Abstract
Restrictions on antimicrobial use in food animal production have been imposed due to concerns over residue accumulation and the development of antibiotic resistance. Thus, there is a need to find potential and safe alternatives to antimicrobials. Some of these natural alternatives include yeasts and their derivatives. Yeasts are single-cell facultative anaerobic ascomycetous eukaryotic fungi that are comprehensively incorporated into poultry nutrition for their potential beneficial effects. They are available as probiotics (whole living yeast cells) or as prebiotics (bioactive derivative components, such as mannan-oligosaccharides, β-glucans, or chitin), along with nucleotides found in distillery yeast sludge or hydrolyzed yeast. The beneficial effects of yeasts and their derivatives stem from their ability to enhance production performance, stimulate immune responses, modulate gut microbiota, and reduce oxidative stress. This review explores the potential roles of yeasts and their derivatives in poultry nutrition. Their effects on productive performance (in broilers, layers, and breeders), carcass traits, immune response, gut health, and oxidative stress are investigated.
1. Introduction
Sustainable poultry production is crucial to meet the growing protein demands of an increasing population. One of the major challenges the poultry industry faces worldwide is optimizing production efficiency. To meet this challenge, there is a growing interest in using poultry feed additives to promote the utilization of nutrients, enhance the immune system, and improve performance [1].
The past decade has seen increasingly stringent limitations on veterinary antibiotic use, implemented in response to public health concerns regarding drug residues’ accumulation in animal products and the emergence of antimicrobial resistance [2]. While functional feed ingredients show promise, they require further systematic investigation. Therefore, there is a great demand to identify safe and effective antibiotic alternatives capable of enhancing the production performance parameters and gut health and promoting the immune response in livestock. Several alternatives, including acids, probiotics, prebiotics, synbiotics, phytobiotics, paraprobiotics, and postbiotics, have been recently introduced into poultry production systems with successful results [3,4,5,6,7,8,9,10].
Yeasts and their fermentation byproducts are also potential alternatives in livestock production [11]. Yeasts are single-cell facultative anaerobic ascomycetous or basidiomycetous eukaryotic fungi [12]. They are ubiquitous in the soil and the skin of humans and animals. Approximately, there are 100 known yeast species, exhibiting remarkable diversity in their shapes and colors, along with numerous yeast derivatives or products. Yeast species are generally ellipsoid in shape, with greatly varying sizes according to the species and the environmental conditions [13]. Saccharomyces cerevisiae (S. cerevisiae), also known as baker’s yeast, and S. boulardii are the most common probiotic cultures introduced into poultry feed formulations [14,15,16,17,18].
Numerous studies have investigated the role of yeasts and their derivatives in animal and poultry nutrition [19,20,21,22]. To ensure antibiotic-free production, yeasts and their byproducts are crucial feed ingredients in the poultry production system [23]. They are used as an alternative protein source to soybean and fish meals in the poultry diet [24,25]. Moreover, they have been incorporated into poultry rations to improve the production performance, immune response, and gut health [25].
Based on the nutritional and health benefits, characteristics, and composition, yeast supplements can be broadly categorized into active dry yeast, yeast culture, and nutritional yeast. The different forms of yeasts and their derivatives are illustrated in Figure 1. Several yeast derivatives and yeast-based feed ingredients, including yeast culture, yeast-fermented products, and yeast extracts, are used [26]. Yeasts are available either in a viable form (probiotic) [27] or in a non-digestible form, including fractionated cell wall glucans and mannans (prebiotics) [18,28]. The new term “postbiotics” refers to the products or metabolic byproducts that are specifically secreted by S. cerevisiae or released after fermentation or lysis, with physiological benefits to the host [29,30]. In addition to live and dry yeasts, cell wall components (prebiotics) like mannooligosaccharides (MOSs) (mannoproteins, 40%), fructooligosaccharides (FOSs), glucooligosaccharides, β-D-glucans (glucose, 60%), and N-acetylglucosamine (chitin, 2%), distillery yeast sludge (DYS), and hydrolyzed yeast (HY), like nucleotides, vitamins, and other compounds, are the most popular yeast products [23,31,32,33,34,35]. DYS is a byproduct of the brewing industry that principally possesses S. cerevisiae, which is characterized by its high protein content. It consists of sedimented yeast cells and residual fermentation metabolites following molasses fermentation, containing proteins and macro- and micronutrients. HY is developed through an enzymatic treatment of S. cerevisiae [36], and it is rich in peptides, amino acids, glucans, mannans, and nucleotides with many benefits to the health, productivity, and immunity of poultry [37,38,39,40,41,42,43]. Yeast culture comprises yeast cells and metabolites, such as oligosaccharides, amino acids, peptides, organic acids, flavor/aroma ingredients, and unidentified growth factors. Moreover, it has phytase, an enzyme that enhances feed digestion [44]. Yeast autolysate is an important source of proteins, nucleotides, vitamins, fiber, and micronutrients [22]. It results from a degradation process by stimulating the yeast’s autolytic enzymes (nucleases and proteases) to solubilize cell wall proteins and nucleic acids. Whole-cell, inactive Candida guilliermondii or Meyerozyma guilliermondii (also known as Pichia guilliermondii (P. guilliermondii)) is a novel yeast spp. It is a citric acid byproduct, showing probiotic and prebiotic properties [45].
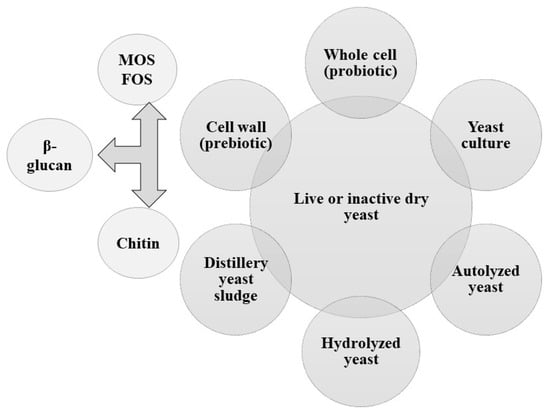
Figure 1.
The different forms of yeasts and their derivatives.
This review article aims to highlight some potential uses of yeasts and their byproducts in poultry nutrition. Their effects on the productive performance of broilers, layers, and breeders, as well as carcass traits, immune response, gut health, and oxidative stress, were investigated.
2. The Different Effects of Yeasts as Dietary Feed Additives for Poultry (Figure 2)
The different impacts of yeasts and their derivatives on the broilers, layers, and breeders are represented in Table 1 and Table 2, respectively.

Table 1.
The different impacts of yeasts and their derivatives on broilers’ production.

Table 2.
The different impacts of yeasts and their derivatives on layers and breeders’ production.
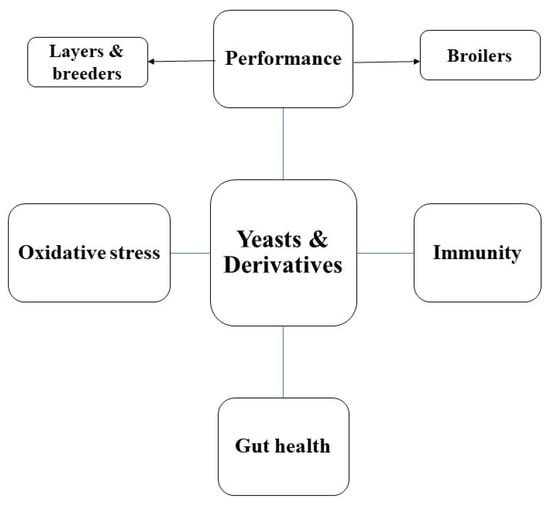
Figure 2.
The different effects of yeasts and their derivatives as dietary feed additives for poultry.
2.1. Productivity
2.1.1. Growth Performance of Broilers
The inoculation of viable or nonviable yeast cells in the diets of broilers has shown promising potential for improving performance and health [85,86,87,88,89,90] and reducing mortality [17,78,91,92] in broiler chickens. The different possible modes of action of yeasts and their derivatives in broilers are illustrated in Figure 3.
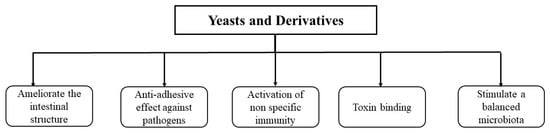
Figure 3.
The different possible modes of action of yeasts and their derivatives in broilers.
The feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR) were improved by the dietary supplementation of broiler chickens with S. cerevisiae, MOS, or HY during the starter period [68,93] or the grower period [41,51,94,95,96] of production. The dietary supplementation with yeast cell wall improved the FCR of broiler chickens at day 44 [35] and Eimeria spp.-infected broilers [77,97]. An average 5.4% increase in BWG and a 2% decrease in FCR were reported in broilers fed on MOS due to improved digestion and absorption [98]. In addition, Hooge and Connolly [99] found that the dietary incorporation of MOS improved BWG by 1.48% and FCR by 2.11% and decreased mortality by 0.76% in broiler chickens. MOSs are rich in mannans and glucans (30% for each) and mannoproteins (12.9%), which have beneficial influences on the growth of broiler chicks. The dietary addition of HY or autolyzed yeast (AY) ameliorates the growth performance parameters in broiler chickens [38,39,43,67,68,100,101,102,103] and quails [104]. Generally, yeasts have been reported to improve the multiplication of helpful intestinal microbiota, activate the innate immune response, stimulate digestive enzyme production and activity, and compete with pathogens for adhesion sites in the gut, thus preventing their multiplication in the intestine.
Yeasts and their derivatives play a key role in ameliorating the intestinal structures and modulating the gut microbiota, which directly contributes to enhanced nutrient utilization and growth performance in broilers [20,56]. The benefits of using yeasts to improve the productivity of broilers may be associated with the presence of glucans and MOSs that modulate the intestinal microbiome and increase the production of short-chain fatty acids, which act as a source of energy for the enterocytes [96]. Yeast cells could stimulate the brush border disaccharidases, including lactase, maltase, and sucrose, in the host gut [105]. The metabolic products of yeast culture could act synergistically with phytase to increase nutrient utilization, decrease phytate content, and, finally, improve growth performance [22]. Moreover, S. cerevisiae could enhance the bioavailability of nutrients by increasing the production of lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, and creatine phosphokinase [106].
Yeast may reduce the microbial population and promote the gut’s nutrient retention and digestibility by decreasing the pH to the optimum range and improving the intestinal health and broilers’ performance status [44]. The supplementation with yeast cell wall has a trophic effect on gut health, with major effects on the digestion, absorption, and metabolism of nutrients, which may positively improve growth performance. M’Sadeq [77] showed improved jejunal histomorphology in yeast cell wall-treated broilers. Moreover, the yeast supplementation may be linked with the reduced energy partitioning toward tissue turnover [107,108,109]. Yeasts may protect the villus structure by beneficially modifying the intestinal microbiota. Yeast supplementation may increase villus height (VH) by activating the growth of anaerobic and cellulolytic bacteria, which enhances the utilization of lactate and sustains a satisfactory intestinal pH, thereby leading to better nutrient retention, digestibility, and growth [110,111]. The microbial flora can promote lactate production and gut utilization, hence keeping a favorable pH of the intestine for the multiplication of their type; however, the unwanted microbial species are unable to maintain themselves. Jazi et al. [112] demonstrated that supplementation of Salmonella typhimurium (S. typhimurium)-infected broiler chickens with MOS mitigated the adverse effects of infection and promoted broiler performance. Similar results were obtained in broiler chickens fed on MOS and challenged with Clostridium perfringens (C. perfringens) [113].
