Abstract
Background/Objectives: To investigate the impact of a routine botulinum toxin type A (BoNT-A) injection in combination with outpatient therapy on the daily activities of stroke survivors with upper extremity spasticity and to facilitate patient-centred assessment focusing on individual needs during daily life. Methods: Design: Observational study across one treatment cycle (3 months). Setting: Spasticity outpatient clinic of a neurorehabilitation hospital in Germany. Participants: Adult stroke survivors (n = 27) with upper extremity spasticity receiving routine BoNT-A treatment. Interventions: Participants received one BoNT-A injection and outpatient therapies as part of their routine management. Augmented assessment was conducted directly before the injection (T0), and at 4 to 6 weeks (Tmax1) and 12 to 14 weeks (T2) following the injection. Main outcome measures: The Canadian Occupational Performance Measure (COPM), Goal Attainment Scaling (GAS), and Arm Activity Measure (ArmA). Secondary outcome measures: The Resistance to Passive Movement Scale (REPAS), Motricity Index (MI), SF-12v2 Health Survey (SF-12v2), Global Clinical Impression (GCI), and importance of and satisfaction with the BoNT-A treatment. Results: Performance of individually selected daily activities and satisfaction with their performance (COPM), passive care tasks (ArmA, part A), and resistance to passive movement (REPAS) significantly improved from T0 to Tmax1. Improvements largely remained at T2. Individual goals were all set at the activities and participation levels of the International Classification of Functioning, Disability and Health. These improved for 75% of participants and were fully attained by 33.3% at Tmax1. Responder analysis indicated that COPM and ArmA improvements were clinically significant for up to 50% of participants. Active upper extremity use (ArmA, part B), health-related quality of life (SF-12v2), and upper extremity strength (MI) remained unchanged. Conclusions: Our results indicate that BoNT-A in combination with routine outpatient therapy positively influenced the individually valued daily activities of stroke survivors. COPM, GAS, and ArmA are suitable for facilitating a patient-centred and daily life-oriented spasticity management post-stroke.
1. Introduction
During daily life, stroke survivors with upper extremity spasticity and their carers are confronted with stiff muscles, resisted or even restricted passive range of motion, and joint deformities, which in turn may, for example, cause pain and difficulties during dressing and personal hygiene, skin maceration, and hamper reaching for objects as well as grasping and releasing them actively []. As a consequence, daily activities can become demanding, time-consuming, and frustrating [].
A recommended therapeutic option for treating spasticity is the injection of botulinum toxin type A (BoNT-A) [,]. Robust evidence demonstrates its efficacy in reducing resistance to passive movement, especially for wrist and finger flexors, and in improving the ability to care for the affected upper extremity []. Significant positive effects are also reported for spasticity-related upper extremity pain, passive range of shoulder motion, associated reactions, and caregiver burden [].
However, despite these benefits, a specific gap remains: the extent to which routine BoNT-A treatment influences meaningful, patient-/carer-identified daily activities within real-world environments is less well understood. Prior research has predominantly emphasized clinical impairments, such as muscle tone or joint mobility, while giving limited consideration to meaningful activities, patient/carer experiences, and individual needs defined by the patient/carers themselves [,]. Evidence from controlled studies often fails to capture outcomes that occur within the contextual realities of home and community life. Although goal-oriented assessment has been addressed in some previous studies, emphasis has often been placed on impairment-level outcomes or standardized functional measures, with comparatively limited focus on activities.
Consequently, evidence remains limited regarding the influence of routine BoNT-A treatment on the lived experience of daily activities. Likewise, the understanding of how goal-oriented and patient-centred approaches are translated into everyday clinical contexts remains limited. Thus, there is a need to examine BoNT-A therapy beyond standardized research environments, addressing patient perspectives as determinants of satisfaction and real-world functional impact and integrating practical aspects of treatment delivery.
To address this gap, we adopted an explicitly patient-centred and occupation-focused approach. Occupations can be defined as activities that provide meaning, structure, and purpose in daily life. They include the range of tasks an individual needs, wishes, or is expected to perform, such as work, leisure, domestic responsibilities, and self-care []. Given their inherently individualized nature, occupations offer a sensitive framework for understanding personal priorities, perceived difficulties, goal setting, and contextual influences. We therefore prioritized assessments at the ‘activities and participation’ levels of the International Classification of Functioning, Disability and Health (ICF) []. However, we also incorporated objective as well as quality of life outcome measures and an assessment performed at the ICF level of ‘body functions’ to ensure a holistic view [,,], without shifting the emphasis away from activity-oriented, patient-rated outcomes. We paid particular attention to practical considerations regarding the application of the assessments, as these aspects are crucial for integrating patient-centred assessment effectively into routine clinical practice.
Therefore, the objective of this study was to evaluate the individualized, real-world benefits of routine outpatient BoNT-A management for stroke survivors with upper extremity spasticity through the implementation of a patient-centred, daily life-oriented, and holistic approach. The study was intended to facilitate a BoNT-A treatment strategy focused on individual patient/carer needs for the performance of concrete meaningful daily activities and to evaluate the suitability of different outcome measures for their use in routine clinical practice.
2. Materials and Methods
2.1. Eligibility Criteria and Study Procedures
This observational study was performed in a spasticity outpatient clinic. Ethical approval was obtained from the local ethics committee (Universitätsmedizin Greifswald).
All adult patients (≥18 years) attending the outpatient clinic to receive BoNT-A injections for upper extremity spasticity following stroke (ischemic stroke, intracerebral or subarachnoidal haemorrhage) were eligible to participate in the study. There were no exclusion criteria relating to chronicity of stroke, number of BoNT-A injections in the past, or additional antispastic medication.
Written informed consent was obtained from patients or their legal representatives, with written assent from the patient where possible.
In line with standard clinic care, participants received BoNT-A from one of two experienced neurologists. Muscle selection, BoNT-A brand, and dosage were individualized based on clinical presentation and treatment goals. Injections were guided by electromyography and electrical stimulation.
Participants also completed a structured interview and assessment with an occupational therapist experienced in stroke rehabilitation. Data were collected over one injection cycle (12–14 weeks) on three study visits. The first (T0) and third (T2) visits coincided with routine BoNT-A treatment appointments, with study procedures scheduled beforehand. The second visit (Tmax1), at peak drug efficacy [], occurred 4–6 weeks post-injection. If participants could not attend the clinic (for Tmax1) or had limited time during T0 or T2, some procedures were offered at home, by phone, or in the hospital.
