Integrated Care in Patients with Atrial Fibrillation and Optimal Medical Treatment for Heart Failure: Results from the Heart failuRe ObsErvational Study (HEROES)
Abstract
1. Introduction
2. Materials and Methods
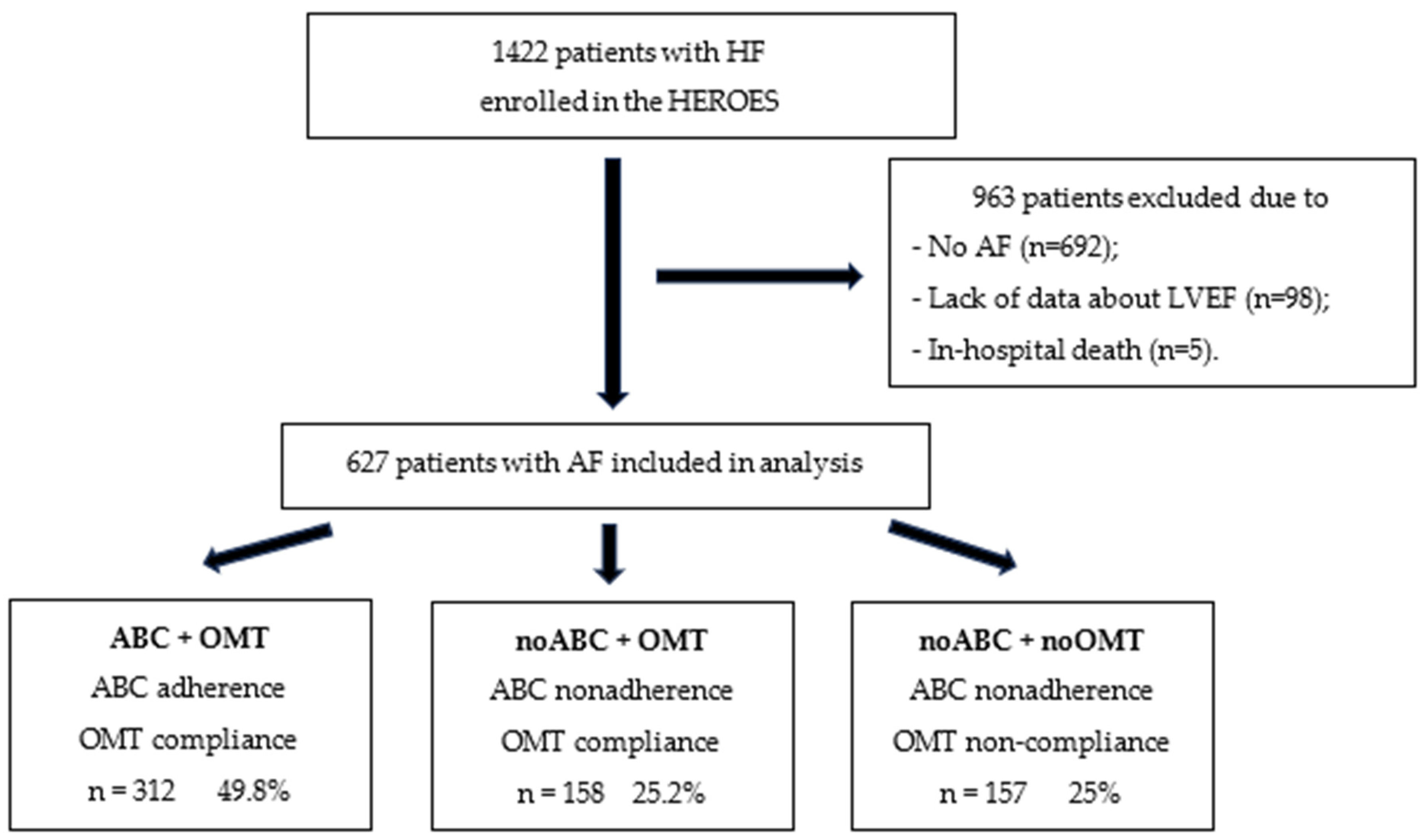
2.1. Study Population
2.2. Covariates
2.3. Atrial Fibrillation Better Care (ABC) Pathway Evaluation
- For hypertension: Controlled blood pressure (≤140/90 mmHg) recorded at baseline;
- For coronary artery disease: Treatment with angiotensin-converting enzyme inhibitors, beta-blockers, and statins;
- For peripheral artery disease: Treatment with statins;
- For previous stroke/transient ischemic attack: Treatment with statins;
- For HF: Treatment according to the guideline recommendations for the specific HF subtype [9].
