Abstract
Rare earth elements were extensively employed for many years to improve plant growth in farming. However, their effect on plant’s behavior relies on their concentration and the plant species. The impact of low doses of lanthanum (La; 1–10 µM) on plant growth, mineral uptake, and the production of secondary metabolites was assessed in two Brassicaceae species (Cakile maritime and Brassica juncea) after 14 days of La exposure. The La accumulation potential was also evaluated. Results showed that both species were able to maintain good dry biomass production under La. C. maritima plants accumulated more La than B. juncea, and a higher accumulation was noticed in the roots (in both plant species). Accordingly to La accumulation in plant tissues, nutrient absorption was affected in C. maritima shoots and roots, whereas no severe effect on nutrient contents was noticed in B. juncea. Phenolic compounds increased in the aerial and underground parts of both species; thus, the accretion was more notorious in shoots of the highest La concentrations. The studied Brassicaceae species showed an ability to survive in a La-contaminated medium. However, according to tolerance index values, C. maritima was found to be more tolerant of La than B. juncea.
1. Introduction
Rare earth elements (REEs) are non-essential metallic cations for living organisms [1,2], but they have been widely used in farming for a long time to improve crop growth (more than 100 cultivated crops have been fertilized with varying concentrations of REEs). These elements have been applied in different ways, such as seed preliminary treatments, foliar sprays, and incorporated into root fertilizers (which can be in the form of solids or liquids) as plant growth regulators [3,4,5]. Indeed, the addition of suitable quantities of REEs can stimulate seed germination, increase chlorophyll content, raise root growth, improve resistance, and subsequently increase crop yield [6]. Fertilizers enriched with REEs contain an average of 19.8, 4.6, 5.4, 1.9, 0.4, and 0.1% of La2O3, CeO2, Nd2O3, Pr6O11, Sm2O3, and Eu2O3, respectively [7]. Therefore, REEs can accumulate in soils, bioaccumulate in plants, and eventually enter the food chain [8], threatening human health [9].
The different processes may affect REE mobility in soils, such as the adsorption onto soil particles and colloids [10,11], the precipitation with phosphates [12], the formation of complexes with both organic and inorganic ligands such as carbonates, sulfates, and organic acids [13], and the interactivities with Mn- and Fe-oxides [11,14].
Lately, the effect of REEs on increasing plant resilience to environmental stress has been the subject of special attention, especially lanthanum (La), due to its distinctive chemical and physical characterization and its high abundance in the environment compared to other REEs [15]. Lanthanum is the major REE that could be found in fertilizers [16]; this fact facilitates its accumulation into the ecosystem and increases La phytotoxicity [17].
Plants absorb metals such as La via their roots and translocate them into several plant tissues, triggering physiological changes [18]. High concentrations of metals can alter the physiological status of the plant through the suppression of germination and growth [19], affecting cellular mitosis and elongation, the disturbance of nutrient uptake, and the change in metabolism, as well as transpiration rate [20]. The presence of metals can be harmful to plants, specifically when exposed to high metal concentrations, as they can raise the generation of reactive oxygen species (ROS) in plant cells [21,22].
However, experimental results showed that the toxicity of each element differs depending on the tested organism and the toxicological endpoint, as well as the period of treatment and the concentrations applied. Previous research demonstrated that a low La concentration has the potential to enhance physiological activities and promote plant growth and, at moderate concentration, La-enhanced photosynthesis and stomatal conductance in rice species [23]; protected soybean seedlings against increased UV-B radiation [24], promoted growth and enhanced soluble sugar and ascorbic acid levels in Brassica rapa [25]. In contrast, the application of medium or high concentrations of La affected wheat root development [26], inhibited nitrogen assimilation processes of soybean roots [27], affected rice photosynthesis [23], and reduced the mineral element content of rice [28]. Liu and Hasenstein [29] revealed that including La at concentrations lower than 1 μM resulted in increased root growth in Z. mays, while concentrations higher than 100 μM inhibited the development of this plant organ. Hence, investigating the impact of pollutants on plant growth can yield valuable insights that can be subsequently applied in assessing ecosystem risk [30].
The current study investigated the effect of low concentrations of La on two Brassicaceae species, Cakile maritima (halophyte) and Brassica juncea (glycophyte). B. juncea, an edible species, is used in oil production and various food applications [31], while C. maritima is an interesting species for traits related to economic potential (seed oil contents) [32]. These two Brassicaceae were chosen in this study due to their ability to tolerate and accumulate multiple metal elements when grown on metal-contaminated soils [33,34].
This research work was conducted to appraise the influence of increasing La concentrations on the growth of C. maritima and B. juncea plants and to assess the La accumulation potential in these two Brassicaceae in order to explore and identify new plant species that could be used in the phytoremediation of RREs polluted soils. The effects of La on nutrient uptake (K, Ca, and Mg) and the biosynthesis of secondary metabolites (phenolic compounds and flavonoids) were also examined.
2. Materials and Methods
2.1. Plant Culture
Cultures of C. maritima and B. juncea were carried out in a closed and semi-controlled greenhouse (natural photoperiod of 14/10 h day/night, mean temperature; 26 ± 4 °C, relative humidity range; 60–90% day/night).
Prior to cultivation, the seeds of the two plant species were sterilized in Ca(ClO)2 5% for 10 min. The disinfected Brassicaceae seeds were immersed in demineralized water for two hours and prepared to germinate in plastic receptacles filled with an inert substrate mixture (gravel/perlite (1:2, v/v)) and were daily hydrated with faucet water. After germination, plants were watered with Hewitt’s nutrient solution (T) [35]. Plantlets of C. maritima and Br. juncea (obtained after 4 weeks of germination) were transferred into a hydroponic system (dark-walled tubes of 150 mL). After 5 days of acclimatization, identical plants were selected for the La treatment. They were exposed to different La concentrations, 0, 1, 2.5, 5, and 10 µM, supplied in the form of La3+ that was added to the nutrient solution (T), for 14 days. All treatment solutions were renewed daily. A schematic representation of the La treatment setup is shown is Figure 1.
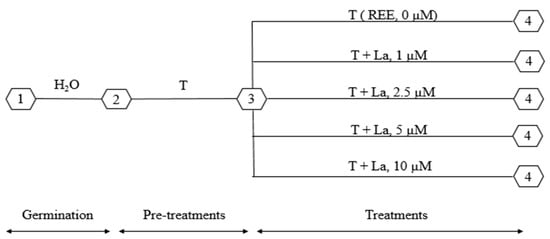
Figure 1.
Schematic representation of the experimental setup of the treatment of Cakile maritima and Brassica juncea with lanthanum (La). 1—germination; 2—pre-treatments: irrigation with Hewitt’s nutrient solution (T) + acclimatization period; 3—beginning of treatments; and 4—end of treatments.