2.2. Baseline Characteristics and Treatment Information
Participants’ age, sex, time since stroke, degree of disability (Oxford Handicap Scale, OHS []), BoNT-A injection details, and type and frequency of physio-and/or occupational therapy during the study were documented.
2.3. Outcome Measures
2.3.1. Primary Outcome Measures
We used three primary outcome measures, all reflecting the ICF level activities and participation.
The Canadian Occupational Performance Measure (COPM) [] was chosen to identify the most important (up to five) individual everyday occupational performance issues for BoNT-A treatment by means of a semi-structured interview at T0. The current baseline performance of the selected daily activities and the level of satisfaction with their performance were also evaluated from the participants’ and/or carers’ perspective and re-assessed 4 to 6 and 12 to 14 weeks later (Tmax1 and T2, respectively). A scale ranging from 1 to 10 (performance: 1 = ‘extremely poor/cannot do’, 10 = ‘can do extremely well’; satisfaction: 1 = ‘not satisfied at all’, 10 = ‘extremely satisfied’) was employed.
Goal Attainment Scaling (GAS) [] was used to select one individual meaningful daily activity at T0 as a primary goal for treatment. For these activities/goals, six graded observable and SMART (specific, measurable, achievable, realistic/relevant, timed) [] behaviour levels were developed. Goal attainment was rated at Tmax1 and T2 using the previously defined individual 6-point GAS scale, where a score of 0 implies ‘the goal has been attained as expected’, +1 = ‘somewhat more than expected’, +2 = ‘much more than expected/best possible outcome for the selected goal’, −1 = ‘less than expected/the participant has improved but did not fully attain the goal’, −2 = ‘no change from baseline’, and −3 = ‘worsening compared to baseline’.
The Arm Activity Measure (ArmA) [,] formed the basis for a structured interview to evaluate the (passive) care activities of the hemiparetic arm (part A) and active use of the affected upper extremity (part B) within participants’ usual settings and situations over the preceding seven days. This measure was developed specifically for patients receiving focal spasticity treatments. Accordingly, the amount of difficulty for the eight passive and thirteen active function items (activities) was rated by participants and/or carers at T0, Tmax1, and T2 using a 5-point Likert scale, where 0 points refers to ‘no difficulty’ and 4 points to ‘unable to do activity’.
We also calculated responder rates (i.e., the proportion of participants demonstrating a clinically significant response) for the three primary outcome measures. Participants were categorized as responders if they obtained a difference of ≥2 points for the COPM [], a GAS score of ≥0, or an improvement of 3.0 points for part A of the ArmA or 2.5 points for part B [].
2.3.2. Secondary Outcome Measures
The Resistance to Passive Movement Scale (REPAS) [] is a summary rating scale based on the Ashworth Scale [], a measure at the ICF level of body functions. It consists of a set of standardized arm (part A) and leg (part B) assessments, each using a 5-point Likert scale, where 0 points represents ‘no increase in muscle tone’ and 4 points ‘limb rigid in flexion or extension’. We used the REPAS to evaluate resistance to eight full-range passive upper extremity joint movements.
The Motricity Index (MI) [,] was deployed at T0, Tmax1, and T2 to document the degree of paresis/strength at the ICF level of body functions. Two 6-point Likert scales, ranging from 0 = ‘no movement’ to 33 = ‘normal pinch grip’/‘normal power’, were applied for three upper and lower extremity movements.
The SF-12v2 Health Survey (SF-12v2) [] is a questionnaire comprising 12 items to address health-related quality of life, a measure at the ICF level of activities and participation. Items are assessed with 5- or 3-point Likert scales. A physical component summary (PCS) and a mental component summary (MCS) are calculated, each ranging between 0 (indicating the lowest self-reported health-related quality of life) and 100 points. The acute version (1-week recall) was used during all three study visits.
The treatment benefit of the BoNT-A injection, i.e., the patient’s condition in comparison to the condition at admission to the project, was assessed by participants/carers and investigators separately using Global Clinical Impression (GCI) at Tmax1 and T2. GCI was also collected from treating outpatient therapists for Tmax1. We applied a 4-point Likert scale, ranging from 1 = ‘very good’ to 4 = ‘bad’ []. When more than one rating was available, for example, from both the participant and their carer, the average was used for analysis.
Visual Analogue Scales (VAS) were employed to document the importance of and satisfaction with the BoNT-A treatment from the participants‘/carers’ perspective at T2. We used a horizontal line without markings, 100 mm in length, with the boundaries ‘not at all important’/‘not at all satisfied’ and ‘most important’/‘most satisfied’.
2.4. Data Analyses
Data were transferred to MS Excel and analysed using IBM SPSS Statistics (versions 23 and 24). The significance level was set at 0.05. Nonparametric statistics were applied to account for the ordinal nature of the outcome measures and the relatively small sample size. Here, nonparametric tests are considered robust without requiring that normal distribution or variance homogeneity assumptions are met. The Friedman test was used for those measures with three measurement time-points (COPM, ArmA, MI, REPAS, and SF12v2). Wilcoxon signed-rank tests were used for pairwise comparisons (with Bonferroni correction) in cases of statistical significance and for GAS and GCI. Statistical analyses excluded those participants with incomplete data for the respective outcome measure. T-scores were calculated for SF12v2 using a suggested validated algorithm [].
3. Results
3.1. Participants
All patient records within our outpatient spasticity clinic were screened. Twenty-seven eligible patients (or their legal representatives) consented to participate (Figure 1).
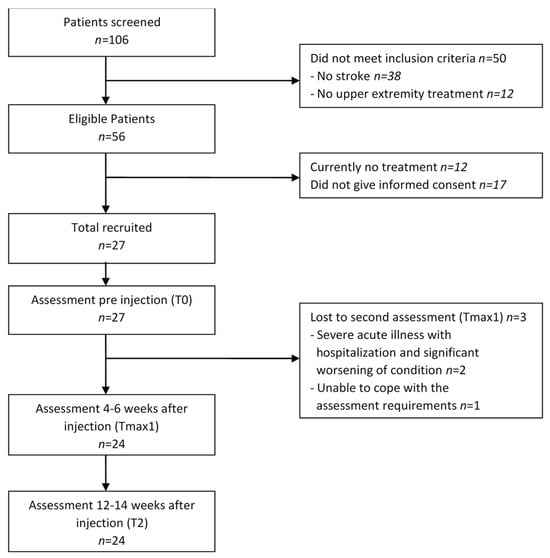
Figure 1.
