Abstract
In light of the phenomenon of colony collapse disorder, there has been a growing interest in finding natural and ecological ways for improving honeybee health. The aim of this scientific research was the isolation and characterization of LAB, which in the future could show the potential to construct a protective preparation for honeybees. After performing MALDI-TOF analysis, of a total of 76 bacterial strains isolated from flowers and honeybee products, 31 were identified as Pediococcus pentosaceus, 26 as Pediococcus acidilactici, and 19 as Lactiplantibacillus plantarum. The characterization of the isolated LAB displayed that CO2 production was present in 52 strains. The highest biomass productivity was observed in the case of strain 9/1 isolated from red clover (Trifolium pratense L.) with biomass productivity equal to 2.100. All isolated bacterial strains showed the ability to produce lactic acid. The strain 13/3 isolated from small-leaved lime (Tilia cordata L.) displayed the highest lactic acid production capacity in 100 mL of culture, i.e., 1.903 g of lactic acid. The carbohydrate assimilation pattern was examined using API 50 CH tests. All isolated strains were able to utilize esculin, D-ribose, D-galactose, D-glucose D-fructose, and D-mannose. It was also noted that the reduction of sugars is a strain-dependent ability and is specific for individual strains.
1. Introduction
Lactic acid bacteria (LAB) form a diverse and common bacterial group, which are naturally present in milk, plants, fermented dairy and vegetable products, and the gastrointestinal tract (GIT) of humans and animals, as well as in water and soil. Typical LAB do not form spores, are nonmotile, catalase-negative, cytochrome-free, anaerobic, and most commonly in the form of Gram-positive cocci and rods [1]. These bacteria are especially known for their ability to produce lactic acid, which is the final product of carbohydrate fermentation [2]. LAB classified as homofermentative produce 2 mols of lactic acid out of 1 mole of glucose, whereas LAB classified as heterofermentative produce lactic acid, acetic acid, carbon dioxide, and lactate acid. Heterolactic fermentation is associated with LAB which lack the enzyme fructose-1,6-biphosphate aldolase and, therefore, are unable to metabolize hexoses via the Embden–Mayeroff pathway. Heteronormative bacteria are also able to utilize pentoses, whereas not all homofermentative LAB are able to perform such a reaction [3]. Particular attention has recently been paid to nondairy LAB due to their metabolic activities [4]. Lactobacillales bacteria consists of the families Lactobacillaceae, Aerococcaceae, Enterococcaceae, Streptococcaceae, Leuconostocaceae, and Arnobacteriaceae. The most common LAB genera are Weissella, Lacticaseibacillus, Lactococcus, Pediococcus, Carnobacterium, Streptococcus, Oenococcus, Enterococcus, and Leuconostoc [5].
Many LAB strains are considered as probiotics [6]. Probiotics are microorganisms that are characterized by their beneficial properties when consumed in appropriate amounts. The International Scientific Association for Probiotics and Prebiotics’ consensus proposed a more correct definition of probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [7]. This definition applies to many microorganisms and at the same time emphasizes the importance of probiotics [8]. They regulate systemic immunity and ensure a nourishing balance in the digestive tract. Probiotic microorganisms should have the ability to withstand acidic pH, enzymes, and bile salts in the gastrointestinal environment of the host [9]. An important feature of probiotic microorganisms is their ability to adhere to epithelial cells in order to avoid the possibility of being excreted from the body [10]. The probiotic properties of LAB are determined by their metabolism related to the utilization of carbohydrates to acids, which lower the pH of the GIT [11]. Some LAB show bacteriostatic properties due to the secretion of lactocins (e.g., lactacin, nisin, lactocidin), and H2O2, which inhibit the growth of pathogens [12]. In humans, LAB show therapeutic properties such as managing antitumor activity, possibly preventing cancer, and improving the balance of the host microbiota [13]. The proteolytic activity of the LAB on caseins may result in an elevation of the antimicrobial peptide (e.g., k-casecidin) obtained by hydrolysis of the k-casein oligopeptide [3]. LAB can also produce bacteriocins, proteinaceous molecules that interfere with the growth of most bacteria [14]. Due to their potential, probiotics have been used for hundreds of years, but gained economic importance in the XX century [15].
LAB also inhabit bee bodies, making up a significant part of their GIT microbiota. In the honeybee GIT, microaerophilic conditions dominate with the temperature of 35 °C and presence of flower nectar sugars, which is the ideal niche for LAB [16]. Along with Snodgrassella alvi, Gilliamella apicola, and Bifidobacterium spp., LAB represent the typical core-bacteria inhabiting honeybee GIT [16]. LAB that populate the GIT of bees prefer fructose as the substrate needed for their growth. As a result, they are more likely to inhabit the fructose-rich niches [17]. The metabolic properties of microbiota provide bees with a barrier against pathogens, and, therefore, play a key role in the immunity and health of these insects [18]. Lactic acid produced by LAB also reduces the environmental pH of wounds, which prevents the spread and multiplication of pathogens that threaten bees [19]. The importance of LAB for bees is a relatively recent and prominent topic, and research conducted on strains inhabiting the bodies of the bees suggests that LAB increase the bees’ resistance to pathogens and insecticides [20,21]. For example, Forsgren et al., in their study, demonstrated that feeding bee larvae with a cell suspension consisting of the 11 hbs-LAB (honeybee-specific LAB) mixture (apart from Bombilactobacillus mellifer Bin4N and Apilactobacillus apinorum Fhon13N) reduced the mortality of larvae infected with the pathogen Paenibacillus larvae [22]. Lamei et al. presented that most of the inhibitory effect on pathogens was related to the extracellular fraction and hbs-LAB secretome blocked the vegetative development of P. larvae [23].
