The Effects of Two New Fertilizers on the Growth and Fruit Quality of Actinidia eriantha Benth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Characterization
2.2. Experimental Design and Treatments
2.3. Plant Biomass
2.4. Determination of Fruit Growth Parameters
2.5. Analysis of Fruit Pigment Parameters
2.6. Measurement of Fruit Quality
2.7. Measurement of Fruit Antioxidants
2.8. Statistical Analysis
3. Results
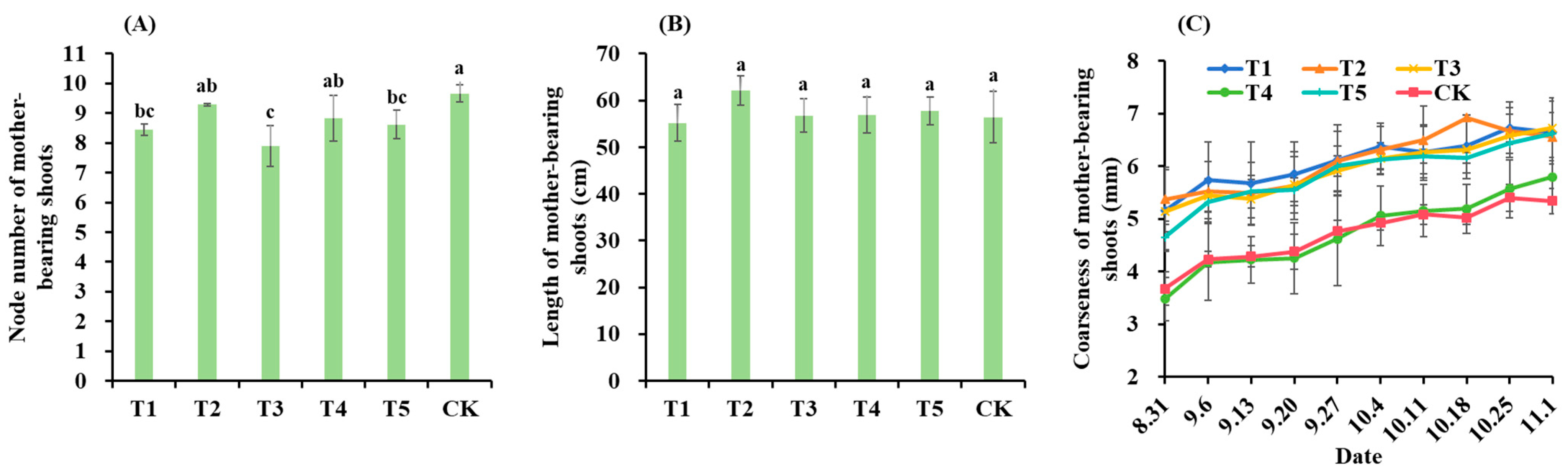
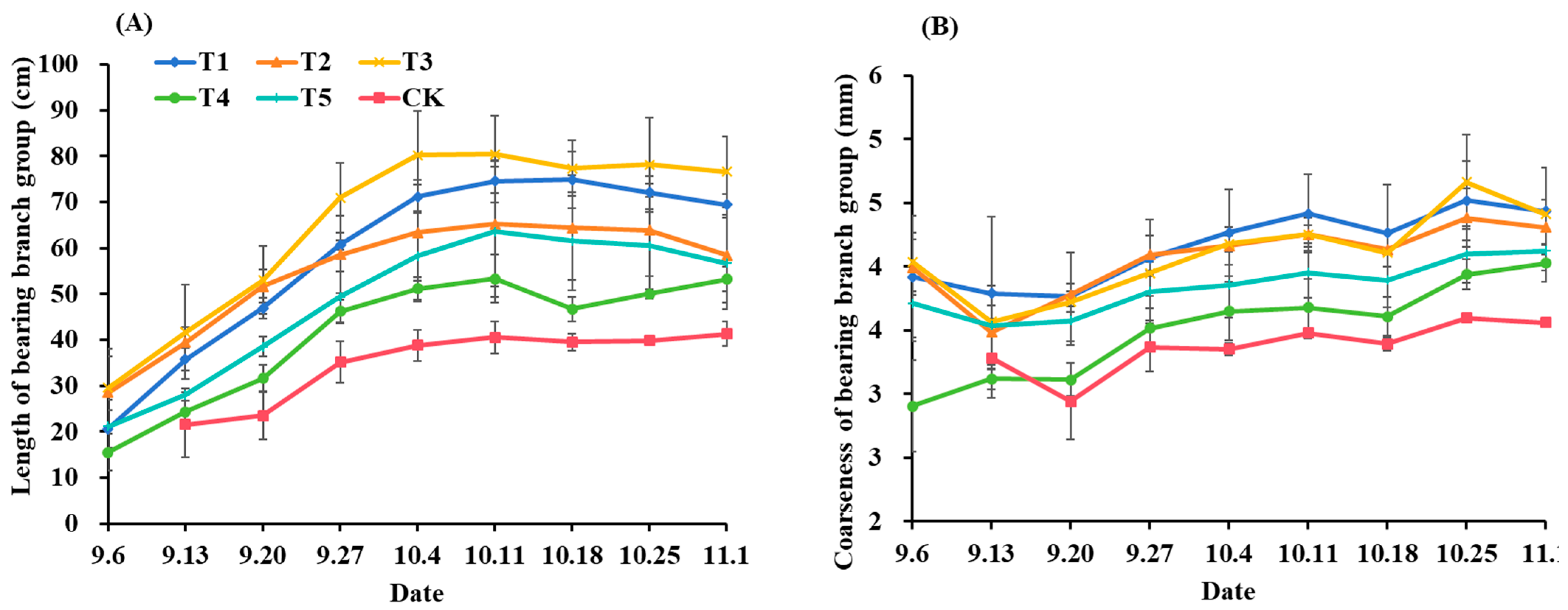
3.1. Growth of Mother-Bearing Shoots and Bearing Branch Group
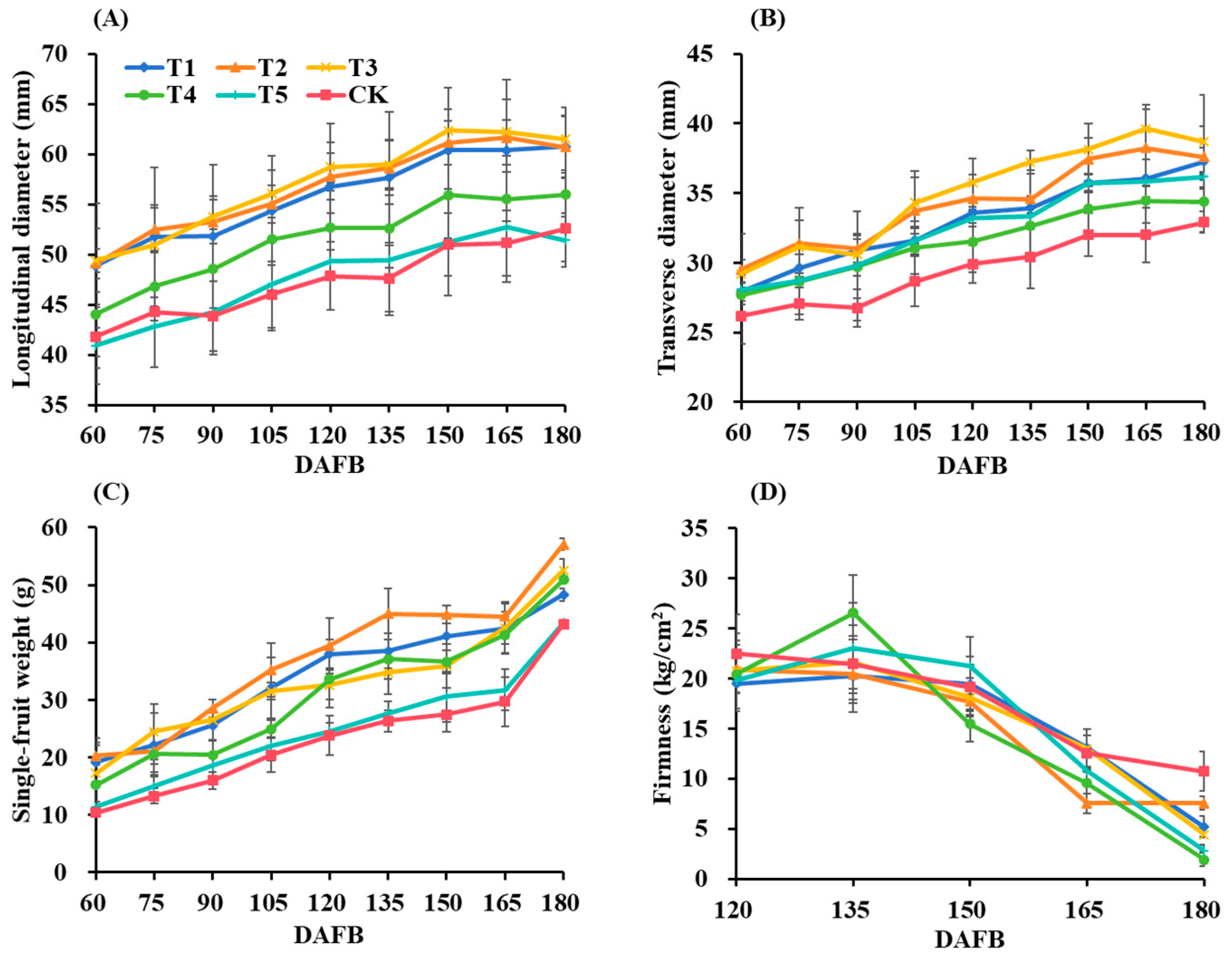
3.2. Fruit Growth Parameters
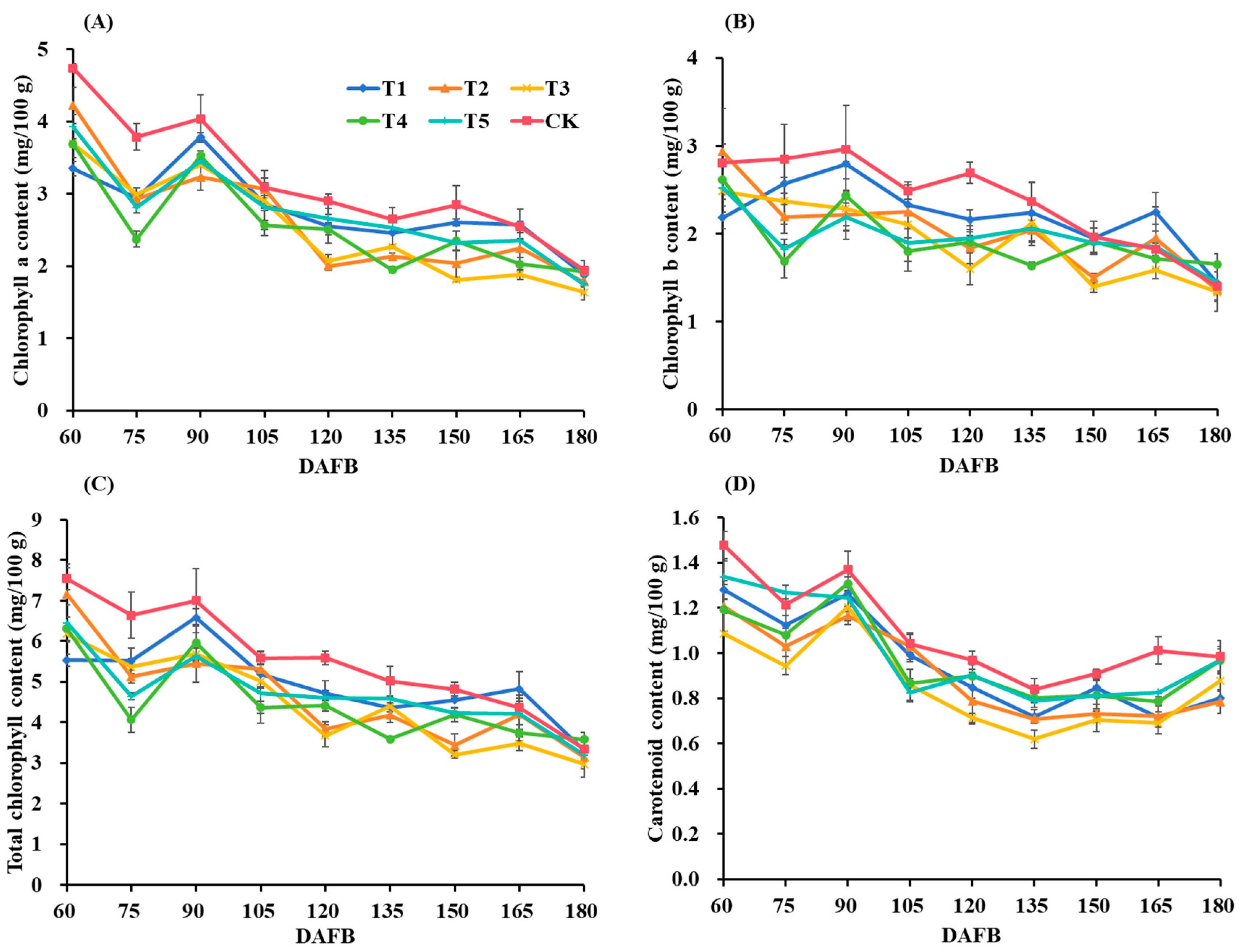
