Abstract
Laifeng ginger (Zingiber officinale cv. Fengtoujiang) is a famous Geographical Indication (GI) ginger variety, which grows specifically in Laifeng County, Hubei, China. In recent years, it faced a serious food safety issue of lead (Pb) exceedance in the rhizomes even though the Pb content in the soil remains at a safe level. This problem severely hinders the local ginger’s market growth. In the present study, a field study across 37 Laifeng ginger farms revealed a connection between the occurrence of Pb exceedance and the choices of fertilizers. Cultivation experiments demonstrated that with more organic fertilizer (OF) applied, the Pb of rhizome effectively declined, and the branching and longitudinal growth were enhanced. The OF application facilitated Pb translocation from rhizomes to stems and leaves. Furthermore, we showed that OF improved the soil properties by altering the pH and the composition of soil microbial communities at the genus level, which was likely to be associated with reduced the Pb content in the ginger rhizomes. This research tackles the critical industry issue of Pb exceedance in Laifeng ginger, providing a basis for the fertilization of root and tuber plants with excessive heavy metal levels, and establishes a foundation for sustainable GI product development.
1. Introduction
Agricultural products covered under the China–EU Geographical Indications (GIs) Agreement are widely recognized for their superior quality and significant added value. However, in recent years, growing concerns have emerged regarding the food safety incidents associated with these GI products. Laifeng ginger’s (Zingiber officinale cv. Fengtoujiang) tubers have been consumed in Laifeng County, Enshi Prefecture, Hubei Province in China for more than 300 years. In 2007, Laifeng ginger was registered as an agricultural GI agricultural product by the General Administration of Quality Supervision (AQSIQ) in China [1,2,3,4,5]. Despite the abundance of local characteristic ginger cultivar in China, Laifeng ginger has gained popularity among Chinese consumers due to its renowned crispy texture, low fiber content, and rich nutrients. The Laifeng ginger industry has become an important part of the local agriculture in Laifeng County. According to national statistics, the standardized ginger planting base in Laifeng County currently covers over 1333 hectares, with an annual output of nearly 4 million kilograms and a comprehensive annual output value exceeding 300 million RMB (Renminbi) [6]. However, in recent years, reports of Pb exceedance in different cultivars of gingers have emerged with increasing frequency, based on the detection of the Ministry of Agriculture and Rural Affairs (MARA) of the People’s Republic of China [7]. From our preliminary study, we found that nearly half of the ginger samples collected from 37 independent farmers in Laifeng County had Pb content exceeding 0.2 mg/kg, i.e., the highest safety level according to the China National Standard (GB 2762-2022) [8] of Pb content in tuber vegetables. This food safely issue has harmed the trade of Laifeng ginger severely and thus has attracted attention in agricultural and food science research in academia in recent years in order to solve the problem.
Lead (Pb) has long been recognized as a critical contaminant in food safety protocols due to its severe threats to human health [9,10]. As a potent neurotoxin and carcinogen, chronic exposure to even trace levels of Pb—through contaminated crops or processed foods—can induce irreversible neurological deficits, developmental disorders in children, and systemic organ damage [11,12,13]. This persistent bio-accumulation risk necessitates Pb’s strict regulation as a mandatory testing parameter for agricultural raw materials globally [14]. Besides the threats of Pb to human health, Pb accumulation also poses significant negative impacts on plant physiology and agricultural sustainability [15,16,17]. Pb toxicity in tuber crops mainly affects plant growth and development, physiological and biochemical characteristics, and cellular structure [18]. An accumulation of Pb in the plant tissues can lead to a significant reduction in biomass [19]. In general, the main way for ginger to absorb Pb is from the soil, through underground parts such as roots and rhizome tissue. Thus, the distribution of Pb within the plant is often uneven, with higher content in the rhizomes and lower levels in the stems and leaves [20]. The exceedance of Pb levels in underground parts of the plant can interfere with the absorption of other beneficial minerals and nutrients from soil by negatively blocking, or binding with, ion carriers, thereafter reducing the intake of water and nutrients, such as zinc, phosphorus, manganese, magnesium, iron, and calcium [21,22,23]. Due to this lack of certain minerals, several key physiological and biochemical processes necessary for plant survival and growth can be severely interfered with, especially the photosynthesis pathway [15,24,25]. So far, only limited studies can be found in the literature on the mechanistic progress of the accumulation of Pb in ginger. Excessive Pb content in soil could directly increase the amount of absorbed Pb and thereby result in the exceedance of Pb in the rhizomes of ginger. Nevertheless, Wang showed that even when the Pb content was the same in soil, Pb content in ginger could be largely dependent on the agricultural practice including fertilization approaches [26]. This phenomenon is closely related to the ionic forms of Pb present in the soil, as only Pb in ionic or free states is more readily absorbed by the underground parts of plants.
In traditional agricultural practices, organic fertilizers (OFs) were the only approach employed by farmers, and were also the primary fertilization method adopted by local farmers in Laifeng County for centuries [27,28]. Owing to the advantages of chemical/compound fertilizers (CF) on aspects of the economy, such as cost reduction, rapid supplementation of the nutrients required by ginger, and yield enhancement, a growing number of farmers have transitioned to substituting OFs with chemical counterparts [29,30]. Numerous studies have demonstrated that the long-term benefits of OF on soil properties and indirect positive impacts on the health of plants should not be neglected [31,32,33]. The application of OF can improve the physico-chemical properties of the soil, such as enhancing soil’s organic matter content, by combining and passivating heavy metal pollutants [32,33]. The functional groups in humus can undergo complexation or chelation reactions with heavy metal ions (such as Pb, cadmium, mercury, etc.) present in the soil [34,35,36]. These changes can promote the conversion of Pb into insoluble forms, which is generally considered to reduce its mobility and potential uptake by plants. However, the overall effect of OF on heavy metal bioavailability is complex and can vary depending on the composition of the OF and soil conditions. In addition, organic matter can combine with Pb ions by complexation and chelation process to form stable organic matter–lead complexes [37], thereby reducing its bioavailability [38,39,40,41,42,43]. More importantly, organic matter provides a sufficient carbon source for soil microorganisms [44,45]. Due to the increased research concerning the interaction between microbial activity, chemical properties in soil and plant growth, it has been revealed that the Pb absorption could also be influenced by the profile of microorganisms in the soil. Studies have shown that some species of microorganism could produce specific groups of organic acids, enzymes, and extracellular polysaccharides polymers. These components can facilitate microbes in adsorbing and immobilizing Pb ions on the surface of cells, and thus can inhibit Pb to be absorbed by the plant [46,47,48,49]. Microorganisms alter soil redox conditions (Eh) and pH, leading to the conversion of bioavailable lead fractions, such as exchangeable and carbonate-bound forms into less soluble, stable species such as sulfides, phosphate minerals, and residual fractions [16,50,51,52].
The aim of the present study is to investigate the cause of the Pb exceedance issue in the edible tuber of the GI product, Laifeng ginger. The first section was intended to ascertain the prevalence of the Pb exceedance that occurred within the Laifeng ginger producing area, and to find the related factors possibly causing this phenomenon. Thus, a field study including 37 individual farmers was conducted in Laifeng County. Agricultural practices were carefully documented, and the profile of the soil’s physical–chemical properties and ginger Pb content were analyzed. Among the crops, elevated Pb levels are commonly linked to excessive Pb in soil. However, soil Pb content in Laifeng County is well below the safety limit, suggesting that local fertilization practices may contribute to the exceedance in ginger. Analysis showed that among the ginger samples with excessive Pb, CF was used in larger proportion than OF. Therefore, the second section was intended to testify the causative relationship between fertilization approaches and Pb exceedance. Pot experiments were conducted with different ratios of OF and CF, applied under Pb stress conditions. Impacts on the growth performances of ginger, Pb content in rhizomes, and stems and leaves were measured in order to elucidate the translocation pattern of Pb from the soil through the underground parts to the above. Meanwhile, the microbial composition and differences in the fertilized soil were further investigated, through 16S sequencing technology. This study addresses Pb exceedance in Laifeng ginger, guiding the fertilization of contaminated tuber crops, and supporting sustainable GI product development.
2. Materials and Methods
2.1. Samples
A total of 37 paired ginger and soil samples were collected from primary ginger-growing areas in Laifeng County, Enshi Prefecture, in Hubei Province, China, in 2021. The mother ginger samples used for the potting experiment were from Laifeng County. The soil used for the potting experiment wsd from South-Central Minzu University, Wuhan.