Over the last two decades, there has been a significant decline in the number of honeybees and, related to it, honeybee colonies. This phenomenon is called “Colony Collapse Disorder” (CCD) and was first observed during the winter of 2006/2007 in the United States and Europe [24,25]. It is suspected that the main factors contributing to CCD include bee pathogens, pesticides, GMO, antibiotics, viruses, air pollution, and mites. Due to the positive impact of pollinators on the economy and ecology, the decline in their life span is disquieting [26]. Improving the condition and health of bees is an urgent matter as is providing them with additional immunity against factors adversely affecting them.
Due to the above, increasing interest in searching for new natural methods to improve the condition and immunity of honeybees is observed worldwide. One of the alternatives can be LAB, which, as natural inhabitants of the honeybee GIT, fulfill a number of beneficial functions in their body, such as, for example, the digestion of complex compounds from flower pollen, saccharides fermentation to lactic and short-chain fatty acids (SCFA), and antagonistic activity towards honeybee pathogens or xenobiotics detoxification [27,28].
The main purpose of this research was to isolate and characterize the LAB derived from the bee environment and honeybee products, thus basing it on the research material with which bees have direct contact. In order to characterize the isolated LAB, their ability to produce carbon dioxide, biomass, and lactic acid was checked, and their ability to utilize carbohydrates was determined using API 50 CH tests. The novelty of this study was its attempt to identify and isolate LAB directly from the bee environment and honeybee (Apis mellifera L.) products (such as bee bread, royal jelly, bee pollen, flower and honeydew honeys, fermented honey). To the best of our knowledge, while there are some studies on the isolation of LAB from flowers [29,30], there are few past studies involving LAB isolation from honeybee products, such as honey [31,32]. Addressing this topic may prove important in future research on honeybees and their environment.
Characterization of the isolated LAB will enable the selection of appropriate LAB for further research due to the unique properties exhibited by individual strains. In the future, attempts will be made to find LAB strains with specific probiotic properties. An example is the detoxification of insecticides, including neonicotinoids or LAB strains (or their metabolites) showing antagonistic activity against honeybee pathogens such as Paenibacillus larvae or Melissococcus plutonius.
Although this is a preliminary study and focuses only on a few research procedures, this first stage of the research is very intense and important in the construction of later probiotic preparations, because the preparation cannot be created without such basic research. After this stage of the research, in vitro and in vivo tests may be conducted.
2. Materials and Methods
2.1. Research Material
The research material that was used to isolate the LAB consisted of various elements of flowers (pistils, stamens, petals, and receptacles) and honeybee products (e.g., 17 different honey types, bee pollen, bee bread, and royal jelly). Nineteen species of flowers were harvested between May and September 2019 and in May 2020. All flower samples were collected in sterile plastic tubes, and immediately transported to the laboratory for analyses. Honeybee products were purchased between May and August 2019 from apiaries and beekeeping farms. Fermented multiflora honey (freshly harvested) was a gift from a beekeeper from Hajnówka. All biological materials and their place of origin are listed in Table 1.

Table 1.
Place of origin of the research material.
2.2. Isolation of Lactic Acid Bacteria Strains
Two methods of isolation were performed to isolate LAB strains, depending on the research material. The first method involved the use of a stomacher and was mainly intended for material derived from flowers. The biological material (the perianth, i.e., the calyx, corolla, petals, stamens, and pistils) was introduced into a stomacher bag along with 50 mL of sterile PBS (pH 7.2), and the samples were then subjected to treatment in the stomacher twice. The next step was to pour 0.1 and 1 mL sample suspensions onto Petri dishes with deMan-Rogosa-Sharp (MRS) (Merck Life Science, Warsaw, Poland) agar medium supplemented with 3% nystatin (100U, Merck Life Science, Warsaw, Poland). In parallel, 1 mL of samples was added to the liquid MRS medium supplemented with 3% nystatin (100U) in aseptic plastic tubes. Plates and test tubes were incubated under anaerobic conditions at 30 °C for 48–72 h. Then, the mixture of isolates from the test tubes was plated on Petri dishes with MRS agar as described above and incubated for a further 48–72 h. The next step was to select the colonies from the plates considering their morphology, consistency, shape, and color, and transfer them to the liquid MRS medium using the sterile loop (in order to propagate the isolates). Samples were incubated under anaerobic conditions at 30 °C for 48–72 h.
