Abstract
Inflammatory bowel disease (IBD) management stands at the cusp of a transformative era, with recent breakthroughs heralding a paradigm shift in treatment strategies. Traditionally, IBD therapeutics revolved around immunosuppressants, but the landscape has evolved significantly. Recent approvals of etrasimod, upadacitinib, mirikizumab, and risankizumab have introduced novel mechanisms of action, offering renewed hope for IBD patients. These medications represent a departure from the status quo, breaking years of therapeutic stagnation. Precision medicine, involving Artificial Intelligence, is a pivotal aspect of this evolution, tailoring treatments based on genetic profiles, disease characteristics, and individual responses. This approach optimizes treatment efficacy, and paves the way for personalized care. Yet, the rising cost of IBD therapies, notably biologics, poses challenges, impacting healthcare budgets and patient access. Ongoing research strives to assess cost-effectiveness, guiding policy decisions to ensure equitable access to advanced treatments. Looking ahead, the future of IBD management holds great promise. Emerging therapies, precision medicine, and ongoing research into novel targets promise to reshape the IBD treatment landscape. As these advances continue to unfold, IBD patients can anticipate a brighter future, one marked by more effective, personalized, and accessible treatments.
1. Introduction
Crohn’s disease (CD) and Ulcerative Colitis (UC), both chronic inflammatory bowel diseases (IBDs), are conditions characterized by chronic relapsing-remittent inflammation of the gastrointestinal tract, significantly impacting patients’ quality of life. Despite the availability of conventional immunosuppressant-based therapies, managing IBDs remains a challenge due to limitations in their effectiveness and the potential for serious side effects [1]. This has sparked a critical need for novel treatment approaches that can effectively control the disease and improve patient outcomes.
In recent years, the landscape of IBD management has witnessed a promising paradigm shift, driven by the emergence of breakthrough therapies and the growing adoption of precision medicine principles. Precision medicine revolutionizes treatment by tailoring therapies to individual patient characteristics, such as genetic profiles, disease severity, and responses to previous therapies [2]. This personalized approach holds the promise of unlocking improved treatment efficacy and reducing adverse effects, providing hope for patients who have struggled with conventional therapies. Alongside precision medicine, the development of novel therapies with distinct mechanisms of action has further transformed IBD management [3,4]. Despite these advancements, the cost of IBD therapies remains a significant barrier, particularly for biologics, which is a reason why the biosimilar market has expanded in the last year. Ongoing research is dedicated to assessing the cost-effectiveness of these treatments and informing policy decisions to ensure equitable access for all patients [5]. This review aims to provide a comprehensive overview of emerging therapies, the role of precision medicine, and the integration of artificial intelligence in IBD management. By highlighting the most recent advancements, current limitations, and potential future directions, we seek to address gaps in knowledge regarding real-world applications, therapeutic challenges, and accessibility issues that impact patient care.
2. Novel Therapies and Emerging Targets
Significant strides have been made in the development of novel treatments for IBD, each targeting specific pathways implicated in the pathogenesis of involved in CD or UC [6]. The mechanism of action and clinical trials of promising novel therapies in CD and UC are summarized in Table 1 and Figure 1.

Table 1.
Comprehensive review of approved advanced molecules and active phase 2/3 trials in IBD.
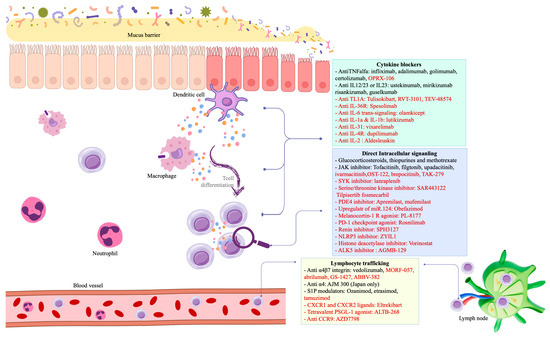
Figure 1.
Interplay between mucosal immunology and pharmaceutical mechanisms in IBD. Drugs written in black are approved for IBD treatment, whereas those in red are currently in active phase 2/3 trials.
Interleukin (IL) inhibitors represent an important class of drugs that selectively target key cytokines involved in IBD inflammation [7]. For instance, risankizumab, guselkumab, and mirikizumab all aim to disrupt the IL-23 pathway [8,9,10]. IL-23 acts via JAK-STAT pathway promoting Th17 lymphocytes differentiation that induces further IL production, innate immune response, and leukocyte migration. Another emerging approach involves the selective inhibition of IL-36, a cytokine from the Il-1 family. IL-36 R signaling is thought to amplify the proliferation of gut cell populations that further promote recruitment and activate intestinal inflammation. Spesolimab, a humanized monoclonal antibody targeting the IL-36 receptor, works by blocking IL-36 signaling, thereby dampening the inflammatory response in UC and CD patients [11].
Selective inhibitors of IL-6 trans-signaling, such as olamkicept (sgp130Fc), offer another avenue for targeted therapy in IBD [12]. By specifically targeting IL-6 trans-signaling, these drugs disrupt the inflammatory cascade while minimizing potential side effects associated with global IL-6 blockade. Preliminary studies have demonstrated the clinical effectiveness of olamkicept in UC patients, paving the way for further investigation in larger clinical trials [13]. In addition, IL-33, a potent inflammatory cytokine, acting as both a pro-inflammatory factor and a transcriptional regulator, could be an interesting objective in future treatments due to its roles in innate and adaptive immunity [14]. Similarly, IL-1α and IL-1β, known pain mediators, play important roles in the pathogenesis of certain autoimmune diseases. Lutikizumab, an anti-IL-1 agent, is currently in phase 2 trials for UC and CD [15].
Therapies targeting TNF-alpha have long been cornerstone treatments for IBD. The TNF family includes a large number of cytokines, of which TNF-alpha is the best known. That being said, the latest advances have focused on tumor necrosis factor-like cytokine 1A (TL1A), another TNF molecule that is involved in the inflammatory cascade. Recent research has revealed that TL1A acts as a regulator mucosal immunity, and is involved in the immunological pathways that contribute to the development of IBD [16]. TL1A levels are elevated in the colonic mucosa of patients with UC and are associated with the severity of the disease. Thus, inhibiting TL1A could serve as a potential therapeutic target for treating inflammatory diseases, as seen in two preliminary studies in patients with UC [17,18]. Additionally, advancements are underway with emerging oral anti-TNF treatments like OPRX-106 and V565, promising new avenues for managing the condition [19,20]. These oral alternatives represent innovative approaches that could offer increased convenience and potentially enhanced effectiveness and treatment adherence for patients dealing with IBD. Several studies have corroborated a preference for oral treatments over other administration methods in patients with IBD. This has driven research efforts toward the development of effective oral therapies [21]. One such approach, currently in an experimental phase, involves the use of a robotic pill. The robotic pill is a novel oral device that navigates the gastrointestinal tract and releases its payload, such ustekinumab, directly to the small intestine [22]. It is nearly fully absorbable, minimizing the need for removal or other interventions. Additionally, in a more advanced stage, JNJ-77242113, a potent oral anti-IL-23 agent has been developed, which is showing promising efficacy in preliminary studies [23].