2.2. Field Study
We performed a systematic field study within the main ginger-producing area of Laifeng County, covering 37 farms in Lvshui (LS), Mansui (MS), Baifusi (BF), Huoshui (HS), and Xiluo (XL) town in 2021. This included fertilization approaches and the geographical environment for gingers.
2.3. Pot Experiment
The potting experiment was conducted in the greenhouse of South-Central MinZu University in June 2023. The mother ginger samples were obtained from Laifeng County, Enshi Prefecture, in Hubei, China. The experimental soil was standardized with a pH of 7.6, an organic matter content of 37.8 g/kg, total nitrogen of 1.15 g/kg, total phosphorus of 0.49 g/kg, potassium content of 0.16 g/kg, available phosphorus of 18 mg/kg, available potassium of 202 mg/kg, and a background Pb of 46.5 mg/kg. Before sowing, the soil was air-dried, sieved through a 10-mesh sieve (approximately 2 mm in diameter), and packed into plastic pots with an upper diameter of 32 cm, a lower diameter of 21 cm, and a height of 28 cm, each containing 5.0 kg of air-dried soil. The ginger rhizomes were germinated until the buds reached approximately 1 cm in length, at which point they were sown.
In order to maximize the effects of fertilization approaches on the Pb absorption in different parts of the ginger, Pb nitrate was applied exogenously at 100 mg/kg, which is twice the background soil level, and, as studies have confirmed, the toxicity threshold of Pb for ginger growth is 500 mg/kg [53]. A control group (CK) was established with solely CF applied. Additionally, the T1 and T2 group received CF and different gradients of OF. The amount of OF applied was set according to previous papers [54,55]. The specific fertilization methods for each group are shown in Table 1. There were 10 pots included in each experimental group and only one ginger tuber was grown at each pot.

Table 1.
Fertilization approach in experimental groups (g/kg).
2.4. Agronomic Traits
In general, after the sowing of ginger “seeds” (mother ginger rhizomes with buds on the surface) into the soil, the growth of ginger can be divided into three stages, including seedling stage, and the initiation and rhizome enlargement stages. The seedling stage takes about 40 days, until the first branch of new grown rhizome is grown out of the bud on the surface of seed. The second stage is about 80 days, until the second branch starts to grow from the first branch. At the rhizome enlargement stage, which occurs around 120 days into the growth cycle, the third-order branches develop (Figure 1).
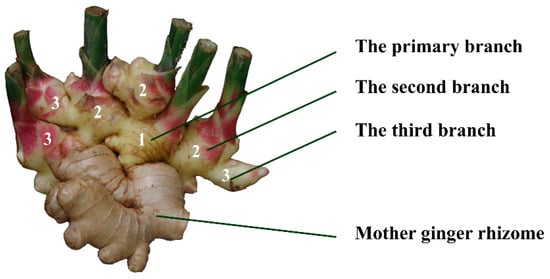
Figure 1.
The branches of ginger rhizome. “1”: the primary branch, “2”: the second branch, “3”: the third branch.
Sampling was conducted at 40, 80, and 120 days throughout the entire 120-day cultivation cycle. According to the specifications of the National Crop Science Data Center (http://www.cgris.net/) [56] for ginger germplasm resources, three plants were randomly selected from each experimental group for agronomic trait measurement each time. The following traits were measured: plant height (cm), stem diameter (cm), number of branches, rhizome length (cm), rhizome width (cm), ginger length (cm), and ginger width (cm). Additionally, the fresh weights of ginger rhizome (g), stem (g), and leaf (g) were recorded, respectively. The measurements were repeated three times for each sample, and the average were used for statistical analysis.
2.5. Determination of Pb Content and Soil pH
Pb concentrations in ginger were determined following GB 5009.12-2017 (National Food Safety Standard—Determination of Pb in Foods, China) [57], while soil Pb analysis adhered to GB/T 17141-1997 (Soil Quality—Determination of Pb–Graphite Furnace Atomic Absorption Spectrophotometry, China) [58]. The pH of the soil suspension was measured using a pH meter.
The regulatory limit for Pb in ginger was set at ≤0.2 mg/kg, according to GB 2762-2022 [8] (National Food Safety Standard—Maximum Levels of Contaminants in Foods). For agricultural soils, Pb risk screening values derived from GB 15618-2018 (Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land) [59] were applied: ≤90 mg/kg for soils with 5.5 < pH ≤ 6.5; ≤120 mg/kg for soils with 6.5 < pH ≤ 7.5; and ≤170 mg/kg for soils with pH > 7.5.
2.6. Absorption and Transfer Coefficients of Pb
To analyze the absorption and translocation patterns of Pb from the soil to different tissues within the ginger, the absolute content of Pb in each plant tissue, the distribution of Pb (D), bioconcentration factor (BCF), and translocation factor (TF) of Pb were calculated as follows:
In the formula, APb (μg): the accumulated content of Pb in different tissue parts of ginger; M (g): the dry weight of different tissues of ginger; and CPb (mg/kg): the Pb content in different tissues of ginger.
D describes the relative content of Pb compared to that of entire plant.
BCF quantifies Pb partitioning to edible organs, determining crop safety under soil contamination. It reflects the biological enrichment capacity of plants for specific metals.
TF is the ratio of heavy metals transferred between a part of the plant and the rhizomes. A larger TF indicates that the plant has a stronger ability to transfer heavy metals from the rhizomes to the above-ground parts (such as the stems, leaves, etc.). Since Pb from the soil is firstly absorbed by the rhizome and then translocated to stems and leaves, the TF describes the ability of stem and leaves to take Pb from rhizomes.
2.7. 16S Ribosomal RNA Sequencing of Rhizosphere Soil Microbiome
At 120 days, the rhizosphere soil of ginger was collected from each experimental group, with three replication pots collected from each group. Nine soil samples of rhizosphere microorganisms were stored at −80 °C and subsequently sent to BENAGEN for full-length 16S rRNA sequencing. After genomic DNA extraction, the isolated DNA was subjected to 1% agarose gel electrophoresis. Primer pairs targeting the V1–V9 region were designed and synthesized, followed by sequencing using PacBio technology. The Amplicon Sequence Variant (ASV) data obtained after sequencing were analyzed using multiple diversity indices, enabling statistical analysis of the microbial community structure at various taxonomic levels. The soil microbial composition, alpha (α) and beta (β) diversity, microbial variation, soil physicochemical–microbial correlations, and gene prediction were analyzed at seven taxonomic levels: kingdom, phylum, class, order, family, genus, and species. The box plots, line plots, and histograms presented in the results section were generated using GraphPad Prism 9.0.2 and RStudio (v.4.2.2). LEfSe (Linear Discriminant Analysis Effect Size) analyses were performed on Galaxy, a web-based analysis platform.
2.8. Statistical Analysis
GraphPad Prism 9 was used to conduct the statistical analysis (GraphPad Software Inc., San Diego, CA, USA). For comparisons between two groups, t-tests were employed, and for comparisons involving more than two groups, one-way analysis of variance (ANOVA) with Tukey’s HSD test were used. The data are shown as the mean ± standard error of the mean (SEM) from three independent experiments. Pearson correlation analysis used RStudio 4.4.1, and the mantel test used the LinkET package (v.0.0.7.4) to analyze p values, which were corrected for multiple testing using the FDR method. Statistical significance is not mark (not significant) for p > 0.05, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
3. Results
3.1. Prevalence of Pb Exceedance in Laifeng Ginger Is Correlated with Fertilization Practice Approaches
In the field study, Pb concentrations contents in ginger, and pH and Pb in soil were measured in soil at each farm located within Laifeng County (Figure 2a). The results are summarized in Table 2. Surprisingly, no exceedance of Pb was found at any of the farms, according to the national standard of Pb safety levels in agricultural soil (GB 15618-2018 [59]). However, 43.24% of the ginger samples exhibited a Pb content above 0.2 mg/kg, which exceeds the safety limit of Pb allowed in vegetables, as regulated in GB 2762-2022 [8] (Figure 2b,c). It is thus reasonable to exclude soil Pb contamination as the dominant factor driving the Pb exceedance in Laifeng ginger. In our survey of agricultural practices in our field investigation, we found that different farms used different fertilization approaches that can be categorized mainly into two types, i.e., with or without the use of OF. We then compared Pb exceedance rates within each of the two fertilization regimes: CF alone versus combined compound/organic fertilizer (CF + OF). As presented in Table 3, the Pb exceedance rate reached 68.75% under the CF treatment, but declined significantly to 23.81% with CF + OF application. These results demonstrate that fertilization approaches are associated with the content of Pb in Laifeng ginger rhizomes, but the causative relationship still remains to be testified, and will be in the next part of the study.