The presence of vitamin B complex, proteins, and minerals in yeast has beneficial effects on the FI of birds. In addition, yeast can maintain a balanced intestinal microbiota that helps maintain the integrity of a healthy gut, which indirectly stimulates FI [114]. Improving the anti-inflammatory responses [115], immunomodulatory effects, and antioxidant properties is another possible mechanism of yeast actions [116].
Sedghi et al. [117] observed no effect of yeast cell wall on the broilers’ performance. A significant reduction in BWG was reported in broilers fed on diets treated with different levels of S. cerevisiae [47,76,78,118]. Also, no effects on the performance parameters were detected after supplementation of broiler chickens and turkeys with S. cerevisiae [66,74,119]. The variability in research findings may be attributed to differences in the breed of bird used, ingredient/diet composition, form or type, and the level of yeast product inclusion in poultry diets.
2.1.2. Production Performance with Layers and Breeders
The dietary supplementation of layers with DYS has shown beneficial effects on overall health and performance [120,121,122]. Also, the dietary addition of HY or AY ameliorates egg production and egg weight in layers [40,123,124,125]. Shashidhara and Devegowda [126] demonstrated that supplementing male breeder flocks with MOSs improved sperm density, hatchability, and maternal antibody titers in progeny. The inclusion of AY in laying hens’ feed increased the total saturated fatty acids and the ratio of saturated/unsaturated fatty acids but decreased the total monounsaturated fatty acids and egg-yolk-cholesterol content [81,124]. Moreover, the supplementation of a probiotic containing P. guilliermondii reduced ammonia production by 46% in layer hens [127]. HY positively affected the body weight at sexual maturity and the development of lymphoid organs in response to the E. coli lipopolysaccharide in broiler breeder pullets [128]. Özsoy et al. [83] detected modulation in the yolk fatty acid composition, especially in the C18:2 n6 content, following dietary feeding on S. cerevisiae. In breeders, the different forms of yeast products can promote gut health and improve feed utilization, which consequently increases the nutrients’ delivery for an embryo’s development and improves the BWG and FCR in hatched chicks [68,101].
2.2. Carcass Traits
The dietary supplementation with yeasts improved the carcass characteristics in broilers [129]. Similarly, AY increased the slaughter weight and the hot and cold carcass weights of quails [104], while it decreased the relative abdominal-fat weight in chickens [93,130]. It has been reported that chitin and β-glucans [131] have hypolipidemic factors that can reduce the amount of body fat in broilers. Moreover, Aristides et al. [61] found that reduced muscle pH and lipid oxidation followed dietary administration of yeast culture. Similarly, Hoque et al. [132] demonstrated no effect of the yeast culture on the pH values of the breast muscles, muscle color parameters (brightness and intensity of yellow and red), water-holding capacity, cooking loss, and shear force of broiler meat. The observed positive effects of the yeast culture on the slaughtering performance and the product quality could be reconducted to ameliorating the gut environment, which leads to better feed utilization and nutrient delivery and enhances the immune response [32,61,133,134].
However, other studies revealed that the administration of a yeast culture induced no effect on the carcass yields in broiler chickens [54,135,136] or turkeys [52]. Also, Ullah et al. [67] could not find any significant contribution of AY to the slaughter performance. These discrepancies in the results could be attributed to many factors, including breed, age, diet composition, environmental conditions, and the duration of the experiment.
2.3. Immune Response
The different types of immune responses were enhanced in birds following supplementation with yeasts or their derivatives, such as MOS, β-glucan, DYS, yeast autolysate, and yeast protein concentrate [81,137,138]. In layers, diets supplemented with yeast autolysate boosted the immune responses and antibody titers [81]. A dietary blend of probiotics, yeast, vitamin E, and vitamin C improved the immune response of laying hens in a high-temperature environment [139].
S. cerevisiae could produce β-glucans or PGG-glucans (betafectin), which can promote the immune response via stimulating white blood cell propagation and function. In addition, S. cerevisiae can promote immunity via the stimulation of neutrophils/heterophils, monocytes, macrophages, and dendritic cells. MOSs and β-glucans stimulate the humoral immune response and, hence, increase the levels of immunoglobulin (Ig)A and IgG [140]. A diet incorporated with a yeast-derived prebiotic (inulin) could increase the expression of IgA in the cecal and mucin mRNA in the jejunum tissues of broiler chickens [141].
Yeast cell walls (mannans) can stimulate the cell-mediated [142] and mucosal immune systems [143] of poultry. Moreover, it can promote innate immune cells, such as macrophages and dendritic cells, and support the production and regulation of cytokines [144], such as interleukin (IL-4) and IL-10 [145], as well as their expression [146]. Therefore, yeast-derived prebiotics could improve the immune system by increasing the expression of anti-inflammatory cytokines but decreasing the expression of pro-inflammatory cytokines. Saccharomyces spp. could attach to receptors on the cell surfaces of monocytes, macrophages, and dendritic cells to upregulate the expression of toll-like receptors and modulate cytokine secretion [147]. Feeding broilers a diet fortified with 0.2% MOS enhanced the expression of cytokines, such as IL-12 and interferon-γ (IFN-γ) [148]. Also, β-glucans in yeast cell walls have been shown to activate the Th1 pathway in resting cells, as evidenced by increased IFN-γ production [149]. Yeast cell wall polysaccharides (glucans, zymosans, and carboxymethylglucan) increase the number and functions of intraepithelial lymphocytes in the gut’s lamina propria. These polysaccharides bind to specific receptors, such as Dectin-1 and CR3, on macrophages, dendritic cells, monocytes, and granulocytes, stimulating the immune response of the gut mucosa [149]. Macrophages have receptors (CR3) for β-1, 3–1, 6-branched glucans. By recognizing specific sugars found in glycoproteins on the epithelial surface, prebiotics would bind to macrophage reception sites, causing a cascading reaction that would activate macrophages and release cytokines, thus triggering an acquired immune response and causing higher antibody responses to antigens [150].
β-Glucans can stimulate the innate and adaptive immunity, activate and promote the phagocytic activity of antigen-presenting cells such as macrophages and dendritic cells [151,152], elevate cytokine and chemokine levels, and enhance oxidative bursts in macrophages, neutrophils, and dendritic cells [140,153]. Moreover, both oligo-mannans and yeast β-glucans can enhance the circulation of new immune cells, increase the number of goblet cells and the production of mucin-2, trigger heightened expression of intestinal tight junctions, and act as anti-inflammatory immunomodulators in poultry [154,155,156].
In the case of bacterial infections, HY showed positive influences on the immune response of broilers against a C. perfringens challenge [157], which may be attributed to the richness of HY with β-glucans and mannans [158] and may potentially have affected the immune system [159]. Purified β-glucan has also been shown to enhance the innate immune system in S. enteritidis-challenged broilers [160]. Similarly, adding MOS to broiler diets promoted the immune response by increasing cytokine mRNA expression and reducing intestinal C. perfringens and Escherichia coli (E. coli) populations [148,161]. The administration of yeast cell walls stimulates the gut microflora and competes with pathogens by competing for nutrients, antimicrobial metabolite production, or adhering to receptor sites that pathogens otherwise would occupy [162]. Moreover, yeast cell walls contain mannans and β-1, 3–1, 6-glucans that can act as microbial molecular templates and modulate the expression of recognition receptors [163]. The dietary inoculation with S. cerevisiae increased the secretion of local mucosal IgA in broilers [97]. Additionally, yeast extracts can enhance the immune response indirectly via changing the population and composition of gut microbiota or probiotics [164,165]. Yeast inoculation in the broiler diet also showed promise in mediating heat shock responses in the epithelial barrier by suppressing the migration of bacterial metabolites and increasing the production of pro-inflammatory cytokines, such as IL-6, which may stimulate the hypothalamic–pituitary–adrenal axis. The small size of P. guilliermondii enables it to chelate the enteric pathogens and modulate the host immunity [166].
The treatment of Eimeria spp.-infected broilers by inactive whole yeast cell products enhanced the performance and nitric oxide production by splenic macrophages, upregulated IL-1 mRNA expression, reduced oocyst shedding, and improved gut architecture [167]. Inflammatory cytokines (IL-1) and macrophage nitric oxide production were increased in broiler chickens fed yeast cell walls, which can be expected to diminish the pathogenesis of Eimeria spp. infection [117,167,168]. Gomez-Verduzco et al. [97] reported that the yeast cell walls can modulate the immune system via increasing humoral and cellular immune responses, decreasing Eimeria spp. shedding in challenged chickens, and enhancing the secretion of mucosal IgA.
The better immune response of supplementing yeast-based products in poultry diets on serum antibodies against viral diseases is due to the beneficial effects of mannans, glucans, and nucleotide contents of yeast [44,47,126,169,170]. Yeasts and their derivatives boosted the antibody titers in terms of cellular, humoral, and mucosal immune response of broilers [171,172], resulting in a better response to vaccination. Broiler chickens fed diets containing xylooligosaccharides showed a higher antibody titer against the highly pathogenic avian influenza virus (AIV H5N1) [173]. Similar results were observed in broilers supplemented with MOS and vaccinated with the AIV (H9) vaccine [174]. In addition, β-glucans enhanced the immune response to the Newcastle disease virus (NDV) vaccine and changed mRNA expression in the spleen of chickens [155,156]. Similarly, El-Manawey et al. [73] reported an increase in the titers against NDV in broilers fed a mixture of S. cerevisiae, yeast cell walls, and yeast extracts. In contrast, the supplementation of yeast could not improve the immunological status of birds against NDV and infectious bursal disease [175], which might be attributed to the adverse surrounding conditions of the experiments and the different doses of inoculated yeast in the diets.
A significant increase in the relative bursa of Fabricius weights was observed in broilers being fed a diet containing yeast powder [47] or Kluyveromyces marxianus [176]. The dietary β-glucans increased the weight of the lymphoid organs and the production of plasma globulin, cytokines, IgA, and IgG in broilers [177]. In addition, β-glucans produced by Schizophyllum commune significantly increased the relative weight of the bursa of Fabricius [165], thus improving the immune response.
2.4. Gut Health
Yeasts have shown promise in maintaining gut health and modulating the intestinal microflora in poultry [26,85,178,179]. Prebiotics are administered in ovo before hatching to increase the gut microbiota population, improve immunity, and, therefore, reduce the need for antibiotics [144]. In addition, the functionality of yeast bioactives as growth promoters makes them attractive for reducing the antimicrobial dependence in poultry production [180]. The supplementation of diets with S. cerevisiae has proved useful in modulating the birds’ intestinal microflora to inhibit the colonization of the gut by bacterial pathogens [118,181]. Modulation of a bird’s gut health by the inclusion of yeast cell walls (MOS and FOS) is mediated by promoting intestinal microbial diversity via favoring the growth of beneficial microbes while inhibiting pathogenic microbial populations and enhancing the intestinal microbial diversity with positive influences on the gut and overall host health [13,32,51,118,182].
