Salvage Chemoradiotherapy for Loco-Regional Recurrence of Esophageal Squamous Cell Carcinoma After Esophagectomy
Abstract
1. Introduction
2. Materials and Methods
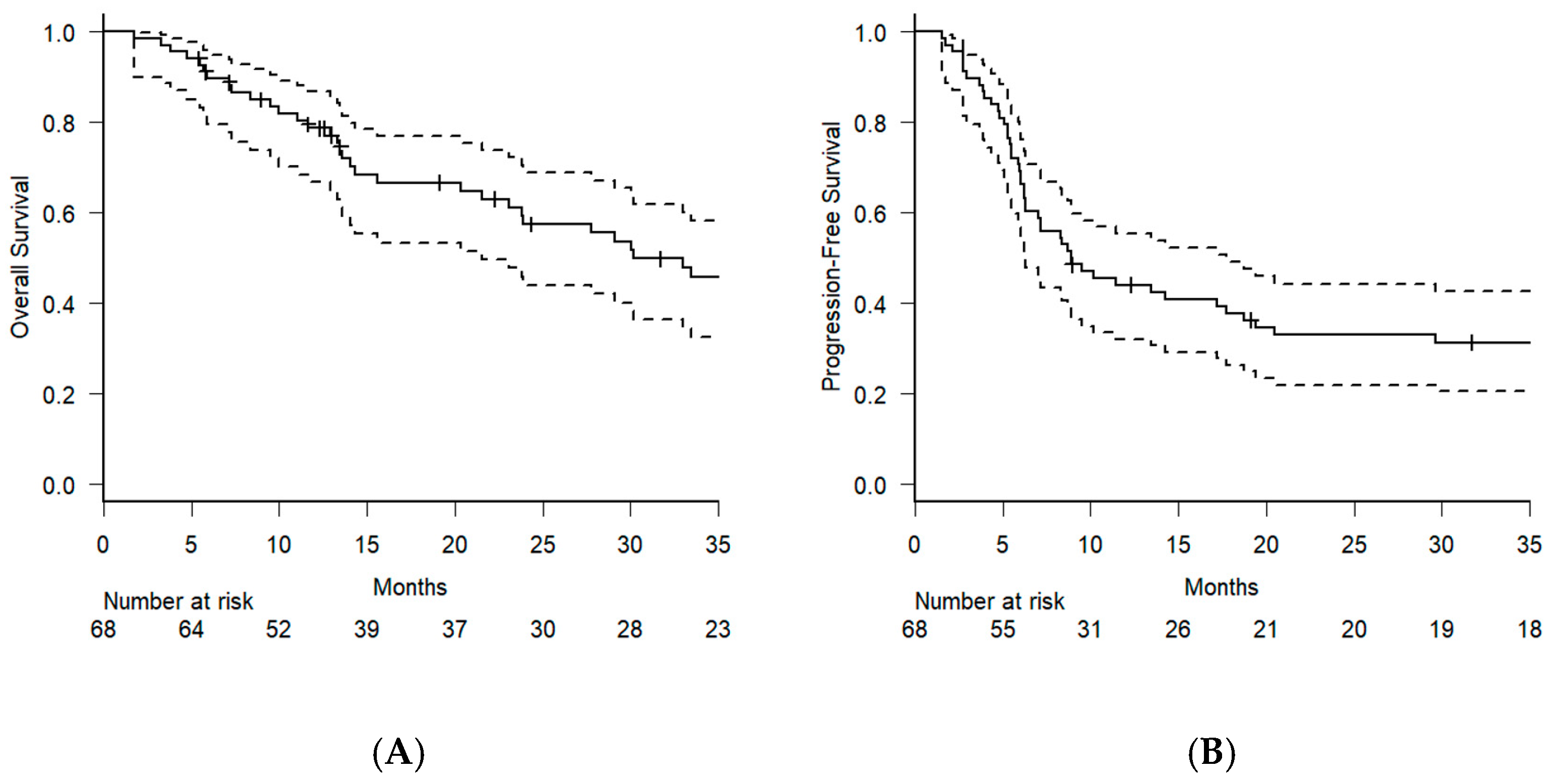
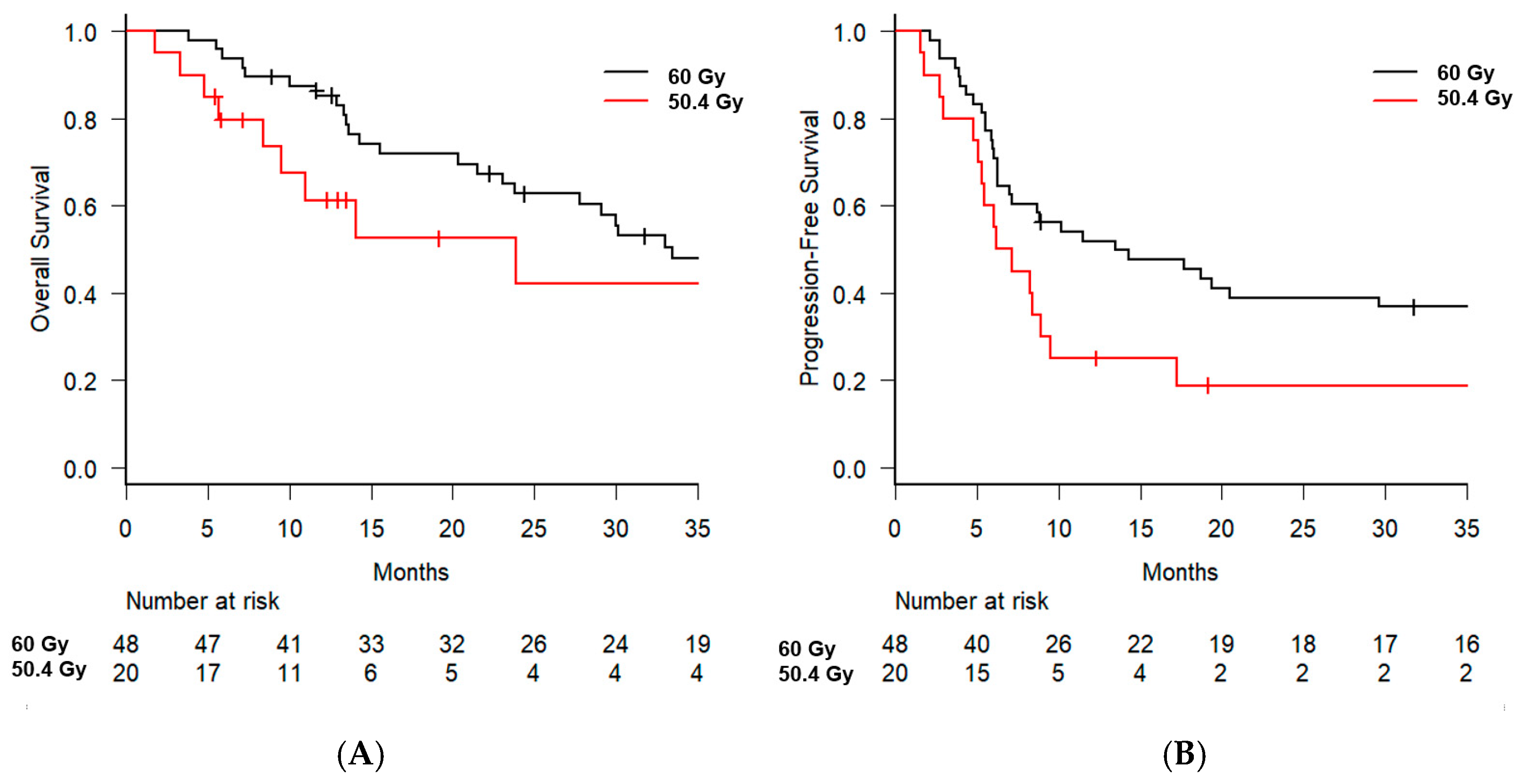
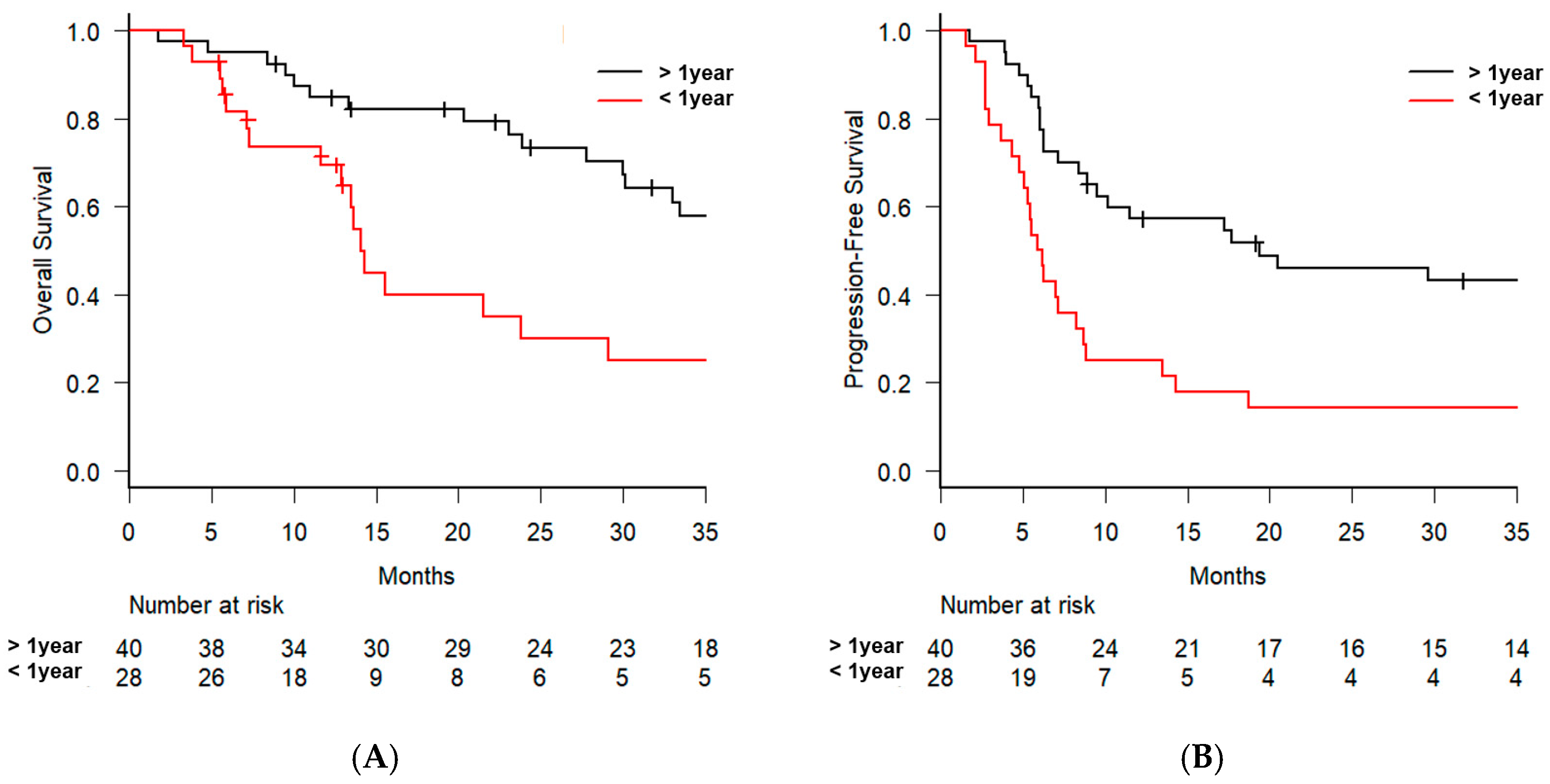
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Zhang, Y.; Peng, L.; Zhang, L. Research Progress on the Predicting Factors and Coping Strategies for Postoperative Recurrence of Esophageal Cancer. Cells 2022, 12, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sudarshan, M. Locoregional and oligometastatic recurrence of esophageal cancer-what are the management strategies? J. Thorac. Dis. 2019, 11, S1643–S1645. [Google Scholar] [CrossRef]
- Su, X.D.; Zhang, D.K.; Zhang, X.; Lin, P.; Long, H.; Rong, T.H. Prognostic factors in patients with recurrence after complete resection of esophageal squamous cell carcinoma. J. Thorac. Dis. 2014, 6, 949–957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parry, K.; Visser, E.; van Rossum, P.S.; Mohammad, N.H.; Ruurda, J.P.; van Hillegersberg, R. Prognosis and Treatment After Diagnosis of Recurrent Esophageal Carcinoma Following Esophagectomy with Curative Intent. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S1292–S1300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugawara, K.; Oka, D.; Hara, H.; Yoshii, T.; Ushijima, H.; Kudo, S.; Fukuda, T. Survival outcomes of esophageal cancer patients with recurrence after curative treatments. BMC Cancer 2023, 23, 1051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitamura, A.; Tsujinaka, S.; Nakano, T.; Sawada, K.; Shibata, C. Treatment Strategies for Locoregional Recurrence in Esophageal Squamous-Cell Carcinoma: An Updated Review. Cancers 2024, 16, 2539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Epistola, R.J.; Chao, J. Systemic therapy for advanced gastroesophageal cancers: Progress and pitfalls. Transl. Gastroenterol. Hepatol. 