Abstract
Background/Objectives: Oral potentially malignant diseases (OPMD) are a group of specific conditions characterized by a variable degree of progression to oral cancer. Although these lesions are generally easily recognizable, clinicians face a difficult challenge in predicting which lesions will undergo malignancy. This fact becomes more pressing when considering that early detection of OPMD significantly influences the survival toll. Our systematic review aims to evaluate current evidence of the mechanism through which salivary microRNAs are involved in OPMD and to study the possibility of using these molecules as a novel biomarker for predicting transition to oral cancer. Methods: A comprehensive search in PubMed, Google Academic, Cochrane and Scopus databases was performed, analyzing studies conducted between 2014 and 2025. The quality of studies was evaluated using Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2). Results: A total of 1046 articles were found; 76 articles were thoroughly examined, but only 33 articles were included in this systematic review. Salivary microRNAs, such as miR-21, miR-34a or miR-320a, were found to be dysregulated in OPMD samples compared to healthy and oral squamous cell carcinoma samples (OSCC), contributing to malignancy through gene expression alteration. Conclusions: Salivary microRNAs were found to be intricately involved in the malignant transformation of OPMD, potentially being promising biomarkers for early detection of oral cancer.
1. Introduction
Despite the progress made in the field of oncology over the last decades, cancer remains a major worldwide health dilemma, with approximately 10 million deaths occurring in 2020 [1]. Being an utterly heterogeneous disease, cancer still encourages ardent controversies in the scientific field regarding the most effective method of inhibiting the anarchic proliferation of cells. Conventional treatments, involving surgery, chemo/radiotherapy and immunotherapy, continue to be the main approaches for treatment, but tumor relapse and adverse effects of the therapies still pose a challenge [2].
As a definition, oral potentially malignant disorders (OPMD) are a group of mucosal disorders with variable degree of progression to oral cancer [3]. These lesions represent an intermediate stage in the progression towards oral cancer, indicating that they have only suffered a small amount of the genetic changes observed in OSCC [4]. A wide range of pathologies such as leukoplakia, erythroplakia, oral lichen planus and submucous fibrosis can be included in the category of premalignant lesions. Even though the majority of these disorders regress or remain stationary, a variable number of OPMD develop genetic alteration and microenvironment changes, which, ultimately, conduct to malignant transformation [5,6]. For instance, leukoplakia carries an associated risk of 3.3% for cancer development over 5 years [7]. Tissue biopsy remains the golden standard for differentiation between premalignant lesions and early OSCC, because it can not only distinguish the histopathological type of neoplasms, but it can also reveal its degree of differentiation [8]. However, in some cases, it becomes difficult to procure biopsy material due to localization of certain tumors, and in eventuality of multifocal lesions, several tissue samples are required. Therefore, there is an acute need to develop novel biomarkers capable of non-invasive identification of malignancy OPMD in order to improve the outcome of patients.
Oral cancer is described as one of the most common malignancies in developing countries, with an elevated incidence in Melanesia, South Central Asia and Eastern Europe [9]. In 2020, nearly 177,757 deaths were recorded with oral cancer as the primary cause [1]. This precarious prognosis, with an overall 5-year survival rate that varies between 40 and 80%, is caused by tardive diagnosis and the frequent tumor recurrence [10]. The most predominant subtype is squamous cell carcinoma (OSCC), with more than 90% of all oral cancers having this origin [11]. Tobacco smoking, alcohol consumption and HPV infection are frequent risk factors that are strongly associated with oral carcinogenesis, disturbing local homeostasis by inducing DNA mutation and genetic alteration [12].
MicroRNAs are short (22–23 nucleotides), non-coding RNAs, capable of regulating gene expression, with an incremental involvement in cancer pathogenesis [13]. There are two main mechanisms by which microRNAs interfere post-transcriptionally with their RNA targets. The first one involves the imperfect complementary binding within the 3′-untranslated regions (3′-UTRs) of target mRNAs, while the second one includes perfect complementarity binding to mRNA sequences, leading the degradation of target mRNAs [14]. By modulating the levels of genes, microRNAs play an important role in cell growth, differentiation, apoptosis and interactions with local environment [15,16]. Circulating microRNAs can be defined as the miRNAs that are secreted extracellularly in various biofluids including serum, plasma, saliva, breast milk, ascites fluid and others [17]. Following transcription and maturation of these molecules, microRNAs are expelled into extracellular medium in 4 possible forms: within the exosomes by active exocytosis, through the export of apoptotic bodies [18], as shedding vesicles [19] or in association with RNA-binding proteins (AGO 1–4) [20] or high-density lipoproteins [21]. Once they enter the external microenvironment, microRNAs continue to be involved in the hallmarks of cancer, regulating proliferation, differentiation, apoptosis, angiogenesis, local invasion and metastasis formation [22].