Patient flow over the study period.
The mean age of participants was 58.2 years (SD 12.0; range 34–82 years); 59.3% were male. The mean period since (first) stroke was 7.1 years (SD 5.3); 59.3% participants had left-side and 40.7% right-side paresis. According to the OHS, all except one of the participants were moderately to severely disabled and required assistance; three of these were totally dependent on others.
3.2. Treatment (Botulinum Toxin A Injections, Physiotherapy, and Occupational Therapy)
Participants were injected with BoNT-A consistent with usual practice. Between two and eight upper extremity muscles were chosen for injection per individual (mean 4.9 muscles, SD 1.6). Details for the BoNT-A treatment at baseline (T0) are shown in Table 1.

Table 1.
Doses used per respective agent and muscle, with number of participants injected (n = 27).
All participants with data over one injection cycle (n = 24) received routine outpatient physiotherapy (PT) and/or occupational therapy (OT): PT, 95.8% (n = 23); OT, 70.8% (n = 17); both, 66.7% (n = 16). Each session typically lasted between 30 and 45 min. One individual participated in inpatient PT and OT during a period of hospitalization between Tmax1 and T2. The remaining participants were engaged between T0 and T2 in a mean number of 34.9 (SD 11.5, range 15–59) outpatient therapy sessions (PT: mean 22.8, SD 6.4, range 9–35; OT: mean 17.7, SD 7.0, range 7–32 sessions).
3.3. Goal Attainment Scaling
Each participant/carer set one specific activity-based goal for BoNT-A treatment during T0 using GAS. The set goals were categorized into eight activity areas (see Figure 2).
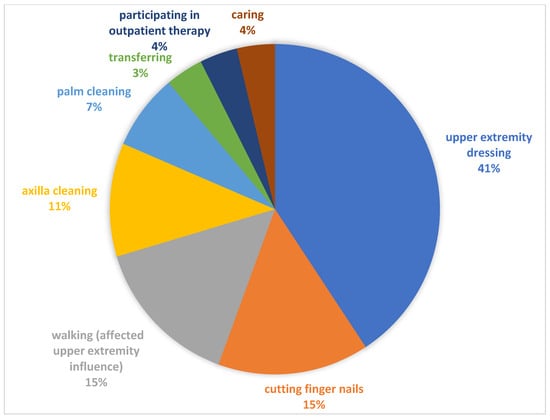
Figure 2.
Categories of identified goals using GAS.
GAS scores four to six weeks after injection (Tmax1) improved in 18 participants (75.0%), with goals fully achieved by 8 participants (33.3%), while 6 participants (25.0%) experienced no change. Twelve to fourteen weeks after injection (T2), 16 participants (66.6%) still showed an improvement, of whom 3 (12.5%) achieved or slightly over-achieved (n = 1) their set goal. Seven participants (29.2%) showed no change, while worsening was experienced by one (4.2%) participant. There was no statistically significant difference between goal attainment at Tmax1 and T2 (p = 0.124).
3.4. Canadian Occupational Performance Measure
Problems relating to occupational performance in the three areas of self-care, productivity, and leisure were identified by all participants/carers. They then rated their importance to them and selected up to four (mean 2.2, SD 0.9) of the most important problems relevant to BoNT-A treatment. Performance of these occupations, as well as satisfaction with performance, improved significantly from T0 to Tmax1 and from T0 to T2, while no significant difference between Tmax1 and T2 was observed (Table 2).

Table 2.
Summary of outcome measure scores and related statistical findings across the study time-points.
3.5. Arm Activity Measure
After BoNT-A treatment, participants/carers reported fewer difficulties during care of the affected upper extremity (passive function subscale). This effect was significant in the T0 to Tmax1 and the T0 to T2 phases, with no effect between Tmax1 and T2 (Table 2).
The active function subscale indicated that almost all participants had remarkable difficulties or were not able to use and manipulate objects with their affected upper extremity. Over time, the median sum scores and IQR for active function were relatively stable. While the Friedman test revealed a significant effect, this was not apparent following post hoc analyses (Table 2).
3.6. Secondary Outcomes
Three participants (11.1%) used the telephone option across the whole study, and one did so for T2; therefore, assessment data requiring physical contact (MI and REPAS) could not be collected for these instances.
Details for the results of the secondary outcome measures are depicted in Table 2.
Resistance to selected upper extremity movements (REPAS) showed a similar pattern to that already observed for COPM and ArmA, with significant improvements from T0 to Tmax1 and T0 to T2, but no significant change between Tmax1 and T2.
Upper extremity strength, as measured by MI, was not influenced by the BoNT-A treatment.
Similarly, quality of life (SF-12v2) did not change over the injection cycle, either for physical or mental health. When the participants’ scores were compared to the U.S. norm with a mean score of 50.0 and a SD of 10, it showed that mental health scores were broadly comparable (mean (SD): T0, 47.6 (8.6); Tmax1, 49.7 (10.3); T2, 49.3 (12.2)), while physical health was markedly reduced (mean (SD): T0: 33.4 (7.9); Tmax1, 36.0 (7.7); T2, 34.7 (7.1)).
BoNT-A treatment benefit (GCI) ratings were obtained for all 24 participants completing the study with full data from participants/carers, study personnel, and outpatient therapists. Ratings ranged from “very good” to “bad”. The median scores for participant/carer as well as physician/therapist estimated both at Tmax1 and T2 correspond to a “good” treatment benefit (median 2.0), while outpatient therapists showed a somewhat more critical but still positive view (median 2.5). A “bad” treatment benefit was observed eight times (Tmax1: n = 5, T2: n = 3) and pertained to six participants. Ratings did not differ significantly between Tmax1 and T2 (p = 0.248).
For most participants/carers, BoNT-A treatment was shown to be of importance, and most ratings for satisfaction with treatment were also at the higher end of the VAS (importance—median 74.0 mm, IQR 55.5–89.5; satisfaction—median 75.0 mm, IQR 60.3–91.5).