2020, 5, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirano, H.; Kato, K. Systemic treatment of advanced esophageal squamous cell carcinoma: Chemotherapy, molecular-targeting therapy and immunotherapy. Jpn. J. Clin. Oncol. 2019, 49, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Itamochi, H.; Kigawa, J. Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer Manag. Res. 2013, 5, 67–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Ren, H.; Li, Z.; Zhang, L.; Shao, Y.; Li, H.; Sun, Y.; Zhang, X.; Wang, Z.; Fu, J. A phase III randomized, controlled trial of nedaplatin versus cisplatin concurrent chemoradiotherapy in patients with cervical cancer. ESMO Open 2022, 7, 100565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, S.; Chen, Z.; Hu, C.; Zhang, J.; Chen, Y.; Song, Y.; Zhao, Q.; Fan, Y.; Wu, G.; Ma, Z.; et al. Nedaplatin Plus Docetaxel Versus Cisplatin Plus Docetaxel as First-Line Chemotherapy for Advanced Squamous Cell Carcinoma of the Lung—A Multicenter, Open-label, Randomized, Phase III Trial. J. Thorac. Oncol. 2018, 13, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T. The role of S-1 in the treatment of gastric cancer. Br. J. Cancer 2008, 98, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, Y.K.; Kim, H.D.; Yook, J.H.; Park, Y.K.; Lee, J.S.; Kim, Y.W.; Kim, J.Y.; Ryu, M.H.; Rha, S.Y.; Chung, I.J.; et al. Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer: Updated Overall Survival Outcomes From Phase III PRODIGY. J. Clin. Oncol. 2024, 42, 2961–2965. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Terazawa, T.; Goto, M.; Tanaka, R.; Kato, T.; Fujitani, K.; Kawakami, H.; Sakai, D.; Kurokawa, Y.; Tsujinaka, T.; et al. Neoadjuvant docetaxel, oxaliplatin and S-1 therapy for the patients with large type 3 or type 4 gastric cancer (OGSG1902): Protocol of a multi-center, phase II study. BMC Cancer 2022, 22, 811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katano, A.; Yamashita, H.; Nakagawa, K. Successful definitive concurrent chemoradiotherapy in a patient with esophageal cancer and Child-Pugh B cirrhosis of the liver. J. Cancer Res. Ther. 2019, 15, 255–257. [Google Scholar] [PubMed]