3.3. Pigment Parameters of Fruit
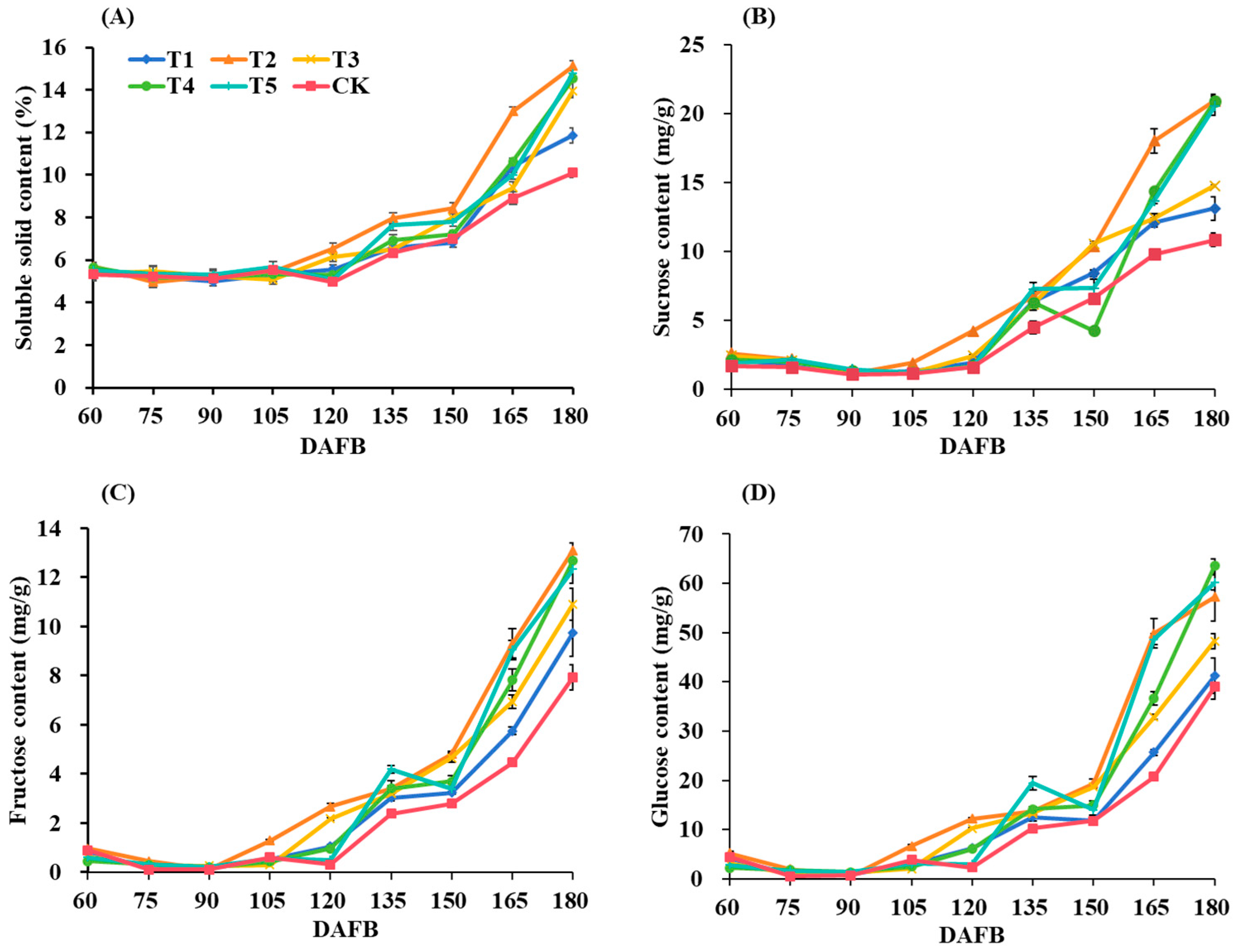
3.4. Sugar Components
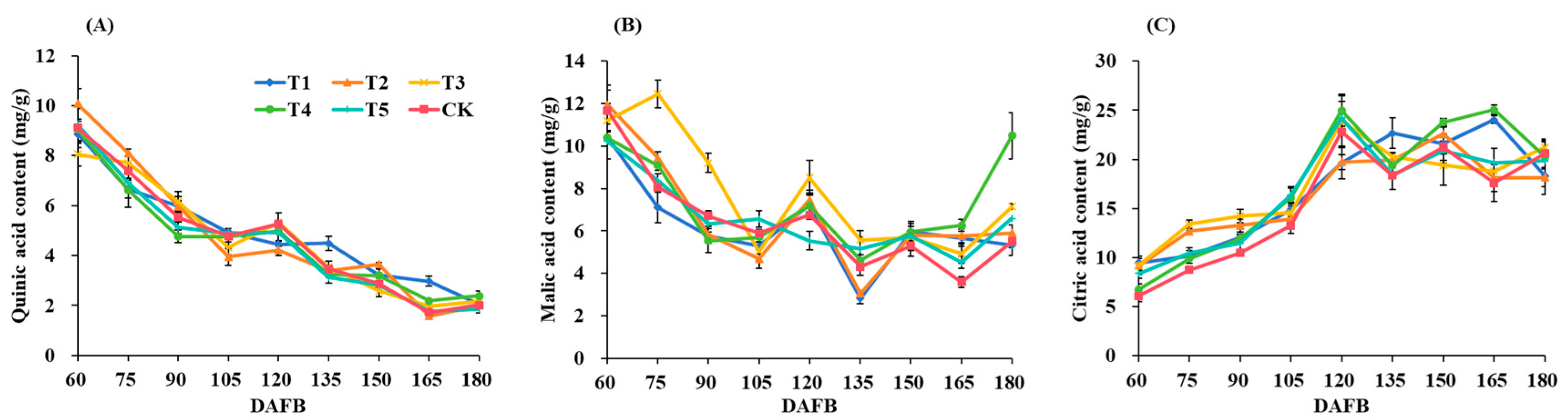
3.5. Organic Acid Profiles
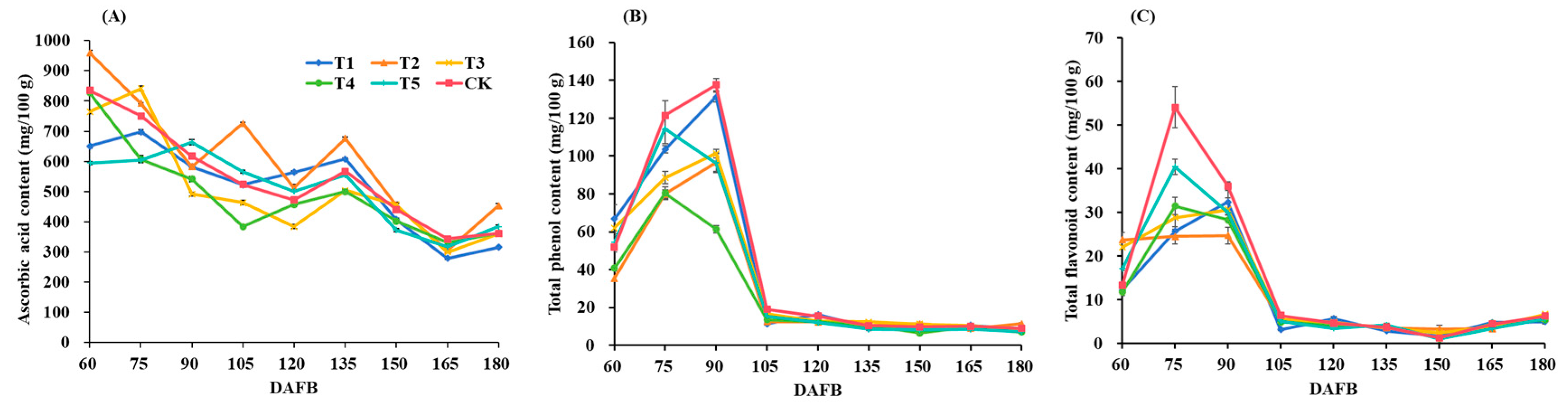
3.6. Fruit Antioxidants
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.J.; Tao, J.J.; Qu, X.Y.; Huang, C.H.; Xu, X.B. Dynamic Variation in Sugar, Acid, and ASA Contents of ‘Ganmi 6′ Kiwifruit (Actinidia eriantha Benth) Fruits. Agric. Sci. Technol. 2015, 16, 2589–2591+2595. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT Database, Production Module. Available online: http://www.fao.org/faostat/en/#data (accessed on 22 November 2024).