3.7. Responder Analyses (i.e., Participants Experiencing a Clinically Significant Response)
Responder rates for GAS, COPM (performance and satisfaction, respectively), and ArmA (active and passive function) illustrate the proportion of participants with a clinically significant treatment result (Figure 3). Such improvement was obtained by 12.5% to 50.0% of participants, at both Tmax1 and T2, with the highest responder rates for COPM satisfaction and ArmA passive care.
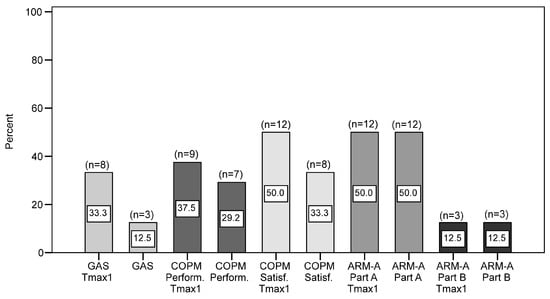
Figure 3.
Clinically significant response rates at Tmax1 and T2 for GAS, COPM, and ARM-A (n = 24). GAS: Goal Attainment Scaling; COPM: Canadian Occupational Performance Measure; Perform.: Performance subscale; Satisf.: Satisfaction subscale; ARM-A: Arm Activity Measure; part A—passive function subscale; part B—active function subscale.
4. Discussion
This observational study provides insights into the process and outcomes in relation to the management of stroke survivors with upper extremity spasticity. It shows how a routine BoNT-A injection in combination with outpatient therapy improves the performance of daily activities and satisfaction with the performance of these activities, in addition to any reduction in the resistance to passive movement. However, effects for active arm/hand use and strength, as well as health-related quality of life, were limited. Despite these limitations, BoNT-A treatment was considered important by most participants/carers. The explicit identification of patient-centred and occupation-focused goals for the BoNT-A treatment, the assessment of goal achievement with outcome measures that specifically capture such benefits, and a responder analysis all indicate that not only statistical, but clinically meaningful changes were induced.
In addition, the results support the notions that the patient-centred and occupation-focused approach can be implemented in routine healthcare, is valued by stroke survivors, and promotes an individually meaningful integration of BoNT-A treatment in spasticity management.
With regard to the assessment tools selected for this research, the main outcome measures (i.e., COPM, GAS, and ArmA) were shown to be valuable for a BoNT-A assessment and treatment focusing on the meaningful everyday needs of stroke survivors with upper extremity spasticity.
This study followed a cohort of patients receiving BoNT-A treatment as part of routine management and applied an individual, holistic, and person-centred assessment approach as recommended by Turner-Stokes et al. []. The aim was to optimize patient management by focusing on the individual daily life challenges experienced by participants/carers and on related outcome measures. The latter were selected to capture the ICF components ‘activities and participation’ and ‘body functions’ and were applied within individual ‘environments’ (usual homes and ordinary life situations). Objective assessment was enhanced by including patient-reported information and complemented by a quality-of-life assessment to maintain a holistic view of participants’ lives, as recommended for evaluation [,]. Such a holistic approach is rather unusual within spasticity management and research, where the focus on clinical signs and symptoms is typical, though some have been critical of this approach [,]. Another strength of our study was the timeframe used for evaluation, which included the observation of the full treatment effect (i.e., four to six weeks after injection; Tmax1). While patients usually return to treatment and assessment when the effect of the drug is waning (often at least three months after injection), this adds to a more complete picture. And lastly, to our knowledge, this is the only study in the field to date that includes views from treating therapists from the community, an important group of healthcare professionals involved in spasticity management.
We used three main outcome measures in our study: GAS, COPM, and ArmA. The GAS is specifically suggested as an outcome measure for spasticity management [] and is widely applied within studies evaluating the effect of BoNT-A for patients with upper extremity spasticity [,,,,,,,,,]; patients value being actively involved in the goal-setting process [,]. While setting one’s own goals may lead to greater autonomy and satisfaction [], increased motivation, and reduced anxiety of lay carers [], the use of GAS may be time-consuming [,,], and defining distinct and SMART goal levels can be difficult []. We had similar experiences, but the predefined SMART and distinct goals made subsequent goal evaluation fast and simple. For routine spasticity assessment, the ‘GAS-light’ model [] may provide an effective alternative, since it was specifically developed to overcome some of the above-mentioned limitations.
The use of the COPM before goal setting with GAS in our study helped us to shorten the goal identification process with GAS considerably. When asked, about half of our participants/carers could not spontaneously identify any spasticity-related challenges during daily life. Similar difficulties were also observed by Law et al. []. Using the COPM allowed us to collect individual difficulties in the three main life areas of self-care, productivity, and leisure in a client-centred and structured manner. While problem identification required considerable time, a point also noted by Toomey, Nicholson, and Carswell [], subsequent re-evaluation was relatively efficient.
While COPM has been shown to be a valuable assessment tool within routine spasticity management, administration requires a fair amount of time and specialist knowledge of the Canadian Model of Occupational Performance and Engagement []. Optimal practice typically requires a regular exchange of information between the injecting staff and therapists to assess, coordinate, adapt, and optimise both BoNT-A and therapeutic treatment.
Finally, our third main outcome measure, ArmA, comprises a range of relevant pre-selected activities. It could be filled in by the patients/carers themselves, and it was relatively easy and quick to use in our study. Most of our participants preferred this outcome measure, although part B, which relates to active function, was partly regarded as being too difficult; this was also reported by ~1/4 of the sample in Ashford et al. []. However, from our experience, the ArmA constitutes a suitable outcome measure for use within outpatient BoNT-A management.
When focusing on the results obtained by the implemented assessments in our study, they were largely in accordance with those of previous systematic reviews/randomized controlled trials (RCTs). This applies to both functional disability (in chronic stroke survivors) and active upper extremity function [,,], resistance to passive movement [,,,], and health-related quality of life (QOL) over one injection cycle [,,,]. The results are also supportive of previously reported disability responder rates []. Observational studies have shown similar results for ease of care/functional disability [,] and resistance to passive upper extremity movement [,,,].