The purpose of the current systematic review is to evaluate the possibility of using the expression level of salivary microRNAs as a tool for early detection of OPMD in order to prevent all the complications associated with the development of OSCC. In addition, this scientific paper aims to present the most relevant mechanism involved in OPMD transition towards OSCC, along with the major advantages of using microRNAs for detecting malignancy of those lesions.
2. Materials and Methods
The PRISMA checklist (Supplementary Materials) was utilized as a guideline to organize and design this systematic review [23,24].
2.1. Eligibility Criteria
This research paper included all studies that evaluated the difference in salivary microRNAs level of expression between healthy individuals, patients with premalignant lesions and patients with OSCC. Also, PICOS terms were used in order to conduct a thorough investigation: participants (patients with malignant or premalignant lesions), intervention (dosage of altered level of salivary microRNAs in saliva of oral potentially malignant disorders or OSCC patients), control (microRNAs expression in healthy subjects) and outcome (difference in salivary microRNAs level of expression between healthy subjects, patients with premalignant lesions and OSCC ones), and the study design included cross-sectional studies, prospective comparative studies, retrospective cohort studies and case–control studies.
Inclusion criteria were as follows:
- (1)
- Studies that analyzed the salivary microRNAs expression in OPMD and oral cancer patients;
- (2)
- Text available in full format;
- (3)
- Articles written in English;
- (4)
- Respecting the structure of IMRAD (introduction, material and method, results, discussions);
Exclusion criteria were as follows:
- (1)
- Reviews, meta-analysis, case reports, case series, opinion articles and letters to the editor;
- (2)
- Articles that incorporate various forms of treatment administration for OPMD a priori to saliva sample acquisition;
- (3)
- Studies that include patients with other oral cavity tumors (non-OSCC) or in other regions of the organism, patients with history or undergoing chemo/radiotherapy and patients with immunosuppression;
- (4)
- Duplicated publications;
- (5)
- Animal experiments;
The process used to determine the eligibility of each study for the planned syntheses involved analyzing study characteristics, including the specific microRNA(s) investigated, detection methods, type of lesion (OPMD or OSCC), presence of a healthy control group, and the reported expression levels. Based on these characteristics, studies were allocated to one or more of the three comparative syntheses: healthy vs OPMD, healthy vs OSCC, and OPMD vs OSCC. A study was included specific synthesis only if it reported microRNA expression levels for both groups required for that specific comparison.
2.2. Publication Search Strategy
An electronic search on Pubmed, Google Academic, Cochrane and Scopus databases was performed. Appropriate keywords and Medical Subject Headings (MeSH) terms were selected and systematically combined using Boolean operators, such as “AND”, “OR”.
Published papers on level of expression of salivary microRNAs in premalignant lesions from 2014 to 2025 were found using the following combination of keywords: (salivary microRNA OR exosome microRNA OR circulating microRNA OR extracellular microRNA) AND (oral cancer OR oral squamous cell carcinoma OR oral premalignant lesions OR leukoplakia OR oral lichen planus OR erythroplakia OR oral submucous fibrosis OR oral potentially malignant disorder). Two independent reviewers (C.O., G.A.) extracted data available on study characteristics (article characteristics, sample types, saliva collection protocol, microRNA extraction and analysis, name of microRNA(s) and type of dysregulation, main study results like sensitivity, specificity and conclusion). All of the inconsistencies were further discussed, and a consensus was obtained. The extracted data focused on identifying changes in microRNA expression levels in both OSCC and OPMD and examining their correlation with the transition from a non-malignant lesion to oral cancer.