The second method of isolating LAB was applied to the samples of honey and honeybee products. The initial step was to mix approx. 10 mL of sterile PBS (7.2 pH) with 1 spoon of biological material and grind it in a mortar (all in aseptic conditions). Then, the same procedure was applied as for the first isolation method. Plates and test tubes were incubated under anaerobic conditions at 37 °C for 48–72 h (plates) and up to 10 days (test tubes). The next step was to select the colonies, taking into account their morphology, consistency, shape, and color, and transfer them to the liquid MRS medium using the sterile loop. Finally, the test tubes were incubated under anaerobic conditions at 37 °C for 24–96 h until a visible growth (biomass) was achieved.
2.3. Cultivation of Isolated Strains of LAB, Propagation, Freezing, and Storage
Bacterial strains isolated from flowers were incubated for 24 h at 30 °C, while strains derived from honeybee products were incubated for 24 h at 37 °C. All strains were cultivated under anaerobic conditions using MRS broth medium, which ensured optimal conditions for growth and multiplication of bacteria. LAB that were subjected to long-term storage were centrifuged after 24 h of cultivation (8694× g, 15 min, 22 °C), and then were stored in Cryobanks™ (Copan Diagnostics Inc., USA, Jefferson Avenue, Murrieta, CA, USA) at −20 °C.
Before experiments, the strains were activated, passaged 2–3 times, and then cultured (3% inoculum) in MRS broth for 24 h at 30 or 37 °C (depending on the origin).
2.4. Assessment of Carbon Dioxide Production Ability
The ability to produce carbon dioxide was assessed by macroscopic observation of air bubbles produced by LAB cultured in a liquid MRS medium. The results were assessed as to whether the carbon dioxide production for a given strain was present or absent.
2.5. Assessment of Biomass Productivity
Bacterial strains were cultured at 30 °C for strains isolated from flowers and at 37 °C for strains isolated from honeybee products. Bacteria were subcultivated by inoculating a 3% inoculum into liquid MRS medium. The above procedure was repeated three times. After 24 h of cultivation and immediately after inoculation, the biomass productivity was assessed by spectrophotometric measurement (wavelength 540 nm) using a Spectroquant® Prove 300 spectrophotometer. The control sample was a sample of a clean, liquid MRS medium, set to 100% light transmittance.
2.6. Assessment of Lactic Acid Production
Bacterial strains isolated from flowers were incubated at 30 °C, while strains isolated from honeybee products at 37 °C. A 0.1M sodium hydroxide solution was prepared and poured into the burette. Then, 2 drops of phenolphthalein were added to the 24 h bacterial culture prepared in advance. Finally, the prepared cultures were titrated with a sodium hydroxide solution until the color turned pink.
2.7. API 50 CH Biochemical Tests
API 50 CH biochemical tests were purchased from the bioMerieux company in order to assess the ability of LAB to utilize carbohydrates and create an individual biochemical profile for an individual strain. Sterile PBS buffer was introduced into the API 50 CH wells to provide suitable conditions for the test. Bacterial strains were cultivated for 24 h in liquid MRS medium and then centrifuged (3864× g, 10 min). The centrifuged biomass was suspended in API 50 CHL medium. The turbidity of the resulting suspension was adjusted to the McFarland 2.0 standard, and then, it was introduced into 50 test wells. Sterile mineral oil was added to maintain anaerobic conditions. After 24 and 48 h of incubation at 30 °C (for strains isolated from flowers) and 37 °C (for strains isolated from honeybee products), the results of the tests were read. A change in the color of bromocresol purple to yellow indicated a positive test result causing acidification. In the case of esculin (contained in well no. 25), a positive test result was demonstrated by a color change to black. Negative results were marked as “−”, positive results as “+”.
2.8. MALDI-TOF Mass Spectrometry Analysis
First, the isolated bacteria were Gram-stained to ensure that each strain was gram-positive. The cell morphology of the bacteria was assessed under a phase-contrast microscope (Nikon Eclipse Ci H600L, Tokyo, Japan).
MALDI-TOF MS analysis was performed in order to identify the isolated bacterial strains to the genus and species. The initiation step was the incubation of the isolates at 30 °C (for strains isolated from flowers) and at 37 °C (for strains isolated from honeybee products). The cultivated bacteria were then transferred onto Petri dishes containing MRS agar medium using a sterile loop. Simultaneously, 300 µL of sterile HPLC purity water was transferred to an Eppendorf tube. The isolated bacterial colonies were transferred from the culture plate to water using a sterile loop. Then, 900 µL of sterile ethanol was added to the test tube and mixed thoroughly. The microbial material was centrifuged in a tabletop centrifuge (20,879× g, 2 min). The supernatant was removed with a pipette (avoiding contact with microbial material). The microbial material was then recentrifuged in a tabletop centrifuge (20,879× g, 2 min) and the residual ethanol was removed with a sterile pipette. The pellet was allowed to air dry for 5 min at room temperature. Then, 25 µL of a 70% aqueous formic acid solution was added, and the pellet was dissolved by pipetting the solution and discharging it several times. The next step was to add 25 µL of 100% acetonitrile to the test tube. The tube was centrifuged in a tabletop centrifuge (20,879× g, 2 min). Subsequently, 1 µL of the supernatant was applied to the empty sample position on the cleaned MALDI plate. The MALDI plate was allowed to air dry at room temperature. Then, 1 µL of α-cyano-4-hydroxycinnamic acid matrix solution was applied to each sample position. The samples with the matrix applied were allowed to air dry at room temperature for 5 min. MALDI plate was placed on a mass spectrometer to collect the MALDI-TOF spectrum and identify isolated bacteria.