2.4. Optimal Medical Treatment for Heart Failure
2.5. Study Design
- -
- ABC + OMT group: Patients with ABC pathway adherence and with OMT for HF;
- -
- noABC + OMT group: Patients without ABC pathway adherence and with OMT for HF;
- -
- noABC + noOMT group: Patients without ABC pathway adherence and without OMT for HF.
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Comparison of Patients Across ABC Pathway Adherence and OMT Compliance
3.3. Adherence to the ABC Pathway
3.4. Adherence to the OMT
3.5. Outcome According to Spectrum of Left Ventricular Ejection Fraction
3.6. Impact of ABC Pathway Adherence and OMT on Outcome, Including Interaction and Stratified Analyses
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorczyca-Głowacka, I.; Galas, A.; Tymińska, A.; Byczkowska, K.; Furman-Niedziejko, A.; Tkaczyszyn, M.; Stefański, A.; Major, A.; Klimczak-Tomaniak, D.; Hamala, P.; et al. Atrial fibrillation in patients with heart failure with reduced, mildly reduced, and preserved ejection fraction: A report from the HEart failuRe ObsErvational Study (HEROES). Kardiol. Pol. 2025, 83, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, U.; Dahlström, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, J.; Sohns, C.; Andresen, D.; Siebels, J.; Sehner, S.; Boersma, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Schunkert, H.; et al. Atrial Fibrillation Burden and Clinical Outcomes in Heart Failure: The CASTLE-AF Trial. JACC Clin. Electrophysiol. 2021, 7, 594–603. [Google Scholar] [CrossRef]
- Wu, J.; Tao, G.; Xie, S.; Yang, H.; Qi, F.; Bao, N.; Li, Z.; Chang, G.; Xiao, H. Prediction of three-year all-cause mortality in patients with heart failure and atrial fibrillation using the CatBoost model. BMC Cardiovasc. Disord. 2025, 25, 466. [Google Scholar] [CrossRef] [PubMed]
- Lenarczyk, R.; Mitręga, K.; Mazurek, M.; Janion, M.; Opolski, G.; Drożdż, J.; Streb, W.; Fuglewicz, A.; Sokal, A.; Laroche, C.; et al. Polish and European management strategies in patients with atrial fibrillation. Data from the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot). Pol. Arch. Med. Wewn. 2016, 126, 138–148. [Google Scholar] [CrossRef]
- Zuin, M.; Bertini, M.; Vitali, F.; Turakhia, M.; Boriani, G. Heart Failure-Related Death in Subjects With Atrial Fibrillation in the United States, 1999 to 2020. J. Am. Heart Assoc. 2024, 13, e033897. [Google Scholar] [CrossRef]
- Schupp, T.; Schmitt, A.; Reinhardt, M.; Abel, N.; Lau, F.; Abumayyaleh, M.; Dudda, J.; Weidner, K.; Ayoub, M.; Akin, M. Prognosis and treatment strategies for atrial fibrillation in heart failure with mildly reduced ejection fraction. Eur. J. Prev. Cardiol. 2024, 31, 1372–1384. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Kozieł, M.; Yang, P.S.; Guo, Y.; et al. Adherence to the “atrial fibrillation better care” pathway in patients with atrial fibrillation: Impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb. Haemost. 