Abstract
Quorum sensing (QS) is a unique form of communication that exists among microbial communities. This system enables microbial cells to achieve behavioral coordination by generating and perceiving specific QS signaling molecules. This “chemical dialogue” allows microorganisms to synchronously express specific genes, thereby regulating group-level functions such as biofilm formation, virulence factor production, antibiotic biosynthesis, and metabolic coordination. Recently, the livestock industry has faced a multitude of challenges, including antibiotic resistance, environmental impact, and production efficiency. QS-based technologies have emerged as novel strategies to address these challenges simultaneously. It is important to note that a key principle of this strategy is that treatments should focus on regulating and modulating microbial QS systems rather than broadly inhibiting them. Therefore, the application of QS-based technologies provides new technical approaches to address core challenges in sustainable livestock production, including alternatives to antibiotics, improved farming efficiency, and environmentally friendly management. Moreover, it contributes to the achievement of carbon neutrality objectives by reducing methane emissions in ruminants through targeted inhibition of methanogen QS. This review systematically examines the biosynthesis mechanisms and regulatory features of the three core QS signaling molecules, with a focus on their practical applications in monogastric animal production, ruminant production, and aquatic animal production. It also explores the interdisciplinary innovative applications of QS-based technologies across multiple fields. By analyzing current research limitations and industrialization bottlenecks, this review outlines key future research directions and development challenges, aiming to provide a reference for the widespread application of QS-based technologies in animal production.
1. Introduction
Quorum sensing (QS) is a key mechanism by which bacteria mediate intercellular communication and cooperatively regulate collective behavior through signaling molecules such as N-acylhomoserine lactones (AHLs) and oligopeptides [1]. As early as 1970, Nealson et al. [2] first discovered this phenomenon in Vibrio fischeri, and it was later confirmed to be widely present in both Gram-negative and Gram-positive bacteria, assisting bacteria in real-time monitoring of population density [3]. From the perspective of current research, international studies began earlier, first exploring the fundamental mechanisms of QS in animal models (such as signaling molecules interfering with the virulence expression of intestinal pathogens) and subsequently advancing to trials in economic animals (e.g., pigs and poultry). For example, the copper-based nanosystem developed by Wang et al. [4]—encapsulated in erythrocyte and platelet membranes—can inhibit the QS mechanisms of Staphylococcus aureus and Pseudomonas aeruginosa. Another study dissected a glyoxal-specific signaling pathway mechanism, revealing that metabolic byproducts can regulate pathogen behavior [5]. Aquaculture and ruminant production have focused on tackling challenges in the livestock industry, including animal health management, disease prevention and control, and adaptation to environmental stressors. Bhuiyan et al. [6] highlighted that biofilm formation, antibiotic resistance, and QS constitute a ‘triple threat’ in bacterial pathogenesis, which further explains the urgency of targeting QS to address bacterial infection and resistance issues in animal production. For example, Xu et al. [7] found that active QS pathways are a key factor influencing the efficient conjugative transfer of resistance genes within pig manure microbial communities, providing insights for breaking the chain of resistance gene transmission. Another study demonstrated that nanomaterials can mitigate the risk of antibiotic resistance gene (ARG) transmission by influencing QS to suppress resistance genes [8]. While these nanomaterial-based QS modulation strategies show promise for mitigating ARG transmission, their practical application in livestock production faces unresolved issues: biosafety concerns, insufficient cost-effectiveness for large-scale farming, and low public acceptance of nanotechnology in food-related sectors. This review provides the first comprehensive application framework for QS-based technology, systematically evaluating its potential to enhance gut health, optimize rumen function, and prevent aquatic diseases, while critically assessing the path to industrialization.
2. Types of Signaling Molecules of QS
According to the differences in signaling molecules and ways of recognition, the bacterial QS systems can be classified into three major types. AHL-mediated QS system is predominant in Gram-negative bacteria, with AHLs as core signals [9]. AHLs are synthesized by LuxI-family synthases using S-adenosylmethionine (SAM) and acyl carrier protein (ACP)-derived acyl chains as substrates. When extracellular AHL concentrations reach a threshold, they bind to LuxR proteins to form AHL-LuxR complexes, which specifically activate or inhibit target gene transcription. This regulation governs multiple bacterial collective behaviors, including bioluminescence [10], production of virulence factors [11], biofilm formation [12], and biosynthesis of antibiotics [13]. Another type is the autoinducing peptide (AIP)-mediated QS system, whose most significant difference from the AHL-mediated system lies in the specificity of signaling molecules—it uses AIPs as signals with high species specificity, and its regulation relies on a two-component system [14]. Specifically, peptides that have been synthesized to be AIP precursors are initially synthesized and released into the cell; when the concentration of these synthesized peptides reaches a threshold value, they bind to histidine kinase receptors on the cell wall and mediate functions of transmission of signals to the intracellular response-regulating proteins via phosphate groups, and finally accomplish the regulation of target gene transcription. In addition, autoinducer-2 (AI-2) is another signaling molecule. AI-2 is widely distributed in bacteria and serves as a universal language for intra- and interspecies communication [15]. AI-2 synthesis involves two steps: S-ribosylhomocysteine (SRH), a precursor of SAM, is first cleaved by the enzyme LuxS, followed by further modification to form mature AI-2.
3. Application of QS in Monogastric Animal Production
Research on QS in vitro validation has advanced to animal models and initial application studies, with considerable potential for controlling intestinal pathogens in agricultural applications. However, the composition of autoinducer molecules and modes of action of these systems, as well as the crosstalk between different QS systems, are still unknown. To the best of our knowledge, comprehensive investigations have been performed only for a small number of representative bacterial strains up to now. Furthermore, most of the investigations focused on the gut microbiome and paid limited attention to the bacterial QS phenomena in other parts of the digestive tract (e.g., stomach and upper small intestine of farm animals). In addition, most of the investigations focused on single aspects of the topic.