Yeast cell wall-derived mannose and MOS act as decoy receptors for the binding of lectins in bacteria and prevent pathogen colonization [183]. Therefore, yeasts containing prebiotics are able to inhibit colonization of the poultry gut and reduce the competition between pathogens and host cells for nutrients [184]. Saccharomyces spp. can produce acetic acid, which has an inhibitory effect on E. coli [185]. In addition, S. boulardii produces fatty acids, like capric acid, caprylic acid, caproic acid, and 2-phenylethanol [186], polyamines [187], and antimicrobial peptides, such as leucocin C [188]. Therefore, S. boulardii can inhibit the growth of C. perfringens [189], S. typhimurium [190], Campylobacter jejuni (C. jejuni) [191,192], and Listeria [186]. The fermentation products, short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, can change the bacterial ecosystem by decreasing the pH, which is intolerant to pathogenic enteric bacteria. Furthermore, they offer energy to the gut and may effectively substitute for antibiotics. Diets incorporated with prebiotics showed increased SCFAs and antibody production [193] in broilers with necrotic enteritis [95]. Moreover, SCFAs are included to regulate cell proliferation in the gut mucosa [169,194]. They reduce the pH of the brush border microenvironment and block the adhesion of pathogenic bacteria [163]. Yeasts have a role in diminishing the gut pH through the production of different organic acids, resulting in an acidic intestinal environment, the crucial inhibition of pathogenic bacterial growth, and increased abundance of beneficial bacteria [21,195,196].
Adding MOS to broilers’ diets reduced the populations of intestinal C. perfringens and E. coli [197,198], as well as Salmonella spp. [199,200]. Mannans and MOSs inhibit the intestinal epithelium attachment of enteric pathogens expressing type-I fimbriae, such as C. perfringens [95,201], Salmonella spp. [183], E. coli [202], and C. jejuni [191]. MOSs can mediate the mass of goblet cells in the villus membrane of the intestinal epithelium [179,203], which is very specialized for synthesizing glycoprotein mucin, which blocks the pathogens at the entry portal [204]. Additionally, mucin has a specific mannosyl-containing receptor, which competitively binds with imH of type-1 fimbriae in Gram-negative bacteria and, thus, helps to eliminate pathogens from the host intestine. This results in the passage of bacteria through the intestine without being colonized [199,203,205]. During feed fermentation, the beneficial bacteria, which yield lactic acid or acetic acid, reduce the number and inhibit the proliferation of putrefactive and pathogenic bacteria, such as Clostridia, Campylobacter, and Salmonella spp. [32,196,198,206]. Further, prebiotics (MOS or FOS) favor the proliferation and increase the abundance of beneficial endogenous gut microflora, such as Lactobacillus crispatus, Lactobacillus salivarius, and Bifidobacteria, which prevent the adhesion and colonization of Salmonella [207] and Eimeria spp. [208]. In addition, yeast derivatives, such as MOS, can enhance the population of beneficial Bifidobacteria by enhancing the synthesis of mucin from epithelial goblet cells. Bifidobacteria depend on glycoprotein mucin, which is produced by goblet cells via secreting the 1,2-α-L-fucosidase and endo-α-N-acetylgalactosaminidase [209]. These beneficial bacteria can compete against pathogenic types for space and resources and diminish pathogenic load by a competitive exclusion mechanism [210]. Moreover, Lactobacilli are regarded as beneficial lactic acid-producing bacteria that produce bacteriocins [211], whereas Bifidobacteria secrete some organic acids and bactericidal substances with a bactericidal effect [212]. Maina et al. [43] reported that diets containing HY tended to have higher levels of acetic acid in the caecum of chickens compared to the control diet, which is likely attributed to the bioactive compounds in HY acting as prebiotics. These compounds improve the beneficial cecal bacterial proliferation involved in acetic acid fermentation. Moreover, the enhanced nutrient absorption and gut health associated with HY contribute to the increased fermentable substrates available for these bacteria, thus supporting further acetic acid production in the ceca.
Yeast cells may inhibit the binding of pathogens to enterocytes by steric hindrance due to their larger size. In addition, increasing lysozyme production mediated by yeast cultures can break the polysaccharide wall of different bacterial types, which protects the poultry from diseases [44]. The in vitro study of Cardozo et al. [166] showed that P. guilliermondii exhibits higher aggregation of S. enterica and E. coli compared to S. cerevisiae and hydrolyzed S. cerevisiae. Additionally, P. guilliermondii showed in vitro anticoccidial infection, especially against Eimeria tenella (E. tenella), E. acervulina, E. maxima, and E. praecox [213]. The secreted proteins of P. guilliermondii disturb the wall of Eimeria oocysts, causing the release of sporocysts and, hence, preventing coccidiosis. Moreover, S. cerevisiae cell walls (MOSs and glucans) can bind mycotoxins in poultry feed and reduce their toxic effects and improve digestion and absorption [214,215]. Overall, the yeast cell wall of MOS or β-glucan components can decrease the colonizing and adhering of pathogens in the intestine and increase intestinal goblet cells, thus improving the intestinal morphology and growth performance [216].
The modulation of epithelial microarchitecture and the enhancement of colonization of lactic acid-producing bacteria in the intestinal tract of broilers are some mechanisms by which yeasts can maintain gut health [88,217]. The dietary incorporation of live yeast has revealed modulation of the gut morphology, as increased ileal villus width and surface area were reported in supplemented chickens [218]. Increasing the VH and crypt depth (CD) increased the diffusion pathway and the surface area available for microbial activity, which, in turn, improved the nutrient absorption [44,219,220]. Likewise, broilers fed a basal diet containing yeast protein concentrate pellets efficiently developed more VH [118]. Yeasts have been shown to enhance ileal epithelial tissue maturation, just like bacitracin methylene disalicylate, in broilers (Fasina and Thanissery, 2011) [111]. A nucleotide-rich yeast extract ameliorated the destructive effects of Eimeria spp. infection in terms of improved BWG, FCR, and VH in broilers [221]. These modulations in gut tissues were mediated by less cell renewal and turnover, leading to lower energy expenditure for intestinal epithelial maintenance. Moreover, longer villi secrete more enzyme, which accelerates the nutrient digestion and absorption [222]. Hussein et al. [223] reported that a coccidial challenge reduced the VH and VH:CD ratio and increased the CD ratio, villus base, tip width, and jejunum muscle thickness, which were ameliorated by the treatment with a probiotic yeast. The higher villous base and tip width caused an increased surface area. Similar results were obtained by Sedghi et al. [117], who noticed that yeast cell walls improved the VH, CD, and VH:CD ratio in broiler chickens. Also, Alkhulaifi et al. [224] demonstrated that chickens fed yeast cell wall diets had increased VH and better intestinal health under the C. perfringens and S. typhimurium challenges. The dietary supplementation with nucleotide-rich yeast alleviated the lesion scores, improved the intestinal morphology and barrier function, and increased intestinal IgA production in C. perfringens-challenged broiler chickens [225].
2.5. Oxidative Stress
Yeast cells and their derivatives can reduce the oxidative stress in poultry by improving the activities of antioxidant enzymes and reducing the reactive oxygen species and lipid peroxidation production. β-Glucan is a highly potent free radical scavenger [226]. In addition, yeast cells and their wall components can stimulate the production of superoxides by macrophages and neutrophils and increase their phagocytic activity [227]. Additionally, yeast cells can upregulate the expression of enzymatic and non-enzymatic antioxidants, including superoxide dismutase and glutathione, respectively, thus preventing damage to the structure and function of cell membranes, proteins, and nucleic acids [140]. Al-Afifi et al. [106] demonstrated that incorporating S. cerevisiae into broiler diets improved the activity of glutathione peroxidase and superoxide dismutase enzymes in poultry. In addition, HY may show antioxidative properties [36], which may play a role in removing free radicals, potentially even in the gut, when administered orally. Therefore, controlling oxidative stress using yeasts or their derivatives may have a direct positive effect on poultry production parameters.
3. Conclusions
Yeasts and their derivatives are increasingly recognized as valuable feed additives in poultry nutrition. Dietary yeasts have demonstrated significant benefits, such as improving growth performance parameters in broilers, layers, and breeders and carcass characteristics; boosting immunity; promoting gut microbiome, development, integrity, and nutrient absorption/utilization; and enhancing the antioxidant status in poultry. The diverse and potential biological properties of yeasts or byproducts make them auspicious natural feed additives to replace frequently used antimicrobial growth promoters. This review article provides insights into the multiple functional roles of yeasts and their derivatives in poultry nutrition and highlights their significance as a viable substitute for feeding antibiotics in the poultry feed industry.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares that there are no conflicts of interest regarding the publication of this paper.