2022, 122, 406–414. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Chao, T.F.; Joung, B.; Takahashi, Y.; Lim, T.W.; Choi, E.K.; Chan, Y.H.; Guo, Y.; Sriratanasathavorn, C.; Oh, S.; Okumura, K.; et al. 2021 focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: Executive summary. Thromb. Haemost. 2021, 22, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Drożdż, J.; Morawiec, R.; Drozd, M.; Krzesiński, P.; Wożakowska-Kapłon, B.; Grabowski, M.; Leszek, P.; Kuch, M.; Kasprzak, J.D.; Janion, M.; et al. Rationale, objectives, and design of the HEart failuRe ObsErvational Study of the Polish Cardiac Society (HEROES). Kardiol. Pol. 2025, 83, 321–324. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.J.; Liu, J.; Lam, K.; Maunder, A.; Pandey, C.; Cave, A.E.; O’Fee, A.; Yang, G.; Mousa, A.; Ee, C. The effectiveness and safety of integrative medicine for chronic heart failure: An umbrella review. Complement. Ther. Med. 2025, 91, 103182. [Google Scholar] [CrossRef]
- Lin, T.T.; Chen, T.Y.; Cheng, J.F.; Lin, L.-L.; Wu, C.-K. Chronotropic Incompetence and Cardiovascular Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2025, 14, e037290. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Treewaree, S.; Lip, G.Y.H. Components of the Atrial fibrillation Better Care pathway for holistic care of patients with atrial fibrillation: A win-ratio analysis from the COOL-AF registry. Europace 2024, 26, euae237. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, B.; Calvert, P.; Liu, Y.; Gue, Y.; Gupta, D.; McDowell, G.; Azariah, J.L.; Namboodiri, N.; Unni, G.; et al. Phenotypes of South Asian patients with atrial fibrillation and holistic integrated care management: Cluster analysis of data from KERALA-AF Registry. Lancet Reg. Health Southeast Asia 2024, 31, 100507. [Google Scholar] [CrossRef]
- Bonini, N.; Proietti, M.; Romiti, G.F.; Vitolo, M.; Fawzy, A.M.; Ding, W.Y.; Imberti, J.F.; Fauchier, L.; Marin, F.; Nabauer, M.; et al. Optimal Medical Therapy for Heart Failure and Integrated Care in Patients With Atrial Fibrillation: A Report From the ESC-EHRA EORP Atrial Fibrillation Long-Term General Registry. J. Am. Heart Assoc. 2025, 14, e030499. [Google Scholar] [CrossRef]
- Kozieł, M.; Simovic, S.; Pavlovic, N.; Kocijancic, A.; Paparisto, V.; Music, L.; Trendafilova, E.; Dan, A.R.; Kusljugic, Z.; Dan, G.A.; et al. Adherence to the ABC (Atrial fibrillation Better Care) pathway in the Balkan region: The BALKAN-AF survey. Pol. Arch. Intern. Med. 2020, 130, 187–195. [Google Scholar] [CrossRef]
- Mitrega, K.; Sredniawa, B.; Sokal, A.; Streb, W.; Kowalczyk, J.; Opolski, G.; Grodzicki, T.; Rewiuk, K.; Kazmierczak, J.; Wierucki, L.; et al. Does atrial fibrillation still increase the risk of death? One-year follow-up results of the NOMED-AF study. Pol. Arch. Intern. Med. 2024, 134, 16619. [Google Scholar] [CrossRef]