2.3. Qualitative Evaluation
In order to minimize the risk of bias, all of the included research papers were subjected to a qualitative assessment using Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2). This tool consists of four key domains: Patients Selection, Index test(s), Reference Standard and Flow and Timing. Every article included was thoroughly analyzed by the two independent examinators (C.O., G.A.), and every domain was rated either “high”, “unclear” or “low” according to their bias risk. QUADAS-2 tool provides a series of “signaling” questions which guide the researchers in proper evaluation of bias risk. If all of the signaling questions in a domain are answered “yes,” the risk of bias is judged as low. Conversely, if signaling questions are answered “no”, the domain is considered to have a potential risk of bias. In addition, using QUADAS-2 tool, the examiners performed an applicability assessment of the reviewed articles [25]. Further details regarding the steps involved in qualitative evaluation of studies can be found in Table 1.

Table 1.
The QUADAS-2 tool signaling questions that assist researchers in systematically assessing the quality and risk of bias in the selected studies.
3. Results
3.1. Literature Search and Study Selection
After the initial search, a total of 1046 scientific articles were retrieved from databases (577 from Pubmed, 324 from Google Academic, 11 from Cochrane database, 134 from Scopus). The duplicates were removed (232), and the other articles were analyzed by two independent authors (C.O., G.A.) according to the inclusion and exclusion criteria. A total of 76 articles were declared potentially admissible and underwent an integral text examination, but only 33 articles entirely met our criteria and were included in this systematic review. Further details regarding the main reasons for study exclusions can be found in Figure 1.
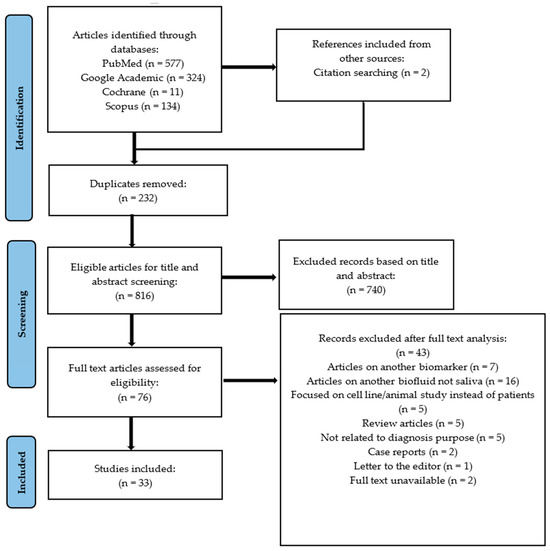
Figure 1.
PRISMA flow-chart diagram for the selection of 33 papers included in the review.
3.2. Characteristics of the Included Studies
The design of the studies was slightly different: 10 studies focused on highlighting the distinct salivary microRNA expression between premalignant lesions and healthy subjects, 9 studies focused on salivary microRNA levels between OSCC and healthy individuals, whereas 14 articles compared the levels of microRNA from the saliva of patients with premalignant lesions to patients with OSCC, in conjunction with saliva sampled from healthy individuals. A detailed presentation of the included studies is provided in Table 2. Moreover, no statistical methods such as subgroup analysis or meta-regression were used to explore heterogeneity, as a meta-analysis was not feasible. Potential sources of heterogeneity were instead examined qualitatively by comparing differences in study design, sample collection protocols, microRNA detection techniques and reporting formats across studies.

Table 2.
General characteristics of the included papers.
3.3. Quality Assessment
The articles underwent a qualitative assessment through QUADAS-2 tool analysis. Following the analysis, a significant number of articles raised concerns related to Patient Selection domain (Table 3). The most frequent issues encountered in the patient selection domain were the small sample size (usually less than 50 patients) [26,27,29,33,34,35,36,43,45,51,55,57], which could impact the statistical stability and the confidence intervals. Another issue observed was the demographic imbalance, as the OPMD and OSCC population were significantly older than the controls and predominantly male. The main concerns regarding the index test were the selective reporting (only miRNA with significant results were presented), the lack of blinding and post hoc derivation of diagnostic cut-offs. The reference standard and flow and timing domains did not, for the most part, induce a significant risk of bias.

Table 3.
Risk assessment of bias and clinical applicability.