- Zhang, L.H.; Li, S.F.; Liu, X.H.; Song, C.L.; Liu, X. Effects of ethephon on physicochemical and quality properties of kiwifruit during ripening. Postharvest Biol. Technol. 2011, 65, 69–75. [Google Scholar] [CrossRef]
- Jia, D.F.; Gao, H.; He, Y.Q.; Liao, G.L.; Lin, L.T.; Huang, C.H.; Xu, X.B. Kiwifruit Monodehydroascorbate reductase 3 gene negatively regulates the accumulation of ascorbic acid in fruit of transgenic tomato plants. Int. J. Mol. Sci. 2023, 24, 17182. [Google Scholar] [CrossRef]
- Jia, D.F.; Xu, Z.Y.; Chen, L.; Huang, Q.; Huang, C.H.; Tao, J.J.; Qu, X.Y.; Xu, X.B. Analysis of organic acid metabolism reveals citric acid and malic acid play major roles in determining acid quality during the development of kiwifruit (Actinidia eriantha). J. Sci. Food Agr. 2023, 103, 6055–6069. [Google Scholar] [CrossRef]
- Xu, X.B.; Liao, G.L.; Huang, C.H.; Jia, D.F.; Zhong, M.; Qu, X.Y.; Liu, Q.; Gao, H. A novel sweet aromatic cultivar of Actinidia eriantha ‘Ganlü No. 1′. J. Fruit Sci. 2024, 41, 358–361. [Google Scholar]
- Liao, G.L.; Xu, X.B.; Huang, C.H.; Zhong, M.; Jia, D.F. Resource evaluation and novel germplasm mining of Actinidia eriantha. Sci. Hortic. 2021, 282, 110037. [Google Scholar] [CrossRef]
- Wu, L.; Lan, J.B.; Xiang, X.X.; Xiang, H.Y.; Jin, Z.; Khan, S.; Liu, Y.Q. Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis). PLoS ONE 2020, 15, e0240355. [Google Scholar] [CrossRef]
- Bi, Y.Y.; Qiao, C.K.; Han, L.J.; Xie, H.Z.; Xu, Y.J.; Wu, D.; Zhuang, M.; Lv, X.R.; Cao, M.Y. Key metabolites and mechanistic insights in forchlorfenuron controlling kiwifruit development. Food Res. Int. 2023, 164, 112412. [Google Scholar] [CrossRef]
- Wan, H.L.; Kong, X.B.; Liu, Y.H.; Jin, F.; Han, L.X.; Xu, M.; Li, X.M.; Li, L.; Yang, J.; Lai, D.N.; et al. Residue analysis and effect of preharvest forchlorfenuron (CPPU) application on quality formation of kiwifrut. Postharvest Biol. Technol. 2023, 195, 112144. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Duan, M.; Yan, S.; Liu, Z.F.; Wang, Q.; Fu, J.; Tong, Y.A. Effects of different fertilizations on fruit quality, yield and soil fertility in field-grown kiwifruit orchard. Int. J. Agric. Biol. Eng. 2017, 10, 162–171. [Google Scholar] [CrossRef]
- Raiesi, T.; Shiri, M.A.; Mousavi, S.M. The fruit quality and nutrient content of kiwifruit produced by organic versus chemical fertilizers. J. Sci. Food Agric. 2024, 104, 6821–6830. [Google Scholar] [CrossRef]
- Zhang, M.H.; Sun, D.Y.; Niu, Z.R.; Yan, J.X.; Zhou, X.L.; Kang, X. Effects of combined organic/inorganic fertilizer application on growth, photosynthetic characteristics, yield and fruit quality of Actinidia chinesis cv ‘Hongyang’. Glob. Ecol. Conserv. 2020, 22, e00997. [Google Scholar] [CrossRef]
- Bao, C.Y.; Zhao, J.; He, Z.X.; Fan, Y.F.; Jiang, C.L.; Deng, L.; Zhou, J.; Wang, L.C. Effects of different kinds of microbial fertilizer and amounts on kiwifruit quality. Soil Fertil. Sci. China 2021, 2, 262–269. [Google Scholar] [CrossRef]
- Patel, V.B.; Singh, S.K.; Asrey, R.; Nain, L.; Singh, A.K.; Singh, L. Microbial and inorganic fertilizers application influenced vegetative growth, yield, leaf nutrient status and soil microbial biomass in sweet orange cv. Mosambi. Indian J. Hort. 2009, 66, 163–168. [Google Scholar]
- Tomić, J.; Stajić, Z.K.; Pešaković, M.; Paunović, S.M.; Milinković, M.; Rilak, B.; Korićanac, A. Fruit quality of strawberry cultivars (Fragaria ananassa Duch.) affected by mineral and microbiological fertilizers. Voćarstvo 2018, 52, 67–76. [Google Scholar]
- Liu, Y.Y.; Xu, X.M.; Fu, H.Y.; Zhao, M.Y.; Chen, W. Effects of microbial fertilizer on apple fruit quality. IOP Conf. Ser. Earth Environ. Sci. 2020, 446, 032102. [Google Scholar] [CrossRef]
- NY/T 2009-2011; Agricultural Industry Standards: Determination of Fruit Firmness. Ministry of Agriculture: Beijing, China, 2009.
