-linked monoterpenes (e.g., D-limonene), consistent with lipoxygenase-mediated PUFA oxidation [] and macroalgal terpenoid intake. This ensemble underpins ‘fresh-green’, ‘citrus’, and ‘marine’ notes and aligns with prior GC-MS/GC-O reports in sea urchins [,,]. In contrast, male gonads are dominated by saturated/branched hydrocarbons and long-chain alcohols with high odor thresholds, indicating a more structural lipid profile with limited direct sensory impact [,]. Minor aromatic hydrocarbons (e.g., styrene; 1,3-bis(1,1-dimethylethyl)-benzene) should be treated as potential environmental markers rather than endogenous aroma drivers []. Overall, this dichotomy explains the stronger and more complex bouquet typically observed for females and reinforces the multivariate separation.

Table 2.
Comparative overview of VOC profiles in female and male gonads of Paracentrotus lividus.
4. Discussion
The volatile organic compounds (VOCs) profile of Paracentrotus lividus gonads reveals clear chemical dimorphism between sexes, highlighting sex-linked differences in lipid metabolism, oxidative processes and dietary assimilation. Female gonads reveal a chemically rich matrix dominated by aldehydes, alcohols, and terpenes, whereas male gonads were characterized by saturated hydrocarbons and long-chain alcohols with limited aromatic activity. These findings support the view that reproductive physiology and metabolic orientation determine the sensory and biochemical signatures of gonadal tissues in sea urchins
Female gonads of Paracentrotus lividus showed a strong enrichment in oxygenated volatiles, particularly aldehydes (hexanal, heptanal, and 3-mthyl-butanal), alcohols (1-penten-3-ol, 1-octen-3-ol), and the monoterpene D-limonene. The suit of compounds collectively contributes to a fresh, sweet, and marine aroma profile [,,,].
The predominance of D-limonene is especially relevant, as this compound, likely derived from the ingestion of terpenoid-rich macroalgae, provides citrus and fruity notes associated with higher sensory acceptance in other edible echinoids such as Mesocentrotus nudus and Evechinus chloroticus [,]. The ability of females to accumulate such lipid-soluble volatiles likely reflects their greater lipid turnover during gonadal maturation, when energetic demands for oogenesis and yolk formation are maximal [,].
The presence of 1-penten-3-ol and 1-octen-3-ol further supports the role of oxidative lipid metabolism in shaping gonadal aroma. These compounds are generated via lipoxygenase-mediated oxidation of polyunsaturated fatty acids (PUFAs), particularly linoleic and linolenic acids, producing green and earthy-mushroom aromatic notes, respectively. Previous work has shown that higher PUFA levels correlate with enhanced aroma complexity in female gonads [,]. This suggests that oxidative metabolism, while potentially promoting lipid peroxidation, is also a key biochemical pathway underlying the distinctive sensory profile of females.
Aldehydes such as hexanal and heptanal reinforce this interpretation. As primary oxidation products of PUFAs, they introduce fresh and grassy aromas that enhance perceived freshness in seafood products []. Meanwhile, branched-chain aldehydes like 3-methyl-butanal, derived from leucine catabolism, impart nutty and roasted tones that increase sensory depth []. These compounds, with low odor thresholds, collectively explain why female gonads are often described as richer and more appealing by consumers.
Interestingly, low but consistent levels of N,N-dimethyl-methylamine were also detected in female gonads. While structurally related to spoilage amines, its endogenous presence in fresh tissues may contribute to subtle “marine” or oceanic aroma []. This controlled amine accumulation, rather than reflecting degradation, may originate from nitrogen metabolism or osmolyte pathways, adding a trace of saltiness that enhances product authenticity.
Male gonads, by contrast, displayed a volatile composition dominated by aliphatic hydrocarbons (decane, heptane, nonane, tetradecane) and long-chain fatty alcohols such as n-nonadecanol-1. These molecules, characterized by high olfactory thresholds, are largely odorless and therefore do not directly shape the sensory perception of male gonads. Their prevalence, however, reveals important biochemical features: males appear to maintain a more inert lipid matrix with lower oxidative turnover, consistent with their reproductive physiology. Whereas females mobilize lipid reserves for gametogenesis, males retain a structurally stable gonadal tissue dominated by neutral lipids and saturated hydrocarbons [,].
The detection of aromatic hydrocarbons such as 1,3-bis(1,1-dimethylethyl)-benzene, although potentially linked to environmental contamination, deserves attention. Similar compounds have been associated with plastic-like or phenolic off-notes in lipidic food matrices [,]. Its relatively higher abundance in males could reflect differential bioaccumulation capacity or interactions between lipid composition and environmental exposure. While not a natural metabolite of sea urchins, its presence warrants further investigation as both a possible contaminant marker and a contributor to off flavors.