3.4. Saliva Characteristics and Investigative Techniques
Saliva is considered to be one of the most valuable, non-invasive biofluids of the human body. Being loaded with immunoglobulins, enzymes, mucins, electrolytes and genetic material, saliva fulfills multiple roles starting from being a truly antiviral and antimicrobial agent and ending with being a protective assistant against erosion and demineralization of teeth [59]. Nowadays, more and more scientists focus on a more inconspicuous role of this biofluid, namely on the possibility of using it as a biomarker for non-invasive cancer detection and monitoring [60]. For instance, the presence of increased levels of IL-10 and IL-13 can be an indicative marker for existence of OSCC [61]. The same outcome can be visualized when comparing the expression of salivary miR-365 from an OSCC sample to a control one. Not only an elevated level of salivary miR-365 was found in exosomes from OSCC compared with normal oral cell lining, but it could also be correlated with disease stage [62,63]. Also, it is important to highlight the advantages of using saliva as a liquid biopsy: low-cost, rapid method of sample collection, non-invasive character, does not require special personnel and storage and is less sensitive than other sample types [64].
In scientific papers included in this systematic review, all the experiments were conducted using whole saliva (Table 4). Moreover, 21 studies specified the use of unstimulated saliva detrimental to stimulated saliva [26,28,29,30,32,34,35,37,41,43,44,45,48,49,50,51,52,53,54,55,58]. A possible explanation is that unstimulated saliva is often taken as the average representative of the entire ecosystem of the oral cavity, represented by all three major salivary glands, minor salivary glands as well as gingival crevicular fluid secretions, and that it does not require any previous preparation [65]. In contrast, two studies focused on salivary microRNA identification from oral swirl [38,39]. Requesting the patients to swirl sterile deionized water or PBS for 60 s allows a more uniformized sampling method and significantly reduces the time required for saliva sampling [66]. Moreover, one study used as a primary method for salivary microRNA sampling oral swabbing [42].

Table 4.
Saliva collection and microRNA extraction.
Information regarding accurate instructions provided to patients before saliva collection was extracted from included studies. More specifically, in 19 of the included articles, patients were informed to refrain from consuming food, drinking or performing any oral hygiene procedures for 30 min to 2 h prior to sampling [26,28,31,32,33,35,37,40,41,43,44,48,50,51,52,53,56,57,58]. In addition, in 6 studies patients were asked to abstain from tobacco use for times ranging from 30 min to 1 day before the saliva sampling [40,41,44,48,50,58]. In order to preserve the integrity of RNAs, RNA Protect Saliva Reagent products were used in 9 studies [27,32,37,41,48,50,53,54,57]. Regarding the storage of saliva, the most common temporary storage was at room temperature, the samples being processed in the shortest time [26,30,31,40,43,44,48,49], while the most frequent long-term storage was at −80 °C [26,27,30,31,32,33,34,35,37,43,44,45,46,47,48,51,52,53,54,56,57,58]. Another aspect worth mentioning is the quantity of saliva used for these experiments. Moataz M ELHefny and his team were able to obtain the expression level of salivary microRNAs in different types of samples using only 0,4 mL of saliva, proving that a limited amount of saliva samples contains satisfactory quantity of microRNAs for analysis [58]. In other experiments, an equally reduced volume of saliva was employed between 0.5 and 10 mL [44,48,50,52,53,54,57].
3.5. Salivary MicroRNAs Expression in OPMD vs. Healthy Controls
In the articles included in this systematic review, salivary miRNAs expression were found to be dysregulated in samples analyzed from OPMD patients compared to healthy controls. In more exact terms, salivary miR-21, miR-31, miR-93, miR-142-3p, miR-146a, miR-155, miR-181b, miR-184, miR-196b, miR-375, miR-412-3p and miR-4484 were found to be upregulated, while miR-27b, miR-125a, miR-137, miR-138, miR-320a, miR-424 and miR-3928 were downregulated in the saliva of patients diagnosed with premalignant lesions compared to healthy individuals.