2.2. Plant Harvesting and Growth Parameters Determination
The plants were collected after 14 days of exposure to La. As a first step, the roots were immersed in a CaCl2 solution for 5 min to eliminate any residual traces of the used REEs that had adhered to the surfaces of tissues [36,37,38]. Afterward, all plant tissues (shoots and roots) underwent three rinses with cold deionized water. Subsequently, the plants were separated into their root and shoot components, and their fresh weights were promptly measured. The fresh plant material was oven-dried at 60 °C until constant weight. The shoot/roots dry biomass ratio (S/R) was determined as follows:
S/R = Shoots dry biomass/Roots dry biomass
The metal tolerance index was determined in the entire plant using the following formula as described by Sleimi et al. [39] and Dridi et al. [40]:
TI (%) = (Dry biomass of treated plants/dry biomass of control plants) × 100
2.3. La Accumulation and Minerals Analyses
Macro-nutrients (K, Ca, and Mg) were extracted and determined using dry plant tissues, as described by Sleimi et al. [41], Kouki et al. [42], and Bankaji et al. [43]: approximately 30 mg of dry plant material (reduced to a fine powder in an agate mortar) were digested in Teflon bombs with a volume of 3 mL of acid mixture composed with HNO3:H2SO4:HClO4 (10:1:0.5, v/v/v) for two hours at 110 °C. The digested products were diluted with 50 mL of nitric acid solution at 0.5% and filtered with the Whatman filter (N°1), and mineral contents were determined using atomic absorption spectrometry (AAS; Perkin Elmer PinAAcle900T, Waltham, MA, USA). Lanthanum contents were quantified using the same mineral extract via inductively coupled plasma mass spectrometry (ICP-MS; Perkin-Elmer NexION 2000C, Waltham, MA, USA) following the Brito et al. [44] procedure.
2.4. Total Polyphenols and Flavonoids Contents Determination
Secondary metabolites (total polyphenols (TP) and flavonoids) were extracted from plant dry tissues (shoots and roots) where 30 mg of the dry material (shoots or roots) were kept overnight into 10 mL of methanol/water mixture (80/20; v/v). The macerate was centrifuged at 2500 rpm for 30 min using a centrifuge (MPW-351 R, GmbH & Co.KG Bremen, Germany), and then filtered. The TP content was estimated according to the Folin–Ciocalteu colorimetric assay described by Velioglu et al. [45] and expressed as mg Gallic acid equivalents. The flavonoid content was determined using a spectrophotometric assay based on flavonoid–aluminum chloride (AlCl3) complexation, as previously described by Quitter et al. [46]. Rutine was used as a standard reference for the quantitative estimation of flavonoids.
2.5. Statistical Analysis
Each set of samples underwent analysis with a minimum of six replicates. The mean values and their respective standard deviations (±) are presented on the bars within the figures. To evaluate the impact of REE on the fluctuation of the parameters under investigation, we conducted a single-factor analysis of variance (ANOVA1) using STATISTICA 8.0 software. This statistical approach determines whether a specific factor exerts a significant impact. To compare the averages, Tukey’s HSD test was utilized to identify significant differences, with statistical significance considered at p < 0.05. Additionally, principal component analysis (PCA) was carried out using STATISTICA to examine the relationships among La and all the parameters studied for the two plant species under study.
3. Results
3.1. Plants Growth
In general, the findings indicated that there was no notable change in the dry biomass production of both B. juncea aerial and underground parts as the concentration of La increased in the hydroponic growth medium (Table 1). In comparison to the control, a slight decrease in the dry weight was detected in the aerial and underground parts of plants subjected to 10 μM La, but the variations were not statistically significant (p > 0.05). Adversely, an increase in the dry weight of aerial segments of the halophyte C. maritima was noted in all plants subjected to La when compared to the control plants (Table 1); however, only shoots of 1 µM La manifested a significant increment in the dry biomass (p < 0.05). Likewise, the roots of C. maritima showed a dry biomass enhancement in 1, 2.5, and 5 µM La (Table 1), and the highest value was detected in plants subjected to 2.5 μM La (p < 0.05) as compared to untreated plants. Nevertheless, 10 µM La significantly reduced roots dry biomass by 31% in C. maritima (p < 0.05).

Table 1.
Shoots (S) and roots (R) dry weight (DW), dry biomass ratio of shoot/root (S/R), and entire plant (EP) tolerance index (TI) of C. maritima and B. juncea plants under the impact of different La doses for 14 days. Values are mean ± standard deviation. Distinct letters signify statistically significant differences at p < 0.05.
The shoots/roots ratio (S/R) of both studied species showed no substantial variation (p < 0.05) in the presence of La in the hydroponic medium (Table 1), except in 10 µM La, a significant increase in C. maritima S/R ratio was noticed when compared to the control.
The tolerance index (TI) of B. juncea was minimized by 20% in plants cultivated in a medium containing 10 µM of La. Contrarily, the La tolerance index showed an increase in the entire plant (EP) of C. maritima at all La concentrations, specifically in the lowest La concentrations (up to a 60% and 40% increase in plants subjected to 1 and 2.5 µM La, respectively; Table 1).
3.2. Lanthanum Accumulation
As illustrated in Figure 2, C. maritima and B. juncea accumulated La in their roots more than in the shoots, especially in the highest applied La concentrations (2.5, 5, and 10 µM La). In addition, results showed that La accumulated in underground parts of the contaminated plants followed the increment of La in the hydroponic medium. In contrast, a very low uptake of La was noticed in shoots of B. juncea in all La concentrations. However, C. maritima accumulated an amount of 400 µg.g−1 DW at 10 µM La. A low La translocation from the root into the shoot justified the difference between La contents in the different segments of the studied species (Table 2). We noticed that C. maritima accumulated more La than B. juncea; where the contents were 4- to 7-fold higher in C. maritima.
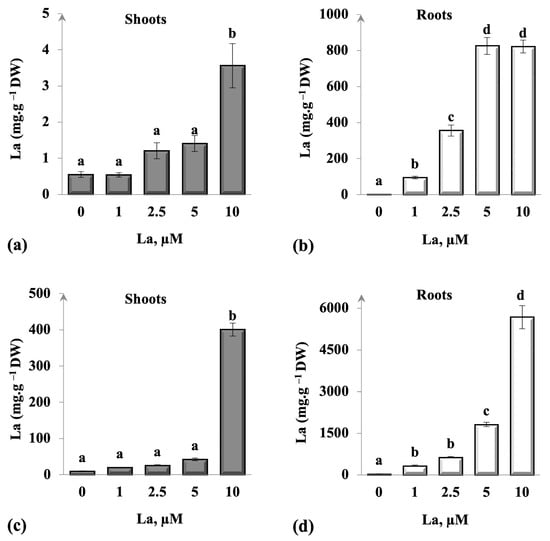
Figure 2.