3. Results and Discussion
3.1. LAB Isolates Obtained from Flowers and Honeybee Products
Flowers and honeybee products constitute a significant niche of sources of LAB due to the high concentration of carbohydrates and slightly acidic pH, thus creating a suitable environment for the development of these bacteria [2]. Most likely, the LAB found in plants come from the surrounding environment or thanks to pollinators and animals. Thus, flower nectar may also contain trace amounts of LAB, resulting in the later content of these bacteria in honeybee products [33]. It is estimated that the LAB population in flowers is in the range of 102 to 104 CFU/g [34]. In order to carry out this study, 27 species of flowers and 12 honeybee products were used. After cultivation on MRS agar medium and careful macroscopic evaluation, it was possible to isolate 76 LAB strains, 51 of which came from flowers and 25 from honeybee products. A greater variability was noticed in the case of LAB isolated from flowers. In the case of honeybee products, bee pollen was the richest in LAB differentiation, from which 6 strains were isolated. The LAB isolation results are shown in Table 2 and Table 3. LAB have been isolated from flowers and honeybee products in previous studies [2,32,35]. Teneva-Angelova and Beshkova (2015), after screening 400 microbial isolates from flowers and after performing confirmation tests, isolated 98 strains that showed a phenotypic LAB identity. Additionally, after conducting these experiments, it was proved that flowers and honeybee products are a natural habitat for microbes, including potentially probiotic LAB [30].

Table 2.
LAB strains were isolated from flowers from which honeybees most often collect nectar. Species of flowers were harvested between May and September 2019 and in May 2020.

Table 3.
LAB strains isolated from honeybee products (harvested from May to August 2019).
As flowers and honeys of different origins are rich sources of D-fructose, in the current study, the attempt to isolate fructophilic lactic acid bacteria (FLAB) was undertaken by the authors according to Sakandar et al. [36]. The isolation media (fructose yeast peptone FYP) contained 1% and 30% D-fructose (and nystatin to inhibit the growth of fungi) and the incubation time was prolonged up to 21 days. Simultaneously, glucose yeast peptone (GYP) was also used. No bacteria were isolated in the case of FYP on the contrary to GYP, where some growth was observed. Unfortunately, the isolates were not able to grow on different agar media in order to receive pure cultures.
3.2. Characteristics of Isolated LAB
Based on the macroscopic observations of LAB strains grown in the liquid MRS medium (presence of gas bubbles), the ability of the LAB to produce carbon dioxide was determined. After analyzing the results, CO2 production was present for 52 bacterial strains. In the remaining 24 strains, gas bubble production was absent. In the case of LAB, the presence of carbon dioxide indicates that they carry out heterolactic fermentation, where glucose is decomposed into lactic acid, ethanol, and CO2 through the phosphoketolase pathway [37,38].
The analysis of the biomass production capacity of LAB was based on the assessment of whether the bacteria displayed the ability to multiply after the next 2 passages.
The production of biomass was calculated according to the formula:
where A24 corresponds to the absorbance measured in the sample after 24 h of cultivation and A0 corresponds to the absorbance of the sample immediately after inoculation.
A = A24h − A0h
The mean biomass productivity (Amean) of the triplicate sample was then calculated to determine which of the strains showed the most efficient multiplication ability after repassage. This step was carried out in order to check the ability of the strains to proliferate after many passages, which is necessary for their potential use in the industry for the construction of probiotic preparations. The highest biomass productivity was observed in the case of strain 9/1 isolated from red clover (Trifolium pratense L.). This strain showed a biomass productivity equal to 2.100. The lowest biomass productivity was observed for the strain 12/1, isolated from small-leaved lime 1 (Tilia cordata L.), which was equal to 1.196. Berisvil and cowriters (2020), in their research, noted a significant fact about individual LAB requirements depending on their metabolic properties [39]. Microorganisms such as LAB may need various essential nutrients such as purines, vitamins, and amino acids. To ensure optimal biomass production, it is recommended to use an appropriate medium that ensures the conditions for growing a given strain. The similarity in the results of the study may indicate similar conditions for the growth of these bacteria and the application of the MRS medium may then be continued for the purpose of their cultivation.
The amount of produced lactic acid was determined by titration with NaOH against the reagent (phenolphthalein). The content of the produced acid was calculated using the following equation:
1 mL of 0.1M NaOH = 0.00906 g of lactic acid
All isolated bacterial strains showed the ability to produce lactic acid. The strain 13/3 isolated from small-leaved lime (Tilia cordata L.) displayed the highest lactic acid production capacity in 100 mL of culture, i.e., 1.903 g of acid. The lowest lactic acid production capacity in 100 mL of the tested sample was displayed by strain 6/1 isolated from brown knapweed (Centaurea jacea L.), and it was equal to 0.604 g of lactic acid. Lactic acid is a safe organic acid often used as a fermentation agent, decontaminant, and antioxidant. This organic molecule is the product of fermentation carried out by various microorganisms with the use of various sources of carbohydrates. Lactic acid production depends on nutrients, pH, temperature, and the LAB strain [40]. In their research, Kylä-Nikkilä et al. demonstrated high productivity and acid tolerance in Lactobacillus strains, thus emphasizing their economic importance in the fermented food industry [41]. The amount of produced acid may also affect the survival of LAB, many species of which do not grow in an environment where the pH is lower than 4 [40]. The highest productivity yields of lactic acid and its purity (>99%) are shown by homofermentative bacteria [42]. The ability to produce lactic acid by LAB may, in the future, be important in the study of the antimicrobial activities of these bacteria towards honeybee pathogens.