Beyond cytokine modulation, therapies targeting adhesion molecules have emerged as promising strategies for the management of IBD. Agents such as vedolizumab, currently approved for the treatment of both UC and CD, as well as investigational drugs like abrilumab, ontamalimab, and AJM-300, disrupt critical pathways involved in inflammation and immune cell trafficking, thereby modulating the disease process [24,25,26].
Looking ahead, the landscape of IBD management is poised for further transformation with the advent of sphingosine-1-phosphate receptor (S1PR) modulators, toll-like receptor (TLR) agonists, and microRNA-based therapies [27]. Drugs like ozanimod and etrasimod [28,29] are both currently approved for UC target S1PR receptors. By modulating S1PR signaling, these drugs have an anti-inflammatory function via sequestration of T cell subsets in the lymphoid tissues and prevention of gut homing in UC patients [30]. In addition, etrasimod recently demonstrated significant improvements versus placebo in patients with isolated proctitis [31].
JAK pathways have been demonstrated as crucial players in IBD [32]. Tofacitinib, filgotinib, and upadacitinib represent a significant advancement in IBD management, offering effective induction and maintenance of remission, especially for patients unresponsive to conventional therapies [33,34,35]. New generation JAK inhibitors, including izencitinib, ivarmacitinib, and peficitinib, have shown promise in clinical trials, mainly for UC [36,37,38].
Researchers are exploring various avenues for the treatment of IBD. Toll-like receptor 9 (TLR9) agonists might be of use in UC patients [39]. Cobitolimod is a synthetic single-stranded DNA molecule containing a CpG motif, a specific DNA sequence that TLR-9 recognizes as bacterial. By binding to TLR-9 on cells like intestinal Treg and B lymphocytes and antigen-presenting cells (APCs), cobitolimod triggers the release of potent anti-inflammatory cytokines, including IL-10 and type I interferons, helping to reduce inflammation [40]. Despite early promising results, the development of cobitolimod has been temporarily suspended, reflecting the complexities of advancing such treatments to broader clinical use. Similarly, phosphodiesterase (PDE) inhibitors, particularly PDE4 inhibitors like apremilast, have garnered attention for their potential to alleviate UC activity. An overexpression of PDE4 isoforms and a defective cAMP-mediated pathway were initially identified in active UC patients. Therapeutic inhibition of PDE4 via apremilast effectively modulated cAMP-dominant signaling through protein kinase A (PKA) and cAMP-response element-binding protein (CREB). This intervention led to clinical improvement in chronic UC, demonstrated by reduced mucosal ulcerations, decreased tissue fibrosis, and diminished inflammatory infiltration [41,42].
Recent studies have also focused on microRNAs (miRNAs) and their role in regulating gene expression in IBD. ABX464 (obefazimod) facilitates the targeted cutting and joining of a specific long non-coding RNA, resulting in the production of an anti-inflammatory microRNA known as miR-124. This new mechanism of action could help in inducing clinical remission in moderate-to-severe UC patients by reversing the expression of inflammatory cytokines [43].
Numerous phase 2 studies are investigating innovative treatments for UC, each with pioneering mechanisms of action. Lanraplenib, a spleen tyrosine kinase (SYK) inhibitor, is being tested for its ability to modulate immune cell signaling in B cells, monocytes, and macrophages, crucial cells in autoimmune disease pathways [44]. Additionally, receptor-interacting serine/threonine kinase inhibitors, such as tilpisertib fosmecarbil and eclitasertib (SAR443122), target RIPK1/2 pathways, which are central to immune responses through the nucleotide-binding oligomerization domain (NOD) and TLRs [45]. RIPK, expressed in antigen-presenting cells like dendritic cells and macrophages, responds to microbe-associated molecular patterns recognized by NOD1, NOD2, and TLRs. This interaction activates RIPK2, leading to the release of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-12/23p40, which are central to the inflammatory response in UC. Another novel approach is rosnilimab, a PD-1 agonist antibody, designed to inhibit T-cell proliferation and cytokine secretion by depleting PD-1high T-cell subsets [46]. SPH3127, which targets the renin-angiotensin system, offers potential anti-inflammatory and antifibrotic effects, and is also under investigation [47]. Eltrekibart, a monoclonal antibody blocking CXCR1/2, aims to disrupt neutrophil extracellular trap formation, and is currently being tested in combination with mirikizumab [48]. Furthermore, leiolizumab (ALTB-268), a PSGL-1 agonist antibody, functions as an immune checkpoint enhancer to reduce T-cell effector activity, encouraging T-cell exhaustion [49]. Lastly, ZYIL1 targets the NLRP3 inflammasome, a complex that activates pro-inflammatory cytokines IL-1β and IL-18, aiming to control inflammation at the cellular level in UC [50]. For patients with mild to moderate UC who do not respond to 5-aminosalicylic acid (5-ASA), therapeutic options are scarce. MH002, a novel biotherapeutic product, consists of a carefully selected consortium of six non-pathogenic, commensal bacteria. These bacteria are well-characterized for their ability to modulate the immune response, promote tissue repair, and reinforce the integrity of the gut barrier, offering a potential new treatment pathway for UC, as seen in a recent randomized clinical trial [51].
New mechanisms of action are being investigated for treating CD, including immune system modulation by agents like vorinostat. Vorinostat, a histone deacetylase inhibitor (HDACi) with anti-cancer properties, has shown potential for regulating immune responses, though its precise mechanisms remain unclear. Studies have indicated that it reduces inflammation by inhibiting monocyte activation, T-cell immune responses, and dendritic cell functions, as well as suppressing Th1/Th17 cells and TNF-α levels, suggesting its utility in autoimmune diseases and conditions such as graft rejection [52]. Additionally, fibrosis-targeting therapies are gaining attention, particularly for fibrostenosing complications. AGMB-129, an oral GI-restricted small molecule inhibitor of ALK5 (TGFβR1), is designed to inhibit TGFβ, a key regulator of fibrosis, specifically within the GI tract [53].
Despite advances in medical therapy, surgery remains essential for refractory IBD or complications such as strictures, fistulas, and colorectal cancer. In CD, laparoscopic ileocecal resection is a viable alternative to anti-TNF therapy in selected cases, with lower recurrence rates [54]. Early bowel resection has also been associated with reduced long-term recurrence and a lower need for postoperative biologics. In UC, total proctocolectomy with ileal pouch–anal anastomosis (IPAA) remains as the preferred surgical approach for medically refractory patients [55]. Minimally invasive techniques, including stricturoplasty and endoscopic balloon dilation, provide alternatives to resection in Crohn’s-related strictures [56]. Optimized perioperative care and multidisciplinary management have significantly improved surgical outcomes in IBD [57].
The evolving landscape of IBD treatment is marked by promising advancements across various therapeutic avenues. Additionally, while challenges persist, such as meeting primary endpoints consistently in clinical trials, the ongoing pursuit of novel treatments underscores the dedication to improving outcomes for patients with IBD. As research progresses and new insights emerge, the hope is to continue refining therapeutic strategies, ultimately enhancing the quality of life for individuals living with these chronic inflammatory conditions.