3.1. Potential Applications in Gut Regulation
As the “universal language” of microbial communication in this system, QS exerts a significant impact on host health and other physiological processes. Given that the gut, as the body’s most important organ for digestion and absorption, is a key site of microbial interaction, it naturally becomes the priority for in-depth QS research. Notably, unlike traditional antibiotics that damage the intestinal barrier while eliminating pathogens, the QS inhibition strategy adopts an entirely distinct regulatory mechanism. Up to now, the main focus of research on QS inhibitors in monogastric animals is on the following two aspects: the first is the use of natural products as quorum-sensing inhibitors (QSIs), which have been summarized in Table 1. Intestinal diseases caused by pathogens like Salmonella and Escherichia coli are one of the limiting factors for the development of the livestock industry [16]. Jha et al. [17] found that Cyclic di-peptide Cyclo (CPP), a cyclic dipeptide from Bacillus subtilis P89, has strong anti-QS activity against Salmonella typhi. Similarly, chlorogenic acid (CA) not only can break QS in harmful bacteria but also can protect the intestinal physical barrier by affecting intestinal epithelial cells [18]. A different level of research is to evaluate chemically synthesized compounds. For instance, Nayak et al. [19] found that the chalcone derivative DC05 showed antibacterial activity against Salmonella typhimurium at a concentration of 80 μM and anti-biofilm activity at the lower concentration of 20 μM; however, the latter (anti-biofilm activity) needed a 75% decrease in compound concentration, indicating that its action on the biofilm was highly targeted and effective [19]. Despite the strong therapeutic effect of these synthesized compounds on livestock, commercialization is still faced with major challenges, including the in vivo metabolic fate of these compounds, toxic side effects on animal hosts, and complex approval procedures for application. It is also important to consider the intestinal micro-environment of the host. Pathogenic bacteria can cause toxic effects on animals through QS, whereas the endogenous microbiota of the host plays an important role in regulating the QS process and QS-mediated collective behaviors of microbiota and colonization resistance against pathogens. For example, Lactobacillus fermentum, a probiotic bacterium enriched in the pig gut, regulates its biofilm formation through the AI-2 signaling system [20]. Moreover, studies have found that the microbial QS signaling molecule N-(3-oxododecanoyl)-L-homoserine lactone (3OC12-HSL) was significantly increased in the intestinal lumen of intrauterine growth restriction (IUGR) porcine; hence, 3OC12-HSL can impair the intestinal barrier by chemically inducing oxidative stress, inducing epithelial cell apoptosis, and disrupting tight junctions [21]. In contrast, the QS signaling molecule indole can establish the intestinal epithelial barrier [22]. Therefore, it may be suggested that not all QS signaling molecules should be suppressed when regulating the gut environment. Many QS signaling molecules can regulate microbial metabolism, such as amino acid metabolism and carbohydrate metabolism, thereby affecting the efficiency of gut absorption and the utilization of various nutrients [23]. These results further suggest that treatments should focus on regulating and modulating instead of broadly inhibiting the microbial QS signaling. Recently, researchers have discovered and characterized a totally novel cell density-dependent chemical signaling system—the N-acyl-cyclolysin (ACL) system in the phylum bacteroidetes [24]. Notably, current studies also found that exogenous substances, such as artificial sweeteners, can suppress QS and further disrupt microbial metabolism and induce intestinal damage [25]. These studies revealed the existence of many unknown ways that exist in microbial communication networks. In contrast, Wu et al. [26] have established an innovative method that combines supramolecular switch technology and suppression of QS to identify and eliminate pathogens. This method provides a new approach to discovering antibiotics free from intestinal bacterial infection and other inflammatory diseases [26]. Recent advances in the study of QS signals revealed that the majority of microorganisms in the intestine of porcine weanling and finishing pigs showed significant rhythmic variations [27]. This new finding provides a unique perspective on the temporal characteristics of the gut microbial QS and guides the exploration of QS-based strategies to improve gut health and efficiency in swine production. However, existing research has almost exclusively focused on the QS regulation of gut microbiota, leaving significant gaps in studies of other critical regions within the digestive tracts of livestock and poultry. Future research in these areas holds tremendous potential.

Table 1.
Applications of natural substances related to quorum-sensing regulation in animal production.
3.2. Feed Additive Development
QSIs are increasingly utilized as feed additives, where they have demonstrated consistent efficacy in modulating intestinal microbial balance, suppressing pathogen virulence, enhancing host disease resistance, and promoting animal growth performance. There is an urgent need for antibiotic alternatives in broiler production, and QSIs offer a new option as feed additives [43]. For example, dietary supplementation with N-acylhomoserine lactonase significantly lowers the feed conversion ratio and increases the average daily gain in broilers [44]. Moreover, it was corroborated in a number of studies that plant extracts are another important source of QSIs [45], which have been summarized in detail in Table 1. Indicatively, chlorogenic acid, a natural QSI, is a very effective inhibitor of the activity of the AI-2 signaling molecule, and thus, it enhances intestinal barrier functionality [28,29]. Carvacrol acts as a competitive inhibitor by binding to the LuxR protein, which inhibits the reunion of the protein with AHL family QS signaling molecules, that is, N-butyryl-L-homoserine lactone (C4-HSL) and N-hexanoyl-L-homoserine lactone (C6-HSL). These competitive combinations also suppress the expression of both QS-related genes, such as luxR and exopolysaccharide-associated gene, which is an effective inhibitor of biofilm-forming in pathogenic bacteria and promotes a more beneficial environment with microbiota [30]. Individually, cinnamaldehyde shows a protective effect on the intestinal mucosa by its antioxidant activity, suppressing toxin-mediated alteration of the QS systems of the positive bacteria and promoting feed service [31,32]. Moreover, mannan oligosaccharides are the products obtained after the catalysis of the enzyme 2-mannanase that binds the receptors on the surface of pathogenic bacteria, thereby functioning as competitive antagonists. This binding not only prevents QS-mediated aggregation and colonization of bacteria but also helps to balance intestinal microbes and improve feed digestibility in animals [46,47]. The dosage of additives should also be controlled. Ye et al. [33] found that vanillin is a QS inhibitor and that the effect of high concentrations of vanillin also inhibited the methanogenic activity of anaerobic digestion systems. This observation implies that any intervention can be associated with the danger of unintended effects. Future feed additives that can actively detect specific pathogen QS signals, like the supramolecular traps designed by Wu et al. [26], and then synthesize and release precise antagonistic substances or competitive signaling molecules in situ, would provide a new direction for additive development [48]. Notably, nanotechnology has been taken as a fundamental, innovative, and modernizing technology in the sphere of feed additives in order to resolve the above predicaments with its unique characteristics and features like small size scale, high specific surface tension, and localized delivery. It facilitates the evolution of the conventional additives into the world of high efficiency, precision, and greenery, which presents a new avenue in enhancing the quality and efficiency of feed additives.
4. Application of QS in Ruminant Production
Compared to monogastric animals, research on QS in ruminants has gained greater prominence. However, this body of work has predominantly centered on common livestock, namely cattle and sheep, with a strong focus on the rumen environment. Consequently, numerous studies have elucidated the mechanisms of microbial QS and their associated signaling molecules within the rumen, yet investigations under production conditions are markedly limited. Similarly, research exploring QS in other anatomical locations, or QS-mediated functional implications for ruminant immunity, growth, and development, remains scarce.
4.1. Potential Applications of Rumen Regulation
Genes encoding putative proteins of the QS system (e.g., Luxl/LuxR homologs) have been found in many major rumen bacteria (Prevotella, Bacteroides, and Ruminococcus) [49]. For instance, Liu et al. [50] initially found celB-type AI-2 receptors (containing dCache_1 domain) in cellulolytic rumen bacteria. Their results showed that the receptors were associated with signaling proteins: methyl-accepting chemotaxis proteins (MCPs), which guide bacteria to feed zones with high AI-2, and histidine kinases (HKs), which modulate the expression of fiber-degrading enzyme genes—thus improving roughage degradation and energy utilization in ruminants [50]. Previous studies have shown that some chemical compounds can regulate microbial metabolism and enhance the synthesis of volatile fatty acids (VFA). For instance, Wang et al. [51] found that the structure of extracellular polymeric substances of activated sludge was changed by low-dose chloramphenicol treatment, and VFA yield was increased 2.88-fold. In addition, the latest research has also found that the QS-related genes were upregulated by low concentrations of tribromophenol, and the microbial adaptability was enhanced, and the VFA yield was significantly improved [52]. These findings from wastewater systems suggest a potential for similar mechanisms in the rumen, but this remains unexplored. Certainly, it is further supported by the positive correlation between long-chain AHLs and methane production, while the short- and medium-chain AHLs and AI-2 positively correlate with VFA levels [53]. Furthermore, in ruminant breeding, highly concentrated diets can induce the rapid replication of Streptococcus bovis (S. bovis) and result in the accumulation of too much lactic acid, causing ruminal acidosis [54]. The ComRS QS system plays an important role in the pathogenesis of S. bovis, and regulates the expression of genes related to susceptibility to cause the rapid replication of S. bovis and increasing the production of lactic acid [55]. Therefore, suppressing QS signaling to inhibit the replication of streptococci could be a strategy to prevent acidosis caused by the explosive accumulation of lactic acid. Additionally, dietary supplementation with D-ribose can suppress the rumen microbial LuxS/AI-2 QS system, but unexpectedly promote the proliferation of rumen microbes and improve production performance [56]. Conversely, 4-hydroxy-2,5-dimethyl-3(2H)-furanone can enhance the growth, as it enhances the QS system and consequent biofilm formation, which augments the concentration of antimicrobial bacteria [34]. In parallel, a recent study has also shown that indoleacetic acid (IAA) reduces the inhibitory activity of aflatoxin B1 on the important rumen microorganisms, including amylolytic bacteria, Prevotella, and Fibrobacter succinogenes [37]. The addition of IAA to the control group led to a drastic rise in acetate concentration and the acetate to propionate ratio of the fermentation fluid. This fermentation partially reconstituted the profiles of VFA in rumen fermentation, namely an increase in acetate, which is a primary source of energy in ruminants. These results indicate a potential use of IAA supplementation as a successful microbial regulatory measure to achieve rumen homeostasis and more efficient energy management. In turn, this can enhance the production performance (e.g., average daily gain and milk yield), as well as the general health of ruminants.