All the results of the short characteristics of the isolated bacteria are presented in Table 4.

Table 4.
The results of the characterization of lactic acid bacteria isolated from flowers and honeybee products in terms of their ability to produce carbon dioxide, biomass, and lactic acid.
3.3. Identification of Isolated LAB Strains
Isolated LAB strains were initially identified with the use of MS-MALDI TOF analysis. Of a total of 76 bacterial strains, 31 have been identified as Pediococcus pentosaceus, 26 as Pediococcus acidilactici, and 19 as Lactiplantibacillus plantarum. The identification index values were interpreted according to the MALDI Biotyper system by Bruker. The scope of the interpretation was as follows: samples with the identification index ≥2.00 showed high-confidence identification, the identification index 1.70–1.99 displayed low-confidence identification. On the other hand, the identification index within the range of 0.00–1.69 showed that the organism could not be identified. In addition, the shape of the identified bacteria was examined with microscopic observation. The identification index showed a significant majority of high-confidence identification, except for strains 2/2, 6/1, 6/2, 8/1, 8/2, 9/4, 13/2, and 17/2, where the identification index suggested low-confidence identification. LAB identification using mass spectrometry was also used in the case of fruits, and it was possible to identify strains such as Pediococcus pentosaceus, Lactiplantibacillus plantarum or Levilactobacillus brevis, which confirmed the presence of these bacterial species in plants [43].
Genetic-based methods would be ideal to identify and classify isolated strains, but these are preliminary studies and only the most effective strains in relation to probiotic properties, after screening in many future tests, will be selected for the analysis.
The results of the identification of the isolated bacteria are presented in the Table 5 and Figure 1.

Table 5.
Identification of LAB strains from honeybee environment and honeybee products using MALDI-TOF mass spectrometry analysis.
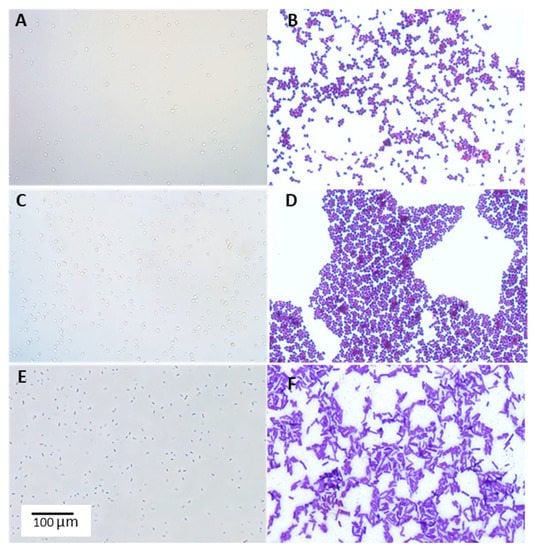
Figure 1.
Example microphotographs of lactic acid bacteria isolates from honeybee environment. (A,C,E)—wet mount (40×); (B,D,F)—Gram staining (100×). (A,B)—Pediococcus acidilactici 4/1 isolated from Robinia pseudoaccacia L.; (C,D)—Pediococcus pentosaceus 16/1 isolated from bee pollen; (E,F)—Lactiplantibacillus plantarum 11/1 isolated from Philadelphus coronarius L. Observed under phase-contrast microscope (Nikon Eclipse Ci H600L, Tokyo, Japan) attached to a digital camera (Nikon Digital Sight DS-U3, Tokyo, Japan) and imaging software (NIS-elements BR 3.0, Nikon, Tokyo, Japan).
3.4. Carbohydrate Assimilation Pattern
The sugar utilization ability of the bacteria was tested using the API 50 CH tests. After testing, it was noticed that all isolated strains were able to utilize esculin, D-ribose, D-galactose, D-glucose, D-fructose, and D-mannose. It was also noted that the reduction of sugars is a strain-dependent ability and is specific for individual strains. The results of the biochemical tests also showed that none of the isolated bacterial strains displayed the potential to utilize glycerol, D-arabinose, L-xylose, D-adonitol, L-sorbose, L-rhamnose, dulcitol, inositol, inulin, D-raffinose, starch, glycogen, xylitol, gentiobiose, D-lyxose, D-fucose, L-fucose, D-arabitol, L-arabitol, gluconate, 2-keto-gluconate, and 5-keto-gluconate (Figure 2).