Variation of La concentration in roots and shoots of Brassica juncea (a,b) and Cakile maritima (c,d) plants exposed to different La concentrations for 14 days. Values were means ± standard deviation. Distinct letters signify statistically significant differences at p < 0.05.

Table 2.
Mean values of translocation factor ratio (TF) of La in Brassica juncea and Cakile maritima under different La concentrations.
3.3. Nutrients Uptake
According to Figure 3, applying different La concentrations affected the mineral absorption in the two species. The results manifested a significant decrease in K amounts in the aerial parts of B. juncea cultivated in the presence of 1 and 10 µM La (p < 0.05) compared to the control. Thus, a significant increment in K content in roots was noted in B. juncea treated with 2.5, 5, and 10 µM, where the K absorption increased by 2.3-fold in 2.5 and 5 µM La and by 1.8-fold in 10 µM La in comparison to the plants that received no treatment (p < 0.05; Figure 3a). Contrarily, La induced a significant decrease in K absorption in C. maritima shoots and roots in all La doses (p < 0.05), where the decline was more notorious in roots when compared to the untreated plants (Figure 3b).
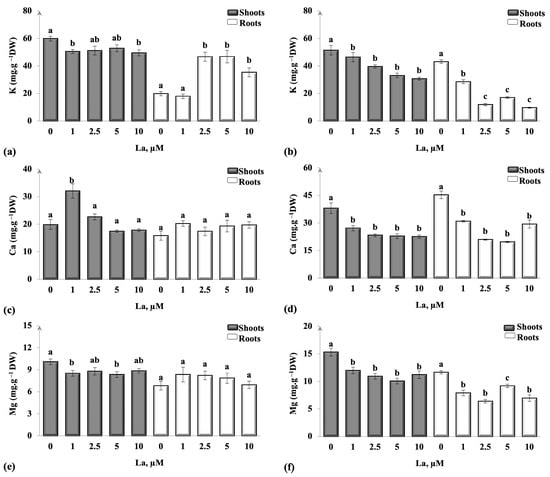
Figure 3.
Mineral elements uptake (K, Ca, and Mg) in roots and shoots of B. juncea (a,c,e) and C. maritima (b,d,f) plants exposed to different La concentrations for 14 days. Values were means ± standard deviation. Distinct letters signify statistically significant differences at p < 0.05.
Brassica juncea exhibited no noteworthy changes in calcium levels within both aerial and underground parts (Figure 3c), except in the case of 1 µM La. In this particular instance, the presence of 1 µM La led to a significant increment in calcium quantity in shoots of the treated plants compared to the others that received no treatment (p < 0.05). However, all La concentrations significantly (p < 0.05) decreased the accumulation of Ca in C. maritima aerial parts and roots as compared to the control (Figure 3d).
The introduction of varying concentrations of La into the medium resulted in a notable reduction in the uptake of Mg in both C. maritima and B. juncea shoots compared to the untreated group (p < 0.05). A different behavior was noted in the roots of the two species treated with La. Lanthanum concentrations did not significantly affect Mg content in B. juncea roots (p < 0.05) (Figure 3e) but significantly decreased Mg content in C. maritima (Figure 3f) when compared to the plants that received no treatment (p < 0.05).
3.4. Total Polyphenols and Flavonoids Production
Regarding the obtained results (Figure 4), TP content significantly increased in shoots of 2.5, 5, and 10 µM La and in roots of 2.5 µM La in B. juncea plants when compared to the untreated ones (p < 0.05; Figure 4a). C. maritima shoots showed a significant increment (p < 0.05) in the TP content in all La concentrations (except in 5 µM La) compared to the control; a clear elevation was only observed in roots of 1 and 2.5 µM La (p < 0.05; Figure 4b).
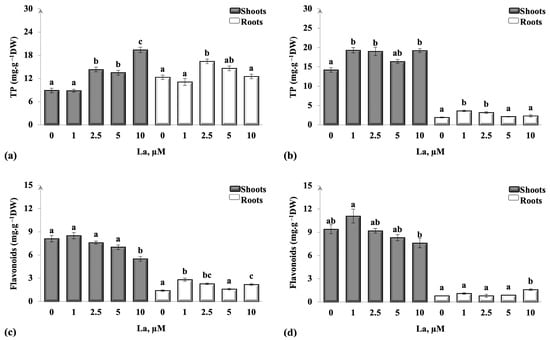
Figure 4.
Fluctuations in the overall total phenolic compounds and flavonoid levels within the shoots and roots of B. juncea (a,c) and C. maritima (b,d) after exposure to different doses of lanthanum (La) for 14 days are illustrated. Values were means ± standard deviation. Values bearing distinct letters signify statistically significant differences at p < 0.05.
The synthesis of flavonoids significantly decreased only in the shoots of the highest La concentration (10 µM) and increased in the roots of 1, 2.5, and 10 µM La in B. juncea treated plants compared to the untreated ones (p < 0.05; Figure 4c). However, no significant variation was noted in flavonoid contents in C. maritima shoots and roots (only a significant increase in root flavonoids of 10 µM La was observed at p < 0.05) in comparison with plants that received no treatment (Figure 4d).
3.5. Principal Component Analysis
We conducted an association analysis among the studied parameters using a correlation circle derived from principal component analysis (PCA), as illustrated in Figure 5. The statistical findings revealed that the multifactorial analysis of the impact of La was primarily influenced by two factors, Factor 1 and Factor 2. In B. juncea, Factor 1 explained 62.53% of the variance in shoots and 45.31% in roots (Figure 5A). Similarly, for C. maritima, Factor 1 accounted for 60.37% of the variance in shoots and 54.56% in roots (Figure 5B). On the other hand, Factor 2 represented a smaller proportion of variance, contributing only 24.54% and 26.80% in B. juncea shoots and roots and 26.17% and 33.84% in C. maritima shoots and roots, respectively. The findings indicated that there was a positive correlation between La and polyphenols, while a negative correlation was observed with flavonoids in shoots of B. juncea (Figure 5A-a). In the roots of the same plant, a positive correlation was found between La and potassium (K) content (Figure 5A-b). Additionally, a clear association between La, magnesium (Mg) amounts, and total polyphenols was observed in the shoots (Figure 5B-c), and a clear association between La and flavonoids was identified in the roots (Figure 5B-d) of C. maritima. However, shoots of C. maritima marked a negative correlation between La and K content (Figure 5B-c).