Regarding the studies included in this systematic review, it has been noted that 5 articles discovered the expression level of salivary miR-21 to be significantly elevated in OPMD samples compared to healthy individuals. Uma Maheswari found that the level of miR-21 was 2.44 times increased in leukoplakia and 2.03 times in OLP in comparison to saliva taken from control subjects [40]. Concerning the sensitivity and specificity of the tests used to identify OPMD, regarding miR-21, Uma Maheswari’s study observed 66% sensitivity and 69% specificity, which may serve as a reference point for predicting the presence of OPMD (Table 5) [40]. Similar results were obtained by F. Zahran and his collaborators, who noticed that miR-21 was significantly upregulated in both OPMD with or without dysplasia compared to healthy controls [28]. The sensitivity and specificity obtained were more promising. MiR-21 was able to identify the OPMD with an accuracy of 90%, whereas the sensitivity was only 60%. A relatively low sensitivity could be attributed to population or technical variability related to saliva collection and normalization procedures. These results align with recent studies on this topic. For instance, Masoumeh Mehdipour identified that miR-21 levels were significantly increased in saliva samples derived from patients with OLP compared to those from healthy controls, being a genuine biomarker panel to recognize OLP lesions [37]. In addition, Shesha R. Prasad demonstrated that miR-21 could also distinguish a patient suffering from OSMF from healthy individuals [41]. On the contrary, the study presented by Dario Di Stasio did not demonstrate any variation in salivary miR-21 between the sample groups [49].

Table 5.
The accuracy of using microRNAs as a method of distinction between OPMD and OSCC vs healthy individuals.
Even though the results for salivary miR-31 were not statistically significant, an upregulation was observed, with a fold increase of 1.6 times and 1.2 times, respectively, for leukoplakia and OLP when compared to controls [40]. Better results were obtained by Masoumeh Mehdipour. He found that the salivary miR-31 levels were higher in dysplastic OLP patients in comparison to the healthy control group (p = 0.01) [37]. Furthermore, Saba Khan reported in his study that miR-31 had significantly higher median fold change of expression level, not only in OLP and leukoplakia as described before, but also in OSMF patients [46]. Regarding the detection of OLP, another two salivary microRNAs could serve as discriminating markers between OLP subjects and healthy ones. For instance, salivary miR-4484 was substantially upregulated (p-value = 0.03545) in OLP patients in a range of 2- to 98-fold out of 14 tested patient samples [29]. Equally, overexpression of salivary miR-142-3p could become an encouraging diagnostic biomarker for OLP, as it was detected overexpressed in the saliva and tissue samples collected from patients with OLP [47]. The salivary concentration of miR-181b was different among the dysplastic and non-dysplastic OPMD compared to controls. An upregulation of the above-mentioned microRNA was found in pathological samples, with 5.14-fold increase in dysplasia lesions and 6.29-fold increase for non-dysplastic lesions [49]. Moreover, salivary miR-184 was also up-regulated in OPMD with or without dysplasia when compared to healthy saliva samples [28].
As for downregulated salivary microRNAs, a considerable decrease in miR-34a expression was observed in leukoplakia patients compared to control [36]. Similar results were obtained by Minoo Shahidi: salivary miR-320a was strongly downregulated in OLP with dysplasia probes compared to healthy controls [32]. The same pattern was followed by salivary miR-27b. An under-expression by 4.52-fold of salivary miR-27b was reported in dysplastic OED compared to healthy saliva samples [49]. The same outcome was achieved by Sana Aghbari but in OLP. Salivary miR-27 expression exhibited a statistically significant downregulation in the OLP group, with the mean value of 5.343 (±4.875), while the control group reached 10.592 (±2.142) [34]. The same author discovered that besides miR-27, miR-137 was also under-expressed in patients with OLP [34]. Moreover, Masoumeh Mehdipour found another microRNA that was dysregulated in OLP. More specifically, miR-125a saliva levels were decreased in patients with OLP (p <0.001). This result is in accordance with the data obtained by research articles focusing on miR-125a levels in oral cancer [67,68]. Furthermore, salivary levels of miR-145 and miR-375 were significantly decreased in cases of OPMD with or without dysplasia [28,48].