- Proietti, M.; Lip, G.Y.H.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.A.; Kalarus, Z.; Tavazzi, L.; et al. Relation of outcomes to ABC (Atrial Fibrillation Better Care) pathway adherent care in European patients with atrial fibrillation: An analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) Registry. Europace 2021, 23, 174–183. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; Cunningham, J.W.; Pedro Ferreira, J.; Zannad, F.; Packer, M.; Fonarow, G.C.; McMurray, J.J.V.; Solomon, S.D. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef]
- Karlström, P.; Pivodic, A.; Dahlström, U.; Fu, M. Modern heart failure treatment is superior to conventional treatment across the left ventricular ejection spectrum: Real-life data from the Swedish Heart Failure Registry 2013–2020. Clin. Res. Cardiol. 2024, 113, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, A.; Pandozi, C.; Bonanni, M.; Mariani, M.V.; Sgarra, L.; Nesti, L.; Pierucci, N.; La Fazia, V.M.; Lavalle, C.; Nardi, F.; et al. Impact of empagliflozin and dapagliflozin on sudden cardiac death: A systematic review and meta-analysis of adjudicated randomized evidence. Heart Rhythm 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Kongmalai, T.; Hadnorntun, P.; Leelahavarong, P.; Kongmalai, P.; Srinonprasert, V.; Chirakarnjanakorn, S.; Chaikledkaew, U.; McKay, G.; Attia, J.; Thakkinstian, A. Comparative cardiovascular benefits of individual SGLT2 inhibitors in type 2 diabetes and heart failure: A systematic review and network meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1216160. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, J.D.; Gorczyca-Głowacka, I.; Sobczak-Kaleta, M.; Barylski, M.; Drożdż, J.; Filipiak, K.J.; Kapłon-Cieślicka, A.; Lelonek, M.; Mamcarz, A.; Ochijewicz, D.; et al. Pharmacotherapy of heart failure A.D. 2023. Expert opinion of Working Group on Cardiovascular Pharmacotherapy, Polish Cardiac Society. Kardiol. Pol. 2023, 81, 537–556. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; ESC Scientific Document Group. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castellá, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Griffin, M.; Mills, M.T. A Holistic Approach to Improving Outcomes in Atrial Fibrillation: The AF-CARE Pathway. Br. J. Hosp. Med. 2025, 86, 15. [Google Scholar] [CrossRef]


| Characteristic | All Patients N = 627 | ABC + OMT Group N = 312 | noABC + OMT Group N = 158 | noABC + noOMT Group N = 157 | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, median (IQR), years | 71.6 (63.8–79.1) | 69.8 (62.7–76.3) | 72.2 (64.3–79.8) | 75.0 (65.6–80.9) | <0.001 |
| Age group, n (%) | |||||
| <65 years | 169 (27.0) | 94 (30.1) | 40 (25.3) | 35 (22.3) | 0.04 |
| 65–74 years | 197 (31.4) | 104 (33.3) | 52 (32.9) | 41 (26.1) | 0.17 |