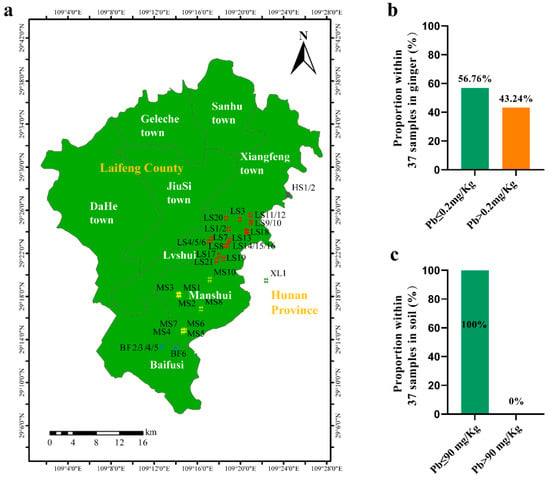
Figure 2.
Sampling map of in the main ginger production areas in Laifeng County (a) and occurrences of Pb exceedance in Laifeng ginger (b) and soil (c). (a) 37 sampling points were included throughout Lvshui (LS), Manshui (MS), Baifusi (BF), Huoshui (HS) and Xiluo (XL) towns; (b) 0.2 mg/kg is the upper limit of Pb allowed in vegetables, according to China National Standard (GB 2762-2022); and (c) 90 mg/kg is the upper limit of Pb allowed in soil with 5.5 < pH ≤ 6.5, according to GB 15618-2018 [59].

Table 2.
37 pairs of ginger and soil samples’ essential information.

Table 3.
The prevalence of Pb exceedance under different fertilization regimes.
3.2. Organic Fertilizer Promotes the Growth of Ginger
OF can reduce Pb stress at all stages of plant growth, and can promote the branching and longitudinal growth of ginger tubers. At the seedling and initiation stages, there were no significant differences among the treatments (Table S1). However, there were significant differences among the treatments at the rhizome enlargement stage. Yield-related indices, such as the number of branches and fresh weight, increased significantly with increased organic manure application, and the length and width of sub-ginger rhizomes also improved. Compared to the CK, the number of branches increased by 18.18% and 69.81% in the T1 and T2 treatments, respectively. Rhizome width increased by 8.21% and 17.20%, rhizome length by 10.58% and 12.37%, and rhizome fresh weight by 14.77% and 29.20%, respectively. (Figure 3d–g). Additionally, above-ground indicators, such as stem fresh weight and leaf fresh weight, also increased significantly with higher quantities of OF application. Compared to the CK treatment, stem fresh weight increased by 4.21% and 3.16% in T1 and T2 treatments, respectively, while leaf fresh weight increased by 13.59% and 28.08% in T1 and T2 treatments, respectively (Figure 3h,i).
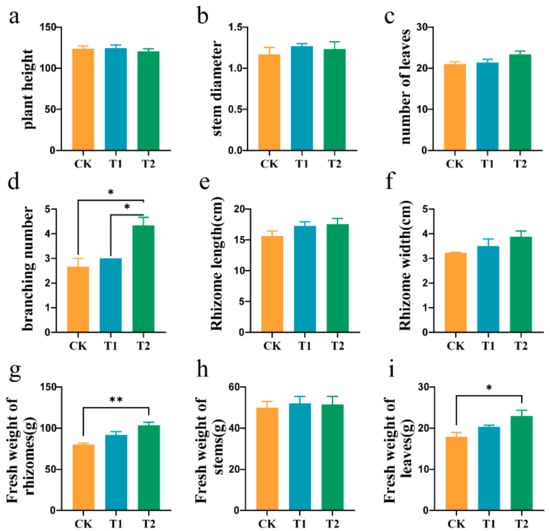
Figure 3.
Agronomic traits of gingers at the rhizome enlargement stage. Plant height (a), stem diameter (b), number of leaves (c), branches (d), rhizome length (e), rhizome width (f), the fresh weights of ginger rhizomes (g), stems (h), and leaves (i) among the different treatment groups. Asterisks indicate significant differences between CK and T1, T2 treatments. * p < 0.05, ** p < 0.01. Error bars stand for the standard error of mean.
3.3. Organic Fertilizer Reduces Pb Content in the Rhizome of Ginger
In each intervention group, the Pb content in rhizomes, stems, and leaves of ginger continuously accumulated during growth, and the trend of Pb content was consistently rhizome > stem > leaf. In contrast, the Pb content in the soil gradually decreased. Across all treatment groups, the Pb accumulation in whole plants showed no significant differences (Figure 4a–c). However, the different fertilization treatments primarily affected the Pb content within plant parts. Specifically, increased application of OF within higher Pb concentrations in the above-ground tissues, particularly the stems and leaves, resulted in a decrease in the Pb content within the underground rhizomes.
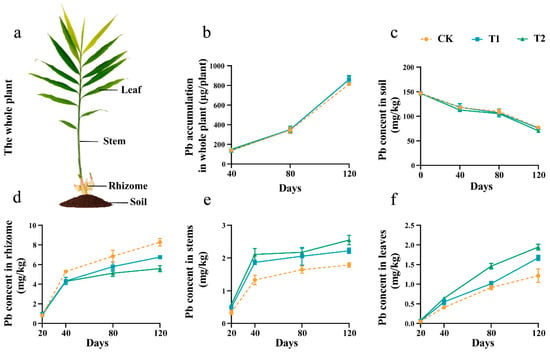
Figure 4.
Pb contents in different parts of ginger in different growth stages. (a) Diagrammatic drawing of different parts in the whole ginger plant. Pb accumulation in whole plant (b), soil (c), rhizome (d), stems (e) and leaves (f). Error bars stand for the standard error of mean.
Under Pb stress, different doses of OF combinations affected the Pb absorption of ginger organs at different growth stages. As shown in Figure 4, Pb levels varied across different tissues in all treatments. The Pb content in ginger rhizome tissues was reduced in all treatment groups under Pb stress, with the most pronounced reduction in T2. Compared to CK, the Pb content in the rhizomes of T1 and T2 was reduced by 18.32% and 32.53%, respectively, after 120 days of growth (Figure 4d). The Pb content in the stem and leaf tissues of the ginger under Pb stress showed an opposite trend compared to the rhizomes. The Pb content in the stem tissues of ginger was higher than in the CK in all treatment groups. A 24.72% and 43.26% increase in Pb content was observed in T1 and T2 stems, respectively, compared to the CK after 120 days of growth (Figure 3a). Similarly, the Pb content in the ginger leaf tissue was higher than in the CK in all treatment groups, with the highest increase in T2, followed by T1. A 36.89% and 59.02% increase in Pb content was observed in T1 and T2 leaves, respectively (Figure 4e,f).
3.4. Pb Accumulation and Distribution Rates Within Different Organs of Ginger Under Pb Stress
In each intervention group, the trend of Pb accumulation and distribution rates were consistent. Different proportions of OF treatments reduced the Pb accumulation and distribution in ginger rhizomes and increased the Pb accumulation and distribution in ginger stems and leaves (Figure 5). The Pb accumulation in rhizomes was lowest in the T2 treatment, followed by the T1. The accumulation of Pb in rhizome tissues was 660.98, 620.00, and 579.33 μg/plant for the CK, T1, and T2 treatment groups, respectively. In contrast, the Pb accumulation in the stem and leaf tissues was higher in the T1 and T2 treatment groups compared to the CK. Pb accumulation in the stem tissues was 88.61, 114.94, and 132.30 μg/plant, and in the leaf tissues, it was 21.75, 33.87, and 44.48 μg/plant for the CK, T1, and T2 treatment groups, respectively.
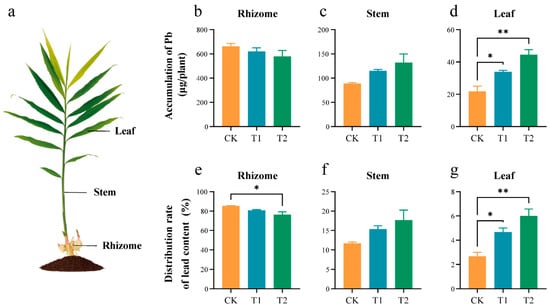
Figure 5.
Pb accumulation and distribution in different organs of ginger at the rhizome enlargement stage. (a) Diagrammatic drawing of different parts in the whole ginger plant. Accumulation of Pb in rhizome (b), stem (c), and leaf (d). Distribution of Pb in rhizome (e), stem (f), and leaf (g). Asterisks indicate significant differences between CK, T1, and T2 treatments. * p < 0.05, ** p < 0.01. Error bars stand for the standard error of mean.