According to our results and those found in the literature, it seems that improved passive joint movement may directly facilitate care tasks, such as putting the affected arm through a sleeve during dressing or washing one’s hands, where the upper extremity is passively involved. However, a reduction in muscle tone and related stiffness seems less likely to improve the performance of those tasks where the affected upper extremity is actively involved and to affect QOL results. There are several factors that may account for this. Firstly, it may be necessary to follow up with patients over extended periods with repeated BoNT-A administrations (at least four injections) to be able to observe an effect [,]. Secondly, the subgroup of patients with a degree of preserved motor control, receiving both repeated BoNT-A injections and rehabilitative therapy, may have a greater potential to improve the execution of active tasks, as Levy et al. [] hypothesize. Thirdly, the lack of a QOL effect after just one injection may be associated with the generic nature of QOL scales and the subsequent diminished sensitivity for detecting change []. Such changes would be more likely with more BoNT-A intervention cycles and/or time for re-evaluation. Accordingly, improvement in QOL has been observed in studies that repeatedly administered injections [,,,]. Fourthly, improvements in active arm and hand use as well as QOL may be more complexly inter-related, with contributing factors including exercise and movement therapy, coping strategies, environmental factors, and compensation, as well as cumulative BoNT-A effects.
In contrast to the largely homogeneous findings in the literature regarding resistance to passive movement, functional disability, active upper extremity function, and QOL over one injection cycle, goal attainment rates differ considerably in the literature (e.g., 23% in Turner-Stokes et al. [] vs. 94% in Ashford and Turner-Stokes []). Study aspects such as the goal-setting process, the BoNT-A treatment used, and the nature of the sample involved vary and may be factors that contribute to the different rates reported. It should be noted that those studies achieving high responder rates often used impairment-based goals such as pain reduction or improvement in range of motion [,,]. In contrast, participant goals in this study focused on the ICF component ‘activities and participation’. (Full) goal attainment in our study was relatively limited at 33%. Turner-Stokes et al. [] observed even smaller attainment rates between 3% and 12% for those goals set at the ICF ‘activities and participation’ levels. Adding to these findings, a large-scale RCT, one of the few studies in the field that included the COPM, did not reveal an effect from BoNT-A for the performance of individual occupations, which can be assigned to the ICF component ‘activities and participation’, or for satisfaction with their performance []. The limited effect at the ‘activities and participation’ level may be explained by the mode of action of the drug, which can be considered to occur at the impairment level. Surprisingly, Nott et al. [] found no differences in goal achievement as a function of ICF level. In this study, set goals were linked to the ICF levels and examined for potentially influencing factors for goal attainment. It should be noted that their sample was small, and their participants’ upper extremity function was less impaired. Further factors may potentially influence goal achievement, such as the number of BoNT-A injections [], initiation of treatment [], and the proportion of goals requiring active arm and hand movements [].
Study Limitations
There are a number of limitations to this study. Firstly, it was conducted in a single centre, and the number of participants was relatively small. These features limit generalizability, as the patient population and care protocols in this setting may differ from those in other clinics or regions. Secondly, compared to standard care elsewhere, our participants had received a relatively high amount of physio- and occupational therapy. It is plausible that the combined effect of BoNT-A treatment and regular physiotherapy, and frequently also occupational therapies, resulted in greater improvements than would have been achieved by BoNT-A alone. This multimodal therapy, while reflective of routine care in this setting, may limit the applicability of the results to other contexts. Thirdly, an inherent disadvantage of observational studies is the lack of a control group. Fourthly, only one BoNT-A injection cycle was observed, while it is possible that some changes may only be observed after multiple injections [,]. Fifth, participants had already received BoNT-A in the past, which may have led to an underestimation of the effect, if a certain reduction in spasticity from the previous administration was still present at injection (T0). Lastly, while patient-reported outcome measures are an integral part of a patient-centred approach, difficulties in differentiating between spasticity and paresis and in remembering accurately may limit their validity.
Variations in outpatient physiotherapy and occupational therapy, BoNT-A treatment parameters, and patient characteristics represent potential confounding (or at least modifying) factors noted in the limitations and underscore the complexity of the clinical context (i.e., routine healthcare). The fact that despite such contextual variability, the patient-centred and occupation-focused integration of BoNT-A treatment in spasticity management induced personally valued and clinically meaningful improvements in a considerable proportion of participants nevertheless supports its usefulness.
We recommend focusing future studies on the collaboration of healthcare providers to improve assessment and treatment to support patients and caregivers in managing daily life issues. Combining a daily living- and patient-centred approach with an earlier treatment initiation [,,] and the use of higher doses of BoNT-A [,,,,,] over a study period of at least 12 months with multiple injection cycles may warrant further investigation. This would allow the analysis of cumulative and sustained effects of treatment, providing deeper insights into optimizing patient outcomes in everyday life. Other potentially influential factors, such as the subjective importance patients attribute to BoNT-A treatment and the role of hopes and beliefs, could merit further investigation to better understand their impact on treatment outcomes.
5. Conclusions
In conclusion, this observational study has shown that patient-centred, holistic, and everyday life-oriented assessment following a process structure that allows stroke survivors (and their caregivers) to identify and assess meaningful occupational performance problems and goals for BoNT-A treatment is possible, but this approach may be challenging and time-consuming. BoNT-A in combination with outpatient occupational and/or physiotherapy clearly has the potential to positively impact daily living, measurable in terms of reducing activity limitations and participation restrictions, and being clinically relevant for at least a proportion of treated patients.
Author Contributions
Conceptualization, T.P.; methodology, T.P., S.R. and D.P.; validation, S.R. and T.P.; formal analysis, S.R. and D.P.; investigation, S.R.; data curation, S.R.; writing—original draft preparation, S.R.; writing—review and editing, D.P. and T.P.; visualization, S.R., T.P. and D.P.; supervision, T.P. and D.P.; project administration, S.R. and T.P.; resources, T.P.; funding acquisition, T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. It was indirectly supported by the BDH Bundesverband Rehabilitation e.V. (charity for neuro-disabilities) by a non-restricted personal grant to TP and in the context of Sybille Roschka’s employment at BDH-Klinik Greifswald, Germany (as member of the research group led by TP). The sponsors had no role in the decision to publish or any content of the publication. Sybille Roschka received a one-month study grant from the University of Birmingham, UK, to spend time in Birmingham to write her master’s thesis as part of her master’s program (which did not include writing the manuscript).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University Medicine Greifswald (int. reg. no. BB092/14, approved on October 21, 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available by contacting the corresponding author via e-mail.