With regard to the sensitivity and specificity of the tests used to identify OPMD, regarding miR-21, Uma Maheswari’s study observed 66% sensitivity and 69% specificity, which may be a base point for predicting local unrest representative to OSCC (Table 5) [40]. For miR-31, poorer accuracy was found, with only 36% sensitivity and 40% specificity [40]. More balanced results were obtained by Dario di Stasio, where the values of sensitivity and specificity of using miR-181b as a biomarker were 94.1% and 81.2%, respectively [49]. Another remarkable data finding was achieved by F. Zahran, where miR-21 was able to identify the OPMD with an accuracy of 90%, whereas the sensitivity was only 60% [28]. On the other hand, miR-145 showed a cutoff point of a 0.6 decrease rate, with 70% specificity and 60% sensitivity, while the cutoff point of miR-184 showed a 3-fold increase, with 75% specificity and 80% sensitivity [28]. Another microRNA with similar characteristics but with inferior precision of recognizing OPMD is miR-375. With 80% sensitivity and 68% specificity, miR-375 could potentially be exploited to detect insidious OPMD in susceptible populations (34). In terms of specific identification of OLP, samples collected from patients suffering from this condition could be diagnosed by using salivary miR-27b, miR-137, and miR-142-3p, with sensitivity ranging from 75% to 100%, and specificity from 80% to 100% [34,47].
These results are encouraging, because they can create the perfect premise for early detection of the disease before the development to OSCC. Even though the potential risk of progression of OPMD to OSCC is relatively reduced, as about 1.36% per year for leukoplakia [69] or 2.7% per year for erythroplakia [70], the risk continues to exist and can lead to insidious development of OSCC. The issue is all the more pressing as tardive detection of OSCC is associated with regional and distant metastasis which diminish the survival rate by 50% [71,72]. Therefore, salivary microRNAs could be used as novel, non-invasive biomarkers capable of detecting OPMD from any part of the oral cavity.
4. Discussions
4.1. Salivary MicroRNAs as Novel Biomarkers for Diagnosis of OPMD and OSCC
Premalignant lesions are in situ modifications of the normal tissue, with a variable tendency to develop into malignant tumors. These lesions can have multiple localizations across the living organism, with vast clinical and histopathological expression [73,74,75]. In the oral cavity, the most commonly described potentially malignant disorders are leukoplakia, erythroplakia, oral submucous fibrosis and oral lichen planus [76]. The most important aspect that endorses the possibility of using microRNAs as biomarkers is the fact that they exhibit the traits of the tissues from which they originate. This fascinating feature is advantageous in case of insidious onset of premalignant lesions. Even though, in most cases, oral potentially malignant disorders are easily diagnosed, there are several regions where these cannot be easily accessed or visualized, being masked by the anatomical elements of the oral cavity [77]. Early diagnosis followed by a proper treatment is crucial, since it may affect the prognosis of the patient [78]. Therefore, salivary microRNAs could be useful in this particular situation.
Several scientific articles addressed the potential role of using salivary microRNAs as innovative biomarkers for cancer detection and prognosis evaluations [79,80,81]. For instance, salivary miR-31 was significantly upregulated in OSCC patients compared to healthy controls, denoting that the usage of that microRNA could discriminate cancerous lesions from normal tissue. In addition, after tumor resection, both plasma and saliva levels of miR-31 were reduced, indicating that an oral tumor was the main origin for altered microRNA production [82]. Another aspect worth mentioning is that salivary microRNAs can be used as a discriminatory tool for assessing the possibility of performing surgical intervention in pancreatic cancer. Hsa-miR-21 could distinguish the patients with unresectable pancreatic cancer from those with pancreatitis, intraductal papillary mucinous neoplasm or cancer-free patients [80]. Moreover, salivary microRNAs such as exosomal miRNA-1307-5p could be used as an indicator for predicting poor survival and impaired patient outcome in oral cancers and can contribute in clinical staging of the salivary gland neoplasms [51,79].
4.2. Salivary MicroRNAs Pathogenesis in Oral Premalignant Lesions Causing Malignant Transformation of OPMD
Histopathological evaluation remains the gold standard for assessing oral potentially malignant lesions. However, the current classification of OPMD with distinct grades of dysplasia cannot precisely predict which of the lesions will suffer a malignant transformation [11]. Thus, salivary microRNAs could be used as an identification method of a subtle turnaround of premalignant lesions towards OSCC.