- Cho, W.K.; Noh, J.M.; Oh, D.; Ahn, Y.C.; Sun, J.M.; Kim, H.K.; Shim, Y.M. Salvage Radiotherapy for Loco-Regional Recurrence of Esophageal Cancer Following Surgery. Cancer Res. Treat. 2024, 57, 165. [Google Scholar] [CrossRef] [PubMed]
- Torii, A.; Tomita, N.; Takaoka, T.; Kondo, T.; Yamamoto, S.; Sugie, C.; Nagai, A.; Miyakawa, A.; Kuno, M.; Uchiyama, K.; et al. Salvage radiotherapy for locoregional recurrence of esophageal cancer after surgery. Jpn. J. Clin. Oncol. 2024, 55, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Nakamura, Y.; Sunakawa, H.; Fujiwara, H.; Hojo, H.; Nakamura, N.; Fujita, T.; Yano, T.; Daiko, H.; Akimoto, T.; et al. Tumor response and survival outcomes of salvage concurrent chemoradiotherapy with three-dimensional conformal radiotherapy and 5-fluorouracil/platinum-based chemotherapy for postoperative locoregional recurrence of esophageal squamous cell carcinoma. Esophagus 2022, 19, 645–652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mummudi, N.; Jiwnani, S.; Niyogi, D.; Srinivasan, S.; Ghosh-Laskar, S.; Tibdewal, A.; Rane, P.; Karimundackal, G.; Pramesh, C.S.; Agarwal, J.P. Salvage radiotherapy for postoperative locoregional failure in esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2022, 35, doab020. [Google Scholar] [CrossRef] [PubMed]
- Katano, A.; Minamitani, M.; Ohira, S.; Yamashita, H. Recent Advances and Challenges in Stereotactic Body Radiotherapy. Technol. Cancer Res. Treat. 2024, 23, 15330338241229363. [Google Scholar] [CrossRef] [PubMed]