Acknowledgments
We would like to thank our participants, their carers, the participating outpatient therapists, and the staff involved in the treatment and in the organization of the treatment sessions within the study. This article is based on the first author’s master’s thesis: Roschka, S. (2019) []. Do stroke survivors with upper extremity spasticity benefit from botulinum toxin? An observational study exploring daily activities. University of Birmingham, UK.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ArmA | Arm Activity Measure |
| BoNT-A | Botulinum toxin type A |
| COPM | Canadian Occupational Performance Measure |
| GAS | Goal Attainment Scaling |
| GCI | Global Clinical Impression |
| ICF | International Classification of Functioning, Disability and Health |
| MI | Motricity Index |
| OHS | Oxford Handicap Scale |
| REPAS | Resistance to Passive Movement Scale |
| SF-12v2 | SF-12 Health Survey, version 2 |
| VAS | Visual Analogue Scale |
References
- Mayer, N.H.; Esquenazi, A.; Childers, M.K. Common patterns of clinical motor dysfunction. Muscle Nerve 1997, 20 (Suppl. S6), S21–S35. [Google Scholar] [CrossRef]
- Bhimani, R.; Anderson, L. Clinical understanding of spasticity: Implications for practice. Rehabil. Res. Pract. 2014, 2014, 279175. [Google Scholar] [CrossRef]
- Francisco, G.E.; Wissel, J.; Platz, T.; Li, S. Post-Stroke Spasticity. In Clinical Pathways in Stroke Rehabilitation, 1st ed.; Platz, T., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 149–173. [Google Scholar]
- Royal College of Physicians; British Society of Rehabilitation Medicine; The Chartered Society of Physiotherapy; Association of Chartered Physiotherapists in Neurology; The Royal College of Occupational Therapists. Spasticity in adults: Management using botulinum toxin. In National Guidelines, 2nd ed.; RCP: London, UK, 2018. [Google Scholar]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum neurotoxins for post-stroke spasticity in adults: A systematic review. Mov. Disord. 2009, 24, 801–812. [Google Scholar] [CrossRef]
- Amini, D.A.; Kannenberg, K.; Bodison, S.; Chang, P.-F.; Colaianni, D.; Goodrich, B.; Mahaffey, L.; Painter, M.; Urban, M.; Handley-More, D.; et al. Occupational Therapy Practice Framework: Domain and Process (3rd Edition). Am. J. Occup. Ther. 2014, 68 (Suppl. S1), S1–S48. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health (ICF); World Health Organization: Geneva, Switzerland, 2001; Available online: https://iris.who.int/handle/10665/42407 (accessed on 1 November 2025).
- Wissel, J.; Ward, A.B.; Erztgaard, P.; Bensmail, D.; Hecht, M.J.; Lejeune, T.M.; Schnider, P.; Altavista, M.C.; Cavazza, S.; Deltombe, T.; et al. European consensus table on the use of botulinum toxin type a in adult spasticity. J. Rehabil. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Manack, A.; Brainin, M. Toward an epidemiology of poststroke spasticity. Neurology 2013, 80 (Suppl. S2), 13–19. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Ward, A.B. The diagnosis and management of adults with spasticity. In Handbook of Clinical Neurology. Neurological Rehabilitation, 1st ed.; Barnes, M.P., Good, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 145–160. [Google Scholar]
- Bamford, J.M.; Sandercock, P.A.; Warlow, C.P.; Slattery, J. Interobserver agreement for the assessment of handicap in stroke patients (letter). Stroke 1989, 20, 828. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; Carswell, A.; McColl, M.A.; Polatajko, H.; Pollock, N. COPM Canadian Occupational Performance Measure, 2nd ed.; Schulz-Kirchner: Idstein, Germany, 2011. [Google Scholar]
- Kiresuk, T.J.; Sherman, R.E. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment. Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef]
- Bovend’Eerdt, T.J.; Botell, R.E.; Wade, D.T. Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef]
- Ashford, S.; Slade, M.; Turner-Stokes, L. Conceptualisation and development of the arm activity measure (ArmA) for assessment of activity in the hemiparetic arm. Disabil. Rehabil. 2013, 35, 1513–1518. [Google Scholar] [CrossRef]
- Ashford, S.; Turner-Stokes, L.; Siegert, R.; Slade, M. Initial psychometric evaluation of the Arm Activity Measure (ArmA): A measure of activity in the hemiparetic arm. Clin. Rehabil. 2013, 27, 728–740. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; Carswell, A.; McColl, M.A.; Polatajko, H.; Pollock, N. Canadian Occupational Performance Measure, 5th ed.; Canadian Association of Occupational Therapists (CAOT): Ottawa, ON, Canada, 2014. [Google Scholar]
- Platz, T.; Vuadens, P.; Eickhof, C.; Arnold, P.; Van Kaick, S.; Heise, K. REPAS, a summary rating scale for resistance to passive movement: Item selection, reliability and validity. Disabil. Rehabil. 2008, 30, 44–53. [Google Scholar] [CrossRef]
- Ashworth, B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964, 192, 540–542. [Google Scholar]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor evaluation in vascular hemiplegia. Eur. Neurol. 1980, 19, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Wade, D. Assessing motor impairment after stroke: A pilot reliability study. J. Neurol. Neurosurg. Psychiatry 1990, 53, 576–579. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Turner-Bowker, D.M.; Gandek, B. User’s Manual for the SF-12v2® Health Survey with a Supplement Documenting SF-12® Health Survey; QualityMetric Incorporated: Lincoln, RI, USA, 2002. [Google Scholar]
- Kaňovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Comes, G.; Grafe, S.; Pulte, I. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post-stroke upper limb spasticity. J. Rehabil. Med. 2011, 43, 486–492. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Ashford, S.; Esquenazi, A.; Wissel, J.; Ward, A.B.; Francisco, G.E.; Lains, J.; Suputtitada, A.; Serrano, S.; Baguley, I.J.; et al. A comprehensive person-centred approach to adult spastic paresis: A consensus-based framework. Eur. J. Phys. Rehabil. Med. 2018, 54, 605–617. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Jacinto, J.; Fheodoroff, K.; Brashear, A.; Maisonobe, P.; Lysandropoulos, A.; Ashford, S.; Baguley, I.; Aggarwal, A.; Olver, J.; et al. Assessing the effectiveness of upper-limb spasticity management using a structured approach to goal-setting and outcome measurement: First cycle results from the ULIS-III study. J. Rehabil. Med. 2021, 53, 2731. [Google Scholar] [CrossRef] [PubMed]
- Ashford, S.; Turner-Stokes, L. Management of shoulder and proximal upper limb spasticity using botulinum toxin and concurrent therapy interventions: A preliminary analysis of goals and outcomes. Disabil. Rehabil. 2009, 31, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Rychlik, R.; Kreimendahl, F.; Schnur, N.; Lambert-Baumann, J. Long-term efficacy and safety of incobotulinumtoxinA and conventional treatment of poststroke arm spasticity: A prospective, non-interventional, open-label, parallel-group study. BMJ Open 2015, 5, e009358. [Google Scholar] [CrossRef]
- Eftekhar, P.; Mochizuki, G.; Dutta, T.; Richardson, D.; Brooks, D. Goal Attainment Scaling in Individuals with Upper Limb Spasticity Post Stroke. Occup. Ther. Int. 2016, 23, 379–389. [Google Scholar] [CrossRef]
- Jost, W.; Hefter, H.; Reissig, A.; Kollewe, K.; Wissel, J. Efficacy and safety of botulinum toxin typ A (Dysport) for the treatment of post-stroke arm spasticity: Results of the German-Asutrian open-label post-marketing surveillance prospective study. J. Neurol. Sci. 2014, 337, 86–90. [Google Scholar] [CrossRef]
- Khan, P.; Riberto, M.; Frances, J.A.; Chueire, R.; Amorim, A.C.F.G.; Xerez, D.; Chung, T.M.; Mercuri, L.H.C.; Longo, A.L.; Lianza, S.; et al. The Effectiveness of Botulinum Toxin Type A (BoNT-A) Treatment in Brazilian Patients with Chronic Post-Stroke Spasticity: Results from the Observational, Multicenter, Prospective BCause Study. Toxins 2020, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Nott, M.T.; Barden, H.L.H.; Baguley, I.J. Goal attainment following upper-limb botulinum toxin-A injections: Are we facilitating achievement of client-centred goals? J. Rehabil. Med. 2014, 46, 864–868. [Google Scholar] [CrossRef]
- Schramm, A.; Ndayisaba, J.P.; auf dem Brinke, M.; Hecht, M.; Herrmann, C.; Huber, M.; Lobsien, E.; Mehnert, S.; Reuter, I.; Stenner, A.; et al. Spasticity treatment with onabotulinumtoxin A: Data from a prospective German real-life patient registry. J. Neural Transm. 2014, 121, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Wissel, J.; Borg, J.; Ertzgaard, P.; Herrmann, C.; Kulkarni, J.; Lindgren, K.; Reuter, I.; Sakel, M.; Säterö, P.; et al. Functional goal achievement in post-stroke spasticity patients: The Botox® Economic Spasticity Trial (BEST). J. Rehabil. Med. 2014, 46, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Fheodoroff, K.; Jacinto, J.; Maisonobe, P. Results from the Upper Limb International Spasticity Study-II (ULIS-II): A large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin a in real-life clinical management. BMJ Open 2013, 3, e002771. [Google Scholar] [CrossRef]
- Holliday, R.C.; Cano, S.; Freeman, J.A.; Playford, E.D. Should patients participate in clinical decision making? An optimised balance block design controlled study of goal setting in a rehabilitation unit. J. Neurol. Neurosurg. Psychiatry 2007, 78, 576–580. [Google Scholar] [CrossRef]
- Young, C.A.; Manmathan, G.P.; Ward, J.C.R. Perceptions of goal setting in a neurological rehabilitation unit: A qualitative study of patients, carers and staff. J. Rehabil. Med. 2008, 40, 190–194. [Google Scholar] [CrossRef]
- Reid, A.; Chesson, R. Goal Attainment Scaling: Is it appropriate for stroke patients and their physiotherapists? Physiotherapy 1998, 84, 136–144. [Google Scholar] [CrossRef]
- Stevens, A.; Beurskens, A.; Köke, A.; Van Der Weijden, T. The use of patient-specific measurement instruments in the process of goal-setting: A systematic review of available instruments and their feasibility. Clin. Rehabil. 2013, 27, 1005–1019. [Google Scholar] [CrossRef]
- Vu, M.; Law, A.V. Goal-attainment scaling: A review and applications to pharmacy practice. Res. Soc. Adm. Pharm. 2012, 8, 102–121. [Google Scholar] [CrossRef]
- Krasny-Pacini, A.; Hiebel, J.; Pauly, F.; Godon, S.; Chevignard, M. Goal Attainment Scaling in rehabilitation: A literature-based update. Ann. Phys. Rehabil. Med. 2013, 56, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L. Goal attainment scaling (GAS) in rehabilitation: A practical guide. Clin. Rehabil. 2009, 23, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Polatajko, H.; Pollock, N.; McColl, M.A.; Carswell, A.; Baptiste, S. Pilot testing of the Canadian Occupational Performance Measure: Clinical and measurement issues. Can. J. Occup. Ther. 1994, 61, 191–197. [Google Scholar] [CrossRef]
- Toomey, M.; Nicholson, D.; Carswell, A. The clinical utillity of the Canadian occupational Performance Measure. Carnadian J. Occup. Ther. 1995, 62, 242–249. [Google Scholar] [CrossRef]
- Townsend, E.A.; Polatajko, H.J. Enabling Occupation II: Advancing an Occupational Therapy Vision for Health, Well-Being & Justice Through Occupation; Canadian Association of Occupational Therapists (CAOT): Ottawa, ON, Canada, 2007. [Google Scholar]
- Ashford, S.; Slade, M.; Nair, A.; Turner-Stokes, L. Arm Activity measure (ArmA) application for recording functional gain following focal spasticity treatment. Int. J. Ther. Rehabil. 2014, 21, 10–17. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, T.; Hu, X.; Wang, T. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zhang, C.; Liu, Y.; Magat, E.; Verduzco-Gutierrez, M.; Francisco, G.E.; Zhou, P.; Zhang, Y.; Li, S. The effects of botulinum toxin injections on spasticity and motor performance in chronic stroke with spastic hemiplegia. Toxins 2020, 12, 492. [Google Scholar] [CrossRef]