Compared with the control, the distribution trends and the accumulation of Pb in each organ were consistent across all treatment groups. The Pb distribution in ginger rhizome tissues was 85.68%, 80.57%, and 76.42% for the CK, T1, and T2 treatment groups, respectively. The Pb distribution in ginger stem tissues was 11.52%, 15.02%, and 17.62%, respectively. The Pb distribution in ginger leaf tissues was 2.80%, 4.42%, and 5.96%, respectively.
3.5. Organic Fertilizer Promotes the Transfer of Pb to the Above-Ground Parts
To further explore the ability, in ginger, of the different parts to accumulate Pb and to transfer between organs, the BCF and TF were calculated for the CK, T1, and T2 groups. The application of OF increases the ability of the above-ground parts to absorb Pb and transfer Pb from the rhizomes to the stems and leaves. As shown in Figure 6, the BCF and TF in different organs of ginger differed significantly between the CF + OF groups and the control group under the same level of Pb stress. Rhizomes are the primary site of Pb exposure in soil. Increasing the OF content significantly reduced the Pb accumulation capacity in ginger rhizomes, with the BCF values ranked from highest to lowest as CK > T1 > T2. However, increasing the OF content significantly enhanced the Pb accumulation capacity in ginger stems and leaves, with the BCF values ranked from highest to lowest as T2 > T1 > CK (Figure 6b–d). Similarly, elevated OF levels significantly increased the ability to transfer Pb from ginger rhizomes to stems and leaves, but had no significant effect on stem-to-leaf transfer. The transfer coefficients from ginger rhizomes to stems and leaves were ranked from highest to lowest as T2 > T1 > CK (Figure 5e,f).
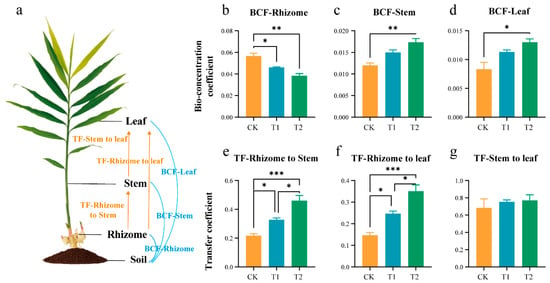
Figure 6.
Bioconcentration Factor (BCF) and Transfer Factor (TF) in different organs of ginger at the rhizome enlargement stage. (a) Diagrammatic drawing of BCF and TF in different organs of ginger. BCF of Pb in rhizome (b), stem (c), and leaf (d). TF of Pb in rhizome to stem (e), rhizome to leaf (f) and stem to leaf (g). Asterisks indicate significant differences between CK, T1, and T2 treatments. * p < 0.05, ** p < 0.01, *** p < 0.001. Error bars stand for the standard error of mean.
3.6. Rhizosphere Soil Microbiome
3.6.1. Diversity Analysis
The microbial α-diversity can be expressed by Chao’s Abundance-based Coverage Estimator (ACE), and Shannon and Simpson indices, where the Chao1 index can be used to estimate the number of ASVs in the sample, the ACE index can be used to estimate species diversity by using rare species, with the higher the value the richer the community is, and the Shannon and Simpson indices can be used to take into account both species richness and species evenness. The Shannon and Simpson indices are rich indicators of community structure. The ACE and Chao1 indices of the T1 and T2 groups were higher than those of the CK treatment. Similarly, the Shannon indices of the T1 and T2 groups were higher than those of the CK treatment. Additionally, Simpson’s inverse index (1/D) was lower in the T1 and T2 treatments than in the CK (Figure 7a–d). The PCA results showed that the β-diversity of the CK, T1 and T2 groups showed a grouping trend, and the points within the groups were closer, while the differences between the groups were more obvious, indicating that there was a significant difference in the microbial community composition among the different groups (Figure 7e).
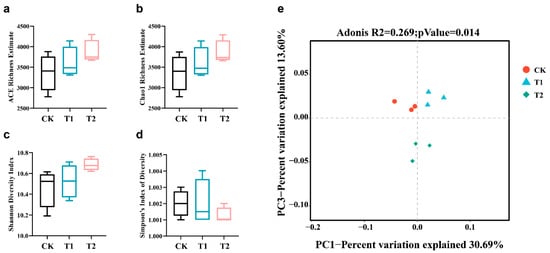
Figure 7.
Diversity of soil microorganisms in groups CK, T1 and T2. Bacterial species richness was estimated using Chao1 (a), ACE (b), Shannon (c), and Simpson’s index (d) at ASV level across all the samples. PCA diagram of weighted unifrac distance matrix (e). Tukey method was used for plotting the whiskers and outliers. Error bars stand for the standard error of mean.
3.6.2. Microbial Composition and Difference Analysis
Significant differences were observed in the soil microbial community composition at the genus level among the treatment groups, while no significant differences were found at the phylum and class levels. At the phylum level, Proteobacteria and Acidobacteriota dominated the bacterial community in the soil amended with CF and OF, accounting for 26.16% and 19.70% of the total bacterial percentage, respectively. Among them, γ-Aproteobacteria, α-Aproteobacteria (belonging to the phylum Ascomycetes), and Vicinarnibateria (belonging to the phylum Acidobacteria) were absolutely dominant in the bacterial community. In addition, Bacteroidota and Gemmatimonadota, of the phylum Bacteroidota, were more predominant, with 8.94% and 6.41%, respectively (Figure 8a). Patescibacteria varied in their proportion in the CK, T1, and T2 groups, with a proportion of 5.00%, 2.68%, and 6.12%, respectively. In addition to the top ten species in terms of abundance, bacteria with significant differences in abundance in the CK, T1, and T2 groups at the class level were also examined (Figure 8b).
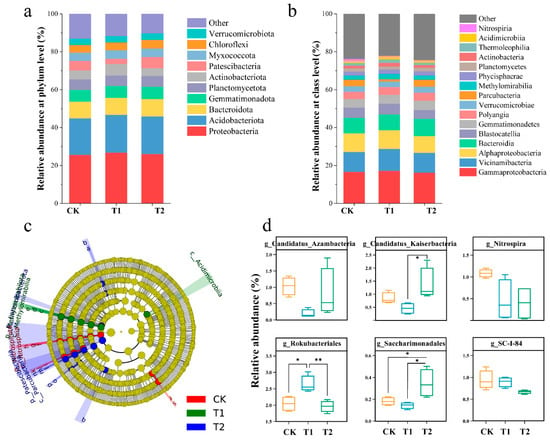
Figure 8.
Changes in microflora composition at phylum (a) and class (b) levels and evolutionary cladistics based on LEfSe analysis (c) and differences in the relative abundance of bacteria in each group at the genus level. The cladogram shows the taxonomic levels represented by rings with phyla at the innermost ring and species at the outermost ring, and each circle as a member at that level. The red, green, and blue circles (a–s) represent the discriminating bacteria enriched in corresponding groups, whereas the yellow circles represent the taxa without significant differences among the three groups. The higher the relative abundance, the larger the circle. (d) Select species with the top10 abundances for display, and the sum of all remaining phylum abundances is displayed as “other”. Asterisks indicate significant differences between CK, T1, and T2 treatments. * p < 0.05, ** p < 0.01. Error bars stand for the standard error of mean.
Among these, the abundance of Acidimicrobiia and Methylomirabilia was significantly higher in the T1 treatment than in the CK and T2 treatments, and the abundance of Nitrospiria and Parcubactcria was significantly lower in the T1 treatment than in the CK and T2 treatments (Figure S1). Furthermore, LEfSe analysis was used to screen for bacteria with significant differences in abundance at the genus level in the CK, T1 and T2 groups. The dominant strains in the CK were Nitrospiria, SC-I-84, and Candidatus_Azambacteria. The dominant species in T1 were Rokubacteriales. The dominant strains in T2 were Candidatus_Kaiserbacteria and Saccharimonadales (Figure 8c,d).
3.7. Correlation Analysis Between Soil Microbial Species with Pb Content of Ginger and Soil
Mantel test analysis revealed statistically significant correlations between the soil microbial community composition and the Pb content present in both ginger plant tissues (rhizome, stem, and leaf) and the surrounding soil. Further investigation using Pearson analysis identified the correlation of the specific microbial groups mentioned in Section 3.6.2 with Pb contents across the rhizome, stem, and leaf of ginger. These correlations encompassed both positive and negative associations, highlighting specific microbial responses to Pb presence in the soil–plant system. Notably, soil pH also significantly correlated with the microbial community, and with the application of OF the soil pH decreased significantly (Figure 9a), suggesting its potential role as a mediating factor in the observed microbial–Pb relationships.