- Katano, A.; Yamashita, H.; Nakagawa, K. Stereotactic body radiotherapy for oligo-recurrence in the liver in a patient with esophageal carcinoma: A case report. Mol. Clin. Oncol. 2017, 7, 1061–1063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, T.; Niibe, Y.; Matsumoto, Y.; Dekura, Y.; Oh, R.J.; Yamashita, H.; Kakuhara, H.; Aoki, M.; Jingu, K. Stereotactic Body Radiotherapy for Pulmonary Oligometastases from Esophageal Cancer: Results and Prognostic Factors. Anticancer Res. 2020, 40, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Seyedin, S.N.; Gannon, M.K.; Plichta, K.A.; Abushahin, L.; Berg, D.J.; Arshava, E.V.; Parekh, K.R.; Keech, J.C.; Caster, J.M.; Welsh, J.W.; et al. Safety and Efficacy of Stereotactic Body Radiation Therapy for Locoregional Recurrences After Prior Chemoradiation for Advanced Esophageal Carcinoma. Front. Oncol. 2020, 10, 1311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schipper, P.H.; Cassivi, S.D.; Deschamps, C.; Rice, D.C.; Nichols, F.C., 3rd; Allen, M.S.; Pairolero, P.C. Locally recurrent esophageal carcinoma: When is re-resection indicated? Ann. Thorac. Surg. 2005, 80, 1001–1005; discussion 1005–1006. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Shan, T.; Zheng, A.; Zhang, Y.; Lu, P.; Zhang, G.; Wang, F.; Xu, Z.; Zheng, G.; Tang, D.; et al. Capecitabine or Capecitabine Plus Oxaliplatin Versus Fluorouracil Plus Cisplatin in Definitive Concurrent Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma (CRTCOESC): A Multicenter, Randomized, Open-Label, Phase 3 Trial. J. Clin. Oncol. 2024, 42, 2436–2445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Ye, J.; Zhu, Z.; Zhao, W.; Zhou, J.; Wu, C.; Tang, H.; Fan, M.; Li, L.; Lin, Q.; et al. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial. J. Clin. Oncol. 2019, 37, 1695–1703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, H.; Makelike, K.; Chen, B.; Xi, M.; Li, Q.; Hu, Y.; Zhu, Y. Definitive concurrent chemoradiotherapy with docetaxel plus cisplatin versus 5-fluorouracil plus cisplatin in patients with esophageal squamous cell carcinoma: Long-term follow-up results of a phase II randomized controlled trial. Radiat. Oncol. 