References
- Abd El-Ghany, W.A.; Abdel-Latif, M.A.; Hosny, F.; Alatfeehy, N.M.; Noreldin, A.E.; Quesnell, R.R.; Chapman, R.; Sakai, L.; Elbestawy, A.R. Comparative efficacy of postbiotic, probiotic, and antibiotic against necrotic enteritis in broiler chickens. Poult. Sci. 2022, 101, 101988. [Google Scholar] [CrossRef]
- Abou-Jaoudeh, C.; Andary, J.; Abou-Khalil, R. Antibiotic residues in poultry products and bacterial resistance: A review in developing countries. J. Infect. Public Health 2024, 17, 102592. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A.; Babazadeh, D. Betaine: A potential nutritional metabolite in the poultry industry. Animals 2022, 12, 2624. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Chitosan: A promising natural polysaccharide feed additive in poultry production systems. Iran. J. Vet. Res. 2023, 24, 301–312. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Applications of organic acids in poultry production: An updated and comprehensive review. Agriculture 2024, 14, 1756. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. The influence of the phytobiotic tulsi (Ocimum sanctum) on broiler production and health. Vet. Stanica 2024, 55, 221–230. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. The impact of dietary guava (Psidium guajava L.) on some livestock production system. CABI Rev. 2024, 19, 1–7. [Google Scholar] [CrossRef]
- Salahi, A.; Abd El-Ghany, W.A. Beyond probiotics, uses of their next-generation for poultry and humans: A review. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1336–1347. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. The different influences of a phytobiotic, green tea (Camellia sinensis L.) on the poultry health and production. J. Appl. Vet. Sci. 2025, 10, 32–45. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. The impact of using bee pollen in poultry systems: Apiculture. Vet. Stanica 2025, 56, 671. [Google Scholar] [CrossRef]
- Fathima, S.; Shanmugasundaram, R.; Sifri, M.; Selvaraj, R. Yeasts and yeast-based products in poultry nutrition. J. Appl. Poult. Res. 2023, 32, 100345. [Google Scholar] [CrossRef]
- Bennett, J.W. Mycotechnology: The role of fungi in biotechnology. J. Biotechnol. 1998, 66, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, O.; Makanjuola, B.; Bankole, T.; Adeyori, A. Yeast culture (Saccharomyces cerevisae) supplementation: Effect on the performance and gut morphology of broiler birds. Glob. J. Sci. Front. Res. Biol. Sci. 2012, 12, 25–29. [Google Scholar]
- Fietto, J.L.; Araujo, R.S.; Valadao, F.N.; Fietto, L.G.; Brandao, R.L.; Neves, M.J.; Gomes, F.C.; Nicoli, J.R.; Castro, I.M. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Cand. J. Microbiol. 2004, 50, 615–621. [Google Scholar] [CrossRef]
- Deraz, S.F. Synergetic effects of multispecies probiotic supplementation on certain blood parameters and serum biochemical profile of broiler chickens. J. Vet. Med. Anim. Health 2018, 6, 27–34. [Google Scholar] [CrossRef]
- Feye, K.M.; Carroll, J.P.; Anderson, K.L.; Whittaker, J.H.; Schmidt-McCormack, G.R.; McIntyre, D.R.; Pavlidis, H.O.; Carlson, S.A. Saccharomyces cerevisiae fermentation products that mitigate foodborne Salmonella in cattle and poultry. Front. Vet. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Mulatu, K.; Ameha, N.; Girma, M. Effects of feeding different levels of baker’s yeast on performance and hematological parameters in broiler chickens. J. World Poult. Res. 2019, 9, 38–49. [Google Scholar] [CrossRef]
- Fornazier, R.; Ribeiro Junior, V.; Albino, L.F.T.; Tavernari, F.C.; Feddern, V.; Silva, D.L.; Serafini, S.; Petrolli, T.G.; Paiano, D.; Calderano, A.A.; et al. Prebiotic composed of yeast (Saccharomyces cerevisiae) cell wall improves performance in broiler diets. Rev. Bras. Zootec. 2024, 53, e20230162. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A.; Awaad, M.H.; Nagwa, S.R. Efficacy of certain feed additives for the prevention of Campylobacter jejuni infection in broiler chickens. Asian J. Anim. Sci. 2015, 9, 427–433. [Google Scholar] [CrossRef]
- Shurson, G.C. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Okoro, V.M.; Mbajiorgu, E.F.; Mbajiorgu, C.A. Yeast (Saccharomyces cerevisiae) and its effect on production indices of livestock and poultry—A review. Comp. Clin. Pathol. 2019, 28, 669–677. [Google Scholar] [CrossRef]
- Perricone, V.; Sandrini, S.; Irshad, N.; Savoini, G.; Comi, M.; Agazzi, A. Yeast-derived products: The role of hydrolyzed yeast and yeast culture in poultry nutrition—A review. Animals 2022, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Bilal, R.M.; Hassan, F.U.; Saeed, M.; Rafeeq, M.; Zahra, N.; Fraz, A.; Saeed, S.; Khan, M.A.; Mahgoub, H.A.; Farag, M.R.; et al. Role of yeast and yeast-derived products as feed additives in broiler nutrition. Anim. Biotechnol. 2021, 34, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Chand, N.; Ihsanuddin, N.; Khan, R.U. Replacement of soybean meal with yeast single cell protein in broiler ration: The effect on performance traits. Pak. J. Zool. 2014, 46, 1753–1758. [Google Scholar]
- Lee, J.; Goo, D.; Kumar Sharma, M.; Ko, H.; Shi, H.; Paneru, D.; Sesha, V.; Choppa, R.; Liu, G.; Kyun Kim, W. Effects of graded yeast cell wall supplementation on growth performance, immunity and intestinal development of broiler chickens raised in floor pens for 42 days. Poult. Sci. 2025, 104, 104695. [Google Scholar] [CrossRef]
- Zhang, A.W.; Lee, B.D.; Lee, S.K.; Lee, K.W.; An, G.H.; Song, K.B.; Lee, C.H. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci. 2005, 84, 1015–1021. [Google Scholar] [CrossRef]
- Vohra, A.; Syal, P.; Madan, A. Probiotic yeasts in livestock sector. Anim. Feed Sci. Technol. 2016, 219, 31–47. [Google Scholar] [CrossRef]
- Leone, F.; Ferrante, V. Effects of prebiotics and precision biotics on performance, animal welfare and environmental impact. A review. Sci. Total Environ. 2023, 901, 165951. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Paraprobiotics and postbiotics: Contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Vet. J. 2020, 10, 323–330. [Google Scholar] [CrossRef]
- Saeed, M.; Afzal, Z.; Afzal, F.; Khan, R.U.; Elnesr, S.S.; Alagawany, M.; Chen, H. Use of postbiotic as growth promoter in poultry industry: A review of current knowledge and future prospects. Food Sci. Anim. Resour. 2023, 43, 1111–1127. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Iji, P.; Choct, M. Dietary modulation of gut microflora in broiler chickens: A review of the role of six kinds of alternatives to in-feed antibiotics. Poult. Sci. J. 2009, 65, 97–114. [Google Scholar] [CrossRef]
- Araujo, L.F.; Bonato, M.; Barbalho, R.; Araujo, C.S.S.; Zorzetto, P.S.; Granghelli, C.A.; Pereira, R.J.G.; Kawaoku, A.J.T. Evaluating hydrolyzed yeast in the diet of broiler breeder hens. J. Appl. Poult. Res. 2018, 27, 65–70. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Bgatova, N.P.; Vetvicka, V. Glucan and Mannan—Two peas in a pod. Int. J. Mol. Sci. 2019, 20, 3189. [Google Scholar] [CrossRef]
- Pascual, A.; Pauletto, M.; Giantin, M.; Radaelli, G.; Ballarin, C.; Birolo, M.; Zomeno, C.; Dacasto, M.; Bortoletti, M.; Vascellari, M. Effect of dietary supplementation with yeast cell wall extracts on performance and gut response in broiler chickens. J. Anim. Sci. Biotechnol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar] [CrossRef]
- Walker, G.K.; Jalukar, S.; Brake, J. The effect of refined functional carbohydrates from enzymatically hydrolyzed yeast on the transmission of environmental Salmonella senftenberg among broilers and proliferation in broiler housing. Poult. Sci. 2018, 97, 1412–1419. [Google Scholar] [CrossRef]
- Kiarie, E.G.; Mohammadigheisar, M.; Schulze, H. Effects of early feeding of enzymatically treated yeast on growth performance, organ weights, intestinal histomorphology, and ceca microbial metabolites in broiler chickens subjected to Eimeria challenge. Poult. Sci. 2022, 101, 101967. [Google Scholar] [CrossRef]
- Alagbe, E.O.; Schulze, H.; Adeola, O. Growth performance, nutrient digestibility, intestinal morphology, cecal mucosal cytokines and serum antioxidant responses of broiler chickens to dietary enzymatically treated yeast and coccidia challenge. J. Anim. Sci. Biotechnol. 2023, 14, 57. [Google Scholar] [CrossRef]
- De Cloet, C.A.; Maina, A.N.; Schulze, H.; Bédécarrats, G.Y.; Kiarie, E.G. Egg production, egg quality, organ weight, bone ash and plasma metabolites in 30-week-old Lohmann LSL lite hens fed corn and soybean meal-based diets supplemented with enzymatically treated yeast. Poult. Sci. 2023, 102, 102527. [Google Scholar] [CrossRef]
- Lin, J.; Comi, M.; Vera, P.; Alessandro, A.; Qiu, K.; Wang, J.; Wu, S.G.; Qi, G.H.; Zhang, H.J. Effects of Saccharomyces cerevisiae hydrolysate on growth performance, immunity function, and intestinal health in broilers. Poult. Sci. 2023, 102, 102237. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Rogiewicz, A.; Kiarie, E.G.; Slominski, B.A. Yeast derivatives as a source of bioactive components in animal nutrition: A brief review. Front. Vet. Sci. 2023, 9, 1949. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Schulze, H.; Kiarie, E.G. Response of broiler breeder pullets when fed hydrolyzed whole yeast from placement to 22 wk of age. Poult. Sci. 2024, 103, 103383. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, H.J.; Yu, S.H.; Wu, S.G.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef]
- Bass, B.E.; Tsai, T.; Yang, H.; Perez, V.; Holzgraefe, D.; Chewning, J.; Frank, J.W.; Maxwell, C.V. Influence of a whole yeast product (Pichia guilliermondii) fed throughout gestation and lactation on performance and immune parameters of the sow and litter. J. Anim. Sci. 2019, 97, 1671–1678. [Google Scholar] [CrossRef]
- Rameshwari, K.S.; Karthikeyan, S. Distillery yeast sludge (DYS) as an alternative feed resource in poultry. Int. J. Poult. Sci. 2005, 4, 787–789. [Google Scholar] [CrossRef]
- Gheisari, A.; Kholeghipour, B. Effect of dietary inclusion of live yeast (Saccharomyces cerevisiae) on growth performance, immune responses and blood parameters of broiler chickens. In Proceedings of the XII European Poultry Conference, Verona, Italy, 10–14 September 2006; p. 6. [Google Scholar]
- An, B.K.; Cho, B.L.; You, S.J.; Paik, H.D.; Chang, H.I.; Kim, S.W.; Yun, C.W.; Kang, C.W. Growth performance and antibody response of broiler chicks fed yeast derived b-glucan and single-strain probiotics. Asian Australas. J. Anim. Sci. 2008, 21, 1027–1032. [Google Scholar] [CrossRef]
- Paryad, A.; Mahmoudi, M. Effect of different levels of supplemental yeast (Saccharomyces cerevisiae) on performance, blood constituents and carcass characteristics of broiler chicks. Afr. J. Agric. Res. 2008, 3, 835–842. [Google Scholar]
