Abstract
Background/Objective: Type 2 Diabetes Mellitus (T2DM) represents a major increasing burden for primary care systems worldwide. Digital health interventions (DHIs) have been proposed as scalable tools to improve glycemic control, yet uncertainty remains regarding which intervention characteristics yield the greatest benefit. To evaluate the effectiveness of DHIs on HbA1c levels in adults with T2DM and to examine whether intervention duration, engagement intensity, glucometer integration, and healthcare provider involvement modify glycemic outcomes. Data Sources: PubMed, Embase, Cochrane Library, and JMIR databases were systematically searched for relevant studies published between January 2020 and May 2025. Study Eligibility Criteria: Randomized controlled trials comparing DHIs plus usual care versus usual care alone in adults with T2DM and reporting HbA1c as the primary outcome. Methods: Data were extracted using the Jadad scale and TIDieR framework. Random-effects meta-analysis estimated pooled mean differences (MD) in HbA1c with 95% CIs. Subgroup analyses examined effects by intervention characteristics. Heterogeneity and sources of variance were assessed through Cochran’s Q, I2, meta-regression, and sensitivity analyses (leave-one-out and trim-and-fill). Results: Thirteen RCTs (n ≈ 20,000) met inclusion criteria. DHIs achieved significant HbA1c reductions (range 0.01% to 1.57%; pooled MD −1.08%; 95% CI −1.18 to −0.99; p = 0.001). Short-term (≤6 months), low-intensity interventions showed the largest effect sizes (MD −1.16%, 95% CI 0.94 to 1.39). Glucometer integration and healthcare provider involvement contributed minimally to additional benefit. Meta-regression confirmed substantial heterogeneity, but no single factor explained variance across studies. Limitations: Considerable heterogeneity across interventions and variability in engagement measurement may limit the generalizability of findings. Conclusions: Short-term, low-intensity DHIs significantly improve glycemic control in primary care populations with T2DM. Advanced meta-analytic techniques confirm the robustness of these effects, providing practical guidance for selecting and implementing effective digital interventions in routine diabetes care.
1. Introduction
Type 2 Diabetes Mellitus (T2DM) is a prevalent chronic condition affecting more than 537 million adults globally and accounting for more than 90% of all diabetic patients worldwide, with significant economic and healthcare burdens [1]. On average, annual healthcare expenses for individuals with diabetes amount to USD 16,752, more than double that of non-diabetic individuals [2]. Managing T2DM requires long-term interventions, often focusing on reducing glycated hemoglobin (HbA1c) levels, a key indicator of glycemic control.
Given the high costs associated with managing diabetes mellitus, healthcare systems worldwide are increasingly turning to high-impact, low-cost strategies, such as mobile health (mHealth) interventions. Mobile interventions have become increasingly sophisticated in their technology and diverse in their approaches to changing and sustaining health behaviors [3]. Over the past decade, the integration of digital health interventions, particularly mobile health (mHealth) applications, has gained momentum as an innovative approach to improve disease management [4]. DHIs, which are therapies given through digital technology like smartphones or websites, have a huge potential to provide efficient, affordable, safe, and scalable interventions to promote healthcare and can be used to optimize outcomes for chronic diseases [5]. Health systems worldwide are increasingly seeking digital health strategies that are both effective and cost-efficient, particularly in developing countries where dedicated digital health departments are often lacking. The key for different governments worldwide to support the use of digital health or mobile health is based on a cost-effectiveness relationship, to be able to implement public policies that allow the design, creation, use, evaluation, and maintenance of software or apps.
The World Health Organization [6] has highlighted digital health as a key component of health system transformation, emphasizing that implementation should be evidence-based and supported by adequate workforce skills and professional competencies [4]. Newer digital health tools increasingly incorporate data-driven personalization features to enhance patient engagement and adherence [7,8].
Despite the increasing use of digital interventions, their effectiveness in reducing HbA1c levels in patients with T2DM remains uncertain. Previous systematic reviews have examined digital health interventions, but many have lacked specificity in focusing on randomized clinical trials (RCTs) and use glycemic control as a primary outcome [9,10,11,12,13]. Moreover, the existing literature presents significant variability in the design, duration, and adherence to digital interventions, making it difficult to draw conclusive evidence regarding their efficacy in T2DM management. This systematic review addresses a specific knowledge gap by providing evidence on the impact of interventions on HbA1c levels in T2DM patients.
2. Materials and Methods
This systematic review follows the PRISMA 2020 guidelines [14], and the completed PRISMA checklist is provided in the Supplementary Materials S1; the protocol was registered on the Open Science Framework (OSF): https://osf.io/kbnwq/, accessed on 7 July 2025. The study aims to evaluate the effectiveness of digital health interventions in reducing HbA1c levels among patients with T2DM, focusing on the characteristics associated with improved outcomes. Specifically, it will (1) assess the impact of intervention duration, comparing short-term (≤6 months) and long-term (>6 months) interventions; (2) examine the role of glucometer integration within the digital intervention (telemonitoring, mobile apps, SMS, and web-based platforms) and its correlation with HbA1c reduction; (3) investigate whether interventions involving healthcare provider support (reported as professionals from any area of health who participated in the design and monitoring of the intervention) differ in effectiveness from those without a reported provider involvement; and (4) analyze the impact of intervention intensity, categorized as low, moderate, or high, on HbA1c reduction, to identify the optimal engagement level for glycemic control.
2.1. Eligibility Criteria
We included studies that met the following criteria: (1) Study design: Randomized controlled trials (RCTs). (2) Participants: Patients included were diagnosed with T2DM. (3) Intervention: Digital interventions, including mobile health (mHealth) applications, aimed at influencing HbA1c levels. (4) Outcome: The primary outcome must be the measurement of HbA1c levels. (5) Comparator: (standard treatment + use of the app) and control group (standard treatment) were compared. (6) Publication Date: Studies published between January 2020 and May 2025 were considered for inclusion, reflecting the period of rapid evolution in mHealth technologies. The rationale for this cutoff is that technological advances in AI and digital health interventions have significantly evolved since 2020, making earlier studies less relevant to current healthcare practices. The post-2020 period also reflects a substantial increase in the development and adoption of mHealth technologies [15]. Language: Studies included were published in English or Spanish.
2.2. Information Sources
A systematic search was conducted in the following electronic databases: PubMed, Embase, Cochrane, and JMIR search portals as bibliographic databases.
2.3. Search Strategy
The search strategy followed the PICO-SD framework: Population (P): Adults with Type 2 Diabetes Mellitus; Intervention (I): Digital health interventions (telemonitoring, mobile apps, SMS, and web-based platforms); Comparator (C): Usual or non-digital care; Outcomes (O): Primary outcome—HbA1c reduction, and secondary—other glycemic indicators; Study Design (SD): Randomized controlled trials, including cluster-RCTs.
A comprehensive search strategy was developed combining free-text terms and Medical Subject Headings (MeSHs), structured around four main concepts: (1) condition of interest (Type 2 Diabetes), (2) digital interventions, (3) study design, and (4) primary outcome. The following terms were used: Condition (Type 2 Diabetes): “type 2 diabetes”, “diabetes mellitus”, “diabetes mellitus type II”, “diabetes type 2”, “type II diabetes mellitus”, “DM2”, “non insulin dependent”, “non-insulin dependent”, “noninsulin dependent diabetes”, “adult onset diabetes”, and “maturity onset diabetes”. Digital Interventions: “digital app”, “digital application”, “mobile health”, “mHealth”, “m-health”, and “diabetes apps”. Study Design: “clinical trial”, “randomized controlled trial”, “RCT”, “efficacy”, and “effectiveness”. Primary Outcome (Glycemic Control): “HbA1c”, “hemoglobin A1c”, “glycosylated hemoglobin”, “glycated hemoglobin”, and “HbA1c value”.
The detailed search strategy, including Boolean operators and database-specific adaptations, is provided in the Supplementary Materials S2.
2.4. Study Selection
Two independent reviewers screened the titles and abstracts of identified studies. Full-text articles were assessed for eligibility based on predefined criteria. Disagreements were resolved through discussion or consultation with a third reviewer. A PRISMA flow diagram was used to illustrate the study selection process.
2.5. Data Collection Process
Data from the included studies were extracted using a standardized data extraction form in Excel. The extracted data included study characteristics (author, year, and country), population demographics, details of the intervention and control groups, study duration, outcome measures (HbA1c levels), and key findings.
Interventions were classified as low intensity (≤1 contact per month or total contact time <3 h), moderate intensity (2–3 contacts per month or 3–10 h in total), or high intensity (≥1 contact per week or >10 h in total) during the intervention period.
To ensure comprehensive reporting of the intervention, we utilized the Template for Intervention Description and Replication (TIDieR) checklist. This tool provides a structured framework for detailing interventions, enhancing transparency, and facilitating replication in future studies. By adhering to TIDieR guidelines, we systematically described the intervention’s components, including its rationale, materials, procedures, delivery methods, and any modifications made during the study. The completed TIDieR checklist is provided in the Supplementary Materials S3.
2.6. Risk of Bias Assessment
The risk of bias for the included RCTs was assessed using the Jadad scale [15], which evaluates three key methodological domains: adequacy of randomization, blinding, and reporting of withdrawals or dropouts. In its modified form, the scale also incorporates stricter criteria by allowing the subtraction of one point if randomization or blinding procedures are judged inadequate, while the total score remains within the 0–5 range. The complete Jadad scale and scoring criteria used in this review are provided in the Supplementary Materials S4.
Trials with a score of ≥3 were considered to have a low risk of bias, whereas those scoring lower were classified as high risk. This threshold is widely accepted as an indicator of methodological soundness. Applying this criterion ensured that the evidence synthesis was based on studies with higher internal validity, thereby enhancing the robustness and credibility of the review findings.
The Jadad scale offers a practical and straightforward approach that facilitates consistent, transparent, and reproducible assessment of study quality in systematic reviews and meta-analyses. Its use in this review contributed to strengthening the methodological rigor and reliability of the conclusions drawn.
2.7. Data Synthesis
A narrative synthesis was first conducted to summarize study characteristics, intervention details, and outcomes not suitable for pooling. This was complemented by a meta-analysis estimating pooled mean differences in HbA1c with 95% Confidence Intervals across eligible trials.