2023, 18, 150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A.; Kelsen, D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhu, S.; Li, J.; Shen, J.; Zhao, Y.; Li, X.; Jia, L.; Li, Q.; Yang, J.; Wu, Y.; et al. High-Dose Versus Standard-Dose Intensity-Modulated Radiotherapy With Concurrent Paclitaxel Plus Carboplatin for Patients With Thoracic Esophageal Squamous Cell Carcinoma: A Randomized, Multicenter, Open-Label, Phase 3 Superiority Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number (Percentage) | |
|---|---|---|
| Age: median [range] | 68 [41–83] | |
| Sex | ||
| Male | 56 (82%) | |
| Female | 12 (18%) | |
| ECOG-PS | ||
| 0 | 56 (82%) | |
| 1 | 12 (18%) | |
| Initial anatomical segments | ||
| Cervical | 6 (9%) | |
| Thoracic | 47 (69%) | |
| Abdominal | 2 (3%) | |
| Unknown | 3 (4%) | |
| Recurrence pattern | ||
| Anastomotic | 13 (19%) | |
| Cervical lymph nodes | 15 (22%) | |
| Thoracic lymph nodes | 27 (40%) | |
| Abdominal lymph nodes | 13 (19%) | |
| Concurrent chemotherapy | ||
| CDDP + 5-FU | 7 (10%) | |
| NDP + 5-FU | 8 (12%) | |
| NDP + S-1 | 51 (75%) | |
| NDP | 2 (3%) | |
| Dose and fractionation | ||
| 50.4 Gy in 28 fractions | 28 (41%) | |
| 60 Gy in 30 fractions | 40 (59%) | |
| Interval from surgery to recurrence | 12.8 months [3.2–227.3] | |
| Covariables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Hazard Ratio [95% CI] | p Value | Hazard Ratio [95% CI] | p Value | ||
| Age | ≤70 vs. >70 years old | 0.646 [0.311–1.344] | 0.242 | 0.633 [0.297–1.350] | 0.236 |
| Sex | Male vs. female | 0.283 [0.087–0.926] | 0.037 | 0.309 [0.088–1.085] | 0.067 |
| ECOG PS | 0 vs. 1 | 0.928 [0.360–2.397] | 0.878 | 1.312 [0.485–3.549] | 0.593 |
| Concurrent chemotherapy | NDP + S-1 vs. Others | 1.395 [0.689–2.824] | 0.355 | 1.309 [0.617–2.779] | 0.483 |
| Radiotherapy dose | 60 Gy vs. 50.4 Gy | 1.515 [0.704–3.261] | 0.289 | 2.414 [1.039–5.610] | 0.040 |
| Recurrence pattern | Anastomotic vs. others | 0.817 [0.337–1.981] | 0.654 | 1.313 [0.516–3.337] | 0.568 |
| Interval of recurrence | >1 vs. ≤1 year | 2.501 [1.284–4.869] | 0.007 | 2.307 [1.118–4.759] | 0.024 |