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with n,n-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kitaya, Y.; Seoka, M.; Kudaka, R.; Yahata, M.; Yamawaki, K.; Shimada, T.; Fujii, H.; Endo, T.; et al. Blue LED light induces regreening in the flavedo of Valencia orange in vitro. Food Chem. 2021, 335, 127621. [Google Scholar] [CrossRef]
- Thacker, J.B.; Schug, K.A. Quantitative determination of fructose, glucose, and sucrose in hard ciders and apple juice by LC–MS/MS. Sep. Sci. Plus 2020, 3, 286–293. [Google Scholar] [CrossRef]
- Flores, P.; Hellin, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- GB 5009.86-2016; National Food Safety Standards: Determination of Ascorbic Acid in Food. National Health and Family Planning Commission: Beijing, China, 2016.
- Chen, W.D.; Xie, C.Y.; He, Q.Q.; Sun, J.X.; Bai, W.B. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Luo, T.; Chen, S.R.; Zhang, H.H.; Jia, S.J.; Wang, J.L. Phytochemical composition and potential biological activities assessment of raspberry leaf extracts from nine different raspberry species and raspberry leaf tea. J. Berry Res. 2020, 10, 295–309. [Google Scholar] [CrossRef]
- Tao, J.J.; Wu, M.T.; Jiao, X.D.; Chen, S.S.; Jia, D.F.; Xu, X.B.; Huang, C.H. Dynamic changes of fruit physiological quality and sugar components during fruit growth and development of Actinidia eriantha. Horticulturae 2022, 8, 529. [Google Scholar] [CrossRef]
- Lee, P.R.; Tan, R.M.; Yu, B.; Curran, P.; Liu, S.Q. Sugars, organic acids, and phenolic acids of exotic seasonable tropical fruits. Nutr. Food Sci. 2013, 43, 267–276. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Tang, Z.; Xu, Q.; Deng, X.X. Isolation, phylogenetic relationship and expression profiling of sugar transporter genes in sweet orange (Citrus sinensis). Plant Cell Tissue Org. 2014, 119, 609–624. [Google Scholar] [CrossRef]
- Zhong, C.H.; Zhang, P.; Jiang, Z.W.; Wang, S.M.; Han, F.; Xu, L.Y.; Huang, H.W. Dynamic changes of carbohydrate and vitamin C in fruit of Actinidia chinensis and A. eriantha during growing season. Plant Sci. J. 2011, 29, 370–376. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Xie, M.; Zhang, C.; Yang, L.Q.; Zhang, Z.; Xiao, J.P.; Zhou, L.Q. Difference in starch accumulation and characterization of sugar metabolism during fruit development of kiwi fruit. Sci. Agric. Sin. 2014, 47, 3453–3464. [Google Scholar] [CrossRef]
- Chen, F.X.; Liu, X.H.; Chen, L.S. Advances in research on organic acid metabolism in fruits. J. Fruit Sci. 2005, 22, 526–531. [Google Scholar]
- Silvia, T.; Elena, D.; Damiano, R.; Rossano, M.; Lucia, G. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Kim, Y.E.; Cho, C.H.; Kang, H.; Heo, H.J.; Cho, Y.S.; Kim, D.O. Kiwifruit of Actinidia eriantha cv. Bidan has in vitro antioxidative, anti-inflammatory and immunomodulatory effects on macrophages and splenocytes isolated from male balb/c mice. Food Sci. Biotechnol. 2018, 27, 1503–1508. [Google Scholar] [CrossRef]
- Du, G.R.; Li, M.J.; Ma, F.W.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Nie, B.; Du, J.Q.; Liu, H.N.; Xu, G.L.; Wang, Z.; He, Y.; Li, B.T. Crowds’ classification using hierarchical cluster, rough sets, principal component analysis and its combination. In Proceedings of the 2009 International Forum on Computer Science-Technology and Applications, ChongQing, China, 25–27 December 2009; IEEE: New York, NY, USA, 2009; Volume 1, pp. 287–290. [Google Scholar]