Minor unsaturated hydrocarbons (e.g., 2,4-dimethyl-1-heptene, 2-undecene) were also identified but, given their limited aromatic potency, their contribution to sensory properties is expected to be negligible. The overall volatile landscape of males thus represents a chemically simpler and less oxidized profile, in line with their function as structural and reproductive tissues rather than as energy-storing organs.
The contrast between the volatile signatures of male and female P. lividus gonads is consistent with patterns observed in other echinoids, such as Strongylocentrotus droebachiensis and Tripneustes gratilla, where females exhibit higher oxidative activity and terpenoid accumulation [,]. These findings suggest that sex-linked metabolic divergence is a widespread feature among regular sea urchins, reflecting distinct physiological demands during reproduction [,].
From an ecological standpoint, such metabolic differentiation may influence not only sensory perception but also trophic interactions. Gonadal composition affects palatability to predators, nutrient flow through benthic food webs, and the nutritional value of sea urchins for higher consumers. Female gonads, being richer in oxygenated lipids and volatile metabolites, could represent a higher-energy and more attractive food source, potentially influencing selective predation or spawning dynamics in natural populations.
From an applied perspective, understanding VOC patterns in sea urchin gonads provides a chemical framework for assessing and enhancing product quality. Volatile profiles could serve as biomarkers for reproductive stage, sex determination, and freshness evaluation in aquaculture. Controlled feeding with selected macroalgae or algal-derived extracts may enhance desirable volatiles such as D-limonene or 1-penten-3-ol, improving organoleptic attributes. Moreover, non-invasive VOC monitoring could contribute to traceability and authentication of sea urchin products [,,], addressing a growing demand for quality assurance in Mediterranean fisheries.
In addition to the chemical differentiation between sexes, it is worth noting that several volatile aromatic hydrocarbons detected (for example, styrene) may signal anthropogenic contamination—albeit at trace levels—and thus raise potential consumer-health considerations. The substance styrene is classified as possibly carcinogenic to humans and its presence in seafood warrants attention, even if the measured levels in gonads of P. lividus were well below established safety thresholds []. Future work should thus complement volatilome profiling with direct quantification of such contaminant-derived volatiles and assess their accumulation or biotransformation in roe tissue.
Despite the detailed chemical insight provided by this study, several limitations remain. The sample size, particularly for females, was constrained by legal protection of the species along the Mediterranean coasts, and sex identification remains feasible only post-mortem. Future research exploring seasonal and dietary influences would clarify how environmental and nutritional factors modulate the volatile landscape of P. lividus.
5. Conclusions
This study demonstrates that the sea urchin (Paracentrotus lividus) gonad volatilome exhibits sex-dependent signatures measurable by HS-SPME-GC-MS. Multivariate modeling (PLS–LDA) and univariate tests (Mann–Whitney with FDR correction) consistently separated females and males, indicating that compositional differences are robust at the metabolite level. Female gonads were enriched in low-odor-threshold oxygenates—notably aldehydes (hexanal, heptanal) and alcohols (1-penten-3-ol, 1-octen-3-ol)—together with diet-linked monoterpenes (e.g., D-limonene), whereas male gonads were dominated by saturated/branched hydrocarbons and long-chain alcohols associated with a more structural lipid profile. These patterns align with a PUFA-LOX/HPL origin for oxygenated volatiles and with macroalgal inputs to terpene composition, providing a mechanistic rationale for the fresher/green–citrus “marine” notes typically attributed to females.
To ensure transparency, minor environmental/artifact markers (e.g., styrene; 1,3-bis(1,1-dimethylethyl)-benzene; chloroform-d; ethylene oxide) were retained in the reporting but excluded from biological interpretation. Together, the family-level grouping, effect-size patterns, and VIP rankings provide a coherent volatilome framework that explains the greater sensory complexity observed for females and corroborates the multivariate separation.
Tentative identifications based on library matches should be consolidated with retention indices and authentic standards; where confidence is low, compounds should remain annotated as Unknown with diagnostic ions. Future work should integrate GC-O and absolute/semi-quantitative calibration to validate odor impact, evaluate seasonality, diet, and maturation stage, and expand to geographical cohorts. From an applied perspective, the sex-dependent volatilome offers candidate markers for quality control, product differentiation, and aquaculture feeding trials aimed at optimizing desirable aroma attributes.