| >74 years | 261 (41.6) | 114 (36.5) | 66 (41.8) | 81 (51.6) | 0.008 |
| Female, n (%) | 206 (32.9) | 95 (30.4) | 51 (32.3) | 60 (38.2) | 0.24 |
| Physical parameters | |||||
| BMI, median (IQR), kg/m2 | 28.4 (25.4–32.3) N = 625 | 29.1 (25.8–32.4) N = 311 | 29.1 (24.9–33.2) N = 158 | 27.1 (24.5–30.7) N = 156 | 0.007 |
| HR, median (IQR), beats/min | 72 (65–80) N = 539 | 72 (65–80) N = 256 | 70 (65–80) N = 145 | 74.5 (65.3–81.8) N = 138 | 0.13 |
| SBP, median (IQR), mmHg | 120 (106–130) N = 539 | 118 (105–128) N = 256 | 123 (110–140) N = 145 | 115.5 (105–130) N = 138 | <0.001 |
| DBP, median (IQR), mmHg | 71 (65–80) N = 539 | 70 (65–80) N = 256 | 75 (68–80) N = 145 | 70 (60.5–79) N = 138 | 0.006 |
| Atrial fibrillation characteristics | |||||
| Form, n (%) | |||||
| Paroxysmal | 255 (40.7) | 136 (43.6) | 66 (41.8) | 53 (33.8) | 0.12 |
| Non-paroxysmal | 372 (59.3) | 176 (56.4) | 92 (58.2) | 104 (66.2) | |
| CHA2DS2-VASc, median (IQR) | 4 (3–5) | 4 (3–5) | 4.5 (3–6) | 5 (3–5) | 0.07 |
| HFpEF, n (%) | 173 (27.6) | 67 (21.5) | 57 (36.1) | 49 (31.2) | 0.002 |
| HFmrEF, n (%) | 130 (20.7) | 69 (22.1) | 33 (20.9) | 28 (17.8) | 0.56 |
| HFrEF, n (%) | 324 (51.7) | 176 (56.4) | 68 (43.0) | 80 (51.0) | 0.02 |
| HF duration, median (IQR), years | 3.8 (0.7–10.7) | 3.6 (0.8–11.8) | 4.4 (0.7–9.9) | 4.1 (0.7–8.9) | 0.75 |
| HF duration, n (%) | |||||
| <1 year | 182 (29.0) | 89 (28.5) | 47 (29.7) | 46 (29.3) | 0.66 |
| 1–3 years | 99 (15.8) | 56 (17.9) | 20 (12.7) | 23 (14.6) | |
| >3 years | 346 (55.2) | 167 (53.5) | 91 (57.6) | 88 (56.1) | |
| Type of visit, n (%) | |||||
| Hospitalization | 539 (86.0) | 256 (82.1) | 145 (91.8) | 138 (87.9) | 0.01 |
| Outpatient | 88 (14.0) | 56 (17.9) | 13 (8.2) | 19 (12.1) | |
| NYHA class, n (%) N = 539 | |||||
| I | 61 (11.3) | 32 (12.5) | 12 (8.3) | 17 (12.3) | 0.40 |
| II | 364 (67.5) | 224 (87.5) | 61 (42.1) | 79 (57.3) | <0.001 |
| III | 106 (19.7) | 0 (0) | 66 (45.5) | 40 (29.0) | <0.001 |
| IV | 8 (1.5) | 0 (0) | 6 (4.1) | 2 (1.4) | 0.004 |
| Medical history, n (%) | |||||
| Hypertension | 446 (71.1) | 228 (73.1) | 117 (74.1) | 101 (64.3) | 0.09 |
| Diabetes mellitus | 251 (40.0) | 120 (38.5) | 72 (45.6) | 59 (37.6) | 0.26 |
| Stroke or TIA | 67 (10.7) | 27 (8.7) | 23 (14.6) | 17 (10.8) | 0.15 |
| Myocardial infarction | 210 (33.5) | 108 (34.6) | 59 (37.3) | 43 (27.4) | 0.15 |
| Stable angina | 200 (31.9) | 98 (31.4) | 53 (33.5) | 49 (31.2) | 0.88 |
| Previous VTE | 19 (3.0) | 11 (3.5) | 7 (4.4) | 1 (0.6) | 0.08 |
| Peripheral arterial disease | 9 (1.4) | 5 (1.6) | 4 (2.5) | 0 (0.0) | 0.14 |
| Chronic kidney disease | 210 (33.5) | 83 (26.6) | 55 (34.8) | 72 (45.9) | <0.001 |
| Alcohol use, n (%) | |||||
| Current | 25 (4.0) | 19 (6.1) | 0 (0) | 6 (3.8) | 0.006 |
| Former | 68 (10.8) | 45 (14.4) | 9 (5.7) | 14 (8.9) | 0.01 |