- Himabindu, A.; Srihari, D.; Raiasekhar, M.; Sudhavani, V.; Subbarammamma, P.; Krishna, K.U.; Paratpara, R.M. Genetie diversity for fruit morphological and biochemical characters of indigenous mango (Mangifera indica L.) cultivars of coastal districts in Andhra Pradesh using principal component analysis. Electron. J. Plant Breed. 2017, 8, 772–778. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, P.; Bhardwaj, A.; Verma, R.B. Principal component analysis approach for comprehensive screening of tomato germplasm for polyhouse condition. J. Exp. Agric. Int. 2021, 43, 67–72. [Google Scholar] [CrossRef]
| Treatments | Fertilizer Name | Dilution Factor |
|---|---|---|
| T1 | Organic water-soluble fertilizer (OWS) | 500× dilution |
| T2 | 1000× dilution | |
| T3 | 1500× dilution | |
| T4 | Microbial fertilizer (MF) | 500× dilution |
| T5 | 1000× dilution | |
| CK | Control | Distilled water |
| Treatments | Longitudinal Diameter (cm) | Transverse Diameter (cm) | Fruit Shape Index | Single-Fruit Weight (g) | Firmness (kg/cm2) |
|---|---|---|---|---|---|
| T1 | 60.73 ± 3.02 a | 37.27 ± 0.97 ab | 1.65 ± 0.07 a | 48.31 ± 1.14 c | 5.18 ± 1.06 c |
| T2 | 60.73 ± 3.09 a | 37.61 ± 2.21 ab | 1.62 ± 0.03 a | 57.07 ± 1.05 a | 7.55 ± 0.68 b |
| T3 | 61.52 ± 3.14 a | 38.69 ± 3.37 a | 1.58 ± 0.04 a | 52.54 ± 1.98 b | 4.44 ± 0.20 c |
| T4 | 55.97 ± 2.21 ab | 34.37 ± 2.14 bc | 1.60 ± 0.09 a | 50.91 ± 0.53 b | 1.95 ± 0.63 d |
| T5 | 51.43 ± 2.67 b | 36.19 ± 0.23 abc | 1.62 ± 0.03 a | 43.40 ± 0.42 d | 2.82 ± 0.63 d |
| CK | 52.58 ± 3.31 b | 32.91 ± 0.78 c | 1.69 ± 0.06 a | 43.16 ± 0.30 d | 10.74 ± 1.93 a |
| Treatments | Chlorophyll a Content (mg/100 g) | Chlorophyll b Content (mg/100 g) | Total Chlorophyll Content (mg/100 g) | Carotenoid Content (mg/100 g) |
|---|---|---|---|---|
| T1 | 1.88 ± 0.06 a | 1.43 ± 0.13 a | 3.31 ± 0.19 ab | 0.80 ± 0.03 b |
| T2 | 1.78 ± 0.07 ab | 1.36 ± 0.05 a | 3.14 ± 0.09 ab | 0.78 ± 0.05 b |
| T3 | 1.64 ± 0.02 b | 1.33 ± 0.09 a | 2.97 ± 0.11 b | 0.88 ± 0.04 ab |
| T4 | 1.92 ± 0.04 a | 1.65 ± 0.02 a | 3.58 ± 0.04 a | 0.97 ± 0.05 a |
| T5 | 1.75 ± 0.22 ab | 1.44 ± 0.33 a | 3.19 ± 0.55 ab | 0.97 ± 0.06 a |
| CK | 1.95 ± 0.13 a | 1.40 ± 0.17 a | 3.34 ± 0.30 ab | 0.98 ± 0.07 a |
| Treatments | Soluble Solid Content (%) | Sucrose Content (mg/g) | Fructose Content (mg/g) | Glucose Content (mg/g) |
|---|---|---|---|---|
| T1 | 11.87 ± 0.35 c | 13.12 ± 0.87 bc | 9.75 ± 0.98 c | 41.20 ± 3.55 d |
| T2 | 15.13 ± 0.25 a | 20.93 ± 0.49 a | 13.11 ± 0.31 a | 57.21 ± 4.81 b |
| T3 | 13.97 ± 0.35 bc | 14.75 ± 0.05 b | 10.91 ± 0.66 b | 48.26 ± 1.58 c |
| T4 | 14.53 ± 0.21 b | 20.90 ± 0.38 a | 12.70 ± 0.27 a | 63.55 ± 1.36 a |
| T5 | 14.80 ± 0.20 ab | 20.52 ± 0.63 a | 12.34 ± 0.57 a | 60.16 ± 1.53 ab |
| CK | 10.10 ± 0.20 d | 10.85 ± 0.50 c | 7.93 ± 0.52 d | 38.98 ± 2.52 d |
| Treatments | Shikimic Acid Content (mg/100 g) | Succinic Acid Content (mg/100 g) | Quinic Acid Content (mg/g) | Malic Acid Content (mg/g) | Citric Acid Content (mg/g) |
|---|---|---|---|---|---|
| T1 | 2.91 ± 0.19 b | 1.05 ± 0.10 b | 2.05 ± 0.07 bc | 5.33 ± 0.50 d | 18.32 ± 1.89 ab |
| T2 | 3.35 ± 0.32 ab | 0.74 ± 0.08 c | 2.01 ± 0.05 bc | 5.90 ± 0.38 cd | 18.13 ± 0.91 b |
| T3 | 2.12 ± 0.20 c | 0.94 ± 0.03 b | 2.17 ± 0.10 ab | 7.14 ± 0.13 b | 21.23 ± 0.70 a |