Given the critical role of QS in mediating microbial community coordination and metabolic synchronization. Chen et al. [57] found that adding AHLs, a typical QS signal, could greatly improve methane production in an anaerobic digestion system. If this is true, then would inhibition of methanogen-specific QS pathways and activation of VFA synthesis pathways simultaneously improve ruminant energy utilization (increase VFA absorption) and reduce methane emissions? The hypothesis stands out as a novel, high-impact research direction with profound implications for sustainable ruminant production. The hypothesis opens avenues to disrupt methanogenic consortia formation and methane biosynthesis without perturbing the rumen’s functional microflora balance. Its innovation also lies in its broader implications: In case of success, it paves the way for the development of QS-based feed additives and rumen modifiers, which aim to achieve the dual goals of improving productivity and promoting environmentally friendly animal farming, aligning with the objectives of carbon neutrality.
4.2. Immunomodulatory Function
Direct applications of QS-based technologies in the immune physiology of ruminants are still in the early stage of experimentation. Current fundamental studies focus on indirect approaches to enhance the immune system by altering rumen microbial homeostasis. Inhibit QS of dangerous bacteria in order to suppress immune stimulation. Indicatively, quercetin, a flavonoid found in plants, has been demonstrated to inhibit Escherichia coli and Staphylococcus aureus growth through a dual-mechanism by disrupting QS and directing bactericidal impact [58]. Since ruminants feed on mostly plant-based food, there may be numerous types of flavonoids naturally present at different concentrations in the digestive tract of ruminants, especially in the rumen. Moreover, Il’shat et al. [59] showed experimentally that non-specific AHL inactivators, which are presumably enzymes or adsorbents, are present in the rumen. These aspects hinder QS mediated by AHL, which may block the colonization and pathogenicity of opportunistic bacteria. All these results suggest that screening of more potent analogs or diet engineering to increase concentrations of such inhibitory agents may create a natural resistance to bacterial QS. Indicatively, tea polyphenols have been established to promote the intestinal mucosal immune barrier through boosting QS by beneficial bacteria. Tea polyphenols supplementation raises the level of these bacteria and enhances their adhesion to the intestinal cells [35]. Moreover, evidence recently showed that the levels of dietary inclusion of tea polyphenols at the dose of 4 or 6 g/kg downregulated the expression of Toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), and nuclear factor-kappa B (NF-κB) in intestinal tissue. The activation of intestinal protective processes is caused by this modulation, as well as strengthening the immune supply of nutrients by the intestinal epithelium [36]. Cold stress results in a significant rise in the propionic and butyric acid concentrations of the cecum in Hulunbuir sheep, the local sheep breed in China that is tolerant to cold, which alters physiological energy stores and natural immunity to enhance cold disposition and cut heat loss [60]. Likewise, Liu et al. [61] discovered that the rumen microbial profile of ruminants supplying with warm drinking water during cold winter changes, reducing the abundance of pro-inflammatory bacteria and enhancing the concentration of beneficial metabolites, including short-chain fatty acids (SCFA). Specific gut microbiome thermal interventions could thus be a new method of immune control in ruminants. An additional researchable approach is that of QSI. Indicatively, sotolon, a natural feranone produced as a result of fermentation by microbes or by plants, has been reported to inhibit biofilm growth as well as virulence factor generation in Pseudomonas aeruginosa [11,42]. There are two benefits of such QS inhibition. It restricts the movement of endotoxins into the bloodstream and reduces nutrient consumption by pathogenic bacteria. Exploring and implementing such analogous QS inhibition strategies could open new avenues for modulating host immunity and overall health.
5. Application of QS in Aquatic Animal Production
5.1. Aquatic Disease Prevention and Control
In aquaculture, a range of bacterial pathogens, such as the Vibrionaceae family, Vibrio, Aeromonas, and Pseudomonas genera, have been reported to use QS systems to regulate the expression of virulence factors [62]. For instance, Li et al. [63] reported that the AHL-mediated QS system in Aeromonas veronii, a pathogen present in the gut of zebrafish (a model organism for research and ornamental aquaculture), enhanced intestinal damage and improved resistance to inhibition of colonization by the gut microbiota of zebrafish. In addition, QS regulation is involved in the pathogenicity of Vibrio parahaemolyticus and Vibrio harveyi to shrimp, which are affected by luminous vibriosis (LV) and acute hepatopancreatic necrosis disease (AHPND) [64,65]. A direct strategy to disrupt this pathogenic signaling is the introduction of quorum quenching enzymes (QQE), which degrade or otherwise interfere with QS signal molecules [66]. For example, Sun et al. [67] isolated a Bacillus velezensis strain DH82 with prominent probiotic potential from the deep-sea Yap Trench (western Pacific Ocean, a high-pressure and low-temperature environment) and evaluated its efficacy in Litopenaeus vannamei aquaculture under challenge with Vibrio parahaemolyticus. The strain DH82 successfully colonized both the aquaculture water and shrimp intestines, where it suppressed the proliferation of pathogenic Vibrio species (e.g., V. parahaemolyticus). In a separate study, Haridas et al. [68] utilized response surface methodology (RSM) to optimize a multi-strain probiotic formulation containing Lactobacillus plantarum, Lactobacillus casei, and two other strains. Administration of this optimized consortium to goldfish (Carassius auratus) resulted in a 91.7% survival rate following challenges with Aeromonas hydrophila. These findings suggest a developmental trajectory for QQ-based probiotic applications toward precisely formulated, multi-strain synergistic consortia, such as consortia, that may enhance QQ efficacy via strain synergy. Concurrently, naturally sourced QSIs demonstrate significant potential, as comprehensively summarized in Table 1. For example, Payam et al. [38] found that sea cucumber saponins (triterpenoid saponins, the major bioactive components of sea cucumbers such as Apostichopus japonicus) can attenuate the virulence of Aeromonas hydrophila, the causative agent of piscine hemorrhagic septicemia (PHS). Specifically, these compounds reduce the activity of key secreted enzymes, including protease, lipase, and most notably, hemolysin, the activity of which was reduced by 44% compared to the A. hydrophila control group without saponin treatment. Similarly, a recent study has found that methyl gallate (MG), a compound isolated from the flowers of Camellia nitidissima (a rare and endemic plant in China belonging to the Theaceae family), also suppresses the activity of QS-associated virulence factors in A. hydrophila [39]. For example, studies by Yang et al. [40] and Lv et al. [41] have identified the dipeptide serine-phenylalanine (Ser-Phe), derived from myosin hydrolysates of the shrimp Litopenaeus vannamei, as a potent endogenous QSI. This dipeptide significantly inhibits the AI-2 QS system in Vibrio parahaemolyticus, consequently downregulating the expression of its virulence genes. In addition to natural extracts and probiotic metabolites, phage display screening technology offers an effective means of precisely developing new QSIs to augment considerably in targeted and efficacious aquatic disease prevention and management. This technology requires a random array of peptide libraries or functional protein fragments in display on the phage surface, targeting QS signaling molecules or directed onto the pathogenic bacteria surface with specific receptors are then used to screen the affinity. It allows the active peptides or protein fragments to be enriched very quickly, and they are capable of specific attachment to the targets, which can be converted into potent QSIs or pathogen-targeted inhibitors [69]. Additionally, Deng et al. [70] found that the LuxS/AI-2 system in Lactobacillus rhamnosus GG alleviates inflammation caused by enterotoxigenic Escherichia coli by promoting biofilm formation and enhancing immune responses, thereby maintaining intestinal balance and promoting fish growth. In contrast, the probiotic Priestia sp. PPB30 disrupts the LuxS/AI-2 QS system of Aeromonas veronii, a typical pathogen of freshwater fish. This disruption reduces the pathogen’s colonization in the intestines of loaches, leading to better health and production yield in this host [71]. Equally, experimental data also support the fact that Bacillus velezensis MT9 helps to maximize gut microbiota structure in Nile tilapia (Oreochromis niloticus) [72]. The observed optimization, facilitated by the control of the intestinal LuxS/AI-2 QS system, has a positive impact on fish growth rates and immunity, which, in the end, increases the productivity of the aquaculture.