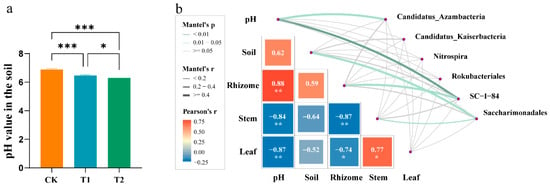
Figure 9.
pH of soil at rhizome enlargement stage (a) and pairwise comparisons of environmental factors are shown (b), with a color gradient denoting Pearson’s correlation coefficient. Soil microbial species were related to each environmental factor by partial Mantel tests. Edge width corresponds to Mantel’s R statistic for the corresponding distance correlations, and edge color denotes the statistical significance. Asterisks indicate significant differences between CK, T1, and T2 treatments. * p < 0.05, ** p < 0.01, *** p < 0.001. Error bars stand for the standard error of mean.
As shown in Figure 9b, soil pH plays an important part in soil–plant systems. pH was positively correlated with the Pb contents in the soil and rhizome, though it was significantly and negatively correlated with the Pb contents in the stem and leaf. Furthermore, the three dominant strains were significantly and positively correlated with ginger parts, pH, and the Pb content in the soil. Specifically, the dominant strain of CK, SC-I-84, showed a significant and positive correlation with both the soil pH and Pb content in ginger rhizomes. The dominant strain Candidatus_Azambacteria exhibited a significant and positive correlation with the soil pH. The dominant strain of Saccharimonadales in T2 was significantly and positively correlated with the soil Pb content in ginger stems.
4. Discussion
In this study, we systematically investigated the regulatory effects of OFs on ginger growth under Pb stress, including Pb accumulation and distribution characteristics, and root-associated microbial communities. The results demonstrated that OF application not only effectively alleviated Pb-induced growth inhibition in ginger, but also reduced Pb content in rhizomes by promoting Pb translocation from rhizomes to stem and leaf tissues. Furthermore, OF altered the soil pH and the dominant microbial communities in Pb-stressed soil.
4.1. Organic Fertilizer Alleviates Pb Stress-Induced Growth Inhibition in Ginger Plants
Under Pb stress, ginger exhibits significant growth inhibition, characterized by reduced plant height, diminished stem diameter, and significantly fewer functional leaves. This response correlates with the Pb-induced suppression of cytokinin biosynthesis and the disruption of vascular bundle development [19,50]. In this study, OF application mitigated these detrimental effects. Representative data from the T2 treatment demonstrate that OF application significantly increased stem fresh weight, leaf fresh weight, and branch number by 3.16%, 28.08%, and 69.81% at the rhizome enlargement stage, respectively, compared to Pb-stressed controls. These morphological recoveries collectively indicate the effective mitigation of Pb-induced growth inhibition. In addition, OF enhanced the length, width, and fresh weight of rhizomes by 12.37%, 17.2%, and 29.20%, respectively. OF indirectly enhanced the efficiency of root nutrient acquisition by increasing soil organic matter, promoting aggregate formation, and improving water and nutrient retention capacity [33]. Moreover, humic acid is the core active component of OF. This improvement is likely mediated through the humic acid stimulation of root growth hormones [40], which expands the absorptive surface area and enhances the plant adaptability under stress conditions [51]. OF elicited organ-specific responses, with above-ground biomass recovery markedly exceeding root recovery. This suggests a post-stress prioritization of assimilate partitioning to photosynthetic tissues, consistent with energy metabolism restructuring during heavy metal detoxification [47,51,60]. It is worth noting that OFs contain abundant carbon sources. Their high carbon-to-nitrogen ratio may temporarily immobilize mineral nitrogen in the soil (required for microbial proliferation), potentially reducing the supply of available nitrogen in the short term [61,62]. The increase in ginger biomass is more likely due to improved soil microenvironments rather than to mere nutrient increment [63,64,65].
4.2. Organic Fertilizer Application Reduces Pb Accumulation in Ginger Rhizomes Under Pb Stress
This study aimed to evaluate the influence of different fertilization methods on Pb accumulation in various tissues of ginger plants. In the pot experiment, a Pb concentration higher than typical field levels was applied to elicit discernible physiological responses and Pb uptake within a relatively short growth period. A Pb level of 100 mg/kg was applied to establish a significant but non-lethal stress environment, as studies show that significant growth inhibition in ginger only occurs at soil Pb concentrations above 500 mg/kg [53]. This study demonstrates that OF application significantly inhibits Pb accumulation in ginger rhizomes (underground parts) under Pb stress, highlighting its potential as an effective strategy for reducing heavy metal contamination risks in edible rhizomes. Compared to the control, the Pb content decreased by 18.32% (T1) and 32.53% (T2) in the rhizomes, respectively. This reduction likely results from interactive mechanisms within the soil–plant system. Firstly, the decomposition of organic matter in sheep manure fertilizer generates organic acids that acidify soil, reducing the pH from 7.0 to 6.3. Although decreased pH typically enhances Pb bioavailability by promoting exchangeable or free Pb2+ speciation [15,66], active components in OF, such as humic and fulvic acids, can mitigate Pb uptake by forming stable complexes through coordination reactions, such as Pb humates. This significantly reduces soluble Pb2+ concentrations in soil solutions, thereby limiting root accumulation [43,67]. However, compared with the CK, the accumulation of the total plant is not significantly decreased in T1 and T2 treatments. Another viewpoint holds that the Pb in the rhizome of the ginger will migrate upwards to the upper part of the plant [42,68,69]. Notably, the T2 treatment increased Pb distribution in stems by 52.95% and in leaves by 112.86% compared to the CK. Therefore, the application of OF did not reduce the total Pb uptake by the plants, while it significantly facilitated the translocation of Pb to the aerial parts. This finding appears to contrast with the conventional view that OFs promote metal immobilization. The different effects can be attributed to the stage-specific nature of OF decomposition [70,71]. In our study, the dominant process likely originated from the rapid release of low-molecular-weight organic acids, such as citric acid and oxalic acid, which form soluble complexes with Pb, thereby enhancing its translocation within the plant [37,38]. This effect outweighed the slower immobilization process mediated by stable humic substances. Therefore, the net outcome of OF application depends on the balance between these two opposing mechanisms, which is influenced by the composition of the OF and the stage of decomposition [72,73,74]. Furthermore, improved soil fertility, such as enhanced nitrogen and phosphorus availability, from OF application promotes the growth of ginger shoots, resulting in increased biomass and higher transpiration rates [75]. Consequently, this intensifies the passive transport of Pb via the xylem to the leaves and stems. This redistribution indicates that enhanced xylem loading capacity facilitates Pb translocation from the roots to the aerial parts [68,69,76]. Mechanistically, this process probably correlates with the upregulated expression of phytochelatin synthesis genes, such as PCS1 [25,38,39,77,78,79]. Collectively, this approach of limiting root uptake and directing Pb to aerial tissues appears to reduce Pb accumulation in edible organs (rhizomes and stems), while potentially facilitating detoxification through sequestration in metabolically non-essential aerial tissues [18,42,43,67]. To ensure food safety, this fertilization method is unsuitable for the local crops harvested from aerial parts, such as leafy vegetables, grains, and fruits, given its significant contamination risk.
4.3. Organic Fertilizer Application Enhances Microbial Species Richness in Soil Under Pb Stress
OF application enhances soil organic matter content, regulates pH, and improves nutrient availability, thereby creating favorable conditions for functional microorganism proliferation [38,39,47]. OFs provide energy for microorganisms. The enzymes secreted by these microbes, such as phosphatase, activate insoluble nutrients in the soil, converting the originally unavailable soil nutrient pool into bioavailable forms. This ‘nutrient mining effect’ does not originate from external fertilizer inputs [80,81]. This study revealed the significant enrichment of heavy-metal-tolerant Proteobacteria (increasing by 5.10% in T1 and 7.55% in T2) and Actinobacteria (4.05% and 9.15% increases, respectively) compared to controls, which is consistent with long term organic amendment effects [48]. At genus level, enhanced abundances of Rokubacteriales, Candidatus_Kaiserbacteria and Saccharimonadales. These microorganisms reduce Pb2+ bioavailability through siderophore and organic acid secretion [46,82]. To be specific, in studies of plant-based remediation for Pb–zinc–slag-contaminated soil, Rokubacteriales has been identified as a key microbial indicator in the rhizosphere. It indirectly influences Pb speciation by enhancing carbon cycling and organic matter degradation. Metabolites produced by this genus, such as organic acids, may facilitate Pb complexation with organic matter, forming insoluble complexes. Crucially, microaggregate formation and metal resistant bacterial consortia, such as Patescibacteria, Saccharimonadales, Microvirga, Pseudomonas collectively govern the heavy metal availability in soil [83], with Saccharimonadales additionally driving phosphorus cycling [84].