One of the most suitable examples that depicts the transition between premalignant and malignant states is the expression level of salivary miR-21, quantified in various grades of OPMD dysplasia. Even though in Uma Maeheswari’s study, no direct, linear correlation between OPMD progression from mild or moderate to severe dysplasia could be established, considerable overexpression of salivary miR-21 was observed in OPMD with severe dysplasia [40]. Dysplastic characteristics of oral squamous epithelium gained by OPMD progression are characterized by cellular atypia and abnormal architecture of the epithelium [83]. Clinical changes are also reflected at the molecular and genomic levels, where modification in keratinocytes proliferation and differentiation is driven by genetic alterations by gene alteration and cell cycle perturbance [84]. Another scientific paper included in this systematic review found the same results. A gradual increase in salivary miR-21 expression was observed from healthy mucosa to OPMD without dysplasia, OPMD with dysplasia and OSCC. However, it is worth noting that the values of salivary miR-21 were significantly increased in OPMD with dysplasia and OSCC compared to other groups, delimiting inflammatory lesions from non-dysplastic ones [28]. Dysregulation of miR-21 is recognized as a notable stage in inflammatory response alteration in many types of cancer [85,86,87]. MiR-21 upregulation in colon cancer can be taken as an example. A positive correlation between miR-21 and Interleukin-6 (IL-6) expression was discovered in colon cancer; miR-21 levels could be enhanced by oncogenic signaling IL-6, leading to NF-kB activation, which maintain an inflammatory and a pro-carcinogenic state [88].
Salivary expression level of miR-320a is another example of a gradual transition from OLP to OSCC. A decreased salivary level of miR-320a was found in dysplastic OLP and OSCC probes compared to non-dysplastic OLP and healthy individuals. MiR-320a could potentiate the oral lichen planus progression towards OSCC by modulating the expression of VEGFR-2 [32]. Described as an angiogenic biomarker, VEGFR-2 not only promotes endothelial proliferation, migration and differentiation, but it also increases the proliferative capacities of cancerous cells [89,90]. Therefore, an increase expression of VEGFR-2 creates a favorable environment for tumor development. Moreover, the expression of miR-320a could be strongly associated with elevated levels of salivary IL-6 and salivary C-reactive protein (CRP). Therefore, the more oncogenic changes that occur in oral tissue, the lower levels of salivary miR-320a, and the higher levels of salivary IL-6 and CRP, will be discovered [32]. IL-6 is a component of the tumor microenvironment (TME), which represents all the non-cancerous host cells in the tumor, such as fibroblasts, endothelial cells and immune cells, along with non-cellular components, including extracellular matrix (ECM), cytokines and growth factors [91]. It is important to mention that multiple cell types in TME do not exist in isolation; instead, they form a complex interaction that contributes to tumor initiation and progression of cancer. It was discovered that in chronic inflammation, normal fibroblasts start a conversion process to cancer-associated fibroblasts (CAF) [92]. Having a new secretory profile, CAF produce elevated levels of IL-6 and other pro-tumorigenic cytokines and interact with tumor cells, which in turn secrete high levels of IL-6. These actions result in STAT3 and MEK/ERK signaling pathways, which stimulate tumor cell proliferation, invasion, growth and decrease apoptosis [93]. Taking into consideration that high levels CRP were found to be strongly associated with cancer severity, salivary IL-6 and CRP, along with miR-320a, could be used as indicators for healthy mucosa transition to OLP and, further, to OSCC [94]. Hence, salivary microRNAs are responsible for the microenvironment modification, which creates the premises of cancer onset and development.
Salivary miR-34a, one of the most studied microRNAs involved in the pathogenesis of malignant tumors, was found to be dysregulated in oral leukoplakia. Therefore, downregulation results in elevated expression of CD44v6 and reduced SYNE1, both in patients with leukoplakia and OSCC [36]. CD44v6 is an isoform of CD44, a transmembrane glycoprotein, that possesses the ability to bind hyaluronan, extracellular matrix proteins and growth factors [95]. Bearing in mind that this molecule increases the accumulation of nuclear β-catenin, and thus, enhances cell proliferation and EMT in cancer, it is possible for CD44v6 to be a subtle element of transition between leukoplakia and OSCC [96]. Another microRNA involved in EMT regulation is miR-203, a significantly upregulated microRNA in oral submucous fibrosis compared to normal oral mucosa, which can become a decisive factor for malignant transformation of oral submucous fibrosis by targeting SFRP4 and TM4SF1 [97]. Moreover, in OLP, upregulated miR-155, targets the miR-155/SOCS1 axis, a pathway with high involvement in immune system and in macrophage differentiation, functioning overall as a tumor-promoting factor [98,99].