5.2. Aquaculture Environmental Management
The cross-regulation between soil microbiomes and adjacent water ecosystems is critical—soil-based pollutants can easily enter the waters of aquaculture of organisms, endangering the well-being of organisms that cope with such releases, as well as the stability of the ecosystems. In this way, the QS signaling molecules will be applicable in the regulation of the soil microbiome towards remediation, which would indirectly benefit the aquaculture sustainability. For γ-hexachlorocyclohexane (γ-HCH)-contaminated soil, a soil bioelectrochemical system (BES) was constructed, and pollutant transformation was enhanced by the modulation of microbial community interactions and electron transfer [73]. Ultimately, the BES-based technology could decrease the migration of γ-HCH from the soil to the adjacent water environment, which alleviated the auto-purification load of aquaculture sediments and prevented γ-HCH-induced toxicity to aquatic organisms. It is worth mentioning that various biochar-related regulatory approaches have different impacts on QS systems and pollutant removal, the effects of which must not be confused. Another research group found that persistent free radicals (PFRs) retained in biochar could disturb the QS system [74]. Conversely, in biochar with the QS signal molecule C4-HSL, the approach to the integrated treatment of the system was synergistic regulation in which C4-HSL specifically triggered the QS pathways of γ-HCH-degrading bacteria, and biochar offered an appropriate microhabitat to these bacteria [73]. For waste treatment, Zhou et al. [75] found that the QS signal molecule 3OC12-HSL could maintain the electrochemical activity of electroactive biofilms (EABs) against high-salinity shocks. This research proposed a new strategy to solve the low efficiency and process stability problems of high-salinity waste treatment in aquaculture. Additionally, recently, a study reported that the exogenous application of N-(3-Oxotetradecanoyl)-L-homoserine lactone (3-oxo-C14-HSL) can induce the expression of nitrogen metabolism and QS genes in biofilm and other nitrogen metabolism genes, thereby enriching for denitrifying bacteria [76]. This is a possible way to solve the problem of biofilm formation and the stability of the framework in aquaculture wastewater treatment. Parallel to this, the emergence of QQ broadens new ideas for environmental remediation. For instance, Yu et al. [77] selected Pseudomonas putida YH-1, whose QQ activity is strong. The activity is mediated by an AHL acyltransferase. The enzyme can preferentially degrade long-chain AHLs. This way can effectively inhibit the biofilm formation of pathogenic bacteria and further attenuate the biofouling of equipment surfaces. While Xue et al. [78] transformed Pseudomonas putida KT2440 and constructed a synthetic QQ gene, which generated an engineered bacterium with strong AHL-degrading ability. This engineered bacterium can not only suppress biofilm formation in activated sludge but also enhance the removal of aromatic pollutants. Therefore, this method can be used for both environmental remediation and disease control. However, the application of QQ bacteria in open aquaculture systems is limited by socio-ethical issues and the long-term ecological effects assessments. Aquaculture facilities constantly emit malodorous, polluting gases. These mainly originate from the anaerobic decomposition of organic matter, including residual feed, organisms’ excreta, and other biological detritus [79]. To control the accumulation of biomass in biofilters through inhibition of microbial QS, a recent study has devised a technology for reducing the accumulation of biological solids in biofilters [80]. Researchers added Rhodococcus sp. BH4 to the gas biofilter, and this strain is capable of inhibiting the QS mechanism. Compared with the control group, this greatly reduced the accumulation of biomass in the biofilter tower [80]. This technology also reduced the possibility of clogging of the packing layer. Importantly, this did not reduce the volatile organic compounds (VOCs) removal efficiency. In principle, this is applicable for the biological treatment of odorous gas from aquaculture, but further study is needed for its long-term effects in field applications. This is because the composition of pollutants from aquaculture is relatively complex, and there may be differences between the laboratory and field conditions. The potential applications of microbial QS in animal production are summarized in Figure 1.
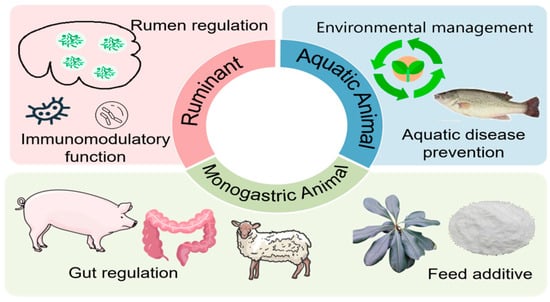
Figure 1.
The summary of the potential application of microbial quorum sensing in animal production.