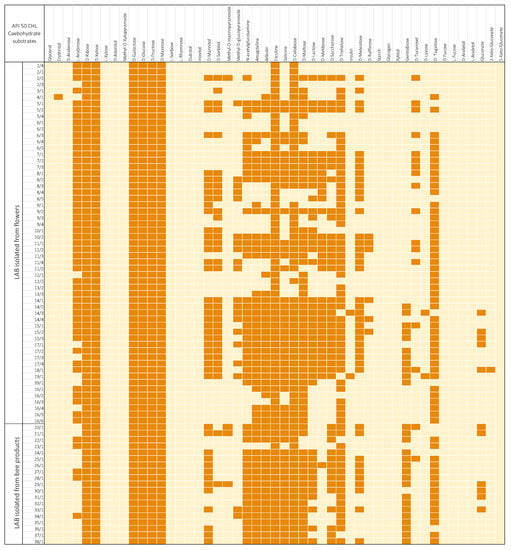
Figure 2.
Utilization of sugars by isolated strains of lactic acid bacteria. The darker color indicates a positive test result, and a lighter one—a negative result.
Due to the presence of unique biochemical features in the isolated LAB, it was possible to notice differences in the utilization of carbohydrates by the LAB depending on their origin (Figure 3). Bacteria were divided into two groups, referring to the research material from which they were isolated: flowers and honeybee products. LAB isolated from flowers more often displayed the ability to utilize carbohydrates such as D-sorbitol, methyl-D-glucopyranoside, D-lactose, D-melibiose, D-melezitose, D-raffinose, D-turanosel, and D-tagatose. LAB isolated from honeybee products more often utilized methyl-D-mannopyranoside, N-acetylglucosamine, amygdaline, arbutin, salicin, D-maltose, D-saccharose, D-trehalose, gentiobiose, and gluconate. However, in the case of carbohydrates such as D-xylose, D-mannitol, D-cellobiose, inulin, and D-xylose, the origin of the bacteria did not significantly affect the result. The highest dissimilarities were noted in the case of D-sorbitol, D-melibiose, and D-tagatose, where the differences between the results were greater than 63% (Figure 2). Enzymatic activities related to the microbiota of the digestive pathway of honeybees break down complex sugars belonging to the diet of these insects. Honeybees collect pollen rich in carbohydrates to provide food and support the entire colony [44]. Buron-Moles et al. (2018) conducted a study on 56 LAB strains and, similar to the studies presented above, all strains were able to degrade D-galactose, D-fructose, D-mannose, and D-glucose. In the case of D-tagatose, the results conducted there differed significantly from the results of our study, indicating the differentiation in LAB metabolism due to the strain and the origin of the isolate [44]. Reduction of monosaccharides and disaccharides was variable and was a strain-dependent trait. D-melezitose was the most degradable polysaccharide among 41 strains, while 6 strains utilized D-raffinose, and 2 isolates reduced inulin. The tested LAB displayed a low ability to metabolize polyols, except for D-mannitol and D-sorbitol, where the number of LAB strains demonstrating the ability to reduce them was 43 and 33 isolates, respectively. The greatest reduced salt was potassium 2-ketogluconate, where 11 LAB strains showed the ability to degrade it. Previous studies also demonstrated the low ability of LAB to metabolize salts and polyols [44,45]. Additionally, the obtained results were divided into two clusters according to the research material from which the isolates were obtained: LAB strains isolated from flowers and strains isolated from honeybee products. The acquired results were compared to each other, and it was noticed, inter alia, that LAB strains isolated from flowers more often degraded polyols such as D-sorbitol or D-mannitol, and bacteria isolated from honeybee products more often showed the ability to utilize salt. Looking at the results obtained, it can be concluded that the LAB origin affects their ability to metabolize carbohydrates.
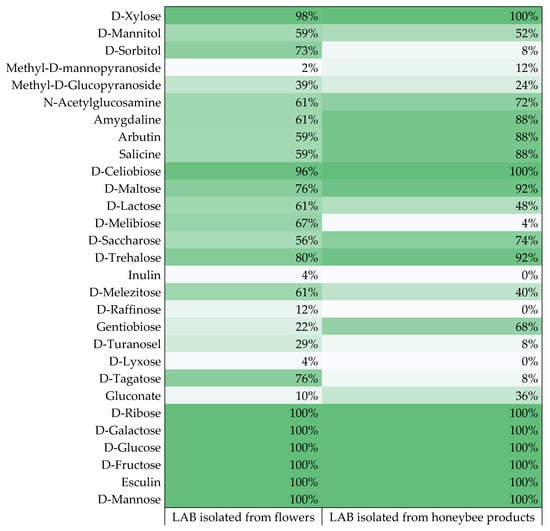
Figure 3.
Percentage of isolated LAB strains capable of utilizing given sugars.
4. Conclusions
As a result of the research, 51 LAB strains were isolated from flowers and 25 strains from honeybee products. So far, there has been a small number of scientific papers dealing with the topic of LAB research in honeybee products. Among 76 isolated LAB, 3 species of bacteria were identified (Pediococcus pentosaceus, Pediococcus acidilactici, and Lactiplantibacillus plantarum). The isolated strains were characterized for their ability to produce carbon dioxide and it was reported that most of them possessed this ability. All LAB were characterized by the capability to produce biomass and lactic acid, but these values were unique for an individual strain of bacteria. API 50 CH tests showed that all isolated strains utilized six carbohydrates. The potential of LAB to degrade sugars turned out to be a strain-dependent trait and often depended on the place of origin of a given strain and strains isolated from flowers more often preferred to utilize different sugars than those isolated from honeybee products.