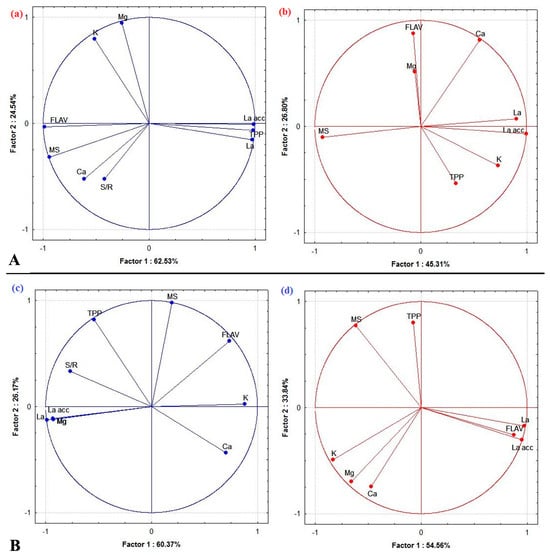
Figure 5.
Correlation circle derived from the principal component analysis (PCA) of lanthanum dose (La), dry biomass (MS), total polyphenols (TPP), flavonoids (FLAV), and nutrients (calcium (Ca), magnesium (Mg), potassium (K)) data of the aerial—(a,c) and underground—(b,d) parts of Brassica juncea (A) and Cakile maritima (B).
4. Discussion
Numerous studies focused on investigating the physiological impacts of applying REEs on plants. Our results showed that C. maritima and B. juncea were capable of maintaining good growth and surviving under metal-induced stress while exposed to various La concentrations in the hydroponic solution. Indeed, there is no significant decline in the biomass production of B. juncea contrarily to C. maritima, which showed a significant increase in shoots and roots dry biomass of plants treated with 1 µM and 2.5 µM La, respectively. In addition, the percentages of the TI of C. maritima exposed to La are higher (p < 0.05) than 100% (Table 1), indicating a considerable tolerance of this species to La-containing medium, especially at the lowest La doses (1, 2.5, and 5 µM). On the contrary, B. juncea TI was lower than 100% at 2.5, 5, and 10 µM La concentrations (Table 1).
Plant biomass production is considered an indicator of the plant’s susceptibility to tolerate metallic constraints. The increase in shoots and root biomass of C. maritima at the lowest La concentrations (1, 2.5, and 5 µM) accorded with previous research where low concentrations of La have also been shown to enhance shoots and root growth of other species. For example, de Oliveira et al. [47] stated an increase in root and shoot biomass of soybean plants contaminated with 5 µM and 10 µM La, respectively. Furthermore, Xiong et al. [48] found that low La doses improved the growth of B. juncea. In contrast, when applying a dose of 1 mg La (≈7 µM), a noticeable decline in the elongation of the primary root was noted, and this effect became more pronounced with higher La concentrations in the nutritive solution. Moreover, an elevated concentration of La (20 µM) negatively affected the growth of soybean [47]. Qin et al. [49] documented that the toxicity induced by metallic cations in plants encompasses the occurrence of chromosome abnormalities associated with the suppression of mitosis. In fact, a mitotoxic effect, characterized by a decline in the mitosis process, was recorded in Allium sativum L. and Triticum durum (Desf.) under the effect of La [26,50].
According to our findings, B. juncea and C. maritima absorbed elevated quantities of La (specifically in their underground parts with a low translocation of La to the shoots). Indeed, Yuan et al. [51] revealed that vascular species generally manifest low REE TFs. Our findings were in concordance with those reported by Xiong et al. [48] and Carpenter et al. [52], where the findings demonstrated that the absorption of REEs by plants was greater in the roots compared to the above-ground tissues of B. juncea. Similar results were observed in the soybean plant [47], ramie (Boehmeria nivea L.) [53], and willows (Salix spp.) [54]. In addition, Dridi et al. (2022) demonstrated that La contents in Helianthus annuus roots were greater than those in shoots [55].
The accumulation of REE in plant tissues can be explained by the fact that REEs might trigger endocytosis within plant cells, helping their deposition [23]. Significant proportions of REEs can also bind to cell walls, while certain ions penetrate the cell membrane and accumulate within organelles as crystalline formations. In fact, the selective accumulation of La in the roots may be attributed to the attraction of La to the cell walls, its precipitation in the root’s apoplast [56], or relatively low permeability through the endodermis [57]. Seregin and Kozhevnikova [58] conveyed that the notable variations in the metal uptake and distribution within different plant parts are contingent upon the metals’ accessibility and entry into plant roots via plasma membrane cation channels. The accumulation of REEs in plant roots suggests that the absorbed elements have likely undergone multiple forms where they can be precipitated, complexed, and inactivated within the root tissues [59]. Additionally, some REEs become localized in root cortical tissues, thereby preventing their translocation to other plant parts, particularly the aerial organs [47]. Moreover, according to Carpenter et al. [52], the absorption of REEs by plant roots can be attributed to their possessing similar ionic radii resembling that of calcium.
Metal accumulation can positively or negatively influence the mineral uptake of plants and thus can disrupt the absorption and distribution of essential minerals in different plant tissues [20]. Our findings indicated that La significantly enhanced (p < 0.05) potassium contents in the roots of B. juncea subjected to 2.5, 5, and 10 µM La. Contrarily, potassium uptake was reduced in C. maritima shoots and root tissues treated with the same La concentrations. Increased potassium levels were detected in other studies; for example, de Oliveira et al. [47] stated a significant increase in K contents in roots and shoots of soybeans contaminated with La, where the obtained results were explained by a synergistic link between La and K. Similarly, Liu et al. [60] reported that La could induce an increase in K accumulation in the plant. However, these results exhibit that La reduced K uptake in C. maritima shoots and roots and B. juncea shoots. This decrease can be attributed to the modification of the vascular structure and a decrease in the diameter and number of the xylem vessels under the effect of metal [61,62].
Calcium is essential for maintaining membrane functions, cell wall integrity, and plant growth [63]. Our results indicated that the presence of La in the hydroponic solution did not affect the absorption of Ca in B. juncea. However, a concentration of 1 µM La significantly increased Ca levels and reduced Mg absorption in the aerial plant parts. Contrarily, La decreased Ca levels in C. maritima shoots and roots. A similar effect on calcium nutrition was reported by Xu et al. [64], where the application of La induced a reduction in Ca accumulation in Hydrocharis dubia. The reduction in Ca content can be due to the accumulation of La in plant tissues. Indeed, due to the chemical and physical similarities between La and Ca (similar ion radii of 0.110 and 0.099 nm, respectively), the action mechanism of these minerals in plants is identical, which allows La to imitate the biological functions of Ca and replace it in proteins and tissues [16,65]. This property generates competition between these two elements because La can replace Ca since they can use identical absorption sites. In general, this substitution induced a change in the cell walls of soybeans at high La concentration [47]. In addition, de Oliveira et al. [47] mentioned that energy-dispersive X-ray microanalysis results indicated that La could be taken up into plant cells and subsequently bound to cell walls, mitochondria, and chloroplast membranes, substituting Ca in cell walls and Ca oxalate crystals. However, our findings indicated an increment in Ca levels in the lowest dose of La (1 µM) in B. juncea. Similar results were observed in the Xie et al. [66] study reporting the increase in Ca in the roots and seeds of rice contaminated with low La concentration.