6. Innovation Research and Development
In modern animal production systems, the main concern is the search for QSIs, and it is the current focus of studies around the world. There are two different kinds of exploratory approaches: the traditional bioprospecting of nature and the high-throughput screening based on modern molecular biology techniques. The study by Jha et al. [17] represents a conventional screening approach, wherein they analyzed cell supernatants from fifteen bacterial isolates. This process led to the identification of a potent QS signal molecule, the cyclic dipeptide cyclo(L-Phe-L-Pro), from Bacillus subtilis P89. The current research has shifted from traditional inhibitor screening to nano-integration technologies. The study published by Zhang et al. [69] developed a high-throughput screening method based on phage display peptide libraries and QS biosensor systems. In their method, the biosensor is used to quantify signal intensity and screen the QS biosensor inhibitors with weak signal from huge peptide libraries. In the future, if we can combine the two approaches, it can speed up the development of new inhibitors. With further studies on QS, more and more new applications are reported in a variety of fields. In the field of microbiology, studies constructed a functional nucleic acid nano-framework platform [81]. They found that intraspecific spatial assembly could greatly enhance the expression of genes encoding surface sensors (e.g., fimbriae, flagellar proteins) of Pseudomonas aeruginosa and the expression of virulence factors, providing a new way to regulate the interaction between microbes. However, it is still challenging to solve the technical and social acceptance problems of nanotechnology application [82]. In materials science, the latest research showed that hydrothermal carbon in a microbial-material hybrid system can regulate microbial QS communication [83]. With this new discovery, for aquaculture environmental management, it is foreseeable that in the future, functional materials could be designed to respond to the environment by selectively adsorbing and accurately releasing QS signaling molecules or their inhibitors in a specific environment to direct microbial collective behavior. Zou et al. [84] designed a multifunctional coating with three lines of defense: anti-adhesion, antibacterial, and anti-QS. This combination strategy was much more effective than either single antibacterial coating or QS inhibition. This provides a new viewpoint to deal with facility contamination, disease transmission, and lower production efficiency caused by pathogenic bacterial biofilms in animal production. In the future, it may also be possible to target the microbial community rather than individual species.
7. Challenges and Limitations
Although QS-based technologies hold great potentials to revolutionize the sustainable animal production, diverse unresolved challenges and intrinsic factors are posing detriment impacts on their large-scale and widespread implementation. These issues require thorough understanding and systematic responses. Among them, the biosafety and environmental risks also take the center stage: nanomaterial-based QS modulation strategies and engineered QQ bacteria have already been shown to be effective in preventing the transmission of antibiotic resistance genes or targeted QS inhibition, although their long-term accumulation into animal tissues could result in unknown toxic effects, and leakage into soil and water systems during production would pose unevaluated ecological risks, an issue aggravated by the low level of acceptance of nanotechnology and genetically modified microorganisms by the general public in food-related activities. Practical use is further hampered by two main factors. First, most natural or synthetic QSIs, such as CA and CPP, degrade under high-pressure, high-temperature feed pelting conditions. Second, the gastrointestinal environment destroys the structural integrity and functional properties of QSIs and QQE before they reach their target sites, as the current delivery system lacks effective protection mechanisms [85]. Natural products are produced in certain environmental niches, and how to realize the large-scale and standardized production of these natural products is still a problem to be solved. There is the further complication of the host-microbe specificity dilemma: non-specific QS regulation can destabilize beneficial microbial QS communication and stimulate pathogenic bacteria, but broad-spectrum QSIs or QQ strategies can inadvertently inhibit these pathways, leading to microbial ecosystem imbalances and impaired host immunity, as exemplified by vanillin’s “off-target effect” of suppressing methanogenic activity at high concentrations. Lastly, industrialization has been restricted by cost-effectiveness and regulation: large scale recovery of natural QSIs (e.g., sea cucumber saponins and tea polyphenols) are prohibited by complicated recovery routes and low yield, synthetic QSIs are prohibited by complex chemical manufacturing at high raw material expense and new QS-based goods, in particular nanomaterials and engineered microorganisms, have been of lengthy, resource-intensive regulatory acceptance, which creates uncertainty that may not translate research success into a practical product.
8. Conclusions and Future Perspectives
Current QS-based technologies have achieved preliminary integration of mechanisms and applications in animal production. The functions of three signal molecules—AHLs, AIPs, and AI-2—have been validated in maintaining the intestinal health of monogastric animals, optimizing rumen function in ruminants, and controlling aquatic pathogens, thereby providing new pathways for antibiotic-free aquaculture. Studies on QSIs have become a topical issue nowadays, yet the question of whether QSIs have an impact on the QS communication of beneficial microbial communities per se and, consequently, on their probiotic actions has become an urgent question to answer. In the meantime, the potential of group-QQEs for the application has evolved into a hot spot of research. However, regarding the tension between specificity and broad-spectrum activity, how can we simultaneously target the QS systems of diverse microbial populations for extensive regulation while precisely targeting specific signaling molecules to avoid disrupting beneficial microbial communities’ QS communication and probiotic functions? This remains a subject requiring further exploration within the scientific community. The further breakthrough of QS regulation technology can center on accuracy, smartness, and industrialization, with technological innovation, experimental validation, and field application as the main drivers: produce QS biosensors for real-time monitoring, unravel the tripartite interaction pathway mechanism using single-cell sequencing, and create heat-resistant, specific-release QS modulators inside nanocarriers to address the issues of industrialization. Over time, the creation of the QS regulation technology will inevitably evolve into the implementation of intervention on individual species to the regulation of the community of microbes in its entirety. QS-based technologies are expected to provide effective solutions for antibiotic substitution in animal production, production efficiency improvement, green breeding mode construction, and ruminant methane emission reduction, and ultimately help build a more efficient and environmentally friendly modern animal production system to promote the realization of carbon neutrality goals [86].