3. The Rise of Precision Medicine in IBD Management
Precision medicine, a rapidly evolving approach to healthcare, aims to tailor treatment to individual patient characteristics, unlocking personalized treatment strategies that optimize efficacy and minimize adverse effects. This personalized approach will very likely transform IBD management [58]. Despite advancements in molecular biology and omics technologies (genomics, proteomics, metagenomics, and metabolomics), understanding IBD’s complexity remains difficult, largely due to the heterogeneity of the data and the lack of standardized analytical pipelines [59].
Artificial intelligence (AI) has emerged as an outstanding tool to address some of these challenges. IBD entails great complexity before diagnosis, and this becomes even more complex once the disease is diagnosed, due to the relapsing course, progressiveness, and lack of response, among other scenarios. Of particular interest is AI’s role in identifying non-invasive biomarkers. AI has been applied to various omics studies in IBD, including genomics, transcriptomics, and microbiomics, with the goal of improving diagnosis, predicting therapeutic response, and understanding disease progression [60]. In genomic studies, AI models leveraging genome-wide association studies’ (GWASs) data have demonstrated better performance than whole-exome sequencing (WES) in distinguishing CD from UC, likely due to the larger sample sizes available in GWASs [61]. Transcriptomic studies using gene expression data from microarrays and RNA sequencing have been effective in differentiating between UC, healthy controls, and other diseases [62,63]. These advancements highlight AI’s potential utility in the diagnostic phases of IBD management by improving accuracy and enabling earlier disease classification.
Genetic testing is being utilized to identify patients with certain genetic variants associated with severe IBD, who may benefit from more intensive therapies [64]. For example, AI models based on single-cell RNA-seq data have been applied to identify inflammatory phenotypes and predict responses to biologic therapies like vedolizumab [65]. Additionally, personalized biologics, designed to selectively target specific immune pathways, are demonstrating remarkable efficacy in treating subsets of patients with IBD [66]. However, in fields like proteomics, AI applications are less advanced, and most studies still rely on traditional statistical approaches.
In the study of the intestinal microbiota, AI has been used to integrate microbiomic data with clinical and demographic parameters, showing potential for predicting disease progression and therapy response [67]. AI is also making strides in histological and endoscopic evaluations, which are crucial for IBD diagnosis and monitoring. AI can accurately identify microscopic disease features, predict histological remission, anticipate flare-ups, and optimize therapeutic management. AI-enhanced endoscopy could improve the detection of subtle mucosal changes, aiding diagnosis, real-time disease activity assessment, and evaluation in clinical trials. However, there remain challenges in developing AI tools that can be broadly applied due to selection bias and data variability [68,69]. In the coming years, international data-sharing initiatives will be key to training AI on comprehensive, unbiased datasets that better reflect the diversity of IBD patient populations.
One of the most validated applications of AI in IBD management is in endoscopic evaluation. AI-assisted systems such as EndoBrain® and CAD-EYE® have been developed to enhance the detection of dysplasia and inflammatory lesions in patients with UC, improving diagnostic accuracy and reducing interobserver variability [70]. Additionally, convolutional neural networks (CNNs) have been trained to identify endoscopic disease activity with accuracy comparable to expert gastroenterologists. Recent studies demonstrate that AI models can automate the classification of endoscopic severity using standardized scoring systems, including the Mayo Endoscopic Score and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS), offering an objective and reproducible assessment of disease progression [71,72].
Another emerging application of AI in IBD management is the use of generative AI models, such as ChatGPT and other large language models (LLMs) (Gemini, LLaMA, Deepseek…), to assist patients and healthcare professionals. These AI-driven tools have been explored for patient education, symptom tracking, and personalized treatment guidance. Chatbots powered by generative AI can provide 24/7 support to patients, answering questions about medications, dietary recommendations, and disease management strategies, improving adherence to treatment and reducing the burden on healthcare providers [73]. Moreover, AI-assisted clinical decision support systems are being developed to integrate patient-reported symptoms, laboratory data, and imaging findings to optimize treatment adjustments in real time [74].
As these tools evolve, standardized AI-based methods are expected to improve histopathology workflows, enabling more precise differentiation between IBD subtypes and between IBD and non-IBD conditions. The integration of multi-omics data with AI offers tremendous potential, but real-world clinical applications are still emerging. Nevertheless, AI’s future role in IBD management looks promising, with the potential to enhance personalized treatment strategies, predict disease progression, and refine diagnostic accuracy. Precision medicine, along with advancements in AI and other novel therapies, offers the potential to improve the quality of life for IBD patients, aiming to reduce the disease’s impact and better manage symptoms.
5. Ensuring Safety in IBD Treatment
As the landscape of IBD management continues to evolve, concerted efforts are essential to strike a delicate balance between advancing therapeutic innovation and ensuring affordability, accessibility and safety. Clinical trials recruitment is becoming more and more complex for a number of reasons, among which placebo use has been a concerning one [84]. In addition, understanding the safety profiles of biologics and small molecules is essential for treatment selection. While anti-TNF agents remain widely used, they are associated with serious infections, malignancies (e.g., melanoma, lymphoma), and immunogenicity, which can reduce their efficacy [85]. In contrast, anti-integrins (vedolizumab) and anti-interleukins (ustekinumab, risankizumab) have a more favorable safety profile, though risks such as respiratory infections should be considered [86]. Recently approved small molecules, including JAK inhibitors (tofacitinib, upadacitinib) and S1PR modulators (ozanimod), provide oral alternatives but come with unique risks, such as cardiovascular events, venous thromboembolism, and serious infections (e.g., herpes zoster reactivation). Treatment selection must be guided by patient-specific factors, such as malignancy history and cardiovascular risk, alongside regular safety monitoring protocols to mitigate adverse effects [87].
Future therapies in IBD are under investigation, targeting new inflammatory pathways beyond current treatments. While promising for refractory cases, their long-term safety remains unknown, requiring rigorous clinical trials and post-marketing surveillance. A risk-stratification approach will be essential to integrate these therapies safely, balancing efficacy and risk in the expanding IBD treatment landscape.
6. Limitations and Future Directions
This review provides an updated perspective on key advances and future directions in IBD management, covering novel therapies, precision medicine, artificial intelligence applications, and healthcare cost challenges. However, several limitations must be acknowledged. Firstly, this is a non-systematic review, meaning it does not follow a structured methodology for literature selection and synthesis. While this approach allows for a broad discussion of cutting-edge aspects, it does not provide a comprehensive analysis of all available evidence, which systematic reviews typically offer. Secondly, although precision medicine and artificial intelligence hold great promise for optimizing IBD management, their real-world implementation remains complex. Genetic variability, disease heterogeneity, and the need for standardization in omics data analysis and AI-driven models present ongoing challenges. Further research is required to ensure the reproducibility and clinical applicability of these technologies.