2.8. Data Analysis
The key outcome of interest was the change in HbA1c levels, expressed as mean differences (MD) between intervention and control groups. A random-effects inverse-variance model with DerSimonian–Laird (DL) estimate of tau2 was used to pool the effect sizes. To assess the robustness of the pooled estimates, sensitivity analyses were conducted using an alternative estimator of between-study variance (restricted maximum likelihood, REML). The direction and magnitude of the pooled effect sizes for HbA1c reduction remained consistent with the primary DerSimonian–Laird model, and no relevant changes were observed in heterogeneity estimates or statistical significance.
Subgroup analysis was performed based on the duration of the interventions, categorizing them into 2 groups: short-term (six months or less) and long-term (more than six months). Other subgroups include the following: use of a glucometer (with or without), the presence of health care providers for the design and follow-up of the interventions (described or not described), and intensity (low, medium, and high) according to the design of the interventions. We reported effect estimates with 95% Confidence Intervals (CIs), and heterogeneity was assessed using Cochran’s Q statistic and I2 values.
Meta-regression analysis was performed to explore potential sources of heterogeneity across the included studies. The goal of the meta-regression was to examine the relationship between the effect sizes (HbA1c reduction) and specific study-level covariates, which could help explain the variation in the effectiveness of digital educational interventions for diabetes management. (1) Standard Error (SE): This accounted for the precision of each study’s effect estimate; (2) Initial HbA1c: Baseline levels of glycated hemoglobin reflected the glycemic control at the start of the study; (3) Total Sample Size: The number of participants in each study was used to assess whether the effect size varied with sample size; (4) Year of Publication (year): The year in which the study was published is relevant for examining whether there was any temporal trend in the reported effect sizes.
We used a random-effects meta-regression model, which incorporates the variability between studies and accounts for both within-study and between-study heterogeneity. The random-effects model was chosen due to the significant heterogeneity observed in the effect sizes across the included studies, as indicated by the high I2 in the initial meta-analysis. The meta-regression was performed using the restricted maximum likelihood (REML) method to estimate between-study variance (tau2). This method provides an unbiased estimate of the variance components in the presence of small samples and accounts for both observed covariates and unexplained heterogeneity.
The heterogeneity was assessed using the following metrics: Tau2 (between-study variance) is an estimate of the between-study variability; I2 is the percentage of residual variation due to heterogeneity, indicating the proportion of total variability in effect estimates that is due to heterogeneity rather than chance.
A leave-one-out sensitivity analysis was performed to assess the robustness of the pooled effect size from the meta-analysis. This analysis involved systematically omitting each study from the meta-analysis and recalculating the pooled effect estimate to evaluate the influence of individual studies on the overall result.
Finally, given the high heterogeneity observed in the meta-analysis, there was a concern that potential publication bias could be contributing to the variability in effect sizes. To address this, a trim-and-fill analysis was conducted. This method estimates the number of potentially missing studies due to publication bias and adjusts the meta-analysis results accordingly. It is widely applied in meta-analyses. This approach detects potential asymmetry in funnel plots and estimates the impact of possibly missing studies by providing an adjusted pooled effect size. The initial analysis was performed using a random-effects model. The trim-and-fill analysis applied a linear trimming estimator to identify any missing studies.
3. Results
3.1. Study Selection
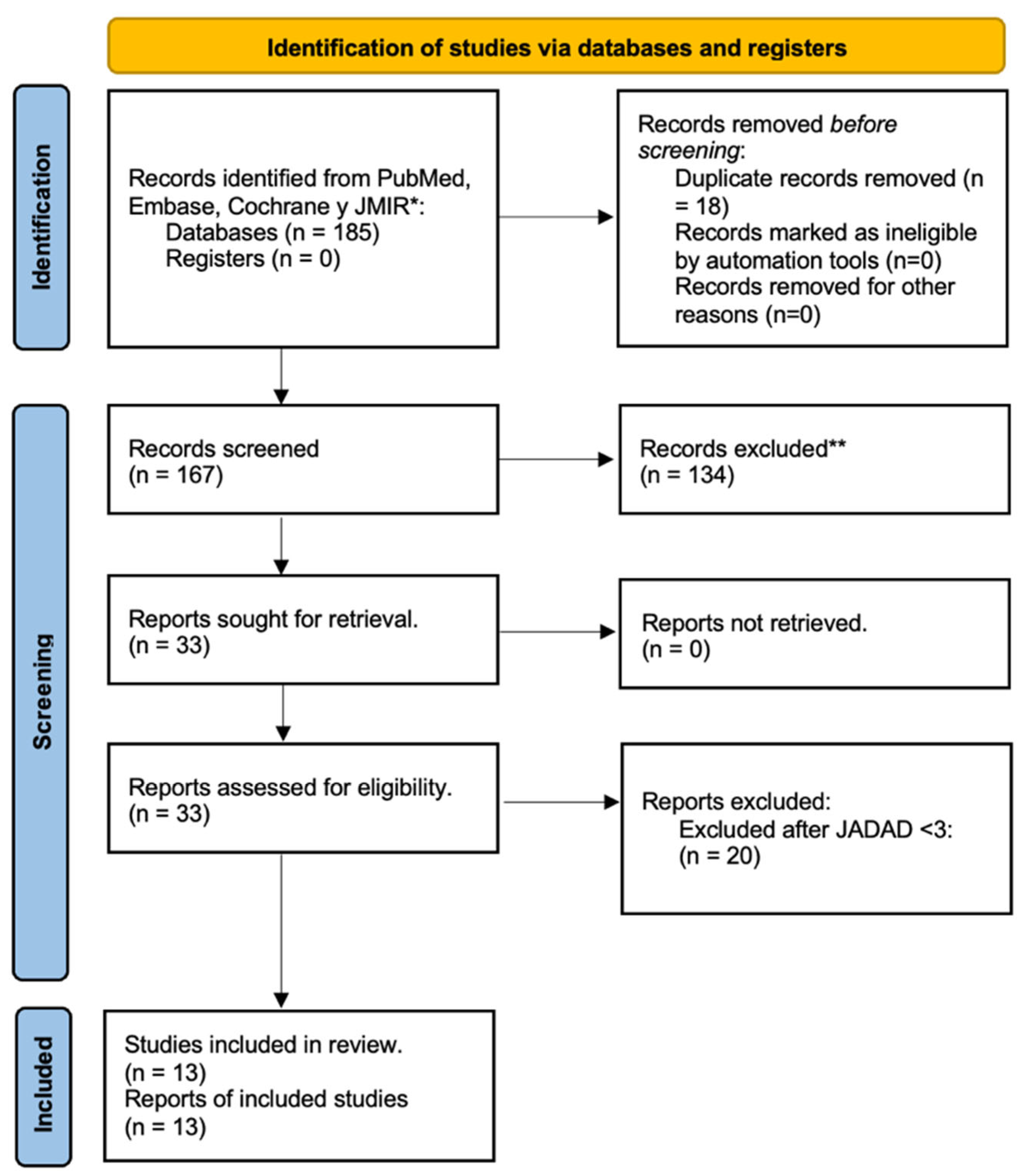
At the end of the search, a total of 185 articles were reviewed, of which 18 duplicates were eliminated for a total of 167.
From this first partial cut, 134 out of 167 studies were eliminated for having assessments of other objectives, a study design different from a randomized clinical trial, systematic reviews, traditional or non-digital health interventions, evaluation of another type of diabetes, pilots, or research protocols were rejected.
Subsequently, these 33 articles were subjected to a specific review according to the main objectives of this study, (Figure 1), and all of them met the following inclusion criteria: (1) study design and randomized clinical trials; (2) digital health diabetes intervention with the use of apps or software based on mHealth; (3) evaluation of effectiveness on Hb1Ac levels; (4) articles published in 2020 to may 2025; and (5) original articles.
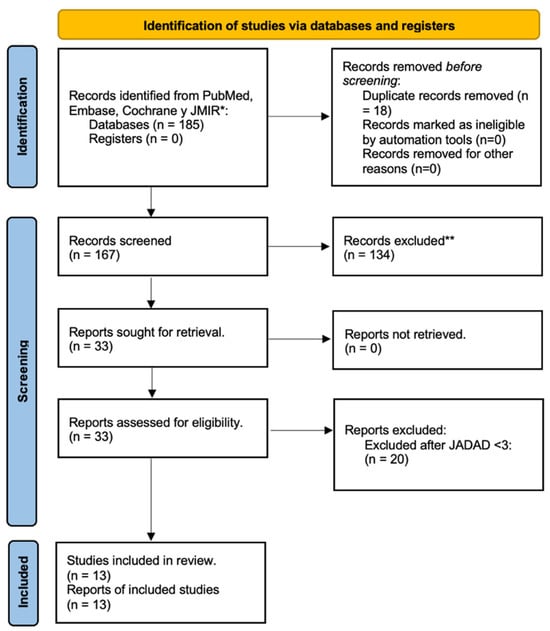
Figure 1.
Diagram PRISMA of identification, screening, eligibility, and inclusion of original articles. * Pubmed (n = 96); Embase (n = 48); Cochrane (n = 24); and JMIR (n = 17). ** Reasons for exclusion: Study design (n = 29); scoping review (n = 24); non-digital interventions (n = 17); T1DM evaluated (n = 15); protocols (n = 10); prediabetes (n = 3); and gestational diabetes (n = 3).
For the final part of the analysis, the Jadad scale was applied to assess the risk of bias [16] for the included RCTs; this approach ensured the inclusion of only 13 high-quality studies.
Description of Studies and Patients’ Characteristics
The 13 included studies were conducted across various countries, including the USA [4], China [5], Spain [1], South Korea [1], Japan [1], and Australia [1], with sample sizes ranging from 50 to over 17,000 participants. The 13 selected articles and their main characteristics are presented in the following table (Table A1 in the Appendix A).