Magnesium serves as the core element within the chlorophyll fragment and acts as a co-factor in numerous enzymes activating the phosphorylation process [63]. Results showed that La negatively affected the Mg content in B. juncea shoots and C. maritima shoots and roots. The decrease in Mg may be caused by the replacement of Mg ions in the chlorophyll molecule by toxic metals, such as La, due to their similar radii. This substitution leads eventually to the destruction of chlorophyll [67].
The mineral uptake was more affected in C. maritima than in B. juncea; K, Mg, and Ca contents were significantly decreased in different plant tissues. Negative correlations were observed between La concentrations and K content in roots, K in shoots, and Ca content in the shoots of C. maritima (Figure 3). These results suggest that this element (La) disrupts K and Ca transport in C. maritima. On the other hand, for B. juncea, there was no clear association between the nutrient contents and the presence of lanthanum. Generally, the addition of La in the hydroponic solution exerts a substantial influence on both plant growth and the absorption of nutrients [48,68]. Wang et al. [68] discovered that these modifications could be attributed to DNA damage and the formation of DNA–protein crossings. Damage in the DNA structure induced by La was shown in Vicia faba L. seedlings [69]. This effect could lead to a disruption of nutritional balance in plants [68,69,70,71].
Metals can be harmful to plants when accumulated in their tissues; thus, plants implement various mechanisms to prevent their accumulation [72], such as the biosynthesis of secondary metabolites under metal stress, attributed to their chelating capacity [73]. The biosynthesis of secondary metabolites is a cellular mechanism to detoxify ROS produced due to oxidative damage of metal ions. In B. juncea, an increase in total polyphenols and flavonoids was noted at 2.5, 5, and 10 µM La, whereas in C. maritima, the increase was significant in all La concentrations. The influence of La on the biosynthesis of secondary metabolites was reported in the literature. Dridi et al. (2022) exposed Helianthus annuus plants to La (1–10 µM) and discovered that the presence of La promoted the production of phenols and flavonoids [55]. In addition, La stimulated taxol production in Taxus yunnanensis cell cultures [10] and induced a general increase in certain phenolic acids in Hypericum perforatum [74].
Due to their influence on membrane permeability, REEs can expedite the exudation of secondary metabolites within the surrounding medium. Phenolic compounds possess the capacity to delay the generation of free radicals [75] in order to protect plants against oxidative stress. Phenols also chelate metal ions due to the existence of hydroxyl and carboxyl groups [76]. REEs promoted the increase in the transcriptions of essential anabolism genes, thus increasing the biosynthesis of secondary metabolites. Ma et al. [77] established a clear connection between the metal content in the culture medium and phenylalanine ammonia-lyase (PAL) activation. As documented by Sen [78], PAL is recognized as a pivotal enzyme in the formation of total polyphenols, including flavonoids and anthocyanins. This enzyme is particularly responsive to various biotic and abiotic stress factors. Certainly, the accumulation of total polyphenols can be attributed to the control of the biosynthesis of phenylpropanoid enzymes [79,80]. This regulation of phenolic compound biosynthesis is closely linked to the adjustment of transcription levels in genes responsible for encoding these biosynthetic enzymes, particularly in response to metal-induced stress. [81,82]. As the primary secondary metabolites, polyphenols, vitamins, and terpenes exhibited the ability to combat ROS and prevent oxidative damage [83]. The chemical characterization of these compounds plays a significant role in their antioxidant properties. For instance, it has been noted that the antioxidant effectiveness of flavonoids is linked to the hydroxyl groups, which facilitate the donation of hydrogen and electrons to radicals, thereby stabilizing them [84].
In this study, La concentrations increased the flavonoid contents in the roots of the two studied Brassicaceae. These results may suggest that both species increased their production of flavonoids as a protective mechanism against metal constraints. Handa et al. [85] revealed that flavonoids were significantly raised in B. juncea under Cr stress; it has been proven that these molecules are capable of elevating the chelation process of metals, which helps to reduce harmful hydroxyl radical levels in plant cells [86,87]. Moreover, flavonoids are recognized for their capacity to eliminate hydrogen peroxide (H2O2) and intervene in the phenol/ascorbate peroxidase cycle [88,89]. These compounds can also suppress lipid peroxidation and increase membrane fluidity and stability, preventing the release of ROS and suppressing the peroxidation reactions [90,91]. In this study, flavonoid contents decreased in the shoots of the two plants. These findings were supported by Babula et al. [74], where flavonoid contents decreased in shoots and roots of Hyperium perforatum under La stress. This fact might be explained by a strategy of saving energy, where plants may instead invest in the phenolic acid (hydroxycinnamic acid) production pathway first and do not trigger the stimulation of genes implicated in the formation of flavonoids and anthocyanins while being able to mitigate stress through the action of phenols [72].
5. Conclusions
Considering these results, we can conclude that low concentrations of lanthanum generally improved the development of C. maritima. Contrary to B. juncea, which remained an ordinary growth in the presence of La. This study also proved that C. maritima can accumulate La more than B. juncea, where the contents were 4- to 7-fold higher in C. maritima. This may explain the fact that mineral uptake was more affected in C. maritima than in B. juncea, where C. maritima’s K, Ca, and Mg contents were found highly decreased in the presence of La. Cakile maritima confirms its halophyte character; it shows an improvement in its growth in the presence of low doses of NaCl. This suggests a better use efficiency of nutrients when La is applied at low concentrations. In addition, the strategy of the two Brassicacea species to tolerate La contaminated medium relies on the biosynthesis of some secondary metabolites as a protective system against metallic constraint; the presence of La amplified total phenolic compounds in B. juncea species and C. maritima. In addition, the roots of the two species can accumulate high levels of lanthanum with a limited translocation factor from roots to shoots. The findings indicate that both of these species exhibited resistance to La (with moderation for B. juncea depending on La concentration in the medium). They were capable of restricting the translocation of the REE into the aerial parts, thereby minimizing La-induced metabolic damage. Consequently, these species are suitable for the phytostabilisation of La within their root systems.