| Never | 534 (85.2) | 248 (79.5) | 149 (94.3) | 137 (87.3) | <0.001 |
| All N = 627 | OMT Compliance N = 470 | OMT Non-Compliance N = 315 | p | |
|---|---|---|---|---|
| A criterion | 557 (88.8) | 434 (92.3) | 123 (78.3) | <0.001 |
| OAC | 557 (88.8) | 434 (92.3) | 123 (78.3) | <0.001 |
| VKA | 113 (18.0) | 83 (17.7) | 30 (19.1) | 0.68 |
| DOAC | 444 (70.8) | 351 (74.7) | 93 (59.2) | <0.001 |
| B criterion * | 513 (81.8) | 398 (84.7) | 115 (73.2) | 0.001 |
| Beta-blocker | 583 (92.9) | 457 (97.2) | 126 (80.3) | <0.001 |
| Digoxin | 99 (15.8) | 74 (15.7) | 25 (15.9) | 0.96 |
| Amiodaron | 111 (17.7) | 85 (18.1) | 26 (16.6) | 0.66 |
| Propafenone | 5 (0.8) | 3 (0.6) | 2 (1.3) | 0.60 |
| AF catheter ablation | 56 (8.9) | 43 (9.1) | 13 (8.3) | 0.74 |
| C criterion | 387 (61.7) | 387 (82.3) | 0 | <0.001 |
| Optimal treatment of | ||||
| Hypertension | 388/446 (87.0) | 300/345 (86.9) | 88/101 (87.1) | 0.96 |
| CAD | 230/296 (77.7) | 202/224 (90.2) | 28/72 (38.9) | <0.001 |
| Stroke/TIA | 48/67 (71.6) | 40/50 (80.0) | 8/17 (47.1) | 0.014 |
| Diabetes mellitus | 232/251 (92.4) | 180/192 (93.8) | 52/59 (88.1) | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorczyca-Głowacka, I.; Molenda, K.; Nadel, M.; Galas, A.; Tymińska, A.; Byczkowska, K.; Siniarski, A.; Furmanek, W.; Stefański, A.; Wożakowska-Kapłon, B.; et al. Integrated Care in Patients with Atrial Fibrillation and Optimal Medical Treatment for Heart Failure: Results from the Heart failuRe ObsErvational Study (HEROES). J. Clin. Med. 2025, 14, 8338. https://doi.org/10.3390/jcm14238338
Gorczyca-Głowacka I, Molenda K, Nadel M, Galas A, Tymińska A, Byczkowska K, Siniarski A, Furmanek W, Stefański A, Wożakowska-Kapłon B, et al. Integrated Care in Patients with Atrial Fibrillation and Optimal Medical Treatment for Heart Failure: Results from the Heart failuRe ObsErvational Study (HEROES). Journal of Clinical Medicine. 2025; 14(23):8338. https://doi.org/10.3390/jcm14238338
Chicago/Turabian StyleGorczyca-Głowacka, Iwona, Karolina Molenda, Maciej Nadel, Agata Galas, Agata Tymińska, Katarzyna Byczkowska, Aleksander Siniarski, Witold Furmanek, Adrian Stefański, Beata Wożakowska-Kapłon, and et al. 2025. "Integrated Care in Patients with Atrial Fibrillation and Optimal Medical Treatment for Heart Failure: Results from the Heart failuRe ObsErvational Study (HEROES)" Journal of Clinical Medicine 14, no. 23: 8338. https://doi.org/10.3390/jcm14238338
APA StyleGorczyca-Głowacka, I., Molenda, K., Nadel, M., Galas, A., Tymińska, A., Byczkowska, K., Siniarski, A., Furmanek, W., Stefański, A., Wożakowska-Kapłon, B., Klimczak-Tomaniak, D., & Morawiec, R. (2025). Integrated Care in Patients with Atrial Fibrillation and Optimal Medical Treatment for Heart Failure: Results from the Heart failuRe ObsErvational Study (HEROES). Journal of Clinical Medicine, 14(23), 8338. https://doi.org/10.3390/jcm14238338