| Covariables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Hazard Ratio [95% CI] | p Value | Hazard Ratio [95% CI] | p Value | ||
| Age | ≤70 vs. >70 years old | 0.751 [0.413–1.364] | 0.347 | 0.612 [0.324–1.154] | 0.129 |
| Sex | Male vs. female | 0.571 [0.267–1.221] | 0.148 | 0.601 [0.266–1.355] | 0.220 |
| ECOG PS | 0 vs. 1 | 1.371 [0.664–2.834] | 0.394 | 1.851 [0.802–4.273] | 0.149 |
| Concurrent chemotherapy | NDP + S-1 vs. others | 1.086 [0.573–2.058] | 0.800 | 1.056 [0.544–2.051] | 0.872 |
| Radiotherapy dose | 60 Gy vs. 50.4 Gy | 1.756 [0.958–3.218] | 0.068 | 2.547 [1.331–4.873] | 0.005 |
| Recurrence pattern | Anastomotic vs. others | 0.983 [0.470–2.053] | 0.963 | 1.233 [0.529–2.874] | 0.627 |
| Interval of recurrence | >1 vs. ≤1 year | 2.494 [1.421–4.377] | 0.001 | 2.877 [1.576–5.250] | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katano, A.; Kiritoshi, T.; Sawayanagi, S.; Yamashita, H. Salvage Chemoradiotherapy for Loco-Regional Recurrence of Esophageal Squamous Cell Carcinoma After Esophagectomy. J. Clin. Med. 2025, 14, 1540. https://doi.org/10.3390/jcm14051540
Katano A, Kiritoshi T, Sawayanagi S, Yamashita H. Salvage Chemoradiotherapy for Loco-Regional Recurrence of Esophageal Squamous Cell Carcinoma After Esophagectomy. Journal of Clinical Medicine. 2025; 14(5):1540. https://doi.org/10.3390/jcm14051540
Chicago/Turabian StyleKatano, Atsuto, Tomoki Kiritoshi, Subaru Sawayanagi, and Hideomi Yamashita. 2025. "Salvage Chemoradiotherapy for Loco-Regional Recurrence of Esophageal Squamous Cell Carcinoma After Esophagectomy" Journal of Clinical Medicine 14, no. 5: 1540. https://doi.org/10.3390/jcm14051540
APA StyleKatano, A., Kiritoshi, T., Sawayanagi, S., & Yamashita, H. (2025). Salvage Chemoradiotherapy for Loco-Regional Recurrence of Esophageal Squamous Cell Carcinoma After Esophagectomy. Journal of Clinical Medicine, 14(5), 1540. https://doi.org/10.3390/jcm14051540