Author Contributions
Conceptualization, Q.Q.; methodology, K.O. and M.Q.; validation, C.T.; formal analysis, C.T.; data curation, C.T. and Q.Q.; writing—original draft preparation, C.T.; writing—review and editing, Q.Q.; supervision, K.O. and M.Q.; project administration, Q.Q.; funding acquisition, Q.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32260861; the Major Discipline Academic and Technical Leaders Training Program of Jiangxi Province, grant number 20243BCE51165; the Jiangxi Provincial Natural Science Foundation, grant number 20232BAB215051; and the Jiangxi Provincial Key Research and Development Program Project, grant number 20232BBF60009.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| QS | Quorum sensing |
| AHLs | N-acylhomoserine lactones |
| QSIs | Quorum-sensing inhibitors |
| AIPs | Autoinducing peptides |
| AI-2 | Autoinducer-2 |
| SAM | S-adenosylmethionine |
| ACP | Acyl carrier protein |
| SRH | S-ribosylhomocysteine |
| CPP | Cyclic di-peptide cyclo |
| CA | Chlorogenic acid |
| Quorum quenching | |
| QQE | Quorum quenching enzymes |
| ACL | N-acyl-cyclolysin |
| IAA | Indoleacetic acid |
| MG | Methyl gallate |
| Ser-Phe | Serine-phenylalanine |
| LV | Luminous vibriosis |
| AHPND | Acute hepatopancreatic necrosis disease |
| PHS | Piscine demorrhagic septicemia |
| EABs | Electroactive biofilms |
| 3OC12-HSL | N-(3-Oxododecanoyl)-L-homoserine lactone |
| 3-oxo-C14-HSL | N-(3-Oxotetradecanoyl)-L-homoserine lactone |
| C4-HSL | N-butyryl-L-homoserine lactone |
| C6-HSL | N-hexanoyl-L-homoserine lactone |
| ARG | Antibiotic resistance gene |
| VFA | Volatile fatty acids |
| SCFA | Short-chain fatty acids |
| RSM | Response surface methodology |
| VOCs | Volatile organic compounds |
| γ-HCH | γ-hexachlorocyclohexane |
| BES | Bioelectrochemical system |
| PFRs | Persistent free radicals |
| RAS | Recirculating aquaculture systems |
| MCP | Methyl-accepting chemotaxis protein |
| HK | Histidine kinase |
| TLR4 | Toll-like receptor 4 |
| MyD88 | Myeloid differentiation primary response 88 |
| NF-κB | Nuclear factor-kappa B |
References
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970, 104, 313–322. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Q.; Yang, Y.; Mai, Q.; Zhou, Y.; Liu, Y.; Liu, Y.; Liu, J. Reshaping bacterial microenvironments: Hybrid biomimetic membrane-coated copper nanosystems combat bacterial biofilm infections by inhibiting bacterial quorum sensing systems. Chem. Eng. J. 2025, 512, 162088. [Google Scholar] [CrossRef]
- Corcoran, C.J.; Cuthbert, B.J.; Glanville, D.G.; Terrado, M.; Valverde Mendez, D.; Bratton, B.P.; Schemenauer, D.E.; Tokars, V.L.; Martin, T.G.; Rasmussen, L.W. A glyoxal-specific aldehyde signaling axis in Pseudomonas aeruginosa that influences quorum sensing and infection. Nat. Commun. 2025, 16, 6616. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I. Biofilm, resistance, and quorum sensing: The triple threat in bacterial pathogenesis. Microbe 2025, 9, 100578. [Google Scholar] [CrossRef]
- Xu, J.; Wen, X.; Wang, S.; Worrich, A.; Ma, B.; Zou, Y.; Wang, Y.; Wu, Y. Identification of key species and molecular mechanisms driving conjugative transfer of antibiotic resistance genes in swine manure-derived bacterial communities. J. Hazard. Mater. 2025, 497, 139638. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guan, W.; Fan, Y.; He, Q.; Guo, D.; Yuan, A.; Xing, Q.; Wang, Y.; Ma, Z.; Ni, J. Zinc/carbon nanomaterials inhibit antibiotic resistance genes by affecting quorum sensing and microbial community in cattle manure production. Bioresour. Technol. 2023, 387, 129648. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Quadri, L.E.; Kuipers, O.P.; De Vos, W.M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol. Microbiol. 1997, 24, 895–904. [Google Scholar] [CrossRef]
- Dunlap, P.V. Quorum regulation of luminescence in Vibrio fischeri. J. Mol. Microbiol. Biotechnol. 1999, 1, 5–12. [Google Scholar]
- Sánchez-Jiménez, A.; Llamas, M.A.; Marcos-Torres, F.J. Transcriptional regulators controlling virulence in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2023, 24, 11895. [Google Scholar] [CrossRef]
- Dickschat, J.S. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 2010, 27, 343–369. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Varga, J.; Chandler, J.R.; Peterson, S.B.; Herman, J.P.; Churchill, M.E.; Parsek, M.R.; Nierman, W.C.; Greenberg, E.P. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J. Bacteriol. 2009, 191, 3909–3918. [Google Scholar] [CrossRef]
- Sturme, M.H.; Kleerebezem, M.; Nakayama, J.; Akkermans, A.D.; Vaughan, E.E.; De Vos, W.M. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie. Leeuwenhoek 2002, 81, 233–243. [Google Scholar] [CrossRef]
- Mitsumori, M.; Xu, L.; Kajikawa, H.; Kurihara, M.; Tajima, K.; Hai, J.; Takenaka, A. Possible quorum sensing in the rumen microbial community: Detection of quorum-sensing signal molecules from rumen bacteria. FEMS. Microbiol. Lett. 2003, 219, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; El-Adawy, H.; Abdelwhab, E.M. A comprehensive review of common bacterial, parasitic and viral zoonoses at the human-animal interface in Egypt. Pathogens 2017, 6, 33. [Google Scholar] [CrossRef]
- Jha, N.K.; Kumar, L.L.; Sivasankar, C.; Gopu, V.; Devi, P.B.; Murali, A.; Shetty, P.H. Cyclic di-peptide Cyclo (L-Phe-L-Pro) mitigates the quorum-sensing mediated virulence in Salmonella typhi and biofilm formation in poultry and plastic system. Food Biosci. 2024, 60, 104391. [Google Scholar] [CrossRef]
- Dong, W.; Liang, G.; Gang, G.; Lu-fang, D. Research progress on role of chlorogenic acid and its application in animal production. Feed Res. 2024, 47, 163. [Google Scholar]
- Nayak, S.P.R.R.; Pohokar, P.; Das, A.; Dhivya, L.S.; Pasupuleti, M.; Soundharrajan, I.; Almutairi, B.O.; Kumaradoss, K.M.; Arockiaraj, J. Chalcone derivative enhance poultry meat preservation through quorum sensing inhibition against Salmonella (Salmonella enterica serovar Typhi) contamination. Food Control 2025, 171, 111155. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, H.; Zheng, Y.; Wang, Y.; He, Y.; Gu, Y. Use of whole-genome analysis to study the effect of various quorum-sensing inhibitors on the biofilm formation of Lactobacillus fermentum. LWT 2023, 173, 114378. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, Z.; Xiong, Y.; Wu, Z.; Tan, X.; Yang, Y.; Zhang, H.; Zhu, X.; Wei, H.; Tao, S. N-(3-oxododecanoyl)-homoserine lactone disrupts intestinal barrier and induces systemic inflammation through perturbing gut microbiome in mice. Sci. Total Environ. 2021, 778, 146347. [Google Scholar] [CrossRef]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS ONE 2013, 8, e80604. [Google Scholar] [CrossRef]
- Ha, J.-H.; Hauk, P.; Cho, K.; Eo, Y.; Ma, X.; Stephens, K.; Cha, S.; Jeong, M.; Suh, J.-Y.; Sintim, H.O. Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr. Sci. Adv. 2018, 4, eaar7063. [Google Scholar] [CrossRef]
- Linares-Otoya, L.; Shirkey, J.D.; Chhetri, B.K.; Mira, A.; Biswas, A.; Neff, S.L.; Linares-Otoya, M.V.; Chen, Y.; Campos-Florian, J.V.; Ganoza-Yupanqui, M.L. Discovery of a widespread chemical signalling pathway in the Bacteroidota. Nature 2025, 646, 423–432. [Google Scholar] [CrossRef]