Additionally, one notable limitation of this review is the lack of discussion on pediatric IBD. Pediatric IBD often presents with more extensive disease at diagnosis, compared to adults. Growth failure and delayed puberty are major concerns in this population, which were not addressed in our study. Pediatric-onset IBD accounts for 20–30% of diagnoses, and is often more aggressive than adult-onset disease. CD is more common than UC, and pediatric UC frequently presents as extensive pancolitis [88]. Additionally, children have a higher risk of surgery within five years of diagnosis. Furthermore, pediatric IBD has a stronger genetic component, and may require different treatment strategies due to distinct disease progression patterns and long-term safety considerations. Very early-onset IBD is more likely to have a monogenic origin, affecting immune regulation and barrier function [89]. Biologics are increasingly used early, but data on newer therapies in children are limited. Despite advances, surgical rates remain high, often due to growth failure or refractory disease, requiring tailored surgical approaches. Future research should focus on age-specific treatment approaches and long-term outcomes in pediatric IBD, bridging the gap between pediatric and adult management strategies.
The future of IBD management is set to be transformed by advancements in precision medicine, AI, and innovative therapeutic strategies. Looking forward, research should prioritize identifying specific patient subgroups that will benefit most from targeted therapies, optimizing precision medicine approaches [90]. Chimeric Antigen Receptor T-cell Therapy (CAR-T) cell therapy is gaining interest in the treatment of autoimmune diseases, including IBD. CAR-T cell therapy involves genetically engineering a patient’s own T cells to target and eliminate specific immune cells responsible for the autoimmune response. The potential benefits of CAR-T cell therapy include the ability to specifically target and eliminate the autoreactive immune cells responsible for the disease, hypothetically leading to a better management of the disease [91]. This approach may also avoid the need for long-term immunosuppressive therapy, which are not free of side effects [92]. Additionally, microbiome-based therapies, including next-generation probiotics and fecal microbiota transplantation (FMT), are emerging as promising strategies to restore gut homeostasis and improve treatment efficacy [93]. Beyond inflammation control, future therapies will likely target fibrosis modulation, epithelial barrier repair, and the gut–brain axis, addressing complications like strictures and motility disorders [94,95]. The rise of digital health tools, such as wearable biosensors and AI-powered symptom-tracking apps, will enable real-time monitoring and personalized disease management, improving patient adherence and outcomes [96]. As research advances, a multidisciplinary approach integrating precision medicine, novel therapeutics, and digital innovations will reshape the IBD treatment paradigm, offering more effective, personalized, and accessible care.
7. Conclusions
Recent advancements in CD and UC management offer new hope for patients. Precision medicine, AI, and novel therapies targeting specific inflammatory pathways show promise in improving treatment efficacy and minimizing adverse effects. However, the rising costs of biologics present challenges in access and affordability. The introduction of biosimilars and emerging treatments may alleviate this burden, provided robust regulatory frameworks and clinician education are in place. AI-driven tools have the potential to revolutionize IBD management by enhancing diagnostic accuracy, predicting disease progression, and personalizing treatment strategies. The integration of multi-omics data with AI is particularly promising in uncovering disease mechanisms and identifying non-invasive biomarkers, further advancing precision medicine.
Moving forward, collaborative efforts, investment in research, and progressive policies are crucial to ensuring equitable access to effective and affordable IBD treatments. By prioritizing patient-centered care and addressing socioeconomic disparities, we can strive towards a future where all individuals affected by IBD receive optimal care.
Author Contributions
Conceptualization, A.M.C.M. and G.A.C.d.l.F.; methodology, G.A.C.d.l.F.; writing—original draft preparation, A.M.C.M.; writing—review and editing, A.M.C.M. and B.G.; visualization, B.G.; supervision A.M.C.M., B.G. and G.A.C.d.l.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We confirm that no external funding was received for the conduct of this study.
Conflicts of Interest
A.M.C.M. has received fees for lectures, consultancy work, or research support from: Lilly, Abbvie, Johnson & Johnson, Takeda, Pfizer, Alfasigma, Ferring, Farmasierra, Kern, Faesfarma. G.A.C.d.l.F. declare no conflict of interest. B.G. has served as acted as consultant to Galapagos/Alfasigma, Roche, Pfizer and Abbvie and as speaker for Abbvie, Janssen, Takeda, Pfizer and Galapagos/Alfasigma.
Abbreviations
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| TNF | Tumor Necrosis Factor |
| IL | Interleukin |
| JAK | Janus Kinase |
| S1P | Sphingosine-1-Phosphate |
| PDE | Phosphodiesterase |
| SYK | Spleen Tyrosine Kinase |
| TLR | Toll-Like Receptor |
| miRNA | MicroRNA |
| GWAS | Genome-Wide Association Studies |
| WES | Whole-Exome Sequencing |
| AI | Artificial Intelligence |
| CNN | Convolutional Neural Network |
| UCEIS | Ulcerative Colitis Endoscopic Index of Severity |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| MAdCAM-1 | Mucosal Addressin Cell Adhesion Molecule-1 |
| CAR-T | Chimeric Antigen Receptor T-cell Therapy |
| PDE4 | Phosphodiesterase-4 |
| ALK5 | Activin-like Kinase 5 |
| PSGL-1 | P-Selectin Glycoprotein Ligand-1 |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| RIPK1/2 | Receptor-Interacting Protein Kinase 1/2 |
| CXCR1/2 | C-X-C Motif Chemokine Receptor 1/2 |
| HDACi | Histone Deacetylase Inhibitor |
| CCR9 | C-C Motif Chemokine Receptor 9 |
| TYK2 | Tyrosine Kinase 2 |
| PD-1 | Programmed Death-1 |
| IPAA | Ileal Pouch-Anal Anastomosis |
| cAMP | Cyclic Adenosine Monophosphate |
| CREB | cAMP Response Element-Binding Protein |
| Treg | Regulatory T Cells |
| APC | Antigen-Presenting Cell |