- Baker, J.A.; Pereira, G. The efficacy of Botulinum Toxin A for spasticity and pain in adults: A systematic review and meta-analysis using the Grades of Recommendation, Assessment, Development and Evaluation approach. Clin. Rehabil. 2013, 27, 1084–1096. [Google Scholar] [CrossRef]
- Elovic, E.P.; Brashear, A.; Kaelin, D.; Liu, J.; Millis, S.R.; Barron, R.; Turkel, C. Repeated treatments with botulinum toxin type A produce sustained decreases in the limitations associated with focal upper-limb poststroke spasticity for caregivers and patients. Arch. Phys. Med. Rehabil. 2008, 89, 799–806. [Google Scholar] [CrossRef]
- McCrory, P.; Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Sandanam, J.; Davies, L.; Munns, M.; Hughes, A. Botulinum toxin a for treatment of upper limb spasticity following stroke: A multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J. Rehabil. Med. 2009, 41, 536–544. [Google Scholar] [CrossRef]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A multicentre randomized controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–13. [Google Scholar] [CrossRef]
- Elovic, E.P.; Munin, M.C.; Kaňovský, P.; Hanschmann, A.; Hiersemenzel, R.; Marciniak, C. Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity. Muscle Nerve 2016, 53, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Marque, P.; Denis, A.; Gasq, D.; Chaleat-Valayer, E.; Yelnik, A.; Colin, C.; Pérennou, D. Botuloscope: 1-year follow-up of upper limb post-stroke spasticity treated with botulinum toxin. Ann. Phys. Rehabil. Med. 2019, 62, 207–213. [Google Scholar] [CrossRef]
- Gracies, J.-M.; Jech, R.; Valkovic, P.; Marque, P.; Vecchio, M.; Denes, Z.; Vilain, C.; Delafont, B.; Picaut, P. When can maximal efficacy occur with repeat botulinum toxin injection in upper limb spastic paresis? Brain Commun. 2021, 3, fcaa201. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Molteni, F.; Cannaviello, G.; Lansaman, T.; Roche, N.; Bensmail, D. Does botulinum toxin treatment improve upper limb active function? Ann. Phys. Rehabil. Med. 2019, 62, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, R.; Kreimendahl, F.; Schnur, N.; Lambert-Baumann, J.; Dressler, D. Quality of life and costs of spasticity treatment in German stroke patients. Health Econ. Rev. 2016, 6, 27. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Davies, L.; McCrory, P.; Hughes, A. Goal attainment scaling in the evaluation of treatment of upper limb spasticity with botulinum toxin: A secondary analysis from a double-blind placebo-controlled randomized clinical trial. J. Rehabil. Med. 2010, 42, 81–89. [Google Scholar] [CrossRef]
- Gracies, J.M.; O’Dell, M.; Vecchio, M.; Hedera, P.; Kocer, S.; Rudzinska-Bar, M.; Rubin, B.; Timerbaeva, S.L.; Lusakowska, A.; Boyer, F.C.; et al. Effects of repeated abobotulinumtoxinA injections in upper limb spasticity. Muscle Nerve 2018, 57, 245–254. [Google Scholar] [CrossRef]
- Rosales, R.L.; Balcaitiene, J.; Berard, H.; Maisonobe, P.; Goh, K.J.; Kumthornthip, W.; Mazlan, M.; Latif, L.A.; Santos, M.M.D.D.; Chotiyarnwong, C.; et al. Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study. Toxins 2018, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Mach, H.; Frohlich, S.; Behrend, S.; Werner, C.; Melzer, I. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling finger flexor stiffness six months later: A randomized controlled trial. Clin. Rehabil. 2012, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.; Ispoglou, S.; Helliwell, B.; Hicklin, D.; Sturman, S.; Pandyan, A. Can the early use of botulinum toxin in post stroke spasticity reduce contracture development? A randomised controlled trial. Clin. Rehabil. 2021, 35, 399–409. [Google Scholar] [CrossRef]
- Abo, M.; Shigematsu, T.; Hara, H.; Matsuda, Y.; Nimura, A.; Yamashita, Y.; Takahashi, K. Efficacy and Safety of OnabotulinumtoxinA 400 Units in Patients with Post-Stroke Upper Limb Spasticity: Final Report of a Randomized, Double-Blind, Placebo-Controlled Trial with an Open-Label Extension Phase. Toxins 2020, 12, 127. [Google Scholar] [CrossRef]
- Baricich, A.; Picelli, A.; Santamato, A.; Carda, S.; de Sire, A.; Smania, N.; Cisari, C.; Invernizzi, M. Safety Profile of High-Dose Botulinum Toxin Type A in Post-Stroke Spasticity Treatment. Clin. Drug Investig. 2018, 38, 991–1000. [Google Scholar] [CrossRef]
- Intiso, D.; Simone, V.; Bartolo, M.; Santamato, A.; Ranieri, M.; Gatta, M.T.; Di Rienzo, F. High dosage of botulinum toxin type a in adult subjects with spasticity following acquired central nervous system damage: Where are we at? Toxins 2020, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M.; GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke upper limb spasticity. Curr. Med. Res. Opin. 2010, 26, 1983–1992. [Google Scholar] [CrossRef]
- O’Dell, M.W.; Brashear, A.; Jech, R.; Lejeune, T.; Marque, P.; Bensmail, D.; Ayyoub, Z.; Simpson, D.M.; Volteau, M.; Vilain, C.; et al. Dose-Dependent Effects of AbobotulinumtoxinA (Dysport) on Spasticity and Active Movements in Adults With Upper Limb Spasticity: Secondary Analysis of a Phase 3 Study. PM R 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B.; et al. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: The TOWER study. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef]
- Roschka, S. Do Stroke Survivors with Upper Extremity Spasticity Benefit from Botulinum Toxin? An Observational Study Exploring Daily Activities. Master’s Thesis, University of Birmingham, Birmingham, UK, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).