5. Conclusions
Organic fertilizer application effectively mitigated Pb stress in ginger plants, as evidenced by significant enhancements in plant growth parameters, such as rhizome fresh weight, branch number, and rhizome width and length in T1 and T2 groups. Critically, organic fertilizer application reduced Pb accumulation in ginger rhizomes under Pb stress by altering the pH value of the soil and the types and abundances of microorganisms in the soil. Compared to the CK, the accumulation, distribution rate and the BCF reduced for rhizomes in the T1 and T2 groups. In the soil, the pH value significantly decreased, and the relative abundance of Proteobacteria and Actinobacteria increased in the T1 and T2 groups. The dominant strains in the CK were Nitrospira, SC-I-84, and Candidatus_Azambacteria, while the dominant strain in the T1 group was Rokubacteriales and in the T2 group were Candidatus_Kaiserbacteria and Saccharimonadales. Concurrently, organic fertilizer promoted the transfer of lead to the stems and leaves. Compared to the CK, the TF from rhizomes to stems and leaves increased in the T1 and T2 groups. In conclusion, the integration of organic fertilizer into Pb-contaminated soil modified the soil microbiome, facilitating Pb partitioning from ginger rhizomes to aerial parts (stems and leaves). This redistribution significantly decreased Pb accumulation and the bioconcentration factor within the edible rhizomes, thereby reducing Pb toxicity and enhancing the safety profile of the harvested ginger.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15202172/s1. Table S1: Effects of different fertilization combinations on ginger growth. Figure S1: Differences in the relative abundance of bacteria in each group at the class level. (* p < 0.05, ** p < 0.01).
Author Contributions
M.S. wrote the manuscript, performed the experiments and conducted data analysis; H.H. (Hao Huai), J.W., and T.A. conducted data analysis; H.H. (Hongzao He) collected experimental materials; H.L. supervised the manuscript writing and provided critical revisions; R.Q. and J.L. designed the study and managed the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities of South-Central Minzu University (grant number CZZ24003, PTZ25019, XTZ24020 and CZD24002).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the support by Fundamental Research Funds for the Central Universities of South-Central Minzu University (CZZ24003, PTZ25019, XTZ24020 and CZD24002). The authors are very grateful for the assistance provided by Laifeng Xingjia Ecological Agriculture Technology Co., Ltd. during field study sampling.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Liu, G.L.; Zhang, Q.; Yin, G.; Musyimi, Z. Spatial distribution of geographical indications for agricultural products and their drivers in China. Environ. Earth Sci. 2016, 75, 612. [Google Scholar] [CrossRef]
- Qie, H.K.; Chao, Y.D.; Chen, H.; Zhang, F. Do geographical indications of agricultural products promote county-level economic growth? China Agric. Econ. Rev. 2023, 15, 666–681. [Google Scholar] [CrossRef]
- Yang, X.T.; Li, Y.L.; Zhao, S.L.; Zhang, P.; Zhao, Y. Geographical origin authentication of agricultural products in the China-EU Geographical Indications Agreement: A comprehensive review of Chinese products. Trends Food Sci. Technol. 2024, 152, 104679. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Yang, Y.J.; An, Y.J.; Kim, S.Y.; Kwon, C.; Kim, S.H. A case study for geographical indication of organic milk in Korea using stable isotope ratios-based chemometric analysis. Food Control 2020, 107, 106755. [Google Scholar] [CrossRef]
- Liu, W.W.; Chen, Y.; Liao, R.X.; Zhao, J.; Yang, H.; Wang, F.H. Authentication of the geographical origin of Guizhou green tea using stable isotope and mineral element signatures combined with chemometric analysis. Food Control 2021, 125, 107954. [Google Scholar] [CrossRef]
- Fengtou Ginger Harvests Well, Comprehensive Output Value Reaches 350 Million Yuan. 2024. Available online: https://www.sohu.com/a/810802551_121610648 (accessed on 22 September 2024).
- Multiple Batches of Ginger Were Found to Have Excessive Lead Content! Here’s How to Deal with It! Available online: https://www.163.com/dy/article/FQP58A9T0534JZBM.html (accessed on 6 November 2020).
- GB 2762-2022; National Food Safety Standards-Limits of Contaminants in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2023.
- Hernández-Martínez, R.; Navarro-Blasco, I. Estimation of dietary intake and content of lead and cadmium in infant cereals marketed in Spain. Food Control 2012, 26, 6–14. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, X.D.; Wu, P.G.; Han, J.L.; Chen, Q. Heavy metals in vegetables and the health risk to population in Zhejiang, China. Food Control 2014, 36, 248–252. [Google Scholar] [CrossRef]
- Abt, E.; Robin, L.P. Perspective on Cadmium and Lead in Cocoa and Chocolate. J. Agric. Food Chem. 2020, 68, 13008–13015. [Google Scholar] [CrossRef] [PubMed]
- Flegal, A.R.; Odigie, K.O. Distinguishing between Natural and Industrial Lead in Consumer Products and Other Environmental Matrices. J. Agric. Food Chem. 2020, 68, 12810–12819. [Google Scholar] [CrossRef]
- Villa, J.E.L.; Peixoto, R.R.A.; Cadore, S. Cadmium and Lead in Chocolates Commercialized in Brazil. J. Agric. Food Chem. 2014, 62, 8759–8763. [Google Scholar] [CrossRef]
- Gaw, S.K.; Kim, N.D.; Northcott, G.L.; Wilkins, A.L.; Robinson, G. Uptake of ΣDDT, arsenic, cadmium, copper, and lead by lettuce and radish grown in contaminated horticultural soils. J. Agric. Food Chem. 2008, 56, 6584–6593. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manage. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Sengar, R.S.; Gautam, M.; Sengar, R.S.; Garg, S.K.; Sengar, K.; Chaudhary, R. Lead stress effects on physiobiochemical activities of higher plants. Rev. Environ. Contam. Toxicol. 2008, 196, 73–93. [Google Scholar]
- Ashraf, U.; Hussain, S.; Anjum, S.A.; Abbas, F.; Tanveer, M.; Noor, M.A.; Tang, X. Alterations in growth, oxidative damage, and metal uptake of five aromatic rice cultivars under lead toxicity. Plant Physiol. Biochem. 2017, 115, 461–471. [Google Scholar] [CrossRef]
- Ashraf, U.; Tang, X. Yield and quality responses, plant metabolism and metal distribution pattern in aromatic rice under lead (Pb) toxicity. Chemosphere 2017, 176, 141–155. [Google Scholar] [CrossRef]
- Elizabeth George, S.; Wan, Y. Microbial functionalities and immobilization of environmental lead: Biogeochemical and molecular mechanisms and implications for bioremediation. J. Hazard. Mater. 2023, 457, 131738. [Google Scholar] [CrossRef]
- Zanin Lima, J.; Monici Raimondi Nauerth, I.; Ferreira da Silva, E.; José Pejon, O.; Guimarães Silvestre Rodrigues, V. Competitive sorption and desorption of cadmium, lead, and zinc onto peat, compost, and biochar. J. Environ. Manage. 2023, 344, 118515. [Google Scholar] [CrossRef]
- Zeng, G.; Si, M.; Dong, C.; Liao, Q.; He, F.; Johnson, V.E.; Arinzechi, C.; Yang, W.; Yang, Z. Adsorption behavior of lead, cadmium, and arsenic on manganese-modified biochar: Competition and promotion. Environ. Geochem. Health 2024, 46, 86. [Google Scholar] [CrossRef]
- Chen, S.; Sun, L.; Sun, T.; Chao, L.; Guo, G. Interaction between cadmium, lead and potassium fertilizer (K2SO4) in a soil-plant system. Environ. Geochem. Health 2007, 29, 435–446. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Wang, S.T.; Gao, Z.J.; Zhang, Y.Q.; Zhang, H.R.; Wu, Z.; Jiang, B.; Liu, Y.; Dong, H.Z. Source and Health Risk Assessment of Heavy Metals in Soil-Ginger System in the Jing River Basin of Shandong Province, North China. Int. J. Environ. Res. Public Health 2021, 18, 6749. [Google Scholar] [CrossRef]