References
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Jagirdhar, G.S.K.; Perez, J.A.; Perez, A.B.; Surani, S. Integration and implementation of precision medicine in the multifaceted inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 5211–5225. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.Y.; Wang, Y.N.; Huang, Y.H.; Jiang, M.; Dai, C. Effectiveness and safety of upadacitinib for inflammatory bowel disease: A systematic review and meta-analysis of RCT and real-world observational studies. Int. Immunopharmacol. 2024, 126, 111229. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Loftus, E.V., Jr. Risankizumab to treat moderately to severely active Crohn’s disease in adults: An evaluation of trials and data. Expert. Rev. Gastroenterol. Hepatol. 2023, 17, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yassin, N.; Marley, A.; Bellato, V.; Foppa, C.; Pellino, G.; Myrelid, P.; Millan, M.; Gros, B.; Avellaneda, N.; et al. Crossing barriers: The burden of inflammatory bowel disease across Western Europe. Ther. Adv. Gastroenterol. 2023, 16, 17562848231218615. [Google Scholar] [CrossRef]
- Bretto, E.; Ribaldone, D.G.; Caviglia, G.P.; Saracco, G.M.; Bugianesi, E.; Frara, S. Inflammatory Bowel Disease: Emerging Therapies and Future Treatment Strategies. Biomedicines 2023, 11, 2249. [Google Scholar] [CrossRef]
- Neurath, M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef]
- Vuyyuru, S.K.; Shackelton, L.M.; Hanzel, J.; Ma, C.; Jairath, V.; Feagan, B.G. Targeting IL-23 for IBD: Rationale and Progress to Date. Drugs 2023, 83, 873–891. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 185–196. [Google Scholar] [CrossRef]
- Parigi, T.L.; Iacucci, M.; Ghosh, S. Blockade of IL-23: What is in the Pipeline? J. Crohn’s Colitis 2022, 16, ii64–ii72. [Google Scholar] [CrossRef]
- Ferrante, M.; Irving, P.M.; Selinger, C.P.; D’haens, G.; Kuehbacher, T.; Seidler, U.; Gropper, S.; Haeufel, T.; Forgia, S.; Danese, S.; et al. Safety and tolerability of spesolimab in patients with ulcerative colitis. Expert Opin. Drug Saf. 2022, 22, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Aden, K.; Bernardes, J.P.; Conrad, C.; Tran, F.; Höper, H.; Volk, V.; Mishra, N.; Blase, J.I.; Nikolaus, S.; et al. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients with Active Inflammatory Bowel Disease. Gastroenterology 2021, 160, 2354–2366.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, B.; Wang, B.; Chen, H.; Li, Y.; Cao, Q.; Zhong, J.; Shieh, M.J.; Ran, Z.; Tang, T.; et al. Effect of Induction Therapy with Olamkicept vs Placebo on Clinical Response in Patients with Active Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2023, 329, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, C.; Xin, S.; Liu, X.; Zhang, S.; Qiao, B.; Shang, H.; Gao, L.; Xu, J. A Deep View of the Biological Property of Interleukin-33 and Its Dysfunction in the Gut. Int. J. Mol. Sci. 2023, 24, 13504. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Y.; Wang, W.; Jie, S.; Hu, X.; Zhou, J.; Wu, T.; Aili, D.; Long, Z.; Li, Y.; et al. Is Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, efficacious for Osteoarthritis? Results from a bayesian network meta-analysis. BioMed Res. Int. 2020, 2020, 9013283. [Google Scholar] [CrossRef]
- Furfaro, F.; Alfarone, L.; Gilardi, D.; Correale, C.; Allocca, M.; Fiorino, G.; Argollo, M.; Zilli, A.; Zacharopoulou, E.; Loy, L.; et al. TL1A: A New Potential Target in the Treatment of Inflammatory Bowel Disease. Curr. Drug Targets 2021, 22, 760–769. [Google Scholar] [CrossRef]
- Danese, S.; Klopocka, M.; Scherl, E.J.; Romatowski, J.; Allegretti, J.R.; Peeva, E.; Vincent, M.S.; Schoenbeck, U.; Ye, Z.; Hassan-Zahraee, M.; et al. Anti-TL1A Antibody PF-06480605 Safety and Efficacy for Ulcerative Colitis: A Phase 2a Single-Arm Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2324–2332.e6. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sands, B.; Siegel, C.A.; Dubinsky, M.; Longman, R.; Sabinho, J.; Laurent, O.O.; Luo, A.A.; Lu, J.D.; Nguyen, D.; et al. DOP87 The Anti-TL1AAntibody PRA023 Demonstrated Proof-of-Concept in Crohn’s Disease: Phase 2a APOLLO-CDStudy Results. J. Crohn’s Colitis 2023, 17, i162–i164. [Google Scholar] [CrossRef]
- Almon, E.; Shaaltiel, Y.; Sbeit, W.; Fich, A.; Schwartz, D.; Waterman, M.; Szlaifer, M.; Reuveni, H.; Amit-Cohen, B.C.; Alon, S.; et al. Novel Orally Administered Recombinant Anti-TNF Alpha Fusion Protein for the Treatment of Ulcerative Colitis: Results from a Phase 2a Clinical Trial. J. Clin. Gastroenterol. 2021, 55, 134–140. [Google Scholar] [CrossRef]
- A Six Week Efficacy, Safety and Tolerability Study of V565 in Crohn’s Disease—Full Text View—ClinicalTrials. Available online: https://clinicaltrials.gov/ct2/show/NCT02976129 (accessed on 15 January 2024).
- Denesh, D.; Carbonell, J.; Kane, J.S.; Gracie, D.; Selinger, C.P. Patients with inflammatory bowel disease (IBD) prefer oral tablets over other modes of medicine administration. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 1091–1096. [Google Scholar] [CrossRef]
- Myers, J.; Nguyen, S.; Patel, N.; Imran, M.; Hashim, M.A.; Dhalla, A.K. An orally administered robotic pill (RP) reliably and safely delivers an ustekinumab biosimilar with high bioavailability relative to subcutaneous (SC) ustekinumab in healthy human participants. Gastroenterology 2024, 166 (Suppl. S5), 492b–492c. [Google Scholar] [CrossRef]
- Bissonnette, R.; Pinter, A.; Ferris, L.K.; Gerdes, S.; Rich, P.; Vender, R.; Miller, M.; Shen, Y.K.; Kannan, A.; Li, S.; et al. An Oral Interleukin-23-Receptor Antagonist Peptide for Plaque Psoriasis. N. Engl. J. Med. 2024, 390, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Watanabe, M.; Ohmori, T.; Nakajima, K.; Ishida, T.; Ishiguro, Y.; Kanke, K.; Kobayashi, K.; Hirai, F.; Watanabe, K.; et al. AJM300 Study Group. AJM300 (carotegrast methyl), an oral antagonist of α4-integrin, as induction therapy for patients with moderately active ulcerative colitis: A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol. Hepatol. 2022, 7, 648–657. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.R.; Reinisch, W.; Lee, S.D.; Tarabar, D.; Louis, E.; Kłopocka, M.; Klaus, J.; Schreiber, S.; Park, D.I.; Hébuterne, X.; et al. Long-Term Safety and Efficacy of the Anti-Mucosal Addressin Cell Adhesion Molecule-1 Monoclonal Antibody Ontamalimab (SHP647) for the Treatment of Crohn’s Disease: The OPERA II Study. Inflamm. Bowel Dis. 2022, 28, 1034–1044. [Google Scholar] [CrossRef]