These studies are preliminary and further investigations will be completed in order to gain a comprehensive view of potential beneficial effects of LAB isolates. In the future, they will be fully characterized in terms of their antagonistic activity (screening) against honeybee pathogens (e.g., Paenibacillus larvae, Melissococcus plutonius), the ability to produce biofilms, the capability to detoxify insecticides (especially neonicotinoids), survival in the simulated honeybee GIT and sugar syrup, identification of metabolites, and other tests in vitro and in vivo that determine probiotic abilities. Finally, top strains will be identified by genetic methods. The intention of the authors is that the isolated strains, after testing in vitro and in field studies on honeybees for probiotic characteristics, will be able to be applied in the construction of an ecological protective preparation for honeybees.
Since MALDI-TOF generated low-confidence identification for some isolates, of the 76 isolates obtained in this study, we took 51 for further research, considering the others as their clones.
Author Contributions
Investigation, methodology, data curation, formal analysis, writing—original draft preparation, writing—review and editing, resources, software, visualization: A.L.; conceptualization, methodology, project administration, writing—review and editing, supervision: A.N.; methodology: I.M. All authors have read and agreed to the published version of the manuscript.
Funding
Research supported by the project from the Provincial Fund for Environmental Protection and Water Management in Lodz (No. 729/BN/D/2019) entitled: “Selection of microorganisms for the construction of ecological protective preparation for honeybee (Apis mellifera L.)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and are available from the corresponding author upon reasonable request.
Acknowledgments
This work paper has been completed while the first author was the Doctoral Candidate in the Interdisciplinary Doctoral School at the Lodz University of Technology, Poland. We would like to thank the following students: Anita Czupryńska, Paula Janiak, Daria Szczuka, and Marta Wróblewska for help in laboratory experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Teneva-Angelova, T.; Hristova, I.; Pavlov, A.; Beshkova, D. Lactic Acid Bacteria—From Nature Through Food to Health. In Advances in Biotechnology for Food Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 91–133. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.; Mohamed, F.; Bleckwedel, J.; Medina, R.; de Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and Functional Properties of Lactic Acid Bacteria Isolated from Wild Fruits and Flowers Present in Northern Argentina. Front Microbiol. 2019, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front Cell Infect Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Salazar, N.; de los Reyes-Gavilán, C. Exopolysaccharides produced by lactic acid bacteria in food and probiotic applications. In Microbial Glycobiology; Academic Press: Cambridge, MA, USA, 2010; pp. 885–902. [Google Scholar] [CrossRef]
- Azhari Ali, A. Isolation and Identification of Lactic Acid Bacteria from Raw Cow Milk in Khartoum State, Sudan. Int. J. Dairy Sci. 2010, 6, 66–71. [Google Scholar] [CrossRef][Green Version]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Yadav, R.; Puniya, A.; Shukla, P. Probiotic Properties of Lactobacillus plantarum RYPR1 from an Indigenous Fermented Beverage Raabadi. Front Microbiol. 2016, 7, 1683. [Google Scholar] [CrossRef]
- Chen, J.; Pang, H.; Wang, L.; Ma, C.; Wu, G.; Liu, Y.; Guan, Y.; Zhang, M.; Qin, G.; Tan, Z. Bacteriocin-Producing Lactic Acid Bacteria Strains with Antimicrobial Activity Screened from Bamei Pig Feces. Foods 2022, 11, 709. [Google Scholar] [CrossRef]
- Fu, C.; Yang, Z.; Yu, J.; Wei, M. The interaction between gut microbiome and anti-tumor drug therapy. Am. J. Cancer Res. 2021, 11, 5812–5832. [Google Scholar] [PubMed]
- Montalban-Lopez, M.; Sanchez-Hidalgo, M.; Valdivia, E.; Martinez-Bueno, M.; Maqueda, M. Are Bacteriocins Underexploited? NOVEL Applications for OLD Antimicrobials. Curr. Pharm. Biotechnol. 2011, 12, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Feuilloley, M.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera Gastrointestinal Microbiota and Lactic Acid Bacteria for Honeybee Protection—A Review. Cells 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Salminen, S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013, 36, 444–448. [Google Scholar] [CrossRef]
- Kwong, W.; Moran, N. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef]
- Niode, N.; Salaki, C.; Rumokoy, L.; Tallei, T. Lactic Acid Bacteria from Honey Bees Digestive Tract and Their Potential as Probiotics. In Proceedings of the International Conference and the 10th Congress of the Entomological Society of Indonesia (ICCESI 2019), Bali, Indonesia, 6–9 October 2020. [Google Scholar] [CrossRef]