Author Contributions
Conceptualization, N.S. and I.C.; methodology, H.B., N.D., P.B. and N.S.; formal analysis, H.B., N.D. and R.F.; writing—original draft preparation, H.B. and R.F.; writing—review and editing, N.D. and S.H.; visualization, S.H., P.B. and I.C.; supervision, N.S. and I.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no funding for this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors gratefully acknowledge the Fundação para a Ciência e Tecnologia (PTDC/CTA-AMB/31863/2017) and CERENA Strategic Project FCT-UIDB/04028/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, H.; Zhao, B.; Chen, Y.; Ren, C.; Chen, Y. Rare earths (Ce, Y, Pr) modified Pd/La2O3-ZrO2-Al2O3 catalysts used in lean-burn natural gas fueled vehicles. J. Rare Earths 2017, 35, 1077–1082. [Google Scholar] [CrossRef]
- Aaseth, J.; Bjorke-Monsen, A.L. Lanthanum Carbonate—A new phosphate binding drug in advanced renal failure. Curr. Med. Chem. 2018, 24, 113–117. [Google Scholar] [CrossRef]
- Tommasi, F.; d’Aquino, L. Rare earth elements and plants. In Rare Earth Elements in Human and Environmental Health: At the Crossroad between Toxicity and Safety; Pagano, G., Ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2017; pp. 107–125. [Google Scholar]
- Sun, D.; He, N.; Chen, Q.; Duan, S. Effects of lanthanum on the photosystem ii energy fluxes and antioxidant system of Chlorella vulgaris and Phaeodactylum tricornutum. Int. J. Environ. Res. Public Health 2019, 16, 2242. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Cavaliere, F.; Elliani, R.; Pirrone, N.; Sprovieri, F.; Tagarelli, A.; Giglio, A. Agrochemical treatments as a source of heavy metals and rare earth elements in agricultural soils and bioaccumulation in ground beetles. Sci. Total Environ. 2020, 749, 141438. [Google Scholar] [CrossRef]
- Tommasi, F.; Thomas, P.J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanessi, M.; Trifuoggi, M. Review of rare earth elements as fertilizers and feed additives: A knowledge gap analysis. Arch. Environ. Contam. Toxicol. 2021, 81, 531–540. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Peng, A. Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. J. Soils Sediments 2002, 1, 124–129. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.; Karayannis, V.; Baklavaridis, A. Environmental defects and economic impact on global market of rare earth metals. IOP Conf. Ser. Mater. Sci. Eng. 2016, 161, 012069. [Google Scholar] [CrossRef]
- Sandeep, G.; Vijayalatha, K.R.; Anitha, T. Heavy metals and its impact in vegetable crops. Int. J. Chem. Stud. 2019, 7, 1612–1621. [Google Scholar]
- Wu, J.; Wang, C.; Mei, X. Stimulation of taxol production and excretion in Taxus spp. cell cultures by rare earth chemical lanthanum. J. Biotechnol. 2001, 85, 67–73. [Google Scholar] [CrossRef]
- Davranche, M.; Gruau, G.; Dia, A.; Marsac, R.; Pédrot, M.; Pourret, O. Biogeochemical factors affecting rare earth element distribution in shallow wetland groundwater. Aquat. Geochem. 2015, 21, 197–215. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Lyons, W.B.; Stetzenbach, K.J.; Byrne, R.H. The solubility control of rare earth elements in natural terrestrial waters and the significance of PO43− and CO32− in limiting dissolved rare earth concentrations: A review of recent information. Aquat. Geochem. 1995, 1, 157–173. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Y.; Wang, X.; Deng, X. Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 2001, 44, 655–661. [Google Scholar] [CrossRef]
- Herrmann, H.; Nolde, J.; Berger, S.; Heise, S. Aquatic ecotoxicity of lanthanum—A review and an attempt to derive water and sediment quality criteria. Ecotoxicol. Environ. Saf. 2016, 124, 213–238. [Google Scholar] [CrossRef]
- Hu, Z.; Richter, H.; Sparovek, G.; Schnug, E. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: A review. J. Plant Nutr. 2004, 27, 183–220. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Human and veterinary antibiotics induce hormesis in plants: Scientific and regulatory issues and an environmental perspective. Environ. Int. 2018, 120, 489–495. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium exposed chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef]
- Latef, A.A.A. Growth and some physiological activities of pepper (Capsicum annuum L.) in response to cadmium stress and mycorrhizal symbiosis. J. Agric. Sci. Technol. 2018, 15, 1437–1448. [Google Scholar]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Smeets, K.; Cuypers, A.; Lambrechts, A.; Semane, B.; Hoet, P.; Van-Laere, A.; Vangronsveld, J. Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol. Biochem. 2005, 43, 437–444. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Zelinová, V. Heavy metal-induced reactive oxygen species and cell death in barley root tip. Environ. Exp. Bot. 2017, 140, 34–40. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Zhou, Q.; Huang, X. Combined effects of lanthanum (III) chloride and acid rain on photosynthetic parameters in rice. Chemosphere 2014, 112, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J.; Ren, Y.J.; Yan, L.Y. Effects of Spray Application of lanthanum and cerium on yield and quality of Chinese cabbage (Brassica chinensis L) Based on Different Seasons. Biol. Trace Elem. Res. 2014, 160, 427–432. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, L.; De-Pinto, M.C.; Nardi, L.; Morgana, M.; Tommasi, F. Effect of some light rare earth elements on seed germination, seedling growth and antioxidant metabolism in Triticum durum. Chemosphere 2009, 75, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Y.; Wang, L.; Zhou, Q.; Huang, X. Effect of lanthanum(III) on the production of ethylene and reactive oxygen species in soybean seedlings exposed to the enhanced ultraviolet-B radiation. Ecotoxicol. Environ. Saf. 2014, 104, 152–159. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, M.; Sun, Z.; Wang, L.; Zhou, Q.; Huang, X. Combined acid rain and lanthanum pollution and its potential ecological risk for nitrogen assimilation in soybean seedling roots. Environ. Pollut. 2017, 231, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, L.; Li, Y.; Sun, J.; Zhou, Q.; Huang, X. Insight into mechanism of lanthanum (III) induced damage to plant photosynthesis. Ecotoxicol. Environ. Saf. 2016, 127, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hasenstein, K.H. La3+ uptake and its effect on the cytoskeleton in root protoplasts of Zea mays L. Planta 2005, 220, 658–666. [Google Scholar] [CrossRef]