- Hibi, T.; Motoya, S.; Ashida, T.; Sai, S.; Sameshima, Y.; Nakamura, S.; Maemoto, A.; Nii, M.; Sullivan, B.A.; Gasser, R.A., Jr.; et al. Efficacy and safety of abrilumab, an 4 7 integrin inhibitor, in Japanese patients with moderate-to-severe ulcerative colitis: A phase II study. Intest. Res. 2019, 17, 375–386. [Google Scholar] [CrossRef]
- Kitsou, K.; Kokkotis, G.; Rivera-Nieves, J.; Bamias, G. Targeting the Sphingosine-1-Phosphate Pathway: New Opportunities in Inflammatory Bowel Disease Management. Drugs 2024, 84, 1179–1197. [Google Scholar] [CrossRef]
- Danese, S.; Panaccione, R.; Abreu, M.T.; Rubin, D.T.; Ghosh, S.; Dignass, A.; Afzali, A.; Wolf, D.C.; Chiorean, M.V.; Vermeire, S.; et al. Efficacy and Safety of Approximately 3 Years of Continuous Ozanimod in Moderately to Severely Active Ulcerative Colitis: Interim Analysis of the True North Open-label Extension. J. Crohn’s Colitis 2024, 18, 264–274. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Vermeire, S.; Peyrin-Biroulet, L.; Dubinsky, M.C.; Panes, J.; Yarur, A.; Ritter, T.; Baert, F.; Schreiber, S.; Sloan, S.; et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): Two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet 2023, 401, 1159–1171, Erratum in Lancet 2023, 401, 1000. [Google Scholar] [CrossRef] [PubMed]
- Saba, J.; Degagne, E. S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer. Clin. Exp. Gastroenterol. 2014, 7, 205–214. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Dubinsky, M.C.; Sands, B.E.; Panés, J.; Schreiber, S.; Reinisch, W.; Feagan, B.G.; Danese, S.; Yarur, A.J.; D’Haens, G.R.; et al. Efficacy and Safety of Etrasimod in Patients with Moderately to Severely Active Isolated Proctitis: Results From the Phase 3 ELEVATE UC Clinical Programme. J. Crohn’s Colitis 2024, 18, 1270–1282, Erratum in J. Crohns Colitis 2024, 18, 1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caballero-Mateos, A.M.; Cañadas-de la Fuente, G.A. Game changer: How Janus kinase inhibitors are reshaping the landscape of ulcerative colitis management. World J. Gastroenterol. 2024, 30, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Danese, S.; Loftus, E.V., Jr.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef]
- Hardwick, R.N.; Brassil, P.; Badagnani, I.; Perkins, K.; Obedencio, G.P.; Kim, A.S.; Conner, M.W.; Bourdet, D.L.; Harstad, E.B. Gut-Selective Design of Orally Administered Izencitinib (TD-1473) Limits Systemic Exposure and Effects of Janus Kinase Inhibition in Nonclinical Species. Toxicol. Sci. 2022, 186, 323–337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, B.; Zhong, J.; Li, X.; Pan, F.; Ding, Y.; Zhang, Y.; Chen, H.; Liu, F.; Zhang, Z.; Zhang, L.; et al. Efficacy and Safety of Ivarmacitinib in Patients with Moderate-to-Severe, Active, Ulcerative Colitis: A Phase II Study. Gastroenterology 2022, 163, 1555–1568. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Feagan, B.G.; Lichtenstein, G.R.; Zhang, H.; Strauss, R.; Szapary, P.; Johanns, J.; Panes, J.; Vermeire, S.; et al. Peficitinib-UC Study Group. Peficitinib, an Oral Janus Kinase Inhibitor, in Moderate-to-severe Ulcerative Colitis: Results from a Randomised, Phase 2 Study. J. Crohn’s Colitis. 2018, 12, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Reinisch, W.; Peyrin-Biroulet, L.; Scaldaferri, F.; Admyre, C.; Knittel, T.; Kowalski, J.; Neurath, M.F.; Hawkey, C. Clinical efficacy of the Toll-like receptor 9 agonist cobitolimod using patient-reported-outcomes defined clinical endpoints in patients with ulcerative colitis. Dig. Liver Dis. 2018, 50, 1019–1029. [Google Scholar] [CrossRef]
- Atreya, R.; Peyrin-Biroulet, L.; Klymenko, A.; Augustyn, M.; Bakulin, I.; Slankamenac, D.; Miheller, P.; Gasbarrini, A.; Hébuterne, X.; Arnesson, K.; et al. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): A phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol. Hepatol. 2020, 5, 1063–1075. [Google Scholar] [CrossRef]
- AlAmeel, T.; AlMutairdi, A.; Al-Bawardy, B. Emerging Therapies for Ulcerative Colitis: Updates from Recent Clinical Trials. Clin. Exp. Gastroenterol. 2023, 16, 147–167. [Google Scholar] [CrossRef]
- Danese, S.; Neurath, M.F.; Kopoń, A.; Zakko, S.F.; Simmons, T.C.; Fogel, R.; Siegel, C.A.; Panaccione, R.; Zhan, X.; Usiskin, K.; et al. Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients with Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2526–2534.e9. [Google Scholar] [CrossRef] [PubMed]
- Apolit, C.; Campos, N.; Vautrin, A.; Begon-Pescia, C.; Lapasset, L.; Scherrer, D.; Gineste, P.; Ehrlich, H.; Garcel, A.; Santo, J.; et al. ABX464 (Obefazimod) Upregulates miR-124 to Reduce Proinflammatory Markers in Inflammatory Bowel Diseases. Clin. Transl. Gastroenterol. 2023, 14, e00560. [Google Scholar] [CrossRef] [PubMed]
- Blomgren, P.; Chandrasekhar, J.; Di Paolo, J.A.; Fung, W.; Geng, G.; Ip, C.; Jones, R.; Kropf, J.E.; Lansdon, E.B.; Lee, S.; et al. Discovery of Lanraplenib (GS-9876): A Once-Daily Spleen Tyrosine Kinase Inhibitor for Autoimmune Diseases. ACS Med. Chem. Lett. 2020, 11, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Honjo, H.; Watanabe, T.; Kamata, K.; Minaga, K.; Kudo, M. RIPK2 as a New Therapeutic Target in Inflammatory Bowel Diseases. Front. Pharmacol. 2021, 12, 650403. [Google Scholar] [CrossRef]
- Luu, K.; Dahl, M.; Hare, E.; Sibley, C.; Lizzul, P.; Randazzo, B. DOP81 Rosnilimab, a novel PD-1 agonist monoclonal antibody, reduces T cell proliferation, inflammatory cytokine secretion, and PD-1high expressing CD4 and CD8 T cells: Results from a Phase 1 healthy volunteer clinical trial. J. Crohn’s Colitis 2024, 18 (Suppl. S1), i226. [Google Scholar] [CrossRef]
- Salmenkari, H.; Korpela, R.; Vapaatalo, H. Renin-angiotensin system in intestinal inflammation-Angiotensin inhibitors to treat inflammatory bowel diseases? Basic Clin. Pharmacol. Toxicol. 2021, 129, 161–172. [Google Scholar] [CrossRef]
- Xv, Y.; Feng, Y.; Lin, J. CXCR1 and CXCR2 are potential neutrophil extracellular trap-related treatment targets in ulcerative colitis: Insights from Mendelian randomization, colocalization and transcriptomic analysis. Front. Immunol. 2024, 15, 1425363. [Google Scholar] [CrossRef]