- Iorizzo, M.; Letizia, F.; Ganassi, S.; Testa, B.; Petrarca, S.; Albanese, G.; di Criscio, D.; de Cristofaro, A. Functional Properties and Antimicrobial Activity from Lactic Acid Bacteria as Resources to Improve the Health and Welfare of Honey Bees. Insects 2022, 13, 308. [Google Scholar] [CrossRef]
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as Major Modulators of Insect Health: Lactic Acid Bacteria and Honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Forsgren, E.; Olofsson, T.; Vásquez, A.; Fries, I. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie 2009, 41, 99–108. [Google Scholar] [CrossRef]
- Lamei, S.; Stephan, J.; Riesbeck, K.; Vasquez, A.; Olofsson, T.; Nilson, B.; de Miranda, J.R.; Forsgren, E. The secretome of honey bee-specific lactic acid bacteria inhibits Paenibacillus larvae growth. J. Apic. Res. 2019, 58, 405–412. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar]
- Leska, A.; Nowak, A.; Nowak, I.; Górczyńska, A. Effects of Insecticides and Microbiological Contaminants on Apis mellifera Health. Molecules 2021, 26, 5080. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into Diversity and Functionalities of Gut Microbiota in Insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Pernice, M.; Simpson, S.; Ponton, F. Towards an integrated understanding of gut microbiota using insects as model systems. J. Insect Physiol. 2014, 69, 12–18. [Google Scholar] [CrossRef]
- Saleh, G.M. Isolation and characterization of unique fructophilic lactic acid bacteria from different flower sources. Iraqi J. Agric. Sci. 2020, 51, 508–518. [Google Scholar] [CrossRef]
- Teneva-Angelova, T.; Beshkova, D. Non-traditional sources for isolation of lactic acid bacteria. Ann. Microbiol. 2015, 66, 449–459. [Google Scholar] [CrossRef]
- Feizabadi, F.; Sharifan, A.; Tajabadi, N. Isolation and identification of lactic acid bacteria from stored Apis mellifera honey. J. Apic. Res. 2020, 60, 421–426. [Google Scholar] [CrossRef]
- Lashani, E.; Davoodabadi, A.; Soltan Dallal, M.M. Some probiotic properties of Lactobacillus species isolated from honey and their antimicrobial activity against foodborne pathogens. Vet. Res. Forum. 2020, 11, 121–126. [Google Scholar] [CrossRef]
- Alvarez-Pérez, S.; Herrera, C.M.; de Vega, C. Zooming-in on floral nectar: A first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiol. Ecol. 2012, 80, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Kurosawa, K.; Miyazaki, M.; Yagi, C.; Kitajima, Y.; Tanaka, S.; Irisawa, T.; Okada, S.; Sakamoto, M.; Ohkuma, M.; et al. Lactobacillus floricola sp. nov., lactic acid bacteria isolated from mountain flowers. Int. J. Syst. Evol. Microbiol. 2011, 61, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Sakandar, H.; Kubow, S.; Sadiq, F. Isolation and in-vitro probiotic characterization of fructophilic lactic acid bacteria from Chinese fruits and flowers. LWT 2019, 104, 70–75. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Drinan, D.F.; Robin, S.; Cogan, T.M. Citric acid metabolism in hetero- and homofermentative lactic acid bacteria. Appl. Environ. Microbiol. 1976, 31, 481–486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berisvil, A.; Astesana, D.; Zimmermann, J.; Frizzo, L.; Rossler, E.; Romero-Scharpen, A.; Olivero, C.; Zbrun, M.V.; Signorini, M.; Sequeira, G.J.; et al. Low-cost culture medium for biomass production of lactic acid bacteria with probiotic potential destined to broilers. FAVE Sección Cienc. Vet. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Upadhyaya, B.P.; DeVeaux, L.C.; Christopher, L.P. Metabolic engineering as a tool for enhanced lactic acid production. Trends Biotechnol. 2014, 32, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kylä-Nikkilä, K.; Hujanen, M.; Leisola, M.; Palva, A. Metabolic engineering of Lactobacillus helveticus CNRZ32 for production of pure L-(+)-lactic acid. Appl. Environ. Microbiol. 2000, 66, 3835–3841. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Garcia, E.; Xavier, D.; Costa, W.; de Carvalho, R.J.; Campana, E.H.; Picão, R.C.; Magnani, M.; Saarela, M.; de Souza, E.L. Identification of Lactic Acid Bacteria Isolated From Fruits and Industrial Byproducts of Fruits Through the Maldi-Tof Technique. In Proceedings of the XII Latin American Congress on Food Microbiology and Hygiene, Foz do Iguacu, Brazil, 12–15 October 2014. [Google Scholar] [CrossRef][Green Version]
- Buron-Moles, G.; Chailyan, A.; Dolejs, I.; Forster, J.; Mikš, M. Uncovering carbohydrate metabolism through a genotype-phenotype association study of 56 lactic acid bacteria genomes. Appl. Microbiol. Biotechnol. 2019, 103, 3135–3152. [Google Scholar] [CrossRef]
- Ni, K.; Wang, Y.; Li, D.; Cai, Y.; Pang, H. Characterization, Identification and Application of Lactic Acid Bacteria Isolated from Forage Paddy Rice Silage. PLoS ONE 2015, 10, e0121967. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).