Finally, further investigations should extend beyond volatile profiling to consider other contamination vectors relevant to both consumer safety and ecosystem health, including toxic trace metals (such as organomercury compounds) and micro- and nano-plastics. The interactions between microplastics and associated organic pollutants have been shown to increase toxicity in marine invertebrates and may similarly affect bioaccumulation pathways in sea urchin roe []. Incorporating these parameters will strengthen the framework for quality control and sustainable use of P. lividus in gastronomic applications.
Author Contributions
Funding obtained: F.C.M.E. and P.S.-J. Conceived and designed the experiments: R.I.-C., E.C.-G., F.C.M.E. and P.S.-J. Performed the analysis and figures: R.I.-C. and F.C.M.E. Analyzed the data: R.I.-C. and F.C.M.E. Wrote the manuscript: R.I.-C. and F.C.M.E. Reviewed the manuscript: R.I.-C., E.C.-G., F.C.M.E. and P.S.-J. Collected the urchin samples: E.C.-G. and P.S.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONVOCATORIA DEL PROGRAMA PROPIO DEL CENTRO DE GASTRONOMÍA DEL MEDITERRÁNEO (Gasterra 2024-25) UA_DENIA PARA EL FOMENTO DE LA I+D+i EN El ÁMBITO DE LA GASTRONOMÍA (GASTERRA 2025).
Institutional Review Board Statement
This study was conducted under the framework of the GASTERRA projects (2024–2025) and received ethical approval from the Research Ethics Committee of the University of Alicante (Reference: UA-2024-10-24_2).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Pablo Candela and Pilar Blasco (SSTTI, University of Alicante) for technical assistance with HS-SPME-GC-MS experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- James, P.; Noble, C.; Hannon, C.; Stefansson, G.; Thórarinsdóttir, G.; Sloane, R.; Ziemer, N.; Lochead, J. Sea Urchin Fisheries, Management and Policy Review (Activity a4. 2.1 of the Urchin Project). 2016. Available online: https://urchinproject.com/wp-content/uploads/sites/3/2016/05/Report_18-2016.pdf (accessed on 12 November 2025).
- Andrew, N.L.; Agatsuma, Y.; Ballesteros, E.; Bazhin, A.G.; Creaser, E.P.; Barnes, D.K.A.; Botsford, L.W.; Bradbury, A.; Campbell, A.; Dixon, J.D. Status and management of world sea urchin fisheries. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2002; pp. 351–438. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Chapter 26—Paracentrotus lividus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 43, pp. 447–485. [Google Scholar]
- Rocha, F.; Baião, L.F.; Moutinho, S.; Reis, B.; Oliveira, A.; Arenas, F.; Maia, M.R.; Fonseca, A.J.; Pintado, M.; Valente, L.M. The effect of sex, season and gametogenic cycle on gonad yield, biochemical composition and quality traits of Paracentrotus lividus along the North Atlantic coast of Portugal. Sci. Rep. 2019, 9, 2994. [Google Scholar] [CrossRef] [PubMed]
- Ghisaura, S.; Loi, B.; Biosa, G.; Baroli, M.; Pagnozzi, D.; Roggio, T.; Uzzau, S.; Anedda, R.; Addis, M.F. Proteomic changes occurring along gonad maturation in the edible sea urchin Paracentrotus lividus. J. Proteom. 2016, 144, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M. On the relationship between marine plants and sea urchins. Oceanogr. Mar. Biol. Ann. Rev. 1975, 13, 213–286. [Google Scholar]
- Bulleri, F.; Benedetti-Cecchi, L.; Cinelli, F. Grazing by the sea urchins Arbacia lixula L. and Paracentrotus lividus Lam. in the Northwest Mediterranean. J. Exp. Mar. Biol. Ecol. 1999, 241, 81–95. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Bulleri, F.; Cinelli, F. Density dependent foraging of sea urchins in shallow subtidal reefs on the west coast of Italy (western Mediterranean). Mar. Ecol. Prog. Ser. 1998, 163, 203–211. [Google Scholar] [CrossRef]
- Bonaviri, C.; Vega Fernández, T.; Fanelli, G.; Badalamenti, F.; Gianguzza, P. Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Mar. Biol. 2011, 158, 2505–2513. [Google Scholar] [CrossRef]