- Parmar, D.V.; Kansagra, K.A.; Momin, T.; Patel, H.B.; Jansari, G.A.; Bhavsar, J.; Shah, C.; Patel, J.M.; Ghoghari, A.; Barot, A.; et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Oral NLRP3 Inflammasome Inhibitor ZYIL1: First-in-Human Phase 1 Studies (Single Ascending Dose and Multiple Ascending Dose). Clin. Pharmacol. Drug Dev. 2023, 12, 202–211. [Google Scholar] [CrossRef]
- Ambrus-Aikelin, G.; Takeda, K.; Joetham, A.; Lazic, M.; Povero, D.; Santini, A.M.; Pranadinata, R.; Johnson, C.D.; McGeough, M.D.; Beasley, F.C.; et al. JT002, a small molecule inhibitor of the NLRP3 inflammasome for the treatment of autoinflammatory disorders. Sci. Rep. 2023, 13, 13524, Erratum in Sci. Rep. 2023, 13, 20081. [Google Scholar] [CrossRef]
- Vermeire, S.; Dewint, P.; Vansteelant, M.; Peterka, M.; Štěpek, D.; Kierkuś, J.; Wiernicka, A.; Napora, P.; Wolański, Ł.; Kopoń, A.; et al. P658 Safety and efficacy of MH002, an optimized live biotherapeutic product, for the treatment of mild to moderate ulcerative colitis: A first-in-disease, double-blind, randomized clinical trial. J. Crohn’s Colitis 2024, 18 (Suppl. S1), i1254. [Google Scholar] [CrossRef]
- Kwak, M.S.; Hwang, C.-I.; Cha, J.M.; Jeon, J.W.; Yoon, J.Y.; Park, S.B. Single-Cell Network-Based Drug Repositioning for Discovery of Therapies against Anti-Tumour Necrosis Factor-Resistant Crohn’s Disease. Int. J. Mol. Sci. 2023, 24, 14099. [Google Scholar] [CrossRef] [PubMed]
- Pala, D.; Ronchi, P.; Rescigno, D.; Bertani, B.; Capelli, A.M.; Guariento, S.; Marchini, G.; Milioli, M.; Cesari, N.; Federico, G.; et al. Design, Synthesis, and Activity of a Novel Series of Pyridazine-Based ALK5 Inhibitors. ACS Med. Chem. Lett. 2024, 15, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- A Bemelman, W.; Collaborators, S.-E.; Adamina, M.; Buskens, C.; Dhoore, A.; Kotze, P.G.; Oresland, T.; Panis, Y.; Samprieto, G.; Spinelli, A.; et al. Evolving role of IBD surgery. J. Crohn’s Colitis 2018, 12, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO guidelines on therapeutics in ulcerative colitis: Surgical treatment. J. Crohn’s Colitis 2022, 16, 179–189. [Google Scholar] [CrossRef]
- Bettenworth, D.; Gustavsson, A.; Atreja, A.; Lopez, R.; Tysk, C.; van Assche, G.; Rieder, F. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm. Bowel Dis. 2017, 23, 133–142. [Google Scholar] [CrossRef]
- Vieujean, S.; Jairath, V.; Peyrin-Biroulet, L.; Dubinsky, M.; Iacucci, M.; Magro, F.; Danese, S. Understanding the therapeutic toolkit for inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2025. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Booth, C.; Cho, J.; Dubinsky, M.; Griffiths, A.M.; Grimbacher, B.; Hambleton, S.; Huang, Y.; Jones, K.; Kammermeier, J.; et al. Precision medicine in monogenic inflammatory bowel disease: Proposed mIBD REPORT standards. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 810–828. [Google Scholar] [CrossRef]
- Iacucci, M.; Santacroce, G.; Majumder, S.; Morael, J.; Zammarchi, I.; Maeda, Y.; Ryan, D.; Di Sabatino, A.; Rescigno, M.; Aburto, M.R.; et al. Opening the doors of precision medicine: Novel tools to assess intestinal barrier in inflammatory bowel disease and colitis-associated neoplasia. Gut 2024, 73, 1749–1762. [Google Scholar] [CrossRef]
- Cannarozzi, A.L.; Latiano, A.; Massimino, L.; Bossa, F.; Giuliani, F.; Riva, M.; Ungaro, F.; Guerra, M.; Brina, A.L.D.; Biscaglia, G.; et al. Inflammatory bowel disease genomics, transcriptomics, proteomics and metagenomics meet artificial intelligence. United Eur. Gastroenterol. J. 2024, 12, 1461–1480. [Google Scholar] [CrossRef]
- D’Addabbo, A.; Latiano, A.; Palmieri, O.; Maglietta, R.; Annese, V.; Ancona, N. Regularized least squares classifiers may predict Crohn’s disease from profiles of single nucleotide polymorphisms. Ann. Hum. Genet. 2007, 71, 537–549. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, R.; Lau, C.T.; Chung, W.C.; Chan, J.C.P.; Liang, F.; Zhao, C.; Zhang, X.; Bian, Z. Identification of useful genes from multiple microarrays for ulcerative colitis diagnosis based on machine learning methods. Sci. Rep. 2022, 12, 9962. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, M.; Ye, Z.; Wang, Y.; Wang, X.; Chen, Y.-G. Development of a 32-gene signature using machine learning for accurate prediction of inflammatory bowel disease. Cell Regen. 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Chang, C.; Boschetti, G.; Ungaro, R.; Giri, M.; Grout, J.A.; Gettler, K.; Chuang, L.-S.; Nayar, S.; Greenstein, A.J.; et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell 2019, 178, 1493–1508.e20. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Davies, J.M.; A Quintero, M.; Delmas, A.; Diaz, S.; Martinez, C.D.; Venables, T.; Reich, A.; Crynen, G.; Deshpande, A.R.; et al. Transcriptional behavior of regulatory T cells predicts IBD patient responses to vedolizumab therapy. Inflamm. Bowel Dis. 2022, 28, 1800–1812. [Google Scholar] [CrossRef]
- Boardman, D.A.; Wong, M.Q.; Rees, W.D.; Wu, D.; Himmel, M.E.; Orban, P.C.; Vent-Schmidt, J.; Zachos, N.C.; Steiner, T.S.; Levings, M.K. Levings, Flagellin-specific human CAR Tregs for immune regulation in IBD. J. Autoimmun. 2023, 134, 102961. [Google Scholar] [CrossRef]
- Manandhar, I.; Alimadadi, A.; Aryal, S.; Munroe, P.B.; Joe, B.; Cheng, X. Gut microbiome-based supervised machine learning for clinical diagnosis of inflammatory bowel diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G328–G337. [Google Scholar] [CrossRef]
- Cannarozzi, A.L.; Massimino, L.; Latiano, A.; Parigi, T.L.; Giuliani, F.; Bossa, F.; Di Brina, A.L.; Ungaro, F.; Biscaglia, G.; Danese, S.; et al. Artificial intelligence: A new tool in the pathologist’s armamentarium for the diagnosis of IBD. Comput. Struct. Biotechnol. J. 2024, 23, 3407–3417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, C.; Shi, Q.; Li, J.; Jiao, Y.; Xu, A.; Zhou, Y.; Wang, X.; Peng, C.; Zhang, X.; Cui, X.; et al. Pathologist-level diagnosis of ulcerative colitis inflammatory activity level using an automated histological grading method. Int. J. Med. Inform. 2024, 192, 105648. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Yamamoto, T.; Murasugi, S.; Koroku, M.; Yonezawa, M.; Nonaka, K.; Nagashima, Y.; Nakamura, S.; Tokushige, K. Comparison of Endoscopic and Artificial Intelligence Diagnoses for Predicting the Histological Healing of Ulcerative Colitis in a Real-World Clinical Setting. Crohns Colitis 360 2024, 6, otae005. [Google Scholar] [CrossRef]