- Cox, C.; Sumners, L.; Kim, S.; Mcelroy, A.; Bedford, M.; Dalloul, R. Immune responses to dietary beta-glucan in broiler chicks during an Eimeria challenge. Poult. Sci. 2010, 89, 2597–2607. [Google Scholar] [CrossRef]
- Koc, F.; Samli, H.; Oku, A.; Ozduven, M.; Akyurek, H.; Senkoylu, N. Effects of Sacchromyces cerevesiae and/or Mannonoligosaccharides on performance, blood parameters and intestinal microbiota of broilers chicks. Bulg. J. Agric. Sci. 2010, 16, 643–650. [Google Scholar]
- Özsoy, B.; Yalcin, S. The effects of dietary supplementation of yeast culture on performance, blood parameters and immune system in broiler turkeys. Ankara Univ. Vet. Fak. Derg. 2011, 58, 117–122. [Google Scholar] [CrossRef]
- Mujahid, H.; Hashmi, A.; Anjum, A.; Waris, A.; Tipu, Y. Detoxification potential of ochratoxin by yeast sludge and evaluation in broiler chicks. J. Anim. Plant Sci. 2012, 22, 601–604. [Google Scholar]
- Abdelrahman, M.M. Effects of feeding dry fat and yeast culture on broiler chicken performance. Turk. J. Vet. Anim. Sci. 2013, 37, 31–37. [Google Scholar] [CrossRef]
- Bai, S.P.; Wu, A.M.; Ding, X.M.; Lei, Y.; Bai, J.; Zhang, K.Y.; Chio, J.S. Effects of probiotic supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013, 92, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lea, H.; Spring, P.; Pickard, J.T.; Burton, E. A natural carbohydrate fraction Actigen™ from Saccharomyces cerevisiae cell wall: Effects on goblet cells, gut morphology and performance of broiler chickens. J. Appl. Anim. Nutr. 2013, 1, e9. [Google Scholar] [CrossRef]
- Swamy, M.N.; Upendra, H.A. Growth performance, crude protein, ether extract and total ash in the breast muscle of broiler chickens supplemented with probiotics. Int. J. Sci. Environ. Technol. 2013, 2, 1000–1007. [Google Scholar]
- Biswas, A.; Mohan, N.; Raza, M.; Mir, N.A.; Mandal, A. Production performance, immune response and blood biochemical parameters in broiler chickens fed diet incorporated with prebiotics. J. Anim. Physiol. Anim. Nutr. 2014, 103, 493–500. [Google Scholar] [CrossRef]
- Sultan, A.; Uddin, I.; Khan, S.; Khan, N.A.; Khan, R.U. Effect of yeast derived carbohydrate fraction on growth performance, apparent metabolizable energy, mineral retention and gut histomorphology of broilers during starter phase. Pak. Vet. J. 2015, 35, 4. [Google Scholar]
- Chand, N.; Faheem, H.; Khan, R.U.; Qureshi, M.S.; Alhidary, I.A.; Abudabos, A.M. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ. Sci. Pollut. Res. Int. 2016, 23, 14414–14421. [Google Scholar] [CrossRef]
- Aristides, L.G.A.; Venancio, E.J.; Alfieri, A.A.; Otonel, R.A.A.; Frank, W.J.; Oba, A. Carcass characteristics and meat quality of broilers fed with different levels of Saccharomyces cerevisiae fermentation product. Poult. Sci. 2018, 97, 3337–3342. [Google Scholar] [CrossRef]
- Wulandari, S.; Syahniar, T.M. The effect of adding pro biotic Saccharomyces cerevisiae on dietary antibiotic free on production performance and intestinal lactic acid bacteria growth of broiler chicken. IOP Conf. Ser. Earth Environ. Sci. 2018, 207, 012034. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, J.; Wang, X.; Zhang, L.; Sun, D.; Xing, Q.; Pirone, A.; Fronte, B. Effects of dietary yeast beta-1,3-1,6-glucan on growth performance, intestinal morphology and chosen immunity parameters changes in Haidong chicks. Asian Australas. J. Anim. Sci. 2019, 32, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, S.P.; Chandra, G.; Sahu, D.S.; Kumar, R.; Maurya, P.S.; Ranjan, K. Effect of dietary supplementation of yeast (Saccharomyces cerevisiae) on performance and hemato-biochemical status of broilers. Ind. J. Poult. Sci. 2019, 54, 15–19. [Google Scholar] [CrossRef]
- Sousa, R.F.; Dourado, L.R.B.; Lopes, J.B.; Fernandes, M.L.; Kato, R.K.; Nascimento, D.C.N.; Sakomura, N.K.; Lima, S.B.P.; Ferreira, G.J.B.C. Effect of an enzymatic blend and yeast on the performance, carcass yield and histomorphometry of the small intestine in broilers from 21 to 42 days of age. Braz. J. Poult. Sci. 2019, 21, eRBCA-2018. [Google Scholar] [CrossRef]
- Sun, H.Y.; Kim, I.H. Dietary supplementation of mixed yeast culture derived from Saccharomyces cerevisiae and Kluyveromyces maxianus: Effects on growth performance, nutrient digestibility, meat quality, blood parameters, and gut health in broilers. J. Poult. Sci. 2019, 56, 140–147. [Google Scholar] [CrossRef]
- Ullah, M.; Marri, G.M.; Jogi, Q.; Rasheed, M.; Rizwana, H.; Kaleri, R.R.; Goil, J.P.; Khoso, Z.A.; Kumar, D.; Shahab, A.; et al. Dietary effect of autolysed yeast on broilers (Levabon®, Biomin Austria) on broiler growth. Pure Appl. Biol. 2019, 8, 1223–1227. [Google Scholar] [CrossRef]
- Ahiwe, E.U.; Abdallh, M.E.; Chang’a, E.P.; Omede, A.A.; Al-Qahtani, M.; Gausi, H.; Graham, H.; Ade, I.P. Influence of dietary supplementation of autolyzed whole yeast and yeast cell wall products on broiler chickens. Asian Australas. J. Anim. Sci. 2020, 33, 579–587. [Google Scholar] [CrossRef]
- Al-Nasrawi, M.A.; Al-Kassie, G.A.; Ali, N.A. Role of yeast (Saccharomyces cerevisiae) as a source of probiotics in poultry diets. Europ. J. Mol. Clin. Med. 2020, 7, 6611–6617. [Google Scholar]
- Ogbuewu, I.P.; Okoro, V.M.; Mbajiorgu, C.A. Probiotic-yeast improves performance indicators in broiler chickens: Evidence from meta-analysis. Appl. Ecol. Environ. Res. 2020, 18, 2823–2843. [Google Scholar] [CrossRef]
- Rafique, K.; Rahman, A.; Mahmood, M. Effect of dietary supplementation of different levels of Saccharomyces cerevisiae on growth performance and hematology in broiler. Ind. J. Anim. Res. 2020, 54, 59–64. [Google Scholar] [CrossRef]
- Ciurescu, G.; Dumitru, M.; Gheorghe, A. Use of brewer’s yeast (Saccharomyces cerevisiae) in broiler feeds to replace corn gluten meal with or without probiotic additives. Arch. Zootech. 2021, 24, 66–83. [Google Scholar] [CrossRef]
- El-Manawey, M.A.; Yousif, E.Y.; Abo-Taleb, A.M.; Atta, A.M. The effect of dietary inclusion of whole yeast, extract, and cell wall on production performance and some immunological parameters of broiler chickens. World Vet. J. 2021, 11, 257–262. [Google Scholar] [CrossRef]
- He, T.; Mahfuz, S.; Piao, X.; Wu, D.; Wang, W.; Yan, H.; Liu, Y. Effects of live yeast (Saccharomyces cerevisiae) as a substitute to antibiotic on growth performance, immune function, serum biochemical parameters and intestinal morphology of broilers. J. Appl. Anim. Res. 2021, 49, 15–22. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, N.; Siddique, R.A.; Sahu, D.S.; Fahim, A.; Singh, R.; Roy, D. Effect of different levels of mushroom powder (Agaricus bisporus) and probiotics (Saccharomyces cerevisiae) on carcass traits and hematological responses of broiler chickens. J. Entomol. Zool. Stud. 2021, 9, 244–248. [Google Scholar] [CrossRef]
- Mohamed, H.E.; Ibrahim, W.A.; Gaafar, R.E. Impact of Moringa olifera leaves or Saccharomyces supplementation on carcass quality, mRNA of heat shock proteins and antioxidants in broilers exposed to heat stress. SVU-Int. J. Vet. Sci. 2022, 5, 193–227. [Google Scholar] [CrossRef]
- M’Sadeq, S. A Ameliorative effect of yeast cell walls on broiler chickens’ performance and gut health under coccidiosis challenge. Czech J. Anim. Sci. 2023, 68, 346–355. [Google Scholar] [CrossRef]
- Okasha, E.G.; Hassan, H.A.; Ismail, Z.S.H. Effects of yeast (Saccharomyces cerevisiae) supplementations on the blood parameters and productive performance of broiler chickens. SVU-Int. J. Agric. Sci. 2023, 5, 130–140. [Google Scholar] [CrossRef]
- Yalçin, S.; Özsoy, B.; Erol, H. Yeast culture supplementation to laying hen diets containing soybean meal or sunflower seed meal and its effect on performance, egg quality traits, and blood chemistry. J. Appl. Anim. Res. 2008, 17, 229–236. [Google Scholar] [CrossRef]
- Hassanein, S.M.; Soliman, N.K. Effect of probiotic (Saccharomyces cerevisiae) adding to diets on intestinal microflora and performance of Hy-Line laying hens. J. Amer. Sci. 2010, 6, 159–169. [Google Scholar]
- Yalçın, S.; Yalçın, S.; Cakin, K.; Eltan, O.; Dagasan, L. Effects of dietary yeast autolysate (Saccharomyces cerevisiae) on performance, egg traits, egg cholesterol content, egg yolk fatty acid composition and humoral immune response of laying hens. J. Sci. Food Agric. 2010, 90, 1695–1701. [Google Scholar] [CrossRef]
- Koiyama, N.T.G.; Utimi, N.B.P.; Santos, B.R.L.; Bonato, M.A.; Barbalho, R.; Gameiro, A.H.; Araújo, C.S.D.S.; Araújo, L.F. Effect of yeast cell wall supplementation in laying hen feed on economic viability, egg production, and egg quality. J. Appl. Poult. Res. 2017, 27, 116–123. [Google Scholar] [CrossRef]
- Özsoy, B.; Karadaðoðlu, O.; Yakan, A.; Önk, K.; Çelik, E.; Þahin, T. The role of yeast culture (Saccharomyces cerevisiae) on performance, egg yolk fatty acid composition, and fecal microflora of laying hens. Rev. Bras. Zootec. 2018, 47, e20170159. [Google Scholar] [CrossRef]
- Hameed, R.; Tahir, M.; Khan, S.H.; Ahmad, T.; Iqbal, J. Effect of yeast supplementation on production parameters, egg quality characteristics and crude protein digestibility in hens. Adv. Life Sci. 2019, 6, 147–151. [Google Scholar]
- Santin, E.; Maiorka, A.; Macari, M.; Grecco, M.; Sanchezi, J.C.; Okada, T.M.; Myasaka, A.M. Performance and intestinal mucosa development of broiler chickens fed diets containing Saccharomyces cerevisiae cell wall. J. Appl. Poult. Res. 2001, 10, 236–244. [Google Scholar] [CrossRef]
- Benites, V.; Gilharry, R.; Gernat, A.; Murillo, J. Effect of dietary mannanoligosaccharide from Bio-Mos or SAF mannan on live performance of broiler chickens. J. Appl. Poult. Res. 2008, 17, 471–475. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Talha, E.A.; Mojahid, A.A.; Dafaalla, E.M. Effect of dietary yeast (Saccharomyces cerevisiae) supplementation on performance, carcass characteristics and some metabolic responses of broilers. Anim. Vet. Sci. 2015, 3, 5–10. [Google Scholar] [CrossRef]
- Roto, S.M.; Rubinelli, P.M.; Ricke, S.C. An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front. Vet. Sci. 2015, 2, 28. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, S.W.; Wang, H.T. Effect of supplementation of yeast with bacteriocin and Lactobacillus culture on growth performance, cecal fermentation, microbiota composition, and blood characteristics in broiler chickens. Asian Australas. J. Anim. Sci. 2017, 30, 211–220. [Google Scholar] [CrossRef]
- Patane, A.S.; Premavalli, K.; Omprakash, A.V.; John Kirubakaran, J.; Hudson, G.H. Effect of dietary yeast supplementation on the production performance of broilers. Int. J. Adv. Biol. Res. 2017, 7, 222–228. [Google Scholar]
- Thongsong, B.; Kalandakano, S.T.; Chavananikul, V. Effects of the addition of probiotic containing both bacteria and yeast or an antibiotic on performance parameters, mortality rate and antibiotic residue in broilers. Thai J. Vet. Med. 2008, 38, 17–26. [Google Scholar] [CrossRef]