- Coelho, J.P.; Pereira, M.E.; Duarte, A.C.; Pardal, M.A. Contribution of primary producers to mercury trophic transfer in estuarine ecosystems: Possible effects of eutrophication. Mar. Pollut. Bull. 2009, 58, 358–365. [Google Scholar] [CrossRef]
- Gurajala, H.K.; Cao, X.; Tang, L.; Ramesh, T.M.; Lu, M.; Yang, X. Comparative assessment of Indian mustard (Brassica juncea L.) genotypes for phytoremediation of Cd and Pb contaminated soils. Environ. Pollut. 2019, 254, 113085. [Google Scholar] [CrossRef]
- Zarrouk, M.; El Almi, H.; Ben Youssef, N.; Sleimi, N.; Smaoui, A.; Ben Miled, D.; Abdelly, C. Lipid composition of seeds of local halophytes: Cakile maritima, Zygophyllum album and Crithmum maritimum. In Tasks for Vegetation Science; Lieth, H., Mochtchenko, M., Eds.; Cash Crop Halophytes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; Volume 38, pp. 121–124. [Google Scholar] [CrossRef]
- Shehzad, J.; Khan, I.; Zaheer, S.; Farooq, A.; Chaudhari, S.K.; Mustafa, G. Insights into heavy metal tolerance mechanisms of Brassica species: Physiological, biochemical, and molecular interventions. Environ Sci Pollut Res. 2023, 30, 108448–108476. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Sangwan, N.S. Amelioration of cadmium stress in Withania somnifera by ROS management: Active participation of primary and secondary metabolism. Plant Growth Regul. 2019, 87, 403–412. [Google Scholar] [CrossRef]
- Hewitt, E.J. 1966. Sand and water culture methods used in study of plant nutrition. Common Wealth Bur. Hortic. Tech. Commun. 1966, 22, 547. [Google Scholar]
- Sghaier, B.D.; Bankaji, I.; Pedro, S.; Caçador, I.; Sleimi, N. Photosynthetic behavior and mineral nutrition of Tamarix gallica cultivated under Aluminum and NaCl combined stress. Phyton-Int. J. Exp. Bot. 2019, 88, 239–252. [Google Scholar] [CrossRef]
- Bouslimi, H.; Ferreira, R.; Dridi, N.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Effects of barium stress in Brassica juncea and Cakile maritima: The indicator role of some antioxidant enzymes and secondary metabolites. Phyton-Int. J. Exp. Bot. 2021, 90, 145–158. [Google Scholar] [CrossRef]
- Sleimi, N.; Kouki, R.; Hadj Ammar, M.; Ferreira, R.; Perez-Clemente, R.M. Barium effect on germination, plant growth, and antioxidant enzymes in Cucumis sativus L. plants. Food Sci. Nutr. 2021, 9, 2086–2094. [Google Scholar] [CrossRef]
- Sleimi, N.; Abdelly, C. Salt-tolerance strategy of two fodder halophytes species: Spartina alterniflora and Suaeda fruticosa. In Tasks for Vegetation Science; Lieth, H., Mochtchenko, M., Eds.; Cash Crop Halophytes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; Volume 38, pp. 79–85. [Google Scholar] [CrossRef]
- Dridi, N.; Bouslimi, H.; Caçador, I.; Sleimi, N. Lead tolerance, accumulation and translocation in two Asteraceae plants: Limbarda crithmoides and Helianthus annuus. S. Afr. J. Bot. 2022, 150, 986–996. [Google Scholar] [CrossRef]
- Sleimi, N.; Bankaji, I.; Kouki, R.; Dridi, N.; Duarte, B.; Caçador, I. Assessment of extraction methods of trace metallic elements in plants: Approval of a common method. Sustainability 2022, 14, 1428. [Google Scholar] [CrossRef]
- Kouki, R.; Dridi, N.; Vives-Peris, V.; Gomez-Cadenas, A.; Caçador, I.; Pérez-Clemente, R.M.; Sleimi, N. Appraisal of Abelmoschus esculentus L. Response to Aluminum and Barium Stress. Plants 2023, 12, 179. [Google Scholar] [CrossRef]
- Bankaji, I.; Kouki, R.; Dridi, N.; Ferreira, R.; Hidouri, S.; Duarte, B.; Sleimi, N.; Caçador, I. Comparison of digestion methods using atomic absorption spectrometry for the determination of metal levels in plants. Separations 2023, 10, 40. [Google Scholar] [CrossRef]
- Brito, P.; Ferreira, R.; Martins-Dias, S.; Azevedo, O.M.; Caetano, M.; Caçador, I. Cerium uptake, translocation and toxicity in the salt marsh halophyte Halimione portulacoides (L.), Aellen. Chemosphere 2021, 266, 128973. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Quittier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrume sculentum Moench) hulls and four. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.; Ramos, S.J.; Siqueira, J.O.; Faquin, V.; Castro, E.M.; Amaral, D.C.; Techio, V.H.; Coelho, L.C.; Silva, P.H.P.; Schnug, E.; et al. Bioaccumulation and effects of lanthanum on growth and mitotic index in soybean plants. Ecotoxicol. Environ. Saf. 2015, 122, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xiong, Z.; Chen, Y.; Huang, H. Interactive effects of lanthanum and cadmium on plant growth and mineral element uptake in crisped-leaf mustard under hydroponic conditions. J. Plant Nutr. 2006, 29, 1889–1902. [Google Scholar] [CrossRef]
- Qin, R.; Wang, C.; Chen, D.; Björn, L.O.; Li, S. Copper-induced root growth inhibition of Allium cepa var. Agrogarum L. involves disturbances in cell division and DNA damage. Environ. Toxicol. Chem. 2015, 34, 1045–1055. [Google Scholar] [CrossRef]
- Xu, Q.M.; Wang, Y.Z.; Liu, H.; Cheng, J.S. Physiological responses and chromosomal aberration in root tip cells of Allium sativum L. to cerium treatments. Plant Soil 2016, 409, 447–458. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, C.; Liu, W.S.; Guo, M.N.; Morel, J.L.; Huot, H.; Yu, H.J.; Tang, Y.T.; Qiu, R.L. Accumulation and fractionation of rare earth elements (REEs) in the naturally grown Phytolacca americana L. In southern China. Int. J. Phytoremediation 2018, 20, 415–423. [Google Scholar] [CrossRef]
- Carpenter, D.J.; Boutin, C.; Allison, J.E.; Parsons, J.L.; Ellis, D. Uptake and effects of six rare earth elements (REEs) on selected native and crop species growing in contaminated soils. PLoS ONE 2015, 10, e0129936. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Huot, H.; Yang, Y.; Guo, M.; Morel, J.L.; Tang, Y.; Qiu, R. Responses of ramie (Boehmeria nivea L.) to increasing rare earth element (REE) concentration in a hydroponic system. J. Rare Earths 2022, 40, 840–846. [Google Scholar] [CrossRef]