3.2. Intervention Characteristics
A total of 13 interventions were analyzed using the Tidier scale, Table A2 (see Appendix A). Among these, six interventions (46.15%) utilized a glucometer for monitoring and self-management of blood glucose levels [16,17,18,19,20,21]. These interventions typically involved cellular-enabled glucose meters or similar technologies integrated into mobile health systems. In contrast, seven digital health interventions (53.85%) did not include a glucometer, focusing instead on educational programs, app-based coaching, or telemedicine without direct blood glucose monitoring [22,23,24,25,26,27,28].
The interventions were delivered by a variety of providers. In six cases (46.2%), interventions were provided by healthcare professionals such as physicians, nurse educators, dietitians, or psychologists [17,19,20,26,27,28]. In the remaining seven interventions (53.9%), the delivery was automated through mobile apps or telemedicine platforms without direct involvement of healthcare providers [16,18,21,22,23,24,25].
The intensity of the interventions varied. Three interventions (23.1%) were highly intensive, involving daily or bi-weekly monitoring and feedback [16,20,21]. Another five interventions (38.5%) had a moderate intensity, with participants receiving weekly or monthly guidance and monitoring [17,22,26,27,28]. The remaining five interventions (38.5%) were lower in intensity, with participants interacting with the intervention only once or at predefined intervals [18,19,23,24,25].
3.3. Risk of Bias
The overall results indicate a variation in the methodological quality of the studies, as reflected in their Jadad scores (see Supplementary Materials). The majority of studies scored 3 or higher, suggesting an overall moderate to high quality in the assessment of randomization, blinding, and dropout reporting across the studies included in this review. However, certain aspects, particularly blinding and the adequacy of randomization, require attention in future studies to ensure more robust methodological designs.
Two studies, Livongo [17] and My Diabetes Coach [23], had lower quality based on incomplete descriptions of randomization and inadequate or absent blinding. The Livongo study had no blinding or adequate randomization, scoring negatively. Several other studies, such as TangPlan and WeChat [18], Kaisang’s intervention [25], and Indica STUDY [27], also scored 3, mostly due to the lack of blinding and limited reporting on withdrawals and dropouts. Other studies [22,26,29] had Jadad scores of 4 and 5, indicating an improvement in methodology, especially with the appropriate reporting of randomization and some level of blinding, but still lacking in one or more criteria such as blinding adequacy or dropout reporting.
The studies IMB Model [24], EPICC [28], SINOMEDISITE [19], and ROADMAP [21] scored 6, demonstrating high methodological rigor. These studies had clear descriptions of randomization methods, adequate blinding procedures, and proper reporting of withdrawals or dropouts, making them the most methodologically sound studies in this review. The certainty of evidence for the primary outcome (HbA1c reduction) was assessed using the GRADE approach. The complete GRADE Summary of Findings table is provided in the Supplementary Materials S5.
3.4. Meta-Analysis
Primary Analyses
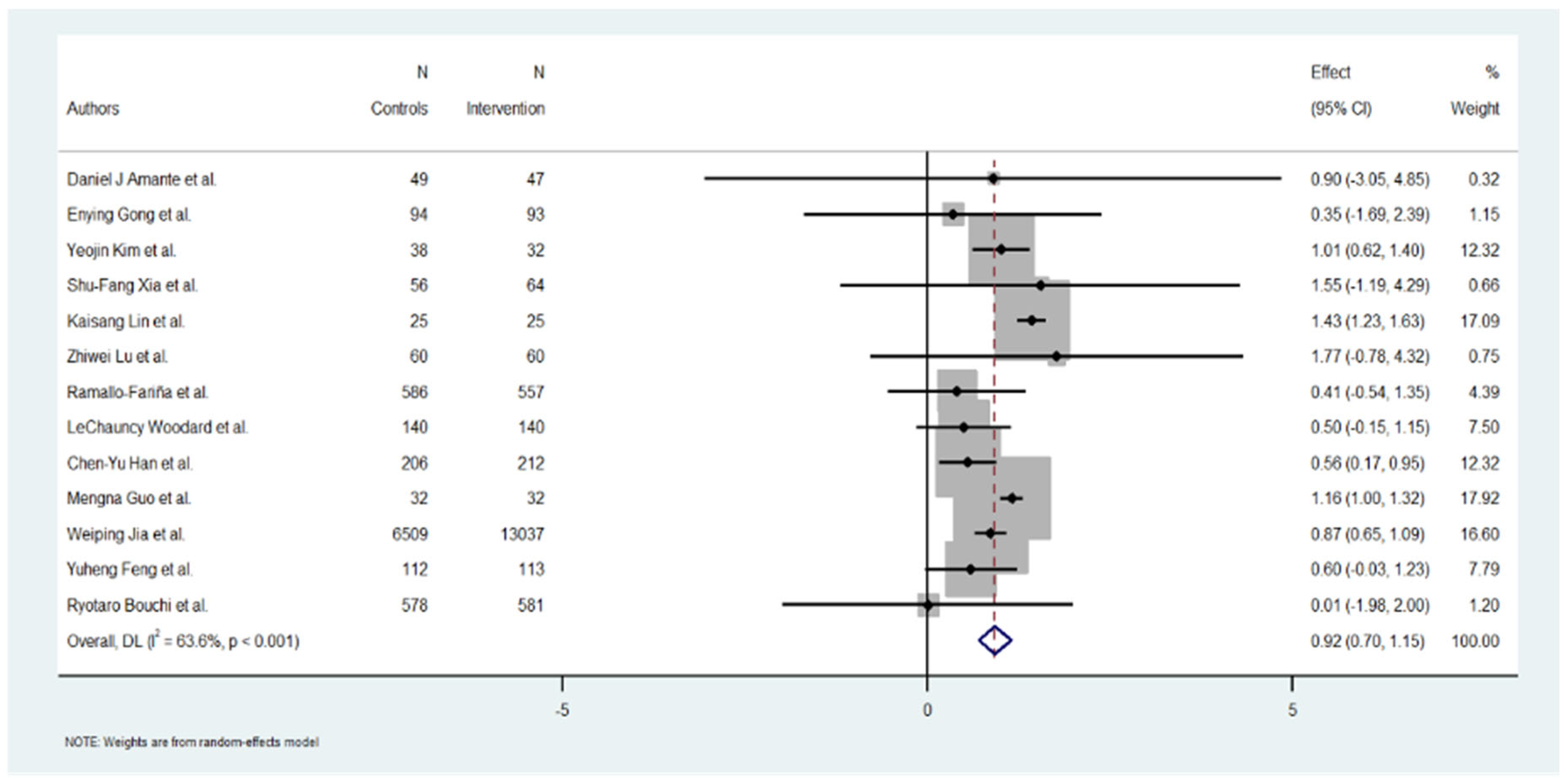
A total of 13 studies were included in the meta-analysis. The overall effect estimate for the change in HbA1c across the studies was −1.08% (95% CI: −1.18 to −0.96), suggesting a positive and significant reduction in HbA1c levels (p = 0.001).
3.5. Study-Level Results
The individual effect estimates varied widely across the included studies. Yeojin Kim et al. [24] reported a significant HbA1c reduction of 1.01 (95% CI: 0.62 to 1.40), contributing 12.32% to the overall weight. Kaisang Lin et al. [25] also reported a significant reduction of 1.43 (95% CI: 1.23 to 1.63), contributing 17.09% to the weight. Mengna Guo et al. [20] showed an effect of 1.16 (95% CI: 1.00 to 1.32), with 17.92% of the weight. Conversely, Ryotaro Bouchi et al. [22] demonstrated a minimum effect of 0.01 (95% CI: −1.98 to 2.00), contributing 1.20% to the total analysis. Several studies, such as those by Daniel J Amante et al. [17], Enying Gong et al. [23], and Zhiwei Lu [26] et al., reported moderate to good results in HbA1c reduction but a low level in the % of weight. Overall, the heterogeneity of the effect sizes was high, as reflected in the heterogeneity measures below (Figure 2).
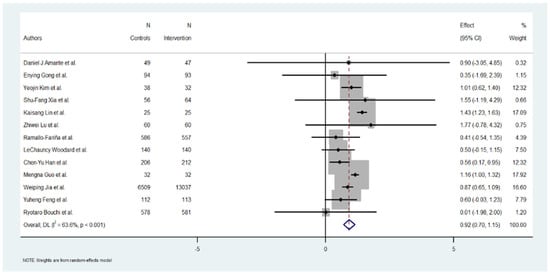
Figure 2.
Effect of Hb1Ac reduction by digital intervention [17,18,19,20,21,22,23,24,25,26,27,28,29].
3.6. Heterogeneity Analysis
The heterogeneity among the included studies was substantial, as evidenced by the following measures: Cochran’s Q = 33.01, with a p-value < 0.001, indicating significant heterogeneity. I2 = 84.68%, suggesting that almost all the observed variability in the effect sizes can be attributed to between-study differences rather than chance.
The heterogeneity variance (tau2) was estimated at 0.0674. The high levels of heterogeneity (I2 > 84.8%) indicate considerable variability across the studies in the reported effects of digital educational interventions on HbA1c reduction. Categorical variables such as provider type and intervention modality were incorporated into subgroup analyses, which help to explore but cannot fully account for the observed heterogeneity. This should be considered when interpreting the variability of effects across trials.
Specifically, the digital interventions evaluated demonstrated highly variable effects on HbA1c reduction, reflecting differences in intervention design and implementation. In addition, the studies encompassed a wide range of sample sizes, from small pilot trials to large-scale randomized controlled trials, which may have disproportionately influenced the pooled estimates. Furthermore, substantial variability was present in the duration of interventions and length of follow-up, thereby increasing between-study variability and contributing to the overall heterogeneity detected.
The high heterogeneity (I2 > 80%) likely reflects differences in population characteristics, intervention design, and follow-up duration, which could not be fully explained by subgroup or meta-regression analyses. These factors should be considered when interpreting the pooled estimates.