- Bilal, R.M.; Hassan, F.; Saeed, M.; Ayasan, T.; Rashed, N.; Akhtar, M.U.; Seidavi, A. Prospects of yeast based feed additives in poultry nutrition: Potential effects and applications. Ind. J. Anim. Sci. 2020, 90, 495–505. [Google Scholar] [CrossRef]
- Yalçın, S.; Eser, H.; Yalçın, S.; Cengiz, S.; Eltan, Ö. Effects of dietary yeast autolysate (Saccharomyces cerevisiae) on performance, carcass and gut characteristics, blood profile, and antibody production to sheep red blood cells in broilers. J. Appl. Poult. Res. 2013, 22, 55–61. [Google Scholar] [CrossRef]
- Muthusamy, N.; Haldar, S.; Ghosh, T.K.; Bedford, M.R. Effects of hydrolysed Saccharomyces cerevisiae yeast and yeast cell wall components on live performance, intestinal histo-morphology and humoral immune response of broilers. Br. Poult. Sci. 2011, 52, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.D.; Wu, S.B.; Choct, M.; Swick, R.A. Effects of yeast cell wall on growth performance, immune responses and intestinal short chain fatty acid concentrations of broilers in an experimental necrotic enteritis model. Anim. Nutr. 2017, 3, 399–405. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Barbosa, J.G.M.; Pereira, R.; Fagundes, N.S.; Rafael, J.M.; Menten, J.F.M. Autolyzed yeast (Saccharomyces cerevisiae) supplementation improves performance while modulating the intestinal immune-system and microbiology of broiler chickens. Front. Sustain. Food Syst. 2018, 2, 85. [Google Scholar] [CrossRef]
- Gomez-Verduzco, G.; Cortes-Cuevas, A.; Lopez-Coello, C.; Avila-Gonzalez, E.; Nava, G.M. Dietary supplementation of mannan-oligosaccharide enhances neonatal immune responses in chickens during natural exposure to Eimeria spp. Acta Vet. Scand. 2009, 51, 11. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.A.; Choct, M. Effects of different dietary levels of mannanoligosaccharide on growth performance and gut development of broiler chickens. Asian Australas. J. Anim. Sci. 2007, 20, 1084–1091. [Google Scholar] [CrossRef]
- Hooge, D.M.; Connolly, A. Meta-analysis summary of broiler chicken trials with dietary Actigen (2009−2011). Int. J. Poult. Sci. 2013, 12, 1–8. [Google Scholar] [CrossRef]
- Gomez, S.; Angeles, M.D.L. Effects of an enzymatically hydrolyzed yeast and yeast culture combined with flavomycin and monensin on finishing broiler chickens. Int. J. Poult. Sci. 2011, 10, 433–439. [Google Scholar] [CrossRef][Green Version]
- Ahiwe, E.U.; Abdallh, M.E.; Chang’a, E.P.; Al-Qahtani, M.; Omede, A.A.; Graham, H.; Iji, P.A. Influence of autolyzed whole yeast and yeast components on broiler chickens challenged with salmonella lipopolysaccharide. Poult. Sci. 2019, 98, 7129–7138. [Google Scholar] [CrossRef]
- Pahlavanzadeh, M.; Sadeghi, A.A.; Mousavi, S.N.; Chamani, M. Influence of spleen meal and hydrolyzed yeast on growth performance, blood cells, antibody titres and IL-2 gene expression in broiler chickens. J. Appl. Anim. Res. 2021, 49, 289–294. [Google Scholar] [CrossRef]
- Sampath, V.; Han, K.; Kim, I.H. Influence of yeast hydrolysate supplement on growth performance, nutrient digestibility, microflora, gas emission, blood profile, and meat quality in broilers. J. Anim. Sci. Technol. 2021, 63, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Bolacali, M.; Irak, K. Effect of dietary yeast autolysate on performance, slaughter, and carcass characteristics, as well as blood parameters, in quail of both genders. S. Afr. J. Anim. Sci. 2017, 47, 460–470. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Al-Afifi, S.H.; Manal, I.; Bayomi, A. Bio chemical effects of Saccharomyces cerevisiae (biological product) and hydrated sodium calcium alimono-silicate “HSCAS” (chemical compound) as anti-aflatoxin in chicken rations. Anim. Health Res. J. 2018, 6, 19–38. [Google Scholar]
- Al-Mansour, S.; Al-Khalf, A.; Al-Homidan, I.I.; Fathi, M. Feed efficiency and blood hematology of broiler chicks given a diet supplemented with yeast culture. Int. J. Poult. Sci. 2011, 10, 603–607. [Google Scholar] [CrossRef]
- Hosseini, S. The effect of utilization of different levels of Saccharomyces cerevisiae on broiler chicken’s performance. Glob. Vet. 2011, 6, 233–236. [Google Scholar]
- Aluwong, T.; Hassan, F.; Dzenda, T.; Kawu, M.; Ayo, J. Effect of different levels of supplemental yeast on body weight, thyroid hormone metabolism and lipid profile of broiler chickens. J. Vet. Med. Sci. 2013, 75, 291–298. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.F. Digestion, neomycin and yeast supplementation in broiler diets under Egyptian summer conditions. J. Egypt. Poult. Sci. 2002, 22, 235–257. [Google Scholar]
- Fasina, Y.; Thanissery, R. Comparative efficacy of a yeast product and bacitracin methylene disalicylate in enhancing early growth and intestinal maturation in broiler chicks from breeder hens of different ages. Poult. Sci. 2011, 90, 1067–1073. [Google Scholar] [CrossRef]
- Jazi, V.; Foroozandeh, A.D.; Toghyani, M.; Dastar, B.; Rezaie-Koochaksaraie, R.; Toghyani, M. Effects of Pediococcusacidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella typhimurium. Poult. Sci. 2018, 97, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.M.E.; Thabet, H.A.; Abdelaziz, M.A.M. Effect of bio-mos utilization in broiler chick diets on performance, microbial and histological alteration of small intestine and economic efficiency. Asian J. Anim. Sci. 2015, 10, 323–334. [Google Scholar] [CrossRef][Green Version]
- Shareef, A.; Al-Dabbagh, A. Effect of probiotic (Saccharomyces cerevisiae) on performance of broiler chicks. Iraq. J. Vet. Sci. 2009, 23, 23–29. [Google Scholar]
- Jensen, G.S.; Patterson, K.M.; Yoon, I. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 487–500. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, B.; Li, X.; Li, L.; Ma, L.; Yan, X. Effects of yeast cell walls on performance and immune responses of cyclosporine A-treated, immune-suppressed broiler chickens. Br. J. Nutr. 2012, 107, 858–866. [Google Scholar] [CrossRef]
- Sedghi, M.; Mohammadi, I.; Sarrami, Z.; Ghasemi, R.; Azarfar, A. Effects of a yeast cell wall product on the performance of broiler chickens and PGC-1α, TLR4, IL-10 and PPARγ genes expression. Ital. J. Anim. Sci. 2022, 21, 263–278. [Google Scholar] [CrossRef]
- Haldar, S.; Ghosh, T.; Bedford, M. Effects of yeast (Saccharomyces cerevisiae) and yeast protein concentrate on production performance of broiler chickens exposed to heat stress and challenged with Salmonella enteritidis. Anim. Feed Sci. Technol. 2011, 168, 61–71. [Google Scholar] [CrossRef]
- Bradley, G.L.; Savage, T.F. The effect of autoclaving a yeast culture of Saccharomyces cerevisiae on turkey poult GAO ET AL.1384 performance and the retention of gross energy, and selected minerals. Anim. Feed Sci. Technol. 1995, 55, 1–7. [Google Scholar] [CrossRef]
- Bilal, M.; Sarwar, M.; Javaid, I.S. Effect of replacing dietary soybean meal with various levels of dried distillery yeast sludge on gut health, immunity, egg quality and performance of layers. Transylv. Rev. 2016, 10, 2626–2638. [Google Scholar]
- Bilal, R.M.; Akhtar, M. Impact of replacement of dietary soybean with dried distillery yeast sludge on performance and health of layer chicks. Gomal Univ. J. Res. 2018, 34, 20–29. [Google Scholar]
- Bilal, R.M.; Akhtar, M.; Javaid, A. Effect of replacement of soybean meal with dried distillery yeast sludge on performance, immunity gut health of layer pullets. Gomal Univ. J. Res. 2018, 34, 7–14. [Google Scholar]
- Marien, M.; De Gussem, M.; Vancraeynest, D. Evaluation of a yeast extract product, containing a guaranteed range of glucans, in free range laying hens. In Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, France, 26–30 August 2007. [Google Scholar]
- Yalçın, S.; Yalçın, S.; Uzunoğlu, K.; Duyum, H.M.; Eltan, Ö. Effects of dietary yeast autolysate (Saccharomyces cerevisiae) and black cumin seed (Nigella sativa L.) on performance, egg traits, some blood characteristics and antibody production of laying hens. Livest. Sci. 2012, 145, 13–20. [Google Scholar] [CrossRef]
- Yalçın, S.S.; Yalçýn, A.; Þahin, H.M.; Duyum, A.; Çalýk, A.; Gümüþ, H. Effects of dietary inactive yeast and live yeast on performance, egg quality traits, some blood parameters and antibody production to SRBC of laying hens. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 345–350. [Google Scholar] [CrossRef]
- Shashidhara, R.G.; Devegowda, G. Effect of dietary mannanoligosaccharide on broiler breeder production traits and immunity. Poult. Sci. 2003, 82, 1319–1325. [Google Scholar] [CrossRef]
- Mi, J.; Chen, X.; Liao, X. Screening of single or combined administration of 9 probiotics to reduce ammonia emissions from laying hens. Poult. Sci. 2019, 98, 3977–3988. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, V. Growth and response to Escherichia coli lipopolysaccharide challenge in Lohmann LSL-Lite pullets when fed a source of omega-3 fatty acids and yeast bioactives from hatch through to 16 wk of age. Poult. Sci. 2023, 102, 102940. [Google Scholar] [CrossRef]
- Eltazi, S.M.; Mohamed, K.A.; Mohamed, M.A. Response of broiler chicks to diets containing live yeast as probiotic natural feed additive. Int. J. Pharm. Res. Allied Sci. 2014, 3, 40–46. [Google Scholar]
- Onifade, A.A.; Odunsi, A.A.; Babatunde, G.M.; Olorede, B.R.; Muma, E. Comparison of the supplemental effects of Saccharomyces cerevisiae and antibiotics in low protein and high fibre diets fed to broiler chickens. Arch. Tierernahr. 1999, 52, 29–39. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhou, T.X.; Ao, X.; Kim, I.H. Effects of β-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize−soybean meal based diets. Livest. Sci. 2012, 150, 419–424. [Google Scholar] [CrossRef]
- Hoque, M.R.; Jung, H.I.; Kim, I.H. Effect of yeast culture (Saccharomyces cerevisiae) supplementation on growth performance, excreta microbes, noxious gas, nutrient utilization, and meat quality of broiler chicken. J. Poult. Sci. 2021, 58, 216–221. [Google Scholar] [CrossRef]
- Firman, J.D.; Moore, D.; Broomhead, J.; McIntyre, D. Effects of dietary inclusion of a Saccharomyces cerevisiae fermentation product on performance and gut characteristics of male turkeys to market weight. Int. J. Poult. Sci. 2013, 12, 141–143. [Google Scholar] [CrossRef]
- Nidamanuri, A.L.; Prince, L.L.L.; Mahapatra, R.K.; Murugesan, S. Effect on physiological and production parameters upon supplementation of fermented yeast culture to Nicobari chickens during and post summer. J. Anim. Physiol. Anim. Nutr. 2022, 106, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Sacakli, P.; Ergun, A.; Koksal, B.H.; Bayraktaroglu, A.G.; Sizmaz, O. Effects of diets supplemented with yeast (Saccharomyces cerevisiae) products or/and hops (Humulus iupulus) on growth performance and intestinal morphology in broilers. Rev. Med. Vet. 2011, 162, 531–537. [Google Scholar]