MiR-181b, overexpressed in patients with OPMD compared to healthy subjects, follows the trend established by the other microRNAs, increasing its salivary level with the increasing degree of dysplasia, supporting its potential as a promising biomarker for tumor risk stratification [49]. On the other hand, miR-181b decreases considerably in OSCC. The difference could be interpreted as a total loss of tissue architecture that occurs in OSCC, which creates the basis for a negative feedback mechanism. In addition, Zhi-Yuan Deng and his team discovered that the expression level of miR-181b was significantly lower in tumor-adjacent tissue compared to oral verrucous carcinoma and normal mucosa. These results suggest that miR-181b expression could be associated with the degree of malignancy, showing that oral verrucous carcinoma is less predisposed to invasion and metastasis than OSCC [100]. These apparently contradictory results, with miR-181b expression increasing with the degree of dysplasia and downregulated in OSCC and oral verrucous carcinoma, from the past two studies presented could also be explained by the distinct study designs (in vivo or ex vivo) and contrasting sampling targets (saliva vs. tissue) [49].
Being a noninfectious, chronic inflammatory disorder, OLP is described as having aberrant miRNA expression levels [101,102]. For instance, the expression of miR-137 was markedly downregulated in the oral mucosa of OLP patients and showed an inverse correlation with CD8 tissue expression, indicating immunomodulatory functions of miR-137 [103]. In a study conducted by Hsi-Feng Tu, luciferase reporter assay results indicate that salivary miR-142-3p could target glucocorticoid receptor α (GRα) gene [48]. GRα is a member of the steroid receptor family, which regulates the action of glucocorticoids [104]. As the predominant isoform, GRα is involved in a wide range of pathological processes, including inflammation, immune response and cellular differentiation [105]. The GRα downregulation produced by miR-142-3p action is a pertinent marker for the disturbance of anti-inflammatory function of the cells, which may support the malignancy of OLP [106].
In another study related to this topic, 45 patients with leukoplakia were treated with 13-cis-retinoic acid and were monitored for approximately 3 years. During this period, 10 patients developed carcinoma in situ or OSCC with an average time of 30.1 months and the other 7 remained at the leukoplakia stage. The other patients were excluded for various reasons [107]. Remarkably is that 25 microRNAs were differentially expressed between progressive and non-progressive leukoplakias.
The included evidence has several limitations, most notably small sample sizes, substantial heterogeneity in saliva collection and microRNA detection methods, and inconsistent or incomplete reporting of quantitative data. Many studies selectively reported only significant microRNAs and lacked precision measures such as confidence intervals, increasing the potential for reporting bias. Demographic imbalances between cases and controls further limit comparability. These issues reduce confidence in the overall evidence base.
5. Conclusions
In summary, the discovery of a tailored treatment for patients with oral premalignant changes, according to their molecular profile, is crucial. The pressing challenge is to determine which OPMD will progress to OSCC and which will not. Salivary microRNAs could represent a solid answer to this matter. Expression changes in microRNAs are increasingly recognized as relevant to distinguish the malignant lesions from the premalignant ones without transformation risk. Having the ability to reflect molecular changes in OPMD, even in non-invasive, inexpensive and easily accessible biofluids such as saliva, microRNAs represent a crucial standpoint in screening and detecting OPMD. In this systematic review, several microRNAs such as miR-21, miR-31 and miR-184 could represent promising biomarkers for salivary diagnosis of OPMD malignancy.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14228128/s1, PRISMA checklist.
Author Contributions
C.O. was responsible for writing the manuscript; I.B.-N. and S.B. provided the working plan and were responsible for the supervision and critical revision of the manuscript; G.A., F.O. and G.B. assessed the manuscript from a medical point of view; C.D. and W.K. offered an objective view of the manuscript and provided English proofreading; M.B. supported the work and made all the critical revision of the work and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Iuliu Hatieganu” University of Medicine and Pharmacy Cluj-Napoca, Romania, Department of Maxillofacial Surgery and Implantology, Doctoral Research Program, through project PCD 777/32/13.01.2025.
Data Availability Statement
Not applicable.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no competing interests or conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OPMD | Oral potentially malignant diseases |
| QUADAS-2 | Diagnostic Accuracy Research Tool |
| OSCC | Oral squamous cell carcinoma |
| OSMF | Oral submucous fibrosis