- Szwejda-Grzybowska, J.; Ropelewska, E.; Wrzodak, A.; Sabat, T. The influence of natural preparations on the chemical composition, flesh structure and sensory quality of pepper fruit in organic greenhouse cultivation. Food Control 2024, 155, 110088. [Google Scholar] [CrossRef]
- Wiessner, S.; Thiel, B.; Krämer, J.; Köpke, U. Hygienic quality of head lettuce: Effects of organic and mineral fertilizers. Food Control 2009, 20, 881–886. [Google Scholar] [CrossRef]
- Liao, F.; Zheng, Y.Y.; Wang, X.F. Promoting the replacement of chemical fertilizer with organic fertilizer: The role of policy incentives and digital technology. Front. Sustain. Food Syst. 2025, 9, 1527913. [Google Scholar] [CrossRef]
- Boberski, P.; Glówka, M.; Torchala, K.; Kulczycki, G.; Kuznik, N. Sustainable Agriculture Solutions: Biodegradable Coatings for Enhanced-Efficiency Fertilizers Using Cellulose and Lignin. J. Agric. Food Chem. 2025, 73, 13105–13124. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.L.; Liu, M.Z.; Lü, S.Y.; Xie, L.H.; Wang, Y.F. Multifunctional Slow-Release Organic-Inorganic Compound Fertilizer. J. Agric. Food Chem. 2010, 58, 12373–12378. [Google Scholar] [CrossRef]
- Tie, J.Z.; Qiao, Y.L.; Jin, N.; Gao, X.Q.; Liu, Y.Y.; Lyu, J.; Zhang, G.B.; Hu, L.L.; Yu, J.H. Yield and Rhizosphere Soil Environment of Greenhouse Zucchini in Response to Different Planting and Breeding Waste Composts. Microorganisms 2023, 11, 1026. [Google Scholar] [CrossRef]
- Maltas, A.; Oberholzer, H.; Charles, R.; Bovet, V.; Sinaj, S. Long-term effect of organic fertilizers on soil properties. Agrar. Schweiz 2012, 3, 148–155. [Google Scholar]
- Li, Y.R.; Wang, K.; Dötterl, S.; Xu, J.M.; Garland, G.; Liu, X.M. The critical role of organic matter for cadmium-lead interactions in soil: Mechanisms and risks. J. Hazard. Mater. 2024, 476, 135123. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liu, S.Q.; Li, J.F.; Li, S.Q. Effects of Several Organic Fertilizers on Heavy Metal Passivation in Cd-Contaminated Gray-Purple Soil. Front. Environ. Sci. 2022, 10, 895646. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Zhang, Z.G.; Chen, Y.C.; An, S.K.; Zhang, L.; Chen, F.L.; Ma, C.N.; Cai, W.Q. Adsorption and desorption of Cd in reclaimed soil under the influence of humic acid: Characteristics and mechanisms. Int. J. Coal Sci. Technol. 2022, 9, 7. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, L.; Wang, X.; Ou, Y.; Liu, H.; Hou, X.; Yan, L.; Li, X. The various effect of cow manure compost on the degradation of imazethapyr in different soil types. Chemosphere 2023, 337, 139325. [Google Scholar] [CrossRef]
- Amjad, M.; Khan, Z.I.; Nadeem, M.; Ahmad, K.; Shah, A.A.; Gatasheh, M.K.; Shaffique, S.; Abbas, T. Accumulation and translocation of lead in vegetables through intensive use of organic manure and mineral fertilizers with wastewater. Sci. Rep. 2024, 14, 12641. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.; Wu, Z.; Zhao, Q.; Wang, Q.; Wang, H.; Singh, B.P.; Wu, W.; Fu, C. Interactions between lead(II) ions and dissolved organic matter derived from organic fertilizers incubated in the field. J. Environ. Sci. 2022, 121, 77–89. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Cai, X.; Li, B.; Zhan, F.; Zu, Y.; He, Y. Organic Materials Promote Rhododendron simsii Growth and Rhizosphere Soil Properties in a Lead-Zinc Mining Wasteland. Plants 2024, 13, 891. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, S.; Yang, W.; Zheng, J.; Ye, L.; Liu, Q.; Yang, J. Organic fertilizer improved the lead and cadmium metal tolerance of Eucalyptus camaldulensis by enhancing the uptake of potassium, phosphorus, and calcium. Front. Plant Sci. 2024, 15, 1444227. [Google Scholar] [CrossRef]
- Wang, X.J.; Chen, J.W.; An, J.H.; Wang, X.P.; Shao, Y. Comparison of the Effects of Different Organic Amendments on the Immobilization and Phytoavailability of Lead. Sustainability 2024, 16, 2981. [Google Scholar] [CrossRef]
- Wu, Y.; Ji, H.; Li, C.; Hou, Z.; Huang, C.; Chen, L.; Wang, Y.; Fu, C.; Zhang, D.; Wu, Z.; et al. Molecular size-dependent compositions and lead (II) binding behaviors of two origins of organic fertilizers-derived dissolved organic matter. Ecotoxicol. Environ. Saf. 2023, 258, 114959. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Sasaki, Y.; Nasukawa, H.; Katahira, M. Recycling potassium from cow manure compost can replace potassium fertilizers in paddy rice production systems. Sci. Total Environ. 2024, 912, 168823. [Google Scholar] [CrossRef]
- Qin, J.; Qian, S.; Chen, Q.; Chen, L.; Yan, L.; Shen, G. Cow manure-derived biochar: Its catalytic properties and influential factors. J. Hazard. Mater. 2019, 371, 381–388. [Google Scholar] [CrossRef]
- Li, Q.J.; Zhang, D.Q.; Song, Z.X.; Ren, L.R.; Jin, X.; Fang, W.S.; Yan, D.D.; Li, Y.; Wang, Q.X.; Cao, A.C. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, C.L.; Lei, M.; Luo, K.; Wang, L.Y.; Liu, R.G.; Li, Y.Y.; Hu, Y.N. Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review. Open Chem. 2022, 20, 793–807. [Google Scholar] [CrossRef]
- Pu, R.F.; Wang, P.P.; Guo, L.P.; Li, M.H.; Cui, X.M.; Wang, C.X.; Liu, Y.; Yang, Y. The remediation effects of microbial organic fertilizer on soil microorganisms after chloropicrin fumigation. Ecotoxicol. Environ. Saf. 2022, 231, 113188. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, H.X.; Dai, Y.C.; Tian, H.X.; Zhou, W.; Lv, J.L. Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci. Total Environ. 2021, 750, 141719. [Google Scholar] [CrossRef]
- Chang Chien, S.W.; Wang, M.C.; Huang, C.C. Reactions of compost-derived humic substances with lead, copper, cadmium, and zinc. Chemosphere 2006, 64, 1353–1361. [Google Scholar] [CrossRef]
- Liu, M.; Tan, X.; Zheng, M.; Yu, D.; Lin, A.; Liu, J.; Wang, C.; Gao, Z.; Cui, J. Modified biochar/humic substance/fertiliser compound soil conditioner for highly efficient improvement of soil fertility and heavy metals remediation in acidic soils. J. Environ. Manage. 2023, 325 Pt A, 116614. [Google Scholar] [CrossRef] [PubMed]
- Řezáčová, V.; Czakó, A.; Stehlík, M.; Mayerová, M.; Šimon, T.; Smatanová, M.; Madaras, M. Organic fertilization improves soil aggregation through increases in abundance of eubacteria and products of arbuscular mycorrhizal fungi. Sci. Rep. 2021, 11, 12548. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Xu, J.M.; Xu, Y.; Wang, K.; Cao, B.L.; Xu, K. Alleviating effects of silicate, selenium, and microorganism fertilization on lead toxicity in ginger (Zingiber officinale Roscoe). Plant Physiol. Biochem. 2019, 145, 153–163. [Google Scholar] [CrossRef]
- Lyu, C.; Li, L.; Liu, X.W.; Zhao, Z.Q. Rape straw application facilitates Se and Cd mobilization in Cd-contaminated seleniferous soils by enhancing microbial iron reduction. Environ. Pollut. 2022, 310, 119818. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Q.; Yang, W.X.; Dinh, Q.T.; Tran, T.A.T.; Zhao, X.D.; Wu, J.T.; Liu, Y.X.; Liang, D.L. DOM derivations determine the distribution and bioavailability of DOM-Se in selenate applied soil and mechanisms. Environ. Pollut. 2020, 259, 113899. [Google Scholar] [CrossRef]
- Specification for Description of Ginger (Zingiber officinale Roscoe) Germplasm Resources. Available online: https://max.book118.com/html/2017/0522/108381220.shtm (accessed on 6 June 2021).