- Fathi, M.M.; Al-Mansour, S.; Al-Homidan, A.; Al-Khalaf, A.; Al-Damegh, M. Effect of yeast culture supplementation on carcass yield and humoral immune response of broiler chicks. Vet. World J. 2012, 5, 651. [Google Scholar] [CrossRef]
- Awaad, M.H.; Atta, A.M.; Elmenawey, M.A.; Gharib, H.B.; Abd El-Ghany, W.A.; Nada, A.A. The effect of a combination of β (1-3) D-glucan and Propionibacterium granulosum on productive performance and immune modulation of immunocompromised and non-immunocompromised broiler chickens. Vet. World J. 2013, 6, 31–38. [Google Scholar] [CrossRef]
- Swiatkiewicz, S.; Waosek, A.; Jozefiak, D. Immunomodulatory efficacy of yeast cell products in poultry: A current review. World’s Poult. Sci. J. 2014, 70, 57–68. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Hosseini, S.A.; Lotfollahian, H.; Shariatmadan, F. Effect of probiotics, yeast, vitamin E and vitamin C supplements on performance and immune response of laying hen during high environmental temperature. Int. J. Poult. Sci. 2007, 6, 895–900. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, K.; Yu, C.Y.; Li, Q.M.; Tong, Y.C.; Wang, C.; Yang, Z.B.; Wang, T. Effects of a yeast-derived product on growth performance, antioxidant capacity, and immune function of broilers. Poult. Sci. 2021, 100, 101343. [Google Scholar] [CrossRef]
- Huang, Q.; Wei, Y.; Lv, Y.; Wang, Y.; Hu, T. Effect of dietary inulin supplements on growth performance and intestinal immunological parameters of broiler chickens. Livest. Sci. 2015, 180, 172–176. [Google Scholar] [CrossRef]
- Pontón, J.; Omaetxebarria, M.J.; Elguezabal, N.; Alvarez, M.; Moragues, M.D. Immunoreactivity of the fungal cell wall. Med. Mycol. 2001, 39, 101–110. [Google Scholar] [CrossRef]
- McCaffrey, C.; Corrigan, A.; Moynagh, P.; Murphy, R. Effect of yeast cell wall supplementation on intestinal integrity, digestive enzyme activity and immune traits of broilers. Br. Poult. Sci. 2021, 62, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.Y.; Kim, W.K. Review: Roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 2018, 5, 245. [Google Scholar] [CrossRef] [PubMed]
- Reis e Sousa, C.R. Activation of dendritic cells: Translating innate into adaptive immunity. Curr. Opin. Immunol. 2004, 16, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Rajput, I.R.; Hussain, A.; Li, Y.L.; Zhang, X.; Xu, X.; Long, M.Y.; You, D.Y.; Li, W.F. Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs mediated signaling to induce immunity by chicken BMDCs. J. Cell. Biochem. 2014, 115, 189–198. [Google Scholar] [CrossRef]
- Yitbarek, A.; Echeverry, H.; Brady, J.; Hernandez-Doria, J.; Camelo-Jaimes, G.; Sharif, S.; Guenter, W.; House, J.D.; Rodriguez-Lecompte, J.C. Innate immune response to yeast-derived carbohydrates in broiler chickens fed organic diets and challenged with Clostridium perfringens. Poult. Sci. 2012, 91, 1105–1112. [Google Scholar] [CrossRef]
- Tsukada, C.; Yokoyama, H.; Miyaji, C.; Ishimoto, Y.; Kawamura, H.; Abo, T. Immunopotentiation of intraepithelial lymphocytes in the intestine by oral administrations of β-glucan. Cell. Immunol. 2003, 221, 1–5. [Google Scholar] [CrossRef]
- Bohn, J.A.; BeMiller, J.M. (1-3)-β-D-glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Chae, B.; Lohakare, J.; Moon, W.; Lee, S.; Park, Y.; Hahn, T.W. Effects of supplementation of β-glucan on the growth performance and immunity in broilers. Res. Vet. Sci. 2006, 80, 291–298. [Google Scholar] [CrossRef]
- Rajapakse, J.R.; Buddhika, M.; Nagataki, M.; Nomura, H.; Watanabe, Y.; Ikeue, Y.; Agatsuma, T. Effect of Sophy β-glucan on immunity and growth performance in broiler chicken. J. Vet. Med. Sci. 2010, 72, 1629–1632. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.H.; Lee, D.N.; Wen, C.M.; Weng, C.F. Effects of beta-glucan supplementation on lymphocyte proliferation, macrophage chemotaxis and specific immune responses in broilers. Asian Australas. J. Anim. Sci. 2004, 17, 1145–1149. [Google Scholar] [CrossRef]
- Schwartz, B.; Vetvicka, V. Review: β-glucans as effective antibiotic alternatives in poultry. Molecules 2021, 26, 3560. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Zhang, J.; Zhang, L.; Huang, K.; Li, J.; Cao, L. Yeast cell wall upregulated cell-mediated immune responses to Newcastle disease virus vaccine. Poult. Sci. 2022, 101, 101712. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, J.; Zhang, J.; Huang, H.; Gui, F.; Xu, W.; Zhang, L.; Bi, S. Beta-glucan enhanced immune response to Newcastle disease vaccine and changed mRNA expression of spleen in chickens. Poult. Sci. 2023, 102, 102414. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rogiewicz, A.; McMillan, E.; Rodriguez-Lecompte, J.C.; Patterson, R.; Slominski, B.A. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on growth performance and local innate immune response of broiler chickens challenged with Clostridium perfringens. Avian Pathol. 2016, 45, 334–345. [Google Scholar] [CrossRef]
- Thomas, S.; Rezoagli, E.; Abidin, I.Z.; Major, I.; Murray, P.; Murphy, E.J. β-Glucans from yeast—Immunomodulators from novel waste resources. Appl. Sci. 2022, 12, 5208. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef]
- Lowry, V.; Farnell, M.; Ferro, P.; Swaggerty, C.; Bahl, A.; Kogut, M. Purified β-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2005, 98, 309–318. [Google Scholar] [CrossRef]
- Liz-Wang, W.; Lv, Z.; Liu, D.; Guo, Y. Bacillus subtilis and yeast cell wall improve the intestinal health of broilers challenged by Clostridium perfringens. Br. Poult. Sci. 2017, 58, 635–643. [Google Scholar] [CrossRef]
- Vandeplas, S.; Dubois Dauphin, R.; Beckers, Y.; Thonart, P.; Thewis, A. Salmonella in chicken: Current and developing strategies to reduce contamination at farm level. J. Food Prot. 2010, 73, 774–785. [Google Scholar] [CrossRef]
- Ferket, P.; Parks, C.; Grimes, J. Benefits of dietary antibiotic and mannanoligosaccharide supplementation for poultry. In Proceedings of the Multi-State Poultry Meeting, Chicago, IL, USA, 14–16 May 2002. [Google Scholar]
- Synytsya, A.; Mickova, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Samudovska, A.; Spisakova, V.; Demeterova, M.; Hresko, S. The influence of fungal β-glucan on nonspecific immunity in broiler chicks. Acta Vet. 2012, 62, 511–519. [Google Scholar] [CrossRef]
- Cardozo, P.; Torras, M.C.; Less, J.; Peisker, M. The aggregation capacity of oligo saccharides from different yeast preparations to bind different pathogenic bacteria. J. Anim. Sci. 2018, 96, 304. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Sifri, M.; Selvaraj, R.K. Effect of yeast cell product (CitriStim) supplementation on broiler performance and intestinal immune cell parameters during an experimental coccidial infection. Poult. Sci. 2013, 92, 358–363. [Google Scholar] [CrossRef]
- Lensing, M.; Van-Der-Klis, J.; Yoon, J.; Moore, D. Efficacy of Saccharomyces cerevisiae fermentation product on intestinal health and productivity of coccidian-challenged laying hens. Poult. Sci. 2012, 9, 1590–1597. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Figueiredo-Lima, D.F.; Faria-Filho, D.E.; Marques, R.H.; Moraes, V.M.B.D. Effect of mannanoligo saccharides and/or enzymes on antibody titers against infectious bursal and Newcastle disease viruses. Arq. Bras. Med. Vet. Zootec. 2009, 61, 6–11. [Google Scholar] [CrossRef]
- Ghosh, T.; Haldar, S.; Bedford, M.; Muthusami, N.; Samanta, I. Assessment of yeast cell wall as replacements for antibiotic growth promoters in broiler diets: Effects on performance, intestinal histomorphology and humoral immune responses. J. Anim. Physiol. Anim. Nutr. 2012, 96, 275–284. [Google Scholar] [CrossRef]
- Ozpinar, H.; Erhard, M.; Ahrens, F.; Kutay, C.; Eseceli, H. Effect of vitamin E, vitamin C and mannanoligosaccharide (Bio-Mos) supplements on performance and immune system in broiler chicks. J. Anim. Vet. Adv. 2010, 9, 2647–2654. [Google Scholar] [CrossRef]
- Zakeri, A.; Kashefi, P. The comparative effects of five growth promoters on broiler chickens humoral immunity and performance. J. Anim. Vet. Adv. 2011, 10, 1097–1101. [Google Scholar] [CrossRef]
- Zhenping, S.; Wenting, L.; Ruikui, Y.; Jia, L.; Honghong, L.; Wei, S. Effect of a straw-derived xylooligosaccharide on broiler growth performance, endocrine metabolism, and immune response. Canad. J. Vet. Res. 2013, 77, 105–109. [Google Scholar]
- Tohid, T.; Hasan, G.; Alireza, T. Efficacy of mannanoligo saccharides and humate on immune response to Avian Influenza (H9) disease vaccination in broiler chickens. Vet. Res. Commun. 2010, 34, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.K.; da Silva, J.D.T.; Torres, K.A.A.; de Faria Filho, D.E.; Hada, F.H.; de Moraes, V.M.B. Humoral immune response of broilers fed diets containing yeast extract and prebiotics in the prestarter phase and raised at different temperatures. J. Appl. Poult. Res. 2009, 18, 530–540. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Lv, Z.; Zhang, B.; Lv, H.; Guo, Y. Effects of Kluyveromyces marxianus supplementation on immune responses, intestinal structure and microbiota in broiler chickens. PLoS ONE 2017, 127, e0180884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Wang, Z. The modulating effect of beta-1, 3/1, 6-Glucan supplementation in the diet on performance and immunological responses of broiler chickens. Asian Australas. J. Anim. Sci. 2008, 21, 237–244. [Google Scholar] [CrossRef]
- Mountzouris, K.; Tsirtsikos, P.; Kalamara, E.; Nitsch, S.; Schatzmayr, G.; Fegeros, K. Evaluation of the efficacy of probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007, 86, 309–317. [Google Scholar] [CrossRef]
- Baurhoo, B.; Ferket, P.R.; Zaho, X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, caecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 2009, 88, 2262–2272. [Google Scholar] [CrossRef]
- Lu, Z.; Thanabalan, A.; Leung, H.; Akbari Moghaddam Kakhki, R.R. The effects of feeding yeast bioactives to broiler breeders and/or their offspring on growth performance, gut development, and immune function in broiler chickens challenged with Eimeria. Poult. Sci. 2019, 98, 6411–6421. [Google Scholar] [CrossRef]
- Javadi, A.; Mirzaei, H.; Safarmashaei, S.; Vahdatpour, S. Effects of probiotic (live and inactive Saccharomyces cerevisiae) on meat and intestinal microbial properties of Japanese quails. Afr. J. Biotechnol. 2012, 11, 12083–12087. [Google Scholar] [CrossRef]
- Baurhoo, B.; Letellier, A.; Zhao, X.; Ruiz-Feria, C.A. Caecal populations of Lactobacilli and bifidobacteria and Escherichia coli populations after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poult. Sci. 2007, 86, 2509–2516. [Google Scholar] [CrossRef]