| T4 | 2.45 ± 0.20 c | 1.08 ± 0.11 b | 2.38 ± 0.21 a | 10.48 ± 1.09 a | 20.27 ± 1.77 ab |
| T5 | 3.27 ± 0.29 ab | 1.43 ± 0.14 a | 1.86 ± 0.15 c | 6.59 ± 0.01 bc | 19.86 ± 1.93 ab |
| CK | 3.44 ± 0.22 a | 1.52 ± 0.14 a | 2.03 ± 0.11 bc | 5.50 ± 0.28 d | 20.62 ± 1.41 ab |
| Treatments | Ascorbic Acid Content (mg/100 g) | Total Phenol Content (mg/100 g) | Total Flavonoid Content (mg/100 g) |
|---|---|---|---|
| T1 | 315.96 ± 1.87 d | 8.91 ± 0.76 b | 5.01 ± 0.54 c |
| T2 | 453.41 ± 7.02 a | 11.32 ± 0.44 a | 5.78 ± 0.31 bc |
| T3 | 360.10 ± 0.91 c | 8.27 ± 0.51 b | 6.67 ± 0.32 a |
| T4 | 363.12 ± 2.71 c | 7.01 ± 0.43 c | 5.43 ± 0.47 bc |
| T5 | 384.45 ± 5.74 b | 7.15 ± 0.65 c | 5.60 ± 0.26 bc |
| CK | 361.61 ± 0.69 c | 8.92 ± 0.85 b | 6.22 ± 0.58 ab |
| Principal Component | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Eigenvalue | 5.979 | 5.716 | 2.931 | 2.052 | 1.323 |
| Variance contribution rate (%) | 33.216 | 31.753 | 16.282 | 11.398 | 7.351 |
| Cumulative variance contribution rate (%) | 33.216 | 64.969 | 81.251 | 92.649 | 100.00 |
| Single-fruit weight | 0.453 | 0.705 | −0.123 | −0.416 | −0.332 |
| Longitudinal diameter | 0.666 | 0.328 | −0.081 | −0.665 | 0.021 |
| Transverse diameter | 0.670 | 0.527 | 0.146 | −0.223 | 0.450 |
| Firmness | −0.819 | 0.483 | 0.041 | −0.200 | 0.234 |
| Chlorophyll a | 0.449 | 0.526 | 0.640 | 0.048 | 0.330 |
| Chlorophyll b | 0.922 | −0.077 | 0.324 | 0.198 | −0.001 |
| Total chlorophyll | 0.750 | 0.245 | 0.594 | 0.104 | 0.119 |
| Carotenoids | 0.808 | 0.260 | −0.446 | −0.269 | 0.093 |
| Soluble solid content | −0.068 | 0.982 | 0.040 | 0.113 | 0.126 |
| Sucrose | −0.318 | 0.884 | −0.098 | 0.312 | −0.098 |
| Fructose | −0.147 | 0.970 | −0.147 | 0.114 | 0.046 |
| Glucose | −0.470 | 0.856 | −0.068 | 0.203 | −0.02 |
| Quinic acid | 0.363 | −0.139 | −0.120 | 0.820 | 0.402 |
| Malic acid | 0.691 | −0.573 | −0.33 | 0.262 | 0.129 |
| Citric acid | −0.408 | −0.189 | 0.876 | −0.094 | −0.145 |
| Ascorbic acid | 0.278 | 0.615 | −0.089 | 0.539 | −0.495 |
| Total phenols | 0.814 | 0.060 | −0.368 | 0.100 | −0.433 |
| Total flavonoids | 0.283 | −0.045 | 0.865 | 0.079 | −0.404 |
| Treatments | Z-Value | Rank |
|---|---|---|
| T1 | −0.503 | 4 |
| T2 | 1.501 | 1 |
| T3 | 1.178 | 2 |
| T4 | −1.089 | 5 |
| T5 | 0.187 | 3 |
| CK | −1.274 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, L.; Xi, D.; Zhang, C.; He, S.; Cheng, D.; Pei, J.; Chen, J. The Effects of Two New Fertilizers on the Growth and Fruit Quality of Actinidia eriantha Benth. Agriculture 2025, 15, 982. https://doi.org/10.3390/agriculture15090982
Liu H, Li L, Xi D, Zhang C, He S, Cheng D, Pei J, Chen J. The Effects of Two New Fertilizers on the Growth and Fruit Quality of Actinidia eriantha Benth. Agriculture. 2025; 15(9):982. https://doi.org/10.3390/agriculture15090982
Chicago/Turabian StyleLiu, Hui, Lan Li, Dujun Xi, Chen Zhang, Shasha He, Dawei Cheng, Jiabo Pei, and Jinyong Chen. 2025. "The Effects of Two New Fertilizers on the Growth and Fruit Quality of Actinidia eriantha Benth" Agriculture 15, no. 9: 982. https://doi.org/10.3390/agriculture15090982
APA StyleLiu, H., Li, L., Xi, D., Zhang, C., He, S., Cheng, D., Pei, J., & Chen, J. (2025). The Effects of Two New Fertilizers on the Growth and Fruit Quality of Actinidia eriantha Benth. Agriculture, 15(9), 982. https://doi.org/10.3390/agriculture15090982