- Boada, J.; Arthur, R.; Alonso, D.; Pagès, J.F.; Pessarrodona, A.; Oliva, S.; Ceccherelli, G.; Piazzi, L.; Romero, J.; Alcoverro, T. Immanent conditions determine imminent collapses: Nutrient regimes define the resilience of macroalgal communities. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162814. [Google Scholar] [CrossRef]
- Guarnieri, G.; Bevilacqua, S.; Vignes, F.; Fraschetti, S. Grazer removal and nutrient enrichment as recovery enhancers for overexploited rocky subtidal habitats. Oecologia 2014, 175, 959–970. [Google Scholar] [CrossRef]
- Ouréns, R.; Naya, I.; Freire, J. Mismatch between biological, exploitation, and governance scales and ineffective management of sea urchin (Paracentrotus lividus) fisheries in Galicia. Mar. Policy 2015, 51, 13–20. [Google Scholar] [CrossRef]
- Phillips, K.; Niimi, J.; Hamid, N.; Silcock, P.; Bremer, P. Sensory and volatile analysis of sea urchin roe from different geographical regions in New Zealand. LWT-Food Sci. Technol. 2010, 43, 202–213. [Google Scholar] [CrossRef]
- Paredes, E.; Costas, D. Non-lethal sex identification of sea urchins: Method and advantages. Lab Anim. 2020, 49, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, J.; Sun, Z.; Liu, B.; Zhao, C.; Chang, Y. Identification of Sex-Specific Markers Through 2b-RAD Sequencing in the Sea Urchin (Mesocentrotus nudus). Front. Genet. 2021, 12, 717538. [Google Scholar] [CrossRef] [PubMed]
- Betancourt-Arango, J.P.; Villaroel-Solis, E.E.; Fiscal-Ladino, J.A.; Taborda-Ocampo, G. Volatilomics: An emerging discipline within Omics Sciences—A systematic review. F1000Research 2024, 13, 991. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Cheli, F.; Chiofalo, V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment—A review. J. Food Eng. 2017, 210, 62–75. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; López-Pedrouso, M.; Gagaoua, M.; Lorenzo, J.M. Foodomics in meat quality. Curr. Opin. Food Sci. 2021, 38, 79–85. [Google Scholar] [CrossRef]
- Brattoli, M.; De Gennaro, G.; De Pinto, V.; Demarinis Loiotile, A.; Lovascio, S.; Penza, M. Odour Detection Methods: Olfactometry and Chemical Sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC–MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef]
- Acquaticci, L.; Angeloni, S.; Baldassarri, C.; Sagratini, G.; Vittori, S.; Torregiani, E.; Petrelli, R.; Caprioli, G. A new HS-SPME-GC-MS analytical method to identify and quantify compounds responsible for changes in the volatile profile in five types of meat products during aerobic storage at 4 °C. Food Res. Int. 2024, 187, 114398. [Google Scholar] [CrossRef]
- Epping, R.; O’Sullivan, M.; Higgins, S.; Cremonesi, M.; Voilley, A.; Cayot, N. Changes in Tuber melanosporum Aroma during Storage: SPME-GC-MS Versus Sensory. Foods 2024, 13, 837. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Vittori, S.; Caprioli, G. An Overview on Truffle Aroma and Main Volatile Compounds. Molecules 2020, 25, 5948. [Google Scholar] [CrossRef]
- Gloaguen, Y.; Riu-Boni, M.; Stark, T.; Schieberle, P. Characterization of the Volatilome of Tuber canaliculatum by HS-SPME-GC-MS. ACS Omega 2022, 7, 26534–26544. [Google Scholar] [CrossRef]
- Brundu, G.; Cannavacciuolo, A.; Nannini, M.; Somma, E.; Munari, M.; Zupo, V.; Farina, S. Development of an efficient, noninvasive method for identifying gender year-round in the sea urchin Paracentrotus lividus. Aquaculture 2023, 564, 739082. [Google Scholar] [CrossRef]
- Takagi, S.; Sato, Y.; Kokubun, A.; Inomata, E.; Agatsuma, Y. Odor-active compounds from the gonads of Mesocentrotus nudus sea urchins fed Saccharina japonica kelp. PLoS ONE 2020, 15, e0231673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal Chim Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Peng, S.L.; Bi, Y.M.; Shan, P.; Hu, X.Y. A New Method Combining LDA and PLS for Dimension Reduction. PLoS ONE 2014, 9, e96944. [Google Scholar] [CrossRef]