- Markus, V.; Paul, A.A.; Teralı, K.; Özer, N.; Marks, R.S.; Golberg, K.; Kushmaro, A. Conversations in the gut: The role of quorum sensing in normobiosis. Int. J. Mol. Sci. 2023, 24, 3722. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Borjihan, Q.; Su, Y.; Bai, H.; Hu, X.; Wang, X.; Kang, J.; Dong, A.; Yang, Y.-W. Supramolecular switching-enabled quorum sensing trap for pathogen-specific recognition and eradication to treat enteritis. J. Am. Chem. Soc. 2024, 146, 35402–35415. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. mSystems 2020, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chu, W.; Ye, C.; Gaeta, B.; Tao, H.; Wang, M.; Qiu, Z. Chlorogenic acid attenuates virulence factors and pathogenicity of Pseudomonas aeruginosa by regulating quorum sensing. Appl. Microbiol. Biotechnol. 2018, 103, 903–915. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Liu, Y.; Xu, M.; Yao, Z.; Zhang, X.; Sun, Y.; Zhou, T.; Shen, M. Effects of chlorogenic acid on antimicrobial, antivirulence, and anti-quorum sensing of carbapenem-resistant Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 997310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, L.; Lu, H.; Zhu, J.; Venkitanarayanan, K.; Liu, X. Transcriptomic analysis of the food spoilers Pseudomonas fluorescens reveals the antibiofilm of carvacrol by interference with intracellular signaling processes. Food Control 2021, 127, 108115. [Google Scholar] [CrossRef]
- Subash-Babu, P.; Alshatwi, A.A.; Ignacimuthu, S. Beneficial antioxidative and antiperoxidative effect of cinnamaldehyde protect streptozotocin-induced pancreatic β-cells damage in wistar rats. Biomol. Ther. 2014, 22, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Celen, S.; Hillaert, U.; van Calenbergh, S.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS ONE 2011, 6, e16084. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Li, L.; Zou, M.; Han, L.; Xu, P.; Gu, Y.; Yuan, R.; Peng, X. Vanillin as a quorum sensing inhibitor: Effect on extracellular polymeric substance production and defoaming potential in anaerobic digestion system. Chem. Eng. J. 2025, 512, 162762. [Google Scholar] [CrossRef]
- Fu, C.; Ge, J.; Qu, M.; Ouyang, K.; Qiu, Q. Effects of 4-hydroxy-2, 5-dimethyl-3(2H)-furanone supplementation on growth performance, serum antioxidant capacity, rumen fermentation characteristics, rumen bacterial quorum sensing, and microbial community in Hu sheep. Anim. Biosci. 2025, 38, 1422–1434. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Xu, Y.; He, X.; Gan, S.; Yin, F. The effects of tea polyphenols in feed on the immunity, antioxidant capacity, and gut microbiota of weaned goat kids. Animals 2025, 15, 467. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Yan, X.; Zhao, Y.; Tan, L.; Miao, X.; Zhao, R.; Huo, W.; Chen, L.; Li, Q. The study on the impact of indoleacetic acid on enhancing the ability of the rumen’s original microecology to degrade aflatoxin B1. bioRxiv 2024, bioRxiv:2024.08.02.606440. [Google Scholar]
- Payam, B.; Soltani, M.; Mehrgan, M.S.; Rajabi Islami, H.; Nazemi, M. Saponins from sea cucumber disrupt Aeromonas hydrophila quorum sensing to mitigate pathogenicity. AMB. Expr. 2025, 15, 43. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Jia, A. Methyl gallate from Camellia nitidissima Chi flowers reduces quorum sensing related virulence and biofilm formation against Aeromonas hydrophila. Biofouling 2024, 40, 64–75. [Google Scholar] [CrossRef]
- Yang, W.; Liu, S.; Liu, X.; Yuan, S.; An, L.; Ren, A.; Bai, F.; Lv, X.; Li, J.; Li, X. Novel quorum-sensing inhibitor peptide SF derived from Penaeus vannamei myosin inhibits biofilm formation and virulence factors in Vibrio parahaemolyticus. LWT 2025, 218, 117542. [Google Scholar] [CrossRef]
- Lv, X.; Yang, W.; Liu, S.; Liu, X.; Yuan, S.; An, L.; Ren, A.; Bai, F.; Li, J.; Li, X. Inhibition effect of marine active peptides SF on dual-species biofilms of Vibrio parahaemolyticus and Aeromonas sobria. Food Biosci. 2024, 61, 104697. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Khafagy, E.-S.; Saqr, A.A.; Alalaiwe, A.; Abbas, H.A.; Shaldam, M.A.; Hegazy, W.A.; Goda, R.M. Tackling virulence of Pseudomonas aeruginosa by the natural furanone sotolon. Antibiotics 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Chen, X.; Zhang, G.M.; Wang, Z.D.; Chen, Z.M.; Chang, W.H.; Chai, H.Y.; Liu, G.H. Effects of N-acylhomoserine lactonase on growth performance, slaughter performance, and nutrient apparent metabolism rate of broiler chickens. Chin. J. Anim. Nutr. 2023, 35, 3607–3616. [Google Scholar]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, Y.; Dou, Y.; Xu, Z.; Tao, W.; Zhou, P. Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 2004, 8, 447–454. [Google Scholar] [CrossRef]
- Asbury, R.E.; Saville, B.A. Manno-oligosaccharides as a promising antimicrobial strategy: Pathogen inhibition and synergistic effects with antibiotics. Front. Microbiol. 2025, 16, 1529081. [Google Scholar] [CrossRef]
- Wang, D.; Cui, F.; Ren, L.; Li, J.; Li, T. Quorum-quenching enzymes: Promising bioresources and their opportunities and challenges as alternative bacteriostatic agents in food industry. Compr. Rev. Food Sci. Food. Saf. 2023, 22, 1104–1127. [Google Scholar] [CrossRef]
- Won, M.; Oyama, L.B.; Courtney, S.J.; Creevey, C.J.; Huws, S.A. Can rumen bacteria communicate to each other? Microbiome 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Q.; Sun, S.; Sun, H.; Wang, Y.; Shen, X.; Zhang, L. Exploring AI-2-mediated interspecies communications within rumen microbial communities. Microbiome 2022, 10, 167. [Google Scholar] [CrossRef]
- Wang, F.; Luo, Y.; Xue, Z.; Feng, Q.; Cao, J.; Wu, Y.; Li, X.; Luo, J. Double-edged sword of extracellular polymeric substances disintegration under chloramphenicol exposure in volatile fatty acids production and antibiotic resistance genes proliferation during sludge fermentation. Chem. Eng. J. 2025, 512, 162569. [Google Scholar] [CrossRef]
- Niu, Q.; Zhang, X.; Wu, Y.; Sun, C.; Zheng, X.; Long, M.; Chen, Y. Protein transformation induced by tribromophenol regulates volatile fatty acids production during sludge fermentation: Interplay between structural modification and metabolic modulation. Chem. Eng. J. 2025, 520, 165971. [Google Scholar] [CrossRef]
- Shah, S.; Park, H.; Park, H.; Kim, J.; Mameda, N.; Choo, K. The relationship between quorum sensing dynamics and biological performances during anaerobic membrane bioreactor treatment. Bioresour. Technol. 2022, 363, 127930. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Wang, H. Metabolic mechanism of acid production by Streptococcus bovis in rumen and its regulation. Chin. J. Anim. Nutr. 2016, 28, 665–673. [Google Scholar]
- Haustenne, L.; Bastin, G.; Hols, P.; Fontaine, L. Modeling of the ComRS signaling pathway reveals the limiting factors controlling competence in Streptococcus thermophilus. Front. Microbiol. 2015, 6, 1413. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, Y.; Zhao, X.; Ouyang, K.; Qu, M.; Qiu, Q. Dietary supplementation with D-ribose enhances growth performance, improves serum antioxidant capacity, and inhibits rumen microbial LuxS/AI-2 quorum sensing of Hu sheep. Anim. Biosci. 2025, in press. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, P.; Li, Y.; Liang, J.; Zhang, G. Genome-centric metagenomic analysis reveals mechanisms of quorum sensing promoting anaerobic digestion under sulfide stress: Syntrophic metabolism and microbial self-adaptation. Sci. Total Environ. 2024, 954, 176240. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Ma, Y.; Yao, Y.; Yu, D.; Shen, J.; Wu, L. The mitigation potential of synergistic quorum quenching and antibacterial properties for biofilm proliferation and membrane biofouling. Water. Res. 2024, 255, 121462. [Google Scholar] [CrossRef]