- Ruan, G.; Qi, J.; Cheng, Y.; Liu, R.; Zhang, B.; Zhi, M.; Chen, J.; Xiao, F.; Shen, X.; Fan, L.; et al. Development and Validation of a Deep Neural Network for Accurate Identification of Endoscopic Images from Patients with Ulcerative Colitis and Crohn’s Disease. Front. Med. 2022, 9, 854677. [Google Scholar] [CrossRef]
- Marin-Santos, D.; Contreras-Fernandez, J.A.; Perez-Borrero, I.; Pallares-Manrique, H.; Gegundez-Arias, M.E. Automatic detection of crohn disease in wireless capsule endoscopic images using a deep convolutional neural network. Appl. Intell. 2023, 53, 12632–12646. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Lin, N.; Zhang, X.; Han, Y.; Wang, X.; Liu, D.; Tan, X.; Pu, D.; Li, K.; et al. Application of Large Language Models in Medical Training Evaluation-Using ChatGPT as a Standardized Patient: Multimetric Assessment. J. Med. Internet Res. 2025, 27, e59435. [Google Scholar] [CrossRef]
- Sciberras, M.; Farrugia, Y.; Gordon, H.; Furfaro, F.; Allocca, M.; Torres, J.; Arebi, N.; Fiorino, G.; Iacucci, M.; Verstockt, B.; et al. Accuracy of Information given by ChatGPT for Patients with Inflammatory Bowel Disease in Relation to ECCO Guidelines. J. Crohn’s Colitis 2024, 18, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K.; Weizman, A.V.; Kuenzig, M.E.; Windsor, J.W.; Kaplan, G.G.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Coward, S.; Jones, J.L.; et al. The 2023 Impact of Inflammatory Bowel Disease in Canada: Treatment Landscape. J. Can. Assoc. Gastroenterol. 2023, 6 (Suppl. S2), S97–S110. [Google Scholar] [CrossRef] [PubMed]
- Catalán-Serra, I.; Ricanek, P.; Grimstad, T. “Out of the box” new therapeutic strategies for Crohn’s disease: Moving beyond biologics. Rev. Esp. Enferm. Dig. 2023, 115, 614–634. [Google Scholar] [CrossRef] [PubMed]
- Saruta, M.; Kawaguchi, I.; Ogawa, Y.; Sanchez Gonzalez, Y.; Numajiri, N.; Tang, X.; Miller, R. Assessing the economics of biologic and small molecule therapies for the treatment of moderate to severe ulcerative colitis in Japan: A cost per responder analysis of upadacitinib. J. Med. Econ. 2024, 27, 566–574. [Google Scholar] [CrossRef]
- Burisch, J.; Vardi, H.; Schwartz, D.; Friger, M.; Kiudelis, G.; Kupčinskas, J.; Fumery, M.; Gower-Rousseau, C.; Lakatos, L.; Lakatos, P.L.; et al. Health-care costs of inflammatory bowel disease in a pan-European, community-based, inception cohort during 5 years of follow-up: A population-based study. Lancet Gastroenterol. Hepatol. 2020, 5, 454–464. [Google Scholar] [CrossRef]
- D’Amico, F.; Solitano, V.; Magro, F.; Olivera, P.A.; Halfvarson, J.; Rubin, D.; Dignass, A.; Al Awadhi, S.; Kobayashi, T.; Queiroz, N.S.F.; et al. Practical Management of Biosimilar Use in Inflammatory Bowel Disease (IBD): A Global Survey and an International Delphi Consensus. J. Clin. Medicine 2023, 12, 6350. [Google Scholar] [CrossRef]
- Jensen, T.B.; Kim, S.C.; Jimenez-Solem, E.; Bartels, D.; Christensen, H.R.; Andersen, J.T. Shift From Adalimumab Originator to Biosimilars in Denmark. JAMA Intern. Med. 2020, 180, 902–903. [Google Scholar] [CrossRef]
- Burisch, J.; Zhao, M.; Odes, S.; De Cruz, P.; Vermeire, S.; Bernstein, C.N.; Kaplan, G.G.; Duricova, D.; Greenberg, D.; Melberg, H.O.; et al. The cost of inflammatory bowel disease in high-income settings: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2023, 8, 458–492. [Google Scholar] [CrossRef]
- Caballero-Mateos, A.M. Gut Feelings: The Psychological Impact of Inflammatory Bowel Disease. J. Clin. Med. 2023, 12, 3867. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Claytor, J.; Hernandez, I.; Hou, J.K.; Kaplan, G.G. The Cost of Inflammatory Bowel Disease Care: How to Make it Sustainable. Clin. Gastroenterol. Hepatol. 2025, 23, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Din, S.; Segal, J.; Blackwell, J.; Gros, B.; Black, C.J.; Ford, A.C. Harms with placebo in trials of biological therapies and small molecules as induction therapy in inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2024, 9, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Chupin, A.; Perduca, V.; Meyer, A.; Bellanger, C.; Carbonnel, F.; Dong, C. Systematic review with meta-analysis: Comparative risk of lymphoma with anti-tumour necrosis factor agents and/or thiopurines in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 1289–1297. [Google Scholar] [CrossRef]
- Marafini, I.; Troncone, E.; Rocchetti, I.; Monteleone, G. Respiratory tract infections in inflammatory bowel disease patients taking vedolizumab: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2020, 11, 585732. [Google Scholar] [CrossRef]
- Bhat, S.; Click, B.; Regueiro, M. Safety and Monitoring of Inflammatory Bowel Disease Advanced Therapies. Inflamm. Bowel Dis. 2024, 30, 829–843. [Google Scholar] [CrossRef]
- Fuller, M.K. Pediatric Inflammatory Bowel Disease: Special Considerations. Surg. Clin. N. Am. 2019, 99, 1177–1183. [Google Scholar] [CrossRef]
- Noel, D.D.; Marinella, P.; Mauro, G.; Tripodi, S.I.; Pin, A.; Serena, A.; Matteo, B.; Giuseppe, F.M.; Patrizia, A.; Stefano, C.; et al. Genetic Variants Assessing Crohn’s Disease Pattern in Pediatric Inflammatory Bowel Disease Patients by a Clinical Exome Survey. Bioinform. Biol. Insights 2021, 15, 11779322211055285. [Google Scholar] [CrossRef]
- Centanni, L.; Cicerone, C.; Fanizzi, F.; D’Amico, F.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Advancing Therapeutic Targets in IBD: Emerging Goals and Precision Medicine Approaches. Pharmaceuticals 2025, 18, 78. [Google Scholar] [CrossRef]
- Arnold, C. Autoimmune disease is the next frontier for CAR T cell therapy. Nat. Med. 2024, 30, 6–9. [Google Scholar] [CrossRef]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Sartor, R.B. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Mateos, A.M. Methodological considerations and prevalence trends in disorders of gut-brain interaction: Lessons from comparative studies. Neurogastroenterol. Motil. 2023, 35, e14662. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, L.V.; Evtushenko, A.A.; Lvova, M.N.; Morozova, K.N.; Kiseleva, E.V. Underneath the Gut–Brain Axis in IBD—Evidence of the Non-Obvious. Int. J. Mol. Sci. 2024, 25, 12125. [Google Scholar] [CrossRef]
- Bar-Mashiah, A.S.; Mason, K.; Marsiglio, M.; Lukin, D.J. Forecasting IBD Activity Using Wearable Devices. Gastroenterology 2025. Online ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).