- Wen, Z.C.; Sun, T.; Geng, X.; Liu, M.H. Discrimination of Pressed and Extracted Camellia Oils by Vis/NIR Spectra Combined with UVE-PLS-LDA. Spectrosc. Spectr. Anal. 2013, 33, 2354–2358. [Google Scholar] [CrossRef]
- Niimi, J.; Leus, M.; Silcock, P.; Hamid, N.; Bremer, P. Characterisation of odour active volatile compounds of New Zealand sea urchin (Evechinus chloroticus) roe using GC-O-FSCM. Food Chem. 2010, 121, 601–607. [Google Scholar] [CrossRef]
- Su, T.; Zhang, G.; Zhao, Y.; Zhang, J.; Shang, X.; Shen, Q.; Hua, S.; Zhu, X. The Biosynthesis of 1-octen-3-ol by a Multifunctional Fatty Acid Dioxygenase and Hydroperoxide Lyase in Agaricus bisporus. J. Fungi 2022, 8, 827. [Google Scholar] [CrossRef]
- Morawicki, R.O.; Beelman, R.B. Study of the Biosynthesis of 1-Octen-3-ol Using a Crude Homogenate of Agaricus bisporus in a Bioreactor. J. Food Sci. 2008, 73, C135–C139. [Google Scholar] [CrossRef]
- Sato, Y.; Takagi, S.; Inomata, E.; Agatsuma, Y. Odor-active volatile compounds from the gonads of the sea urchin Mesocentrotus nudus in the wild in Miyagi Prefecture, Tohoku, Japan. Food Nutr. Sci. 2019, 10, 860–875. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; López-Hernández, J.; González-Castro, M.J.; de la Cruz-García, C.; Simal-Lozano, J. Comparison of volatile components in fresh and canned sea urchin (Paracentrotus lividus, Lamarck) gonads by GC–MS using dynamic headspace sampling and microwave desorption. Eur. Food Res. Technol. 2001, 212, 643–647. [Google Scholar] [CrossRef]
- Hughes, A.D.; Kelly, M.S.; Barnes, D.K.A.; Catarino, A.I.; Black, K.D. The dual functions of sea urchin gonads are reflected in the temporal variations of their biochemistry. Mar. Biol. 2006, 148, 789–798. [Google Scholar] [CrossRef]
- Anedda, R.; Siliani, S.; Melis, R.; Loi, B.; Baroli, M. Lipid metabolism of sea urchin Paracentrotus lividus in two contrasting natural habitats. Sci. Rep. 2021, 11, 14174. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, D.; Shen, Y.; Gao, H.; Yao, X. Mapping gaseous dimethylamine, trimethylamine, ammonia, and their particulate counterparts in marine atmospheres of China’s marginal seas—Part 2: Spatiotemporal heterogeneity, causes, and hypothesis. Atmos. Chem. Phys. 2022, 22, 1515–1528. [Google Scholar] [CrossRef]
- Arafa, S.; Chouaibi, M.; Sadok, S.; El Abed, A. The influence of season on the gonad index and biochemical composition of the sea urchin Paracentrotus lividus from the Golf of Tunis. Sci. World J. 2012, 2012, 815935. [Google Scholar] [CrossRef]
- Machado, A.M.; Fernández-Boo, S.; Nande, M.; Pinto, R.; Costas, B.; Castro, L.F.C. The male and female gonad transcriptome of the edible sea urchin, Paracentrotus lividus: Identification of sex-related and lipid biosynthesis genes. Aquac. Rep. 2022, 22, 100936. [Google Scholar] [CrossRef]
- Martínez, A.; Abanto, M.; Días, N.B.; Olate, P.; Pérez Nuñez, I.; Díaz, R.; Sepúlveda, N.; Paz, E.A.; Quiñones, J. Recent Trends in Food Quality and Authentication: The Role of Omics Technologies in Dairy and Meat Production. Int. J. Mol. Sci. 2025, 26, 4405. [Google Scholar] [CrossRef]
- Cozzolino, D. The role of vibrational spectroscopy as a tool to assess economically motivated fraud and counterfeit issues in agricultural products and foods. Anal. Methods 2015, 7, 9390–9400. [Google Scholar] [CrossRef]
- Banton, M.I.; Bus, J.S.; Collins, J.J.; Delzell, E.; Gelbke, H.P.; Kester, J.E.; Moore, M.M.; Waites, R.; Sarang, S.S. Evaluation of potential health effects associated with occupational and environmental exposure to styrene—An update. J. Toxicol. Environ. Health Part B 2019, 22, 1–130. [Google Scholar] [CrossRef]
- Choi, H.; Hwang, U.-K.; Lee, M.; Kim, Y.-J.; Han, T. Evaluating Toxic Interactions of Polystyrene Microplastics with Hazardous and Noxious Substances Using the Early Life Stages of the Marine Bivalve Crassostrea gigas. Nanomaterials 2025, 15, 349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).