- Il’shat, K.; Galimzhan, D.; Kseniya, I.; Madina, K. Inhibition of bacterial quorum sensing by the ruminal fluid of cattle. Geomate. J. 2017, 13, 88–92. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, Y.; Ji, K.; Feng, M.; Du, X.; Jiao, D.; Wu, X.; Zhong, C.; Cong, H.; Yang, G. Enhanced propionate and butyrate metabolism in cecal microbiota contributes to cold-stress adaptation in sheep. Microbiome 2025, 13, 103. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Dai, J.; Qu, M.; Ouyang, K.; Qiu, Q. Heated drinking water in winter improves growth performance of male Hu sheep by modulating rumen quorum sensing and metabolites, and enhancing serum antioxidant capacity. Anim. Biosci. 2025, 38, 2280–2296. [Google Scholar] [CrossRef]
- Gupta, D.S.; Kumar, M.S. The implications of quorum sensing inhibition in bacterial antibiotic resistance-with a special focus on aquaculture. J. Microbiol. Methods 2022, 203, 106602. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Han, S.; Chen, Z.; Qin, M.; Liu, L.; Jiang, X.; Wang, H. The effect of N-acyl-homoserine lactones-mediated quorum sensing on intestinal colonization and damage by Aeromonas veronii. Aquaculture 2022, 561, 738627. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.M.; Griffin, M.; Francis-Floyd, R.; Baker, S.; Camus, A.; Pelton, C.; Dill-Okubo, J. Vibrio harveyi in a Caribbean spiny lobster (Panulirus argus) with hepatopancreas necrosis. Vet. Pathol. 2023, 60, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, J.; Deng, S.; Li, R.; Lv, W.; Zhou, S.; Tang, X.; Sun, Y.Z.; Ke, M.; Wang, K. Quorum quenching bacteria Bacillus velezensis DH82 on biological control of Vibrio parahaemolyticus for sustainable aquaculture of Litopenaeus vannamei. Front. Mar. Sci. 2022, 9, 780055. [Google Scholar] [CrossRef]
- Haridas, D.V.; Joshy, C.; Pillai, D. Optimization of the multispecies probiotic combination with N-acylhomoserine lactone-degrading ability for increased disease resistance of Carassius auratus using response surface methodology. Aquaculture 2022, 548, 737597. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Yao, Z.; Wang, X.; Chen, J.; Yang, W.; Yao, J.; Lin, Y.; Liu, Z.; Lin, X. A high-throughput screening approach for bacterial quorum sensing inhibitors (QSIs) against Aeromonas hydrophila infection. Aquaculture 2022, 560, 738488. [Google Scholar] [CrossRef]
- Deng, Z.; Hou, K.; Valencak, T.G.; Luo, X.M.; Liu, J.; Wang, H. AI-2/LuxS quorum sensing system promotes biofilm formation of Lactobacillus rhamnosus GG and enhances the resistance to enterotoxigenic Escherichia coli in germ-free zebrafish. Microbiol. Spectr. 2022, 10, e00610-22. [Google Scholar] [CrossRef]
- Gao, C.; Yang, W.; Duan, Y.; Niu, W.; Wang, Y.; Qin, M.; Li, Y. Dietary supplementation of Priestia sp. PPB30 attenuates intestinal colonization and inflammatory response induced by Aeromonas veronii in loach through interfering with the LuxS/AI-2 quorum sensing system. Fish Shellfish Immunol. 2025, 165, 110576. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, M.; Quarta, E.; Sicuro, A.; Pecoraro, L.; Schiavone, R.; Tredici, S.M.; Talà, A.; Corallo, A.; Verri, T.; Stabili, L.; et al. Effect of Bacillus velezensis MT9 on nile tilapia (Oreochromis Niloticus) intestinal microbiota. Microb. Ecol. 2025, 88, 37. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, J.; Su, X.; Xu, J.; He, Y. Conductive material and AHLs addition altered soil microbiome and facilitated γ-HCH dechlorination but inhibited CH4 cumulation. Soil. Biol. Biochem. 2024, 191, 109347. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, K.; Chen, Z.; Li, N.; Wang, M. Biochar reduced the risks of human bacterial pathogens in soil via disturbing quorum sensing mediated by persistent free radicals. Environ. Sci. Technol. 2024, 58, 22343–22354. [Google Scholar] [CrossRef]
- Zhou, S.; An, W.; Zhao, K.; Lin, L.; Yang, S.; Zhang, Y.; Xu, M. Protection of electroactive biofilms against hypersaline shock by quorum sensing. Water. Res. 2023, 233, 119823. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Gong, W.; Zhang, K.; Wang, G.; Xia, Y.; Yu, M.; Xie, W.; Lu, Z.; Cheng, X. Effect of exogenous acylhomoserine lactone 3-oxo-C14-HSL on the performance of biofilm in moving bed biofilm reactor. J. Water. Process. Eng. 2024, 64, 105595. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, Z.; Li, X.; Wan, P.; Zhang, J.; Peng, C. Gamma-caprolactone-based isolation of Pseudomonas sp. YH-1: A novel quorum quenching bacterium for AHL degradation and biofouling control. Chem. Eng. J. 2025, 520, 166280. [Google Scholar] [CrossRef]
- Xue, Y.M.; Wang, Y.C.; Lin, Y.T.; Jiang, G.Y.; Chen, R.; Qin, R.L.; Jia, X.Q.; Wang, C. Engineering a Pseudomonas putida as living quorum quencher for biofilm formation inhibition, benzenes degradation, and environmental risk evaluation. Water. Res. 2023, 246, 120690. [Google Scholar] [CrossRef]
- Xiang, K.; Sun, H.F.; Xu, Y.Q.; Pei, L.W.; Zhao, J.; Ye, Z.Y. Advances in the production and removal of off - flavors in recirculating aquaculture systems (RAS) for the cultivation of freshwater fish. Chin. J. Fish. 2024, 48, 3–15. [Google Scholar]
- Wang, Y.C.; Wang, C.; Han, M.F.; Tong, Z.; Lin, Y.T.; Hu, X.R.; Deng, J.G.; Hsi, H.C. Inhibiting effect of quorum quenching on biomass accumulation: A clogging control strategy in gas biofilters. Chem. Eng. J. 2022, 432, 134313. [Google Scholar] [CrossRef]
- Chen, N.; Xi, J.; Du, N.; Shen, R.; Zhao, R.; He, W.; Peng, T.; Yang, Y.; Zhang, Y.; Yu, L. Framework nucleic acid strategy enables closer microbial contact for programming short-range interaction. Sci. Adv. 2024, 10, eadr4399. [Google Scholar] [CrossRef]
- Rao, L.; Yuan, Y.; Shen, X.; Yu, G.; Chen, X. Designing nanotheranostics with machine learning. Nat. Nanotechnol. 2024, 19, 1769–1781. [Google Scholar] [CrossRef]
- Kang, Z.; Li, N.; Han, X.; Wang, C.; Yang, X.; Yu, H. Constructing hybrid microorganism-material systems to improve pollutant bioremediation: Investigating the improved bioactivity and quorum sensing of a functional strain with acid-added microwave hydrochar. Chem. Eng. J. 2025, 506, 160384. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, C.; Zhang, H.; Wu, Y.; Lin, Y.; Cheng, J.; Lu, K.; Li, L.; Zhang, Y.; Chen, H. Three lines of defense: A multifunctional coating with anti-adhesion, bacteria-killing and anti-quorum sensing properties for preventing biofilm formation of Pseudomonas aeruginosa. Acta Biomater. 2022, 151, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.; Lee, J.-K. Bacterial biofilm inhibitors: An overview. Ecotox. Environ. Saf. 2023, 264, 115389. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Goswami, D.; Saraf, M. Decoding quorum sensing: The language of bacteria for sustainable development. In Climate Change and Soil Microorganisms for Environmental Sustainability; Springer Nature: Singapore, 2025; pp. 23–46. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).