- GB 5009.12-2017; National Food Safety Standard—Determination of Lead in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2017.
- GB/T 17141-1997; Soil Quality—Determination of Lead and Iron. China National Environmentional Monitoring Center: Beijing, China, 1997.
- GB 15618-2018; Soil Environmental Quality—Risk Control Standards for Soil Pollution in Agricultural Land (Trial Version). Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Li, J.; Chang, Y.; Al-Huqail, A.A.; Ding, Z.; Al-Harbi, M.S.; Ali, E.F.; Abeed, A.H.A.; Rekaby, S.A.; Eissa, M.A.; Ghoneim, A.M.; et al. Effect of Manure and Compost on the Phytostabilization Potential of Heavy Metals by the Halophytic Plant Wavy-Leaved Saltbush. Plants 2021, 10, 2176. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Ma, T.F.; Liu, X.Y.; Xu, Y.H.; Gu, J.T.; Gu, Y.F.; Yuan, J.; Wen, T.; Xue, C.; Shen, Q.R. Degradation of Complex Carbon Sources in Organic Fertilizers Facilitates Nitrogen Fixation. J. Agric. Food Chem. 2024, 72, 12988–13000. [Google Scholar] [CrossRef] [PubMed]
- Verenitch, S.; Mazumder, A. Carbon and Nitrogen Isotopic Signatures and Nitrogen Profile To Identify Adulteration in Organic Fertilizers. J. Agric. Food Chem. 2012, 60, 8278–8285. [Google Scholar] [CrossRef]
- Ren, L.J.; Yang, H.; Li, J.; Zhang, N.; Han, Y.Y.; Zou, H.T.; Zhang, Y.L. Organic fertilizer enhances soil aggregate stability by altering greenhouse soil content of iron oxide and organic carbon. J. Integr. Agric. 2025, 24, 306–321. [Google Scholar] [CrossRef]
- Ren, L.J.; Yang, H.; Li, J.Q.; Li, X.Y.; Han, Y.Y.; Zou, H.T.; Zhang, Y.L. Soil Aggregates and Organic Carbon Affected by Bio-Fertilizer in Greenhouse Soil. Commun. Soil. Sci. Plant Anal. 2025, 56, 784–799. [Google Scholar] [CrossRef]
- Wang, H.X.; Xu, J.L.; Liu, X.J.; Zhang, D.; Li, L.W.; Li, W.; Sheng, L.X. Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res. 2019, 195, 104382. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Y.; Zhang, Q.; Lin, S.; Zhang, Y.; Du, M.; Chen, M.; Ye, J.; Wu, Z.; Wang, H. Reasonable deep application of sheep manure fertilizer to alleviate soil acidification to improve tea yield and quality. Front. Plant Sci. 2023, 14, 1179960. [Google Scholar] [CrossRef]
- Yu, B.; Xu, D.; Li, Y.; Wang, W. Influence of Fertilization on Growth and Lead Content of Pepper under Lead Stress. Plants 2023, 12, 2960. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, C.; Kim, M.; Nam, J.; Shim, C.; Shin, J. Evaluation of the Impact of Activated Biochar-Manure Compost Pellet Fertilizer on Volatile Organic Compound Emissions and Heavy Metal Saturation. Int. J. Environ. Res. Public Health 2022, 19, 12405. [Google Scholar] [CrossRef]
- Kim, S.H.; Kumari, S.; Kim, C.J.; Lee, E.Y.; Alam, A.N.; Chung, Y.S.; Hwang, Y.H.; Joo, S.T. Effect of Adding Cultured Meat Tissue on Physicochemical and Taste Characteristics of Hybrid Cultured Meat Manufactured Using Wet-Spinning. Food Sci. Anim. Resour. 2024, 44, 1440–1452. [Google Scholar] [CrossRef]
- Kumari, M.; Sheoran, S.; Prakash, D.; Yadav, D.B.; Yadav, P.K.; Jat, M.K.; Ankit, A. Long-term application of organic manures and chemical fertilizers improve the organic carbon and microbiological properties of soil under pearl millet-wheat cropping system in North-Western India. Heliyon 2024, 10, e25333. [Google Scholar] [CrossRef]
- Zheng, C.M.; Wu, W.; Rui, Y.K. Effects of Organic Fertilizer on the Contents and Distribution of Heavy Metals in Spinach. Asian J. Chem. 2012, 24, 2811–2812. [Google Scholar]
- Zhu, R.; Liu, J.; Zong, H.Y.; Xie, Y.X.; Li, S.J.; Wang, F.L. Effect of Combined Application of Organic and Inorganic Fertilizer and Irrigation on Lead Transport Characteristics in Soil-Wheat Systems. Fresenius Environ. Bull. 2021, 30, 164–170. [Google Scholar]
- Qi, H.; Zhuang, Z.; Liu, J.; Huang, S.Y.; Wang, Q.Q.; Wang, Q.; Li, H.F.; Wan, Y.N. Potential to Ensure Safe Production of Water Spinach in Heavy Metals-Contaminated Soil by Substituting Chemical Fertilizer with Organic Fertilizer. Plants 2024, 13, 2935. [Google Scholar] [CrossRef]
- Khan, I.; Iqbal, M.; Raza, S.H.; Anwar, S.; Ashraf, M.; Shafiq, F. Tartaric acid soil-amendment increases phytoextraction potential through root to shoot transfer of lead in turnip. Chemosphere 2022, 296, 134055. [Google Scholar] [CrossRef]
- Wu, X.S.; Cai, Q.Y.; Xu, Q.; Zhou, Z.; Shi, J.Y. Wheat (Triticum aestivum L.) grains uptake of lead (Pb), transfer factors and prediction models for various types of soils from China. Ecotoxicol. Environ. Saf. 2020, 206, 111387. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.M.; Ashraf, U.; Qiu, B.L.; Ali, S. Transfer of lead (Pb) in the soil-plant-mealybug-ladybird beetle food chain, a comparison between two host plants. Ecotoxicol. Environ. Saf. 2017, 143, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.D.; Hwang, S. Tobacco phytochelatin synthase (NtPCS1) plays important roles in cadmium and arsenic tolerance and in early plant development in tobacco. Plant Biotechnol. Rep. 2015, 9, 107–114. [Google Scholar] [CrossRef]
- Wu, C.J.; Zhang, J.; Chen, M.; Liu, J.K.; Tang, Y.L. Characterization of a Nicotiana tabacum phytochelatin synthase 1 and its response to cadmium stress. Front. Plant Sci. 2024, 15, 1418762. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Ban, Y.H.; Li, Z.; Chen, H.; Yang, R.; Tang, M. Arbuscular mycorrhizal fungi play a role in protecting roots of Sophora viciifolia Hance. from Pb damage associated with increased phytochelatin synthase gene expression. Environ. Sci. Pollut. Res. 2014, 21, 12671–12683. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.Y.; Li, J.W.; Liu, F.H.; Liu, Z.C.; Luo, S.L.; Wu, Y.; Lyu, J.; Yu, J.H. Reduced Chemical Fertilizer Combined With Bio-Organic Fertilizer Affects the Soil Microbial Community and Yield and Quality of Lettuce. Front. Microbiol. 2022, 13, 863325. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Zhang, M.K. Effect of bio-organic fertilizers partially substituting chemical fertilizers on labile organic carbon and bacterial community of citrus orchard soils. Plant Soil 2023, 483, 255–272. [Google Scholar] [CrossRef]
- Yu, Z.; Yao, X.; Yang, M.; Hu, S.; An, X.; Li, C. Co-application of sheep manure and commercial organic fertilizer enhances plant productivity and soil quality in alpine mining areas. Front. Microbiol. 2024, 15, 1488121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wang, K.; Liu, X.R.; Yao, L.G.; Chen, Z.J.; Han, H. Exopolysaccharide-Producing Bacteria Regulate Soil Aggregates and Bacterial Communities to Inhibit the Uptake of Cadmium and Lead by Lettuce. Microorganisms 2024, 12, 2112. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.F.; Liu, Y.; Chen, G.F.; Turatsinze, A.N.; Yue, L.; Ye, A.L.; Zhou, Q.; Wang, Y.; Zhang, M.L.; Zhang, Y.B.; et al. Granular bacterial inoculant alters the rhizosphere microbiome and soil aggregate fractionation to affect phosphorus fractions and maize growth. Sci. Total Environ. 2024, 912, 169371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).