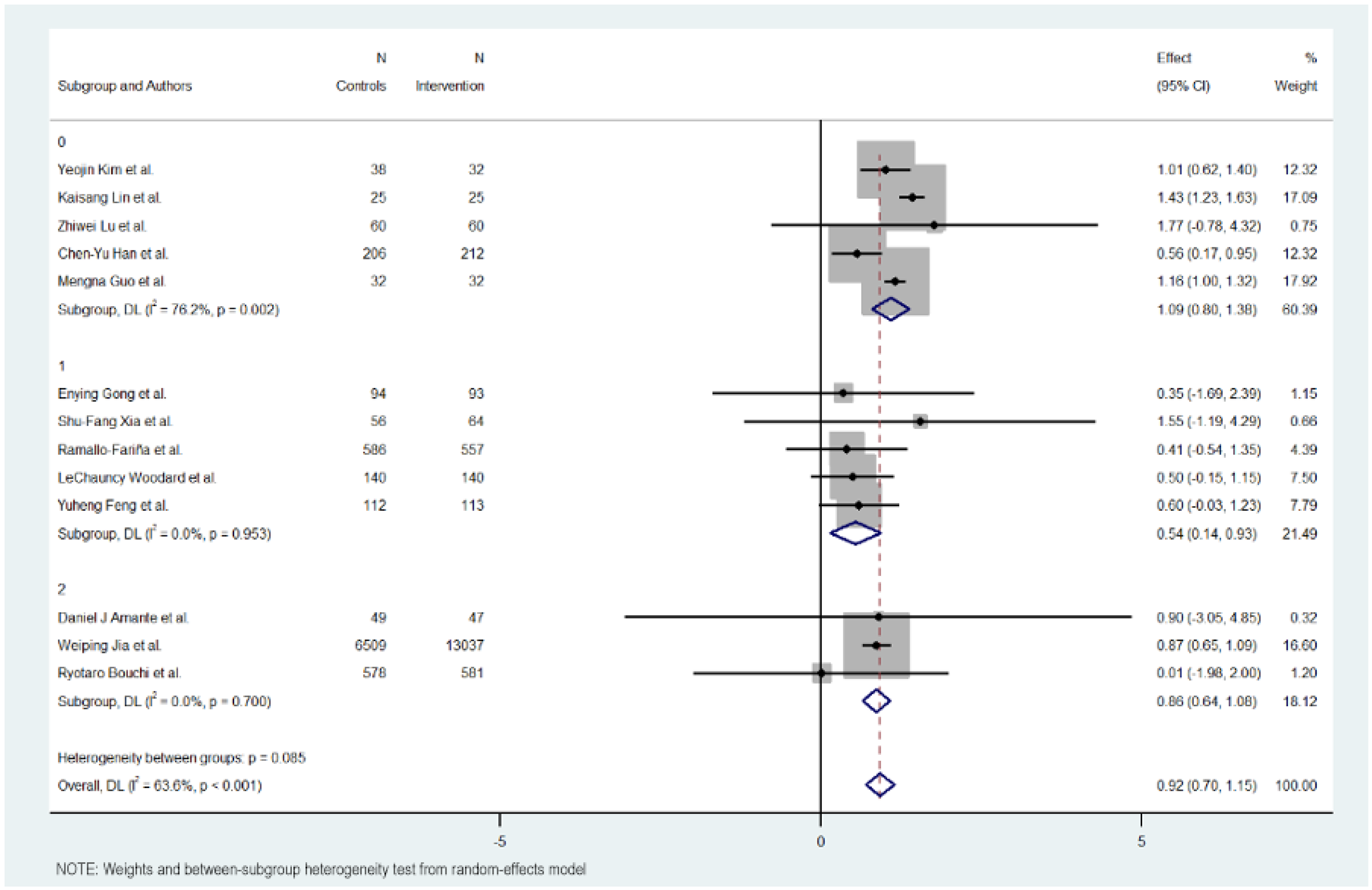
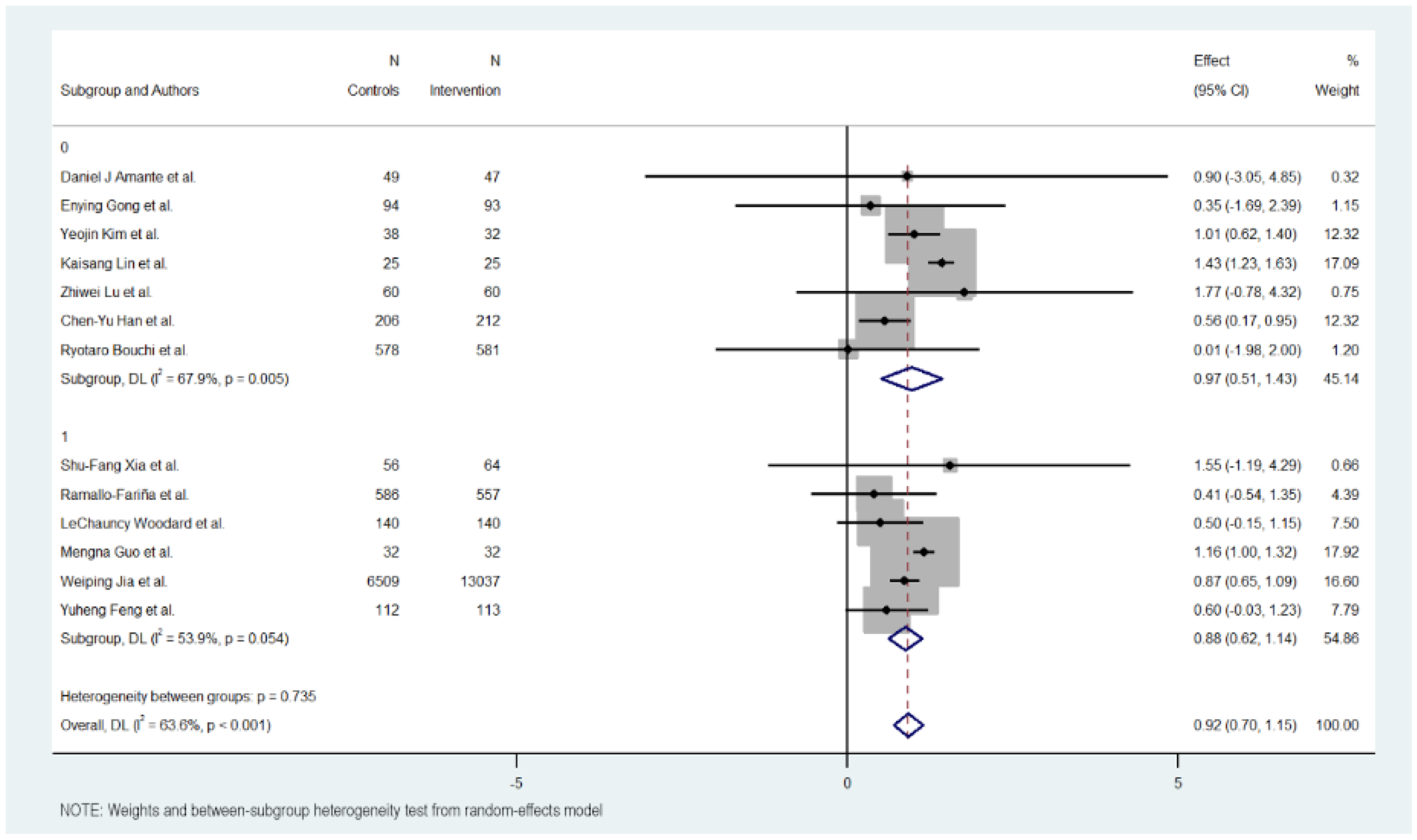
3.7. Subgroup Analysis
Figure 3, Figure 4, Figure 5 and Figure 6 illustrate the impact of key design features of digital health interventions on HbA1c reduction: differences according to intervention duration (short-term vs. long-term) and intensity (low, moderate, and high), the effects of glucometer integration, and the involvement of healthcare providers.
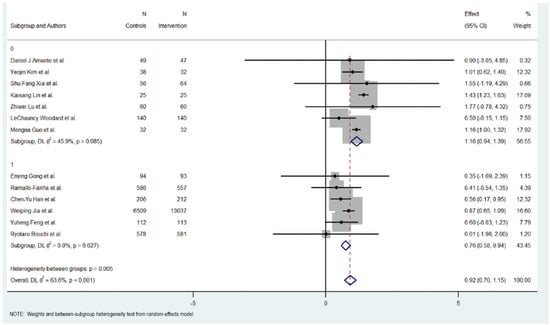
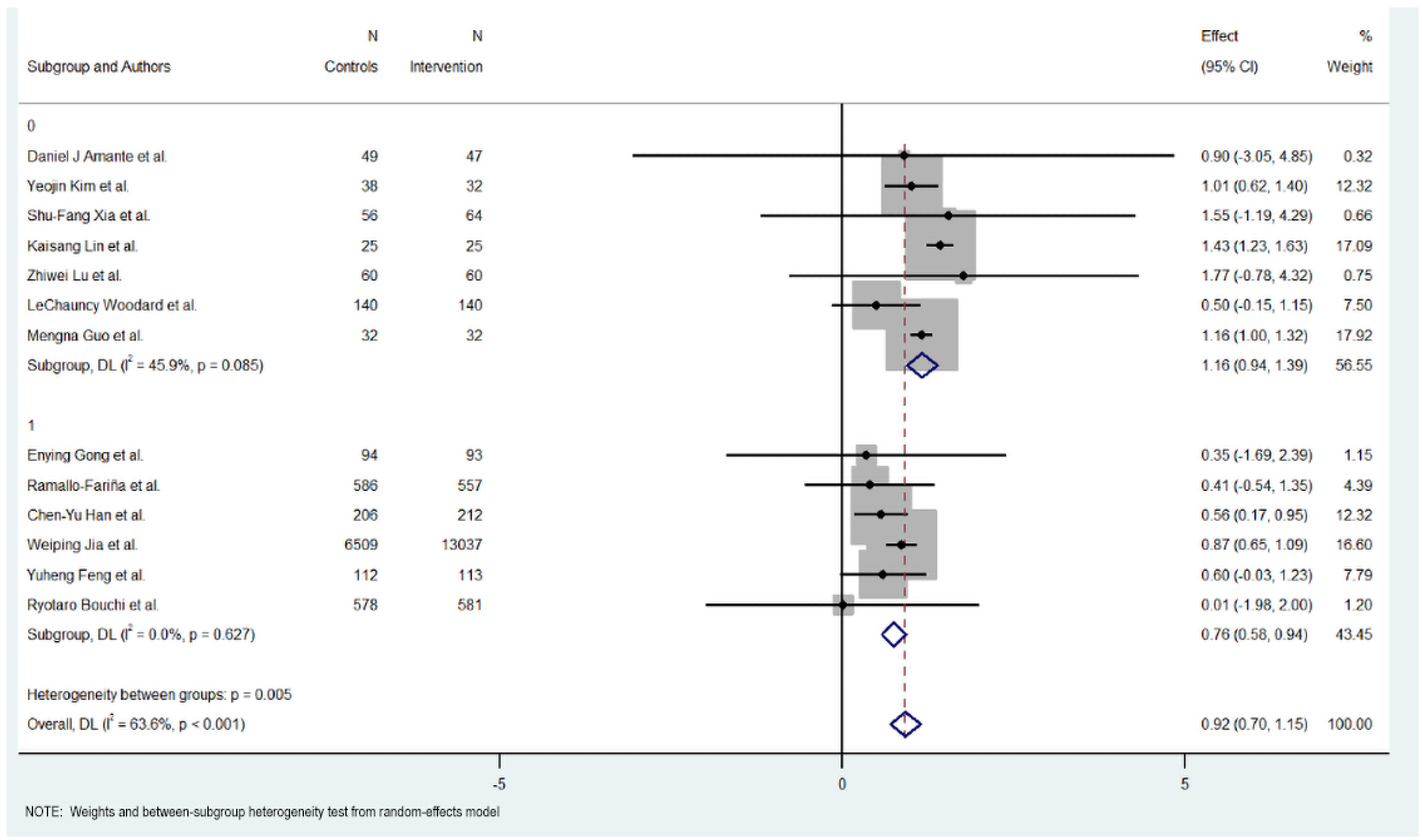
Figure 3.