- Spring, P.; Wenk, C.; Dawson, K.A.; Newman, K.E. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult. Sci. 2000, 79, 205–211. [Google Scholar] [CrossRef]
- Fathima, S.; Shanmugasundaram, R.; Adams, D.; Selvaraj, R.K. Gastrointestinal microbiota and their manipulation for improved growth and performance in chickens. Foods 2022, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Offei, B.; Vandecruys, P.; De Graeve, S.; Foulquie-Moreno, M.R.; Thevelein, J.M. Unique genetic basis of the distinct antibiotic potency of high acetic acid production in the probiotic yeast Saccharomyces cerevisiae var. boulardii. Genome Res. 2019, 29, 1478–1494. [Google Scholar] [CrossRef] [PubMed]
- Murzyn, A.; Krasowska, A.; Stefanowicz, P.; Dziadkowiec, D.; Łukaszewicz, M. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS ONE 2010, 5, e12050. [Google Scholar] [CrossRef] [PubMed]
- Zaouche, A.; Loukil, C.; De Lagausie, P.; Peuchmaur, M.; Macry, J.; Fitoussi, F.; Bernasconi, P.; Bingen, E.; Cezard, J.P. Effects of oral Saccharomyces boulardii on bacterial overgrowth, translocation, and intestinal adaptation after small-bowel resection in rats. Scand. J. Gastroenterol. 2000, 35, 160–165. [Google Scholar] [CrossRef]
- Li, R.; Yassami, S.; Kiviniemi, E.A.; Qiao, W.; Takala, T.M.; Saris, P.E. Listeria decontamination of chicken meat with beer brewed with bacteriocin producing Saccharomyces boulardii. LWT-Food Sci. Technol. 2021, 152, 112323. [Google Scholar] [CrossRef]
- Wang, B.; Hussain, A.; Zhou, Y.; Zeng, Z.; Wang, Q.; Zou, P.; Gong, L.; Zhao, P.; Li, W. Saccharomyces boulardii attenuates inflammatory response induced by Clostridium perfringens via TLR4/TLR15 MyD8 pathway in HD11 avian macrophages. Poult. Sci. 2020, 99, 5356–5365. [Google Scholar] [CrossRef]
- Badia, R.; Brufau, M.T.; Guerrero-Zamora, A.M.; Lizardo, R.; Dobrescu, I.; Martin-Venegas, R.; Ferrer, R.; Salmon, H.; Martınez, P.; Brufau, J. b-Galactomannan and Saccharomyces cerevisiae var. boulardii modulate the immune response against Salmonella enterica serovar Typhimurium in porcine intestinal epithelial and dendritic cells. Clin. Vaccine Immunol. 2012, 19, 368–376. [Google Scholar] [CrossRef]
- Corrigan, A.; Fay, B.J.; Corcionivoschi, N.; Murphy, R.A. Effect of yeast mannan-rich fractions on reducing Campylobacter colonization in broiler chickens. J. Appl. Poult. Res. 2017, 26, 350–357. [Google Scholar] [CrossRef]
- Massacci, F.R.; Lovito, C.; Tofani, S.; Tentellini, M.; Genovese, D.A.; De Leo, A.A.P.; Papa, P.; Magistrali, C.F.; Manuali, E.; Trabalza-Marinucci, M. Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 positively affects performance and intestinal ecosystem in broilers during a Campylobacter jejuni infection. Microorganisms 2019, 7, 596. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Lan, Y.; Xun, S.; Tnmminga, S.; Williams, B.A.; Verstegen, M.W.A.; Eridi, G. Real-time PCR detection of lactic acid bacteria in caecal contents of Eimeria tenella-infected broilers fed soybean oligosaccharides and soluble soybean polysaccharides. Poult. Sci. 2004, 83, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfariane, G.T.; Englyst, H.N. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 2001, 73, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Elghandour, M.; Tan, Z.L.; Abu Hafsa, S.H.; Adegbeye, M.J.; Greiner, R.; Ugbogu, E.A.; Cedillo Monroy, J.; Salem, A. Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: A review. J. Appl. Microbiol. 2020, 128, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.; Kakani, R.; Haq, A.; Byrd, J.A.; Bailey, C.A. Growth promoting effects of prebiotic yeast cell wall products in starter broilers under an immune stress and Clostridium perfringens challenge. J. Appl. Poult. Res. 2015, 24, 66–72. [Google Scholar] [CrossRef]
- Li, Y.B.; Xu, Q.Q.; Yang, C.J.; Yang, X.; Lv, L.; Yin, C.H.; Liu, X.L.; Yan, H. Effects of probiotics on the growth performance and intestinal microflora of broiler chickens. Pak. J. Pharm. Sci. 2014, 27, 713–717. [Google Scholar]
- Fernandez, F.; Hinton, M.; Van-Gils, B. Dietary mannanoligosaccharides and their effect on chicken caecal microflora in relation to Salmonella Enteritidis colonisation. Avian Pathol. 2002, 31, 49–58. [Google Scholar] [CrossRef]
- Kumar, B.S.; Vijayasarath, S.K.; Gowda, R.N.S.; Satyanarayana, M.L. Probiotics in the prevention of experimental fowl typhoid in broilers- a pathomorphological study. Ind. J. Anim. Sci. 2002, 72, 528–531. [Google Scholar]
- Zhang, H.; Zhou, Y.; Xu, H.; Liang, C.; Zhai, Z. Bacillus amyloliquefaciens BLCC1-0238 alone or in combination with mannan-oligosaccharides alleviates subclinical necrotic enteritis in broilers. Prob. Antimicrob. Proteins 2022, 14, 158–168. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.A.; Kocher, A.; Mikkelsen, L.L.; Choct, M. Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic Escherichia coli challenge. Br. Poult. Sci. 2008, 49, 550–559. [Google Scholar] [CrossRef]
- Baurhoo, B.; Goldflus, F.; Zhao, X. Purified cell wall 392 of Saccharomyces cerevisiae increases protection against intestinal pathogens in broiler chickens. Int. J. Poult. Sci. 2009, 8, 133–137. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Hooge, D. Broiler chicken performance may improve with MOS. Feedstuffs 2005, 75, 11–13. [Google Scholar]
- Audisio, M.C.; Oliver, G.; Apella, M.C. Protective effect of Enterococcus faecium J96, a potential probiotic strain, on chicks infected with Salmonella pullorum. J. Food Prot. 2000, 63, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, L.; Doyle, M.P. Salmonellae reduction in poultry by competitive exclusion bacteria Lactobacillus salivarius and Streptococcus cristatus. J. Food Prot. 2007, 70, 874–878. [Google Scholar] [CrossRef]
- Markazi, A.D.; Perez, V.; Sifri, M.; Shanmugasundaram, R.; Selvaraj, R.K. Effect of whole yeast cell product supplementation (CitriStim) on immune responses and cecal microflora species in pullet and layer chickens during an experimental coccidial challenge. Poult. Sci. 2017, 96, 2049–2056. [Google Scholar] [CrossRef]
- Katayama, T.; Fujita, K.; Yamamoto, K. Novel bifidobacterial glycosidases acting on sugar chains of mucin l glycoprotein. J. Biosci. Bioeng. 2005, 99, 457–465. [Google Scholar] [CrossRef]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396–402. [Google Scholar] [CrossRef]
- Joerger, R.D. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003, 82, 640–647. [Google Scholar] [CrossRef]
- Gibson, G.R.; Wang, X. Regulatory effects of Bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 1994, 77, 412–420. [Google Scholar] [CrossRef]
- Dantan-Gonzalez, R.E.; Quiroz-Castaneda, R.E.; Cobaxin-Cardenas, M.; Valle-Hernandez, J.; Gama-Martınez, Y.; Tinoco-Valencia, J.; Serrano-Carreon, L.; Ortiz-Hernandez, L. Impact of Meyerozyma guilliermon dii isolated from chickens against Eimeria sp. protozoan, an in vitro analysis. BMC Vet. Res. 2015, 11, 278. [Google Scholar] [CrossRef]
- Santin, E.; Paulillo, A.C.; Maiorka, A.; Nakaghi, L.S.; Macari, M. Evaluation of the efficacy of Saccharomyces cerevısiae cell wall to ameliorate the. Int. J. Poult. Sci. 2003, 2, 341–344. [Google Scholar] [CrossRef]
- Awaad, M.H.; Atta, A.M.; Abd El-Ghany, A.A.; Elmenawey, M.; Ahmed, K.; Hassan, A.A.; Nada, A.A.; Abdelaleem, G.A. Effect of a specific combination of mannan-oligosaccharides and â-glucans extracted from yeast cell wall on the health status and growth performance of ochratoxicated broiler chickens. J. Am. Sci. 2011, 7, 82–96. [Google Scholar]
- Agazzi, A.; Perricone, V.; Omodei Zorini, F.; Sandrini, S.; Mariani, E.; Jiang, X.R.; Ferrari, A.; Crestani, M.; Nguyen, T.X.; Bontempo, V.; et al. Dietary mannan oligosaccharides modulate gut inflammatory response and improve duodenal villi height in post-weaning piglets improving feed efficiency. Animals 2020, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Pourabedin, M.; Xu, Z.; Baurhoo, B.; Chevaux, E.; Zhao, X. Effects of mannanoligosaccharide and virginiamycin on the cecal microbial community and intestinal morphology of chickens raised under suboptimal conditions. Canad. J. Microbiol. 2014, 60, 255–266. [Google Scholar] [CrossRef]
- Wang, W.; Ren, W.; Li, Z.; Yue, Y.; Guo, Y. Effects of live yeast on immune responses and intestinal morphological structure in lipopolysaccharide-challenged broilers. Canad. J. Anim. Sci. 2016, 97, 136–144. [Google Scholar] [CrossRef]
- Sims, M.; Dawson, K.; Newman, K.; Spring, P.; Hoogell, D. Effects of dietary mannan oligosaccharide, bacitracin methylene disalicylate, or both on the live performance and intestinal microbiology of turkeys. Poult. Sci. 2004, 83, 1148–1154. [Google Scholar] [CrossRef]
- Luquetti, B.C.; Furlan, R.L.; Alarcon, M.F.F.; Macari, M. Saccharomyces cerevisiae cell wall dietary supplementation on the performance and intestinal mucosa development and integrity of broiler chickens vaccinated against coccidiosis. Rev. Bras. Ciên. Avícol. 2012, 14, 89–95. [Google Scholar] [CrossRef]
- Leung, H.; Yitbarek, A.; Snyder, R.; Patterson, R.; Barta, J.R.; Karrow, N.; Kiarie, E. Responses of broiler chickens to Eimeria challenge when fed a nucleotide-rich yeast extract. Poult. Sci. 2019, 98, 1622–1633. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Hussein, S.M.; M’Sadeq, S.A.; Beski, S.S.M.; Mahmood, A.L.; Frankel, T.L. Different combinations of peppermint, chamomile and a yeast prebiotic have different impacts on production and severity of intestinal and bursal abnormalities of broilers challenged with coccidiosis. Ital. J. Anim. Sci. 2021, 20, 1924–1934. [Google Scholar] [CrossRef]
- Alkhulaifi, M.M.; Alqhtani, A.H.; Alharthi, A.S.; Al Sulaiman, A.R.; Abudabos, A.M. Influence of prebiotic yeast cell wall ex tracts on growth performance, carcase attributes, bio chemical metabolites, and intestinal morphology and bacteriology of broiler chickens challenged with Salmonella typhimurium and Clostridium perfringens. Ital. J. Anim. Sci. 2022, 21, 1190–1199. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Hady, M.M.; Kamel, N.F.; Ragaa, N.M. The impact of exogenous dietary nucleotides in ameliorating Clostridium perfringens infection and improving intestinal barriers gene expression in broiler chicken. Vet. Anim. Sci. 2020, 10, 100130. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, L.; Durackova, Z.; Sandula, J.; Sasinkova, V.; Krajcovic, J. Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat. Res. 2001, 497, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ferencik, M.; Kotulova, D.; Masler, L.; Bergendi, L.; Sandula, J.; Stefanovic, J. Modulatory effect of glucans on the functional and biochemical activities of guinea-pig macrophages. Methods Find. Exp. Clin. Pharmacol. 1986, 8, 163–166. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).