- Mohsin, M.; Salam, M.M.A.; Nawrot, N.; Kaipiainen, E.; Lane, D.J.; Wojciechowska, E.; Kinnunen, N.; Heimonen, M.; Tervahauta, A.; Peräniemi, S.; et al. Phytoextraction and recovery of rare earth elements using willow (Salix spp.). Sci. Total Environ. 2022, 809, 152209. [Google Scholar] [CrossRef] [PubMed]
- Dridi, N.; Ferreira, R.; Bouslimi, H.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Assessment of tolerance to lanthanum and cerium in Helianthus annuus plant: Effect on growth, mineral nutrition, and secondary metabolism. Plants 2022, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Duarte, A.C.; de Oliveira, C.; Ramos, S.J.; de Castro, E.M.; Siqueira, J.O.; Guilherme, L.R.G. Lanthanum content and effects on growth, gas exchanges, and chlorophyll index in maize plants. Acta Sci. Biol. Sci. 2018, 40, 38469. [Google Scholar] [CrossRef]
- Telesiński, A.; Snioszek, M.; Smolik, B.; Malinowska, K.; Mikiciuk, M.; Cichocka, J.; Zakrzewska, H. Fuoride uptake in hydroponic culture by different clones of basket willow Salix viminalis L. Fluoride 2011, 44, 255. [Google Scholar]
- Seregin, I.; Kozhevnikova, A. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar] [CrossRef]
- Souri, M.K.; Hatamian, M.; Tesfamariam, T. Plant growth stage influences heavy metal accumulation in leafy vegetables of garden cress and sweet basil. Chem. Biol. Technol. Agric. 2019, 6, 25. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Zhang, X.; Gao, Z. Effects of lanthanum on growth and accumulation in roots of rice seedlings. Plant Soil Environ. 2013, 59, 196–200. [Google Scholar] [CrossRef]
- Barceló, J.; Vázquez, M.D.; Poschenrieder, C. Cadmium induced structural and ultrastructural changes in the vascular system of bush bean stems. Acta Bot. 1988, 101, 254–261. [Google Scholar] [CrossRef]
- Ouzounidou, G. Effect of copper on germination and seedling growth of Minuatia, Silene, Alyssum and Thalaspi. Biol. Plant. 1995, 37, 411–416. [Google Scholar] [CrossRef]
- Tu, C.; Ma, L.Q. Effects of arsenic on concentration and distribution of nutrients in the fronds of the arsenic hyperaccumulator Pteris vittata L. Environ. Pollut. 2005, 135, 333–340. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, Y.; Min, H.; Cai, S.; Sha, S.; Cheng, G. Laboratory assessment of uptake and toxicity of lanthanum (La) in the leaves of Hydrocharis dubia (Bl.) Backer. Environ. Sci. Pollut. Res. 2012, 19, 3950–3958. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gomez, N.C.; Vu, H.N.; Skovran, E. Lanthanide chemistry coordination in chemical complexes shaping our technology to coordination in enzymes shaping bacterial metabolism. Inorg. Chem. 2016, 55, 10083–10089. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.B.; Zhu, J.G.; Chu, H.Y.; Zhang, Y.L.; Zeng, Q.; Ma, H.L.; Cao, Z.H. Effect of lanthanum on rice production, nutrient uptake and distribution. J. Plant Nutr. 2002, 25, 2315–2331. [Google Scholar] [CrossRef]
- Küpper, H.; Šetlik, I.; Spiller, M.; Küpper, F.C.; Prášil, O. Heavy metal-induced inhibition of photosynthesis: Targets of in vivo heavy metal chlorophyll formation. J. Phycol. 2002, 38, 429–441. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; He, M.; Jiang, C.; Tian, L.; Tian, Y.; Wang, X. Mineral nutrient imbalance, DNA lesion and DNA-protein crosslink involved in growth retardation of Vicia faba L. seedlings exposed to lanthanum ions. J. Environ. Sci. 2012, 24, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, X.; Tian, Y.; Cheng, T.; Hu, L.; Chen, F.; Jiang, C.; Wang, X. Lanthanum resulted in unbalance of nutrient elements and disturbance of cell proliferation cycles in V. faba L. seedlings. Biol. Trace Elem. Res. 2011, 143, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Messedi, D.; Sleimi, N.; Abdelly, C. Some physiological and biochemical aspects of salt tolerance of Sesuvium portulacastrum. In Tasks for Vegetation Science; Lieth, H., Mochtchenko, M., Eds.; Cash Crop Halophytes; Kluwer Academic Publishers: Dordrecht, The Netherland, 2003; Volume 38, pp. 71–77. [Google Scholar] [CrossRef]
- Bokri, H.; Kahlaoui, S.; Hcini, K.; Dhoueibi, M.; Harzallah-Skhiri, F.; Stambouli-Essassi, S. Induction of organogenesis and callogenesis in Limbarda crithmoïdes L. (Asteraceae) explants cultured on MS media supplemented with various concentrations of Na+ and K+. Not. Bot. Horti. Agrobo. 2023, 51, 13147. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Babula, P.; Klejdus, B.; Kovacik, J.; Hedbavny, J.; Hlavna, M. Lanthanum rather than cadmium induces oxidative stress and metabolite changes in Hypericum perforatum. J. Hazard. Mater. 2015, 286, 334–342. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Ma, C.; Liu, H.; Guo, H.; Musante, C.; Coskun, S.H.; Nelson, B.C.; White, J.C.; Xing, B.; Dhankher, O.P. Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ. Sci. Nano 2016, 3, 1369–1379. [Google Scholar] [CrossRef]
- Şen, A. Oxidative stress studies in plant tissue culture, antioxidant enzyme. In Biochemistry, Genetics and Molecular Biology “Antioxidant Enzyme”; El-Missiry, M.A., Ed.; IntechOpen Publisher: London, UK, 2012; pp. 59–88. [Google Scholar]
- Zafari, S.; Sharifi, M.; Ahmadian Chashmi, N.; Mur, L.A. Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol. Biochem. 2016, 99, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia Obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Jia, H.; Sun, X.; Shangguan, L.; Mu, Q.; Wang, B.; Fang, J. Comparative transcriptome analysis of grapevine in response to copper stress. Sci. Rep. 2015, 5, 17749. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S. Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress: A review. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Keilig, K.; Ludwig-Mueller, J. Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Bot. Stud. 2009, 50, 311–318. [Google Scholar]
- Kadioglu, A.; Saruhan, N.; Sağlam, A.; Terzi, R.; Acet, T. Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul. 2011, 64, 27–37. [Google Scholar] [CrossRef]
- Dridi, N.; Bouslimi, H.; Brito, P.; Sleimi, N.; Hidouri, S.; Caçador, I. Assessment of lanthanum (La) and cerium (Ce) phytotoxicity in a halophyte species: Limbarda crithmoides L. J. Stress Physiol. Biochem. 2023, 19, 101–113. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).