HbA1c reduction according to duration in digital health interventions [17,18,19,20,21,22,23,24,25,26,27,28,29].
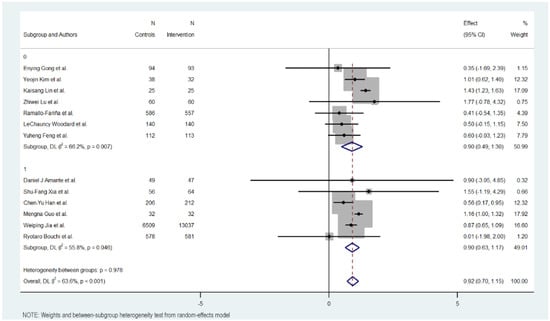
Figure 4.
HbA1c reduction according to intensity in digital health interventions [17,18,19,20,21,22,23,24,25,26,27,28,29].
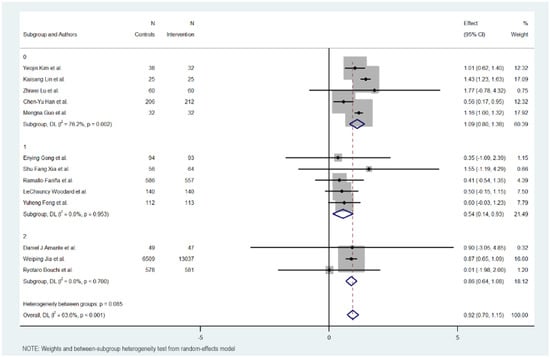
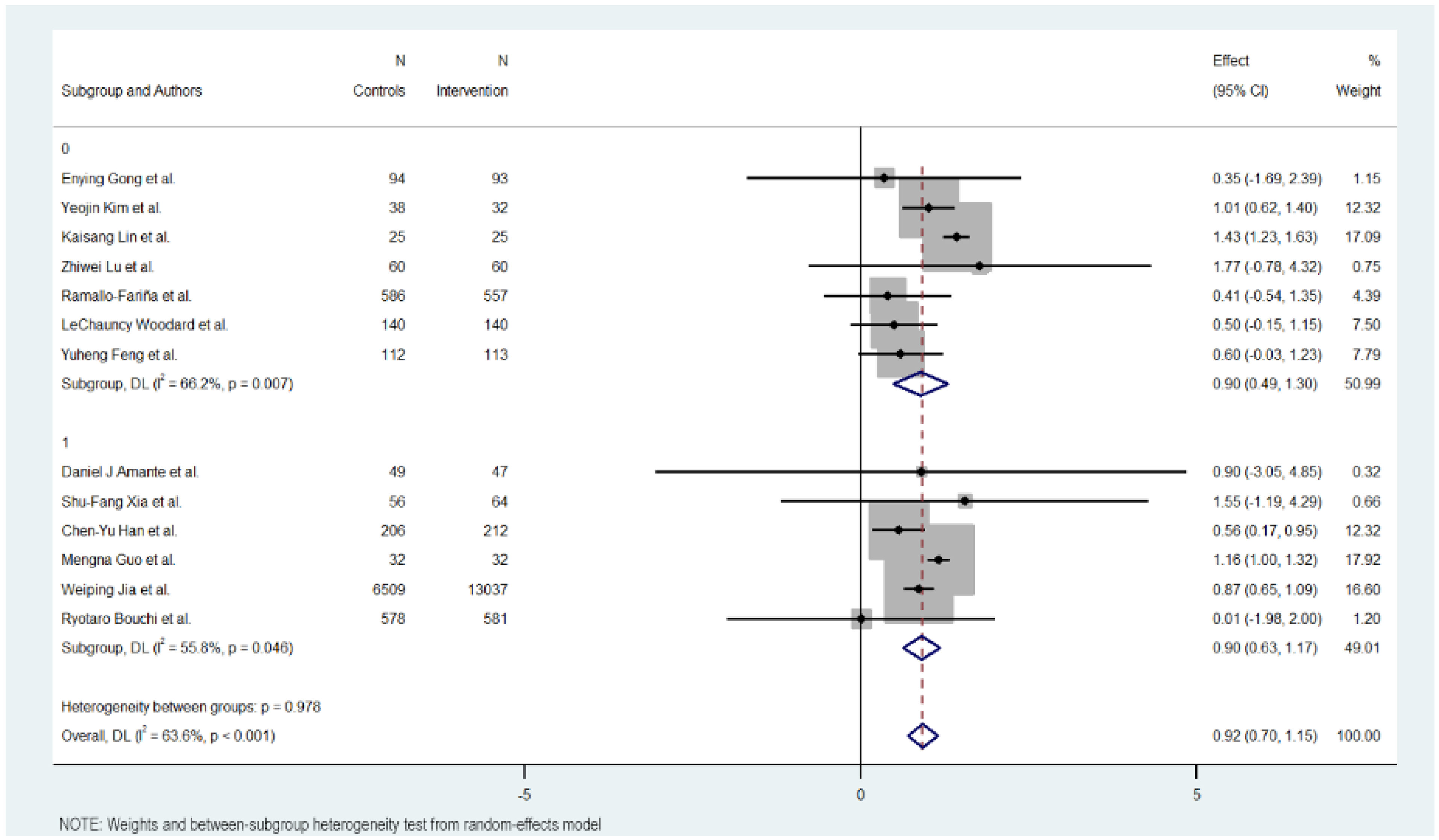
Figure 5.
HbA1c reduction according to glucometer integration in digital health interventions [17,18,19,20,21,22,23,24,25,26,27,28,29].
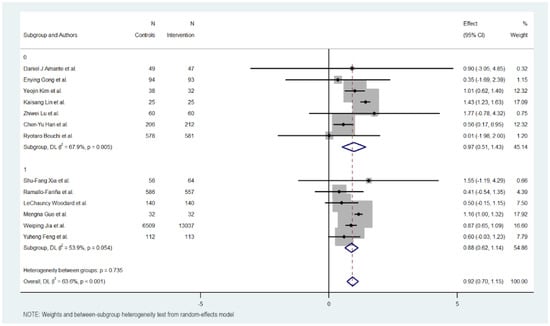
Figure 6.
HbA1c Reduction according to healthcare provider involvement in digital health interventions [17,18,19,20,21,22,23,24,25,26,27,28,29].
3.8. Duration of Intervention
Subgroup analysis revealed differences between short-term (six months or less) and long-term interventions (more than six months). For short-term interventions, the pooled effect size was significant, with an MD of 1.165 (95% CI: 0.94 to 1.390, p < 0.000), indicating a positive impact of the digital interventions on reducing HbA1c levels. The short-term interventions exhibited regular heterogeneity (Cochran’s Q = 11.10, p = 0.085, I2 = 45.9%).
Meanwhile, long-term interventions did not show a statistically significant effect on HbA1c reduction (MD = 0.758, 95% CI: 0.581 to 0.935, p = 0.000). Heterogeneity in this subgroup was low (Cochran’s Q = 3.48, p < 0.627, I2 = 0.0%), suggesting no substantial variability among studies in this group.
Overall, the heterogeneity was moderate and significant with all included studies (Cochran’s Q = 33.01, p < 0.001, I2 = 63.6%), indicating variations in the effects of digital educational interventions on HbA1c reduction. However, significant heterogeneity was also detected between the short-term and long-term intervention subgroups (Cochran’s Q = 7.74, p < 0.005), with short-term interventions showing a more consistent and favorable outcome.
3.9. Intensity of Intervention
The intensity of the groups was also studied in three groups: low, moderate, and high intensity in sending content and messages.
The subgroup with a low intensity of the intervention obtained better results in reducing Hb1Ac, followed by the subgroup with a high intensity. The subgroup with a moderate intensity was the one that showed the least effect in reducing Hb1Ac.
The subgroup with a low intervention intensity had an MD of 1.091 (95% CI: 0.801 to 1.381, p < 0.000), indicating more than 1% on Hb1Ac levels. This subgroup exhibited high heterogeneity (Cochran’s Q = 16.83, p = 0.002, I2 = 76.2%).
The subgroup with a moderate intervention intensity had an MD of −0.540 (95% CI: −0.934 to −0.145; p < 0.007), indicating a modest reduction in Hb1Ac, half the effect compared to the low intensity intervention. The heterogeneity in this group was low (Cochran’s Q = 0.68, p = 0.953, I2 = 0.0%).
The subgroup with an intense intervention intensity had an MD of 0.860 (95% CI: 0.644 to 1.076, p < 0.000), indicating a midpoint effect between low and moderate intensity. The heterogeneity in this group was also low (Cochran’s Q = 0.71, p = 0.700, I2 = 0.0%).
Overall, the heterogeneity was moderate and significant with all included studies (Cochran’s Q = 33.01, p < 0.001, I2 = 63.6%), indicating variations in the intensity of digital educational interventions on HbA1c reduction. In addition, significant heterogeneity was not detected between the level of intensity intervention subgroups (Cochran’s Q = 4.93, p < 0.085), with interventions with lower intensity showing a more favorable outcome compared to the others.
3.10. Use of Glucometer
The use of a glucometer revealed no differences between interventions with or without it. The interventions without using a glucometer had 0.895 effect against 0.902 in the interventions using it.
The interventions without a glucometer had an MD of 0.895 (95% CI: 0.488 to 1.302, p < 0.000), indicating a positive impact of the digital interventions on reducing HbA1c levels. These interventions exhibited moderate heterogeneity (Cochran’s Q = 17.73, p = 0.007, I2 = 66.2%).
Meanwhile, interventions with a glucometer show almost the same effect on HbA1c reduction (MD = 0.902, 95% CI: 0.629 to 1.175, p = 0.000). Heterogeneity in this subgroup was low (Cochran’s Q = 11.30, p < 0.046, I2 = 55.8%).
Overall, the heterogeneity was moderate and significant with all included studies (Cochran’s Q = 33.01, p < 0.001, I2 = 63.6%), indicating variations in the effects of digital educational interventions on HbA1c reduction. In addition, significant heterogeneity was not detected between the short-term and long-term intervention subgroups (Cochran’s Q = 0.00, p < 0.978), with interventions with a glucometer showing a more favorable outcome but not showing a big difference.
3.11. Providers
Analysis revealed little differences between interventions in which it is indicated were designed and supervised by health professionals compared to those that do not specify it.
The group of interventions where the participation of health providers is described showed a lower effect with an MD of 0.877 (95% CI: 0.618 to 1.136, p < 0.000). These interventions exhibited moderate heterogeneity (Cochran’s Q = 10.85, p = 0.054, I2 = 53.9%).
On the other hand, the group of interventions that do not describe the participation of health providers showed a better effect with an MD of 0.968 (95% CI: 0.509 to 1.427, p < 0.000). These interventions exhibited moderate heterogeneity (Cochran’s Q = 18.69, p = 0.005, I2 = 67.9%).
Overall, the heterogeneity was moderate and significant with all included studies (Cochran’s Q = 33.01, p < 0.001, I2 = 63.6%), indicating variations in the effects of provider support interventions on HbA1c reduction. However, significant heterogeneity was not detected between the provider and non-provider intervention subgroups (Cochran’s Q = 0.11, p 0.735), with better results in the subgroup that does not describe the use of health providers for the design and supervision of the intervention.
3.12. Metaregresion
The meta-regression revealed that none of the individual covariates, including initial HbA1c, sample size, or year of publication, significantly explained the variation in effect sizes across the studies. Although the model accounted for about 29% of the between-study variance, a large proportion of the heterogeneity (96.81%) remains unexplained, indicating that other factors may contribute to the variability in the effectiveness of digital interventions for glycemic control in diabetes management.
The coefficient for initial HbA1c was −0.333 (95% CI: −0.9144903 to 0.248321, p = 0.211). This suggests a negative but non-significant relationship between initial HbA1c levels and the effect size. The coefficient for total sample size was 0.1866961 (95% CI: −1.17228 to 1.545672, p = 0.748). This indicates that the total number of participants in the studies had no significant effect on the effect size. The coefficient for the year of publication was 0.1093 (95% CI: −0.2555 to 0.4742, p = 0.491). This suggests a positive relationship between the year of publication and the effect size, although this was not statistically significant.
3.13. Leave-One-Out Sensitivity Analysis
The overall pooled effect estimate from the meta-analysis was 1.084 (95% Confidence Interval [CI]: 0.988 to 1.180). The sensitivity analysis revealed that the pooled effect size remained relatively stable when individual studies were excluded, suggesting that no single study had a disproportionately large influence on the overall estimate.
3.14. Cumulative Meta-Analysis by Year
The cumulative analysis showed variable levels of heterogeneity across the years. Initially, heterogeneity was minimal, with I2 = 0.0% for the first two studies. However, as more studies were included, particularly from 2021 onwards, the heterogeneity increased moderately before dropping again to negligible levels by 2024.
3.15. Meta-Analysis and Trim-And-Fill Analysis
A trim-and-fill analysis was conducted to assess and adjust for publication bias. The linear trimming estimator identified no missing studies; the between-study variance (tau2) is 0.067, indicating no substantial reduction in heterogeneity after adjusting for potential publication bias.
4. Discussion
4.1. Principal Findings and Comparison with Previous Studies
This systematic review aimed to evaluate the effectiveness of digital interventions, specifically mobile health (mHealth) applications, in reducing HbA1c levels in patients with T2DM. The results demonstrated that mHealth interventions have the potential to significantly lower HbA1c levels, with reductions ranging from 0.35% to 1.57%. However, the findings also highlight the substantial variability in the effectiveness of these interventions, depending on factors such as intervention duration, user adherence, and the specific design of the digital tools.
The results of this review are consistent with previous studies that have reported moderate improvements in glycemic control through digital interventions [9,10,11,12]. However, our analysis further emphasizes the critical importance of the duration of the intervention and user engagement. Interventions that lasted six months or less consistently achieved greater reductions in HbA1c levels compared to longer interventions. These findings suggest that sustained engagement with mHealth tools may be essential for achieving optimal outcomes in glycemic control. Other systematic reviews have found a decrease in Hb1Ac levels with better results in interventions of average duration between 12 and 24 weeks [30]. Meanwhile, interventions targeting HbA1c reduction with the support of telemedicine during the COVID-19 pandemic demonstrated a pooled mean decrease of −0.6% relative to usual care [13].
Subgroup analysis revealed that interventions ≤ 6 months with lower intensity achieved the most substantial reductions in HbA1c levels, suggesting that brief, concentrated engagements may optimize glycemic outcomes in Type 2 Diabetes management. These findings align with previous studies indicating that targeted, time-bound interventions can enhance patient adherence and engagement, leading to measurable improvements in glycemic control [17,19]. The second group with higher intensity achieved the second most substantial reductions, and the third group with moderate intensity the lowest, although other studies show better results in interventions with moderate intensity, the results in high- and low-density interventions obtain very similar results [11]. Conversely, the inclusion of glucometer use and direct involvement of healthcare providers showed minimal impact on HbA1c reduction, highlighting that intervention effectiveness may hinge more on the frequency and intensity of patient engagement than on the addition of specific monitoring tools or professional oversight [9,29]. The results change when the digital intervention includes, in addition to the use of the application, the participation of health coaches who carry out continuous monitoring and intervention in patients, where results can be seen not only in the decrease in glycated hemoglobin, but also in other clinical indicators such as high blood pressure and BMI, as well as in behavioral changes in food choices [10]. It is also important to note that the limited additional benefit observed from healthcare provider involvement and glucometer integration may depend on contextual factors such as the intensity of patient engagement and the design of feedback mechanisms. These components may enhance adherence or motivation in some settings but not necessarily translate into greater glycemic improvements across all interventions. During the pandemic, some studies with subgroup analyses indicated greater efficacy in trials implementing frequent and personalized monitoring, as well as in those delivering high-intensity interventions. All this suggests that a scalable, self-directed intervention model focusing on high user engagement and structured, short-term content delivery may serve as an efficient approach for glycemic management in broader healthcare settings [11].
The heterogeneity in the design of the interventions, including differences in the functionalities of the mHealth apps, participation of clinicians, or additional communication tools such as those provided by health coaches, also played a role in the variability of results. While some apps focused on self-management support through reminders and educational content, others incorporated more advanced features such as real-time feedback, artificial intelligence, or telemedicine consultations. As we discussed, the latter group of interventions tended to report better outcomes, reinforcing the value of integrating more personalized and adaptive digital features into diabetes management tools.
4.2. Strengths and Limitations
The findings of this review suggest that mHealth interventions, particularly those incorporating advanced features such as real-time feedback and AI-driven recommendations, can be effective tools for improving glycemic control in patients with Type 2 Diabetes Mellitus (T2DM). Healthcare providers should consider integrating these digital tools into routine diabetes care, especially for patients who require additional support in self-management.
By focusing exclusively on studies with rigorous experimental designs, this review aims to provide a clearer understanding of the effectiveness of digital health technologies in improving glycemic control. This information is essential at a time when the adoption of digital tools in healthcare is growing rapidly. The findings will add to the existing academic literature on digital health interventions and provide practical evidence-based information for healthcare providers and policy makers seeking to implement these technologies to improve diabetes management and control. DHIs encompass all types of interventions with characteristics and different ways in which digital and mobile technologies are being used to support health system needs. Unlike other systematic reviews and meta-analyses, our research is based on studying the most relevant characteristics of digital health interventions in users with T2DM based on randomized clinical trials with a control and experimental group, considering Hb1Ac as the main indicator of efficacy but assessing strategic points of the interventions such as duration, intensity, use of a glucometer, and the participation of health professionals in the design or monitoring of the digital intervention. Features that will allow for the selection of digital tools with significant results, but also enable public decision-makers to select those they consider optimal based on the context and objectives they pursue in public health.
However, the variability in outcomes underscores the importance of personalized approaches, tailoring digital interventions to individual needs and preferences. From a practical perspective, these findings indicate that low- to moderate-intensity interventions of adequate duration may be feasibly implemented in routine diabetes care, particularly in primary care settings with limited resources. Clearly specifying session frequency, follow-up timing, and comparator conditions can help guide providers in selecting and adapting digital health strategies to their patient populations.
Policymakers should also be aware of the potential of digital health technologies to reduce healthcare costs by improving disease management. However, given the variability in outcomes, it is crucial to establish standardized guidelines for the development, implementation, and evaluation of these tools to ensure their effectiveness across diverse populations.
Despite the promising results, this review has several limitations. First, the studies included varied considerably in terms of sample size, intervention duration, and study settings, which may affect the generalizability of the findings. Additionally, most studies were conducted in high-income countries, which may limit the applicability of the results to low- and middle-income settings where access to digital tools and healthcare infrastructure differs. Another limitation is the lack of long-term follow-up data in most studies. While short-term improvements in HbA1c are valuable, diabetes management is a chronic, lifelong process, and it remains unclear whether the benefits observed in these studies can be sustained over the long term. The quality of the selected articles also turned out to be a limitation. Although 33 articles were selected after the bibliographic search and the elimination of exclusion criteria according to the objectives of the study, only 13 were selected after being reviewed with the modified Jadad scale. Finally, we found a limitation in the study of subgroups of health providers, because no description was found in a group of interventions of the participants in the design and follow-up of their RCTs, probably assuming that the design of the interventions and follow-up were carried out by health professionals.
Future research should prioritize long-term evaluations to better assess the durability and sustainability of the effects of digital health interventions in diabetes management. Although no substantial publication bias was detected in the present analysis, the possibility that studies reporting positive outcomes are more likely to be published than those with null or negative results cannot be fully excluded. In addition, while the use of the modified Jadad scale ensured the inclusion of randomized controlled trials with acceptable methodological quality, the inherent subjectivity of risk-of-bias assessment tools may have introduced some variability in study selection.
Further studies should also explore the incremental value of emerging digital features, including machine learning–driven personalization and telemedicine-based support, to determine their added clinical benefit beyond conventional digital interventions. Particular emphasis should be placed on strategies to sustain long-term user engagement, which remains a critical determinant of glycemic outcomes and overall intervention effectiveness.
5. Conclusions
This systematic review and meta-analysis confirm that digital health interventions (DHIs), particularly short-term and low-intensity mHealth applications, significantly reduce HbA1c levels in patients with Type 2 Diabetes. The finding that simple, low-intensity interventions can achieve meaningful glycemic improvements is especially relevant in resource-limited settings, where scalability and cost are critical. The use of glucometers and healthcare provider involvement showed minimal additional benefit, suggesting that effective diabetes management can be achieved through self-directed, digitally supported care models. These findings indicate that intervention duration and intensity are the main factors associated with improved glycemic outcomes.
Given the heterogeneity among studies and the limited long-term follow-up, the results should be interpreted with caution. Future research is needed to confirm the sustainability and applicability of these low-resource digital strategies through long-term, high-quality trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm15031123/s1, Supplementary Materials S1: PRISMA Checklist; Supplementary Materials S2: Searching strategy; Supplementary Materials S3: Template for the checklist for the description and replication of interventions (tidier); Supplementary Materials S4: Jadad scores for selected articles; Supplementary Materials S5: Grade summary.
Author Contributions
Conceptualization, O.E.R.-M., M.d.C.G.-T. and C.B.-T.; Methodology, O.E.R.-M. and C.B.-T.; Software, C.B.-T.; Validation, O.E.R.-M. and C.B.-T.; Formal analysis, C.B.-T.; Investigation, O.E.R.-M. and C.B.-T.; Resources, C.B.-T.; Data curation, O.E.R.-M. and C.B.-T.; Writing—original draft preparation, O.E.R.-M.; Writing—review and editing, M.d.C.G.-T. and C.B.-T.; Visualization, O.E.R.-M. and C.B.-T.; Supervision, C.B.-T. and M.d.C.G.-T.; Project administration, C.B.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Centre for the Development of Industrial Technology (CDTI) under the Multi-Country Projects call of the State Plan for Scientific, Technical and Innovation Research (PEICTI) 2024–2027, co-funded by the Recovery, Transformation and Resilience Plan—NextGenerationEU (Project No. PAIS-20241102).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors would like to thank Camila Higueras Callejón for helping in the search for bibliographic material, reviewing different virtual libraries, and providing advice for the selection of keywords and search strategy. The authors would also like to express their special gratitude to María Sobeida Leticia Blázquez-Morales, who contributed to the initial conceptualization of this systematic review and meta-analysis. Her valuable input and dedication were fundamental during the early stages of this work. We deeply regret her passing and respectfully acknowledge her memory. The authors acknowledge the use of ChatGPT 5.0 (OpenAI, San Francisco, CA, USA) for minor editorial assistance in language polishing and formatting.
Conflicts of Interest
O.E.R.M. is employed by Adhera Health. This work is a systematic review and meta-analysis based exclusively on previously published studies, and the author’s institutional affiliation had no influence on study selection, data extraction, analysis, interpretation, or reporting of the results. The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| CI | Confidence Interval |
| CIBERESP | Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública |
| COVID-19 | Coronavirus Disease 2019 |
| DL | DerSimonian–Laird estimator |
| DHI(s) | Digital Health Intervention(s) |
| DM2/T2DM | Type 2 Diabetes Mellitus |
| eHealth | Electronic Health |
| HbA1c | Glycated Hemoglobin |
| HTN | Hypertension |
| I2 | Higgins’ I-squared statistic |
| IoT | Internet of Things |
| JMIR | Journal of Medical Internet Research |
| MD | Mean Difference |
| mHealth | Mobile Health |
| OSF | Open Science Framework |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT(s) | Randomized Controlled Trial(s) |
| REML | Restricted Maximum Likelihood |
| SE | Standard Error |
| SMS | Short Message Service |
| TIDieR | Template for Intervention Description and Replication |
| USA | United States of America |
| WHO | World Health Organization |
Appendix A

Table A1.
Overview of study characteristics and participants.
Table A1.
Overview of study characteristics and participants.
| Authors | Country | Sample (N) | Women (n) | Men (n) | Mean Age (Years) | HbA1c Inclusion Criteria |
|---|---|---|---|---|---|---|
| Daniel J Amante et al., 2021 [17] | USA | 96 | 63 | 85 | 56.7 | NR |
| Enying Gong et al., 2020 [23] | Australia | 187 | 78 | 109 | 57 | NR |
| Yeojin Kim et al., 2021 [24] | South Korea | 68 | 38 | 30 | 57.2 | NR |
| Shu-Fang Xia et al., 2022 [18] | China | 120 | 43 | 77 | 56.7 | ≥7.0% |
| Kaisang Lin et al., 2022 [25] | China | 50 | 27 | 23 | 53.3 | ≥7.0% |
| Zhiwei Lu et al., 2020 [26] | China | 120 | 54 | 66 | 54.9 | NR |
| Ramallo-Fariña et al., 2020 [27] | Spain Group 3 (only) | 586 | 300 | 286 | 55.2 | NR |
| LeChauncy Woodard et al., 2023 [28] | USA | 280 | 16 | 264 | 67.2 | >8.0% |
| Chen-Yu Han et al., 2023 [19] | China | 418 | 308 | 110 | 51.95 | NR |
| Mengna Guo et al., 2021 [20] | China | 64 | 25 | 39 | 57.3 | NR |
| Weiping Jia et al., 2021 [21] | USA | 17,554 | 10322 | 7232 | 60.5 | ≥7.0% |
| Yuheng Feng et al., 2023 [29] | USA | 225 | 116 | 109 | 65.6 | >7.0% |
| Ryotaro Bouchi et al., 2024 [22] | Japan | 1159 | 256 | 903 | 56.1 | 6.0–8.9% |
Abbreviations: NR, Not reported.

Table A2.
Selected characteristics for the study of subgroups of digital interventions using Tidier tool.
Table A2.
Selected characteristics for the study of subgroups of digital interventions using Tidier tool.
| Authors | Essential Components of the Intervention | Materials/Procedures | Duration | Use of Glucometer | Intervention Provider | Duration | Intensity | Sessions and Duration | Timing of Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Daniel J Amante et al., 2021 [17] | Real-time monitoring of SMBG data for poorly controlled Type 2 Diabetes | Cellular-enabled glucose meter for automatic SMBG uploads | 6 months | Yes | No | 6 months | High | Access to on-demand telephone coaching | Baseline 6 months (each arm) |
| Enying Gong et al., 2020 [23] | Diabetes coaching using feedback and reinforcement via app-based system | My Diabetes Coach app with conversational agent to track HbA1c and HRQoL | 12 months | No | No | 12 months | Moderate | Weekly sessions or appointments | Baseline 6 months 12 months |
| Yeojin Kim et al., 2021 [24] | Self-management intervention based on the Information–Motivation–Behavioral skills model | IMB Model—participants used a smartphone app for weekly data input | 6 months principal + 1 month following | No | No | 6 months principal + 1 month following | Low | Not reported | Baseline 6 months |
| Shu-Fang Xia et al., 2022 [18] | Self-management guidance for diabetes via software | TangPlan, WeChat SMBG, and health education self-management guidance, with data recorded via WeChat | 6 months | Yes | Multidisciplinary team of healthcare professionals | 6 months | Moderate | Access to on-demand | Baseline 6 months |
| Kaisang Lin et al., 2022 [25] | Monitoring and management of metabolic indicators in T2DM patients via IoT | IoT technology-based diabetes management system: follow-up visits using IoT for patient data collection | 3 months | No | No | 3 months | Low | Not reported | Baseline 3 months |
| Zhiwei Lu et al., 2020 [26] | Effect of telemedicine patient management on blood glucose control in Chinese patients with T2DM | Patients received in-hospital pharmacy consultations and follow-up via the online platform | 6 months | No | No | 6 months | Low | Access to on-demand | Baseline 6 months |
| Ramallo-Fariña et al., 2020 [27] | Use of ICT to support decision-making in T2DM patients | Digital interventions for patients, professionals, or both | Over 24 months | No | Physicians, nurses, primary care professionals | Over 24 months | Moderate | 8 face-to-face group sessions one every 3 months | Baseline 3 months 6 months 12 months 24 months |
| LeChauncy Woodard et al., 2023 [28] | Communication and self-management support for diabetes patients | RE-AIM model | 10 months | No | Physicians, nurse educators, dietitians, and psychologists | 10 months | Moderate | 6 group sessions | Baseline 6 months post-intervention |
| Chen-Yu Han et al., 2023 [19] | Telemedicine for structured self-monitoring of blood glucose | SMBG | 12 months | Yes | No | 12 months | Low | Individual coaching on demand (needs-based tele-coaching) | Baseline 3 months 6 months post- intervention |
| Mengna Guo et al., 2021 [20] | mHealth management with an implantable glucose sensor for T2DM patients | Glucose sensor and mobile app mHealth management | 1 month | Yes | General practitioners | 1 month | Low | Not reported | Baseline 1 month |
| Weiping Jia et al., 2021 [21] | Mobile health (mHealth) intervention for better diabetes control in primary care | ROADMAP app and digital platform | 12 months | Yes | Primary care doctors | 12 months | High | At least 2 sessions each month | Baseline 12 months |
| Yuheng Feng et al., 2023 [29] | eHealth family intervention for T2DM patients based on family support | WeChat app modules and eHealth family-based intervention | 12 months | No | Professional healthcare providers | 12 months | Moderate | Not reported | Baseline 12 months |
| Ryotaro Bouchi et al., 2024 [22] | IoT-based approach to support lifestyle changes and improve diabetes care | IoT devices and mobile app IoT devices for self-monitoring and data submission | 12 months | Yes | Doctors | 12 months | High | Automatic feedback | Baseline 12 months |
Abbreviations: SMBG: self-monitoring of blood glucose; HbA1c: glycated hemoglobin; T2DM: Type 2 Diabetes Mellitus; HRQoL: health-related quality of life; IoT: Internet of Things; ICTs: information and communication technologies; mHealth: mobile health; RE-AIM Model: reach, effectiveness, adoption, implementation, and maintenance.
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; Available online: https://fmdiabetes.org/wp-content/uploads/2022/01/IDF_Atlas_10th_Edition_2021-comprimido.pdf (accessed on 11 October 2024).
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2018. Diabetes Care 2018, 41, S13–S27. Available online: http://care.diabetesjournals.org/content/41/Supplement_1/S13 (accessed on 10 October 2024). [CrossRef]
- Dugas, M.; Gao, G.G.; Agarwal, R. Unpacking mHealth interventions: A systematic review of behavior change techniques used in randomized controlled trials assessing mHealth effectiveness. Digit. Health 2020, 6, 2055207620905411. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Intervenciones de Salud Digital Centradas en Los Jóvenes: Un Marco Para Planificar, Desarrollar e Implementar Soluciones Con y Para la Población Joven; PAHO: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Alsaqqa, H.H.; Alwawi, A. Digital intervention for public health: Searching for implementing characteristics, concepts and recommendations: A scoping review. Front. Public Health 2023, 11, 1142443. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Yera, R. Herramientas difusas en sistemas de recomendación: Una encuesta. Rev. Int. Sist. Intelig. Comput. 2017, 10, 776–803. [Google Scholar]
- Bagolle, A.; Casco, M.; Nelson, J.; Orefice, P.; Raygada, G.; Tejerina, L. La Gran Oportunidad de la Salud Digital en América Latina y el Caribe; Inter-American Development Bank: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Kebede, M.M.; Zeeb, H.; Peters, M.; Heise, T.L.; Pischke, C.R. Effectiveness of digital interventions for improving glycemic control in persons with poorly controlled type 2 diabetes: A systematic review, meta-analysis, and meta-regression analysis. Diabetes Technol. Ther. 2018, 20, 767–782. [Google Scholar] [CrossRef]
- Lee, F.; Han-Burgess, E.; Kennedy, A.; Serafini, P.; Pourrahmat, M.M.; Breznen, B. HbA1c reduction with digital health devices in type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Value Health 2023, 26, 1051–1060. [Google Scholar] [CrossRef]
- Kerr, D.; Ahn, D.; Waki, K.; Wang, J.; Breznen, B.; Klonoff, D.C. Digital interventions for self-management of type 2 diabetes mellitus: Systematic literature review and meta-analysis. J. Med. Internet Res. 2024, 26, e55757. [Google Scholar] [CrossRef]
- Nguyen, V.; Ara, P.; Simmons, D.; Osuagwu, U.L. The role of digital health technology interventions in the prevention of type 2 diabetes mellitus: A systematic review. Clin. Med. Insights Endocrinol. Diabetes 2024, 17, 11795514241246419. [Google Scholar] [CrossRef]
- Chiaranai, C.; Chularee, S.; Saokaew, S.; Bhatarasakoon, P.; Umnuaypornlert, A.; Chaomuang, N.; Doommai, N.; Nimkuntod, P. Effectiveness of telehealth on glycemic control in patients with type 2 diabetes mellitus during the COVID-19 pandemic: A systematic review and meta-analysis of randomized controlled trials. Int. J. Nurs. Stud. Adv. 2023, 6, 100169. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Istepanian, R.S.H. Mobile health (m-health) in retrospect: The known unknowns. Int. J. Environ. Res. Public Health 2022, 19, 3747. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.D.; Wells, G.A.; Huët, C.; McAlister, F.A.; Salmi, L.R.; Fergusson, D.; Laupacis, A. Assessing the quality of randomized trials: Reliability of the Jadad scale. Control. Clin. Trials 1999, 20, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Amante, D.J.; Harlan, D.M.; Lemon, S.C.; McManus, D.D.; Olaitan, O.O.; Pagoto, S.L.; Gerber, B.S.; Thompson, M.J. Evaluation of a diabetes remote monitoring program facilitated by connected glucose meters for patients with poorly controlled type 2 diabetes: Randomized crossover trial. JMIR Diabetes 2021, 6, e25574. [Google Scholar] [CrossRef]
- Xia, S.F.; Maitiniyazi, G.; Chen, Y.; Wu, X.Y.; Zhang, Y.; Zhang, X.Y.; Li, Z.Y.; Liu, Y.; Qiu, Y.Y.; Wang, J. Web-based TangPlan and WeChat combination to support self-management for patients with type 2 diabetes: Randomized controlled trial. JMIR Mhealth Uhealth 2022, 10, e30571. [Google Scholar] [CrossRef]
- Han, C.Y.; Zhang, J.; Ye, X.M.; Lu, J.P.; Jin, H.Y.; Xu, W.W.; Wang, P.; Zhang, M. Telemedicine-assisted structured self-monitoring of blood glucose in management of type 2 diabetes mellitus: Results of a randomized clinical trial. BMC Med. Inform. Decis. Mak. 2023, 23, 283. [Google Scholar] [CrossRef]
- Guo, M.; Meng, F.; Guo, Q.; Bai, T.; Hong, Y.; Song, F.; Ma, Y. Effectiveness of mHealth management with an implantable glucose sensor and a mobile application among Chinese adults with type 2 diabetes. J. Telemed. Telecare 2023, 29, 632–640. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, P.; Zhu, D.; Duolikun, N.; Li, H.; Bao, Y.; Li, X.; ROADMAP Study Group. Evaluation of an mHealth-enabled hierarchical diabetes management intervention in primary care in China (ROADMAP): A cluster randomized trial. PLoS Med. 2021, 18, e1003754. [Google Scholar] [CrossRef]
- Bouchi, R.; Izumi, K.; Ishizuka, N.; Uemura, Y.; Ohtsu, H.; Miyo, K.; Tanaka, S.; Satoh-Asahara, N.; Hara, K.; Odawara, M.; et al. Internet of things–based approach for glycemic control in people with type 2 diabetes: A randomized controlled trial. J. Diabetes Investig. 2024, 15, 1287–1296. [Google Scholar] [CrossRef]
- Gong, E.; Baptista, S.; Russell, A.; Scuffham, P.; Riddell, M.; Speight, J.; Bird, D.; Williams, E.; Lotfaliany, M.; Oldenburg, B. My Diabetes Coach, a mobile app–based interactive conversational agent to support type 2 diabetes self-management: Randomized effectiveness–implementation trial. J. Med. Internet Res. 2020, 22, e20322. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, H.; Seo, J.M. Integrated diabetes self-management program using smartphone application: A randomized controlled trial. West. J. Nurs. Res. 2022, 44, 383–394. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, W.; He, F.; Shen, J. Evaluation of the clinical efficacy of treatment of overweight and obesity in type 2 diabetes mellitus by a telemedicine management system based on internet of things technology. Comput. Intell. Neurosci. 2022, 2022, 8149515. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; He, Y.; Zhai, Y.; Wu, J.; Wang, J.; Zhao, Z. Internet-based medication management services improve glycated hemoglobin levels in patients with type 2 diabetes. Telemed. J. E Health. 2021, 27, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ramallo-Fariña, Y.; García-Bello, M.A.; García-Pérez, L.; Boronat, M.; Wägner, A.M.; Rodríguez-Rodríguez, L.; de Pablos-Velasco, P.; Llorente Gómez de Segura, I.; González-Pacheco, H.; Carmona Rodríguez, M.; et al. Effectiveness of internet-based multicomponent interventions for patients and health care professionals to improve clinical outcomes in type 2 diabetes evaluated through the INDICA study: Multiarm cluster randomized controlled trial. JMIR Mhealth Uhealth 2020, 8, e18922. [Google Scholar] [CrossRef]
- Woodard, L.; Amspoker, A.B.; Hundt, N.E.; Gordon, H.S.; Hertz, B.; Odom, E.; Utech, A.; Razjouyan, J.; Rajan, S.S.; Kamdar, N.; et al. Comparison of collaborative goal setting with enhanced education for managing diabetes-associated distress and hemoglobin A1c levels: A randomized clinical trial. JAMA Netw. Open 2022, 5, e229975. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, Y.; Mao, L.; Gu, M.; Yuan, H.; Lu, J.; Zhang, Q.; Zhao, Q.; Li, X. Effectiveness of an eHealth family-based intervention program in patients with uncontrolled type 2 diabetes mellitus in the community via WeChat: Randomized controlled trial. JMIR Mhealth Uhealth 2023, 11, e40420. [Google Scholar] [CrossRef]
- Salgueiro, L.J.; Pereyra-Bencosme, K.; Vargas-Martínez, C.; Marquez, J.O.; Osorio, S.C.; dos Santos, A.R.; Molina, A.C.; Bolaños, A.V.; Bernal, D.O.; de Sousa, G.S.; et al. Digital interventions in type 2 diabetes mellitus. Princ. Pract. Clin. Res. 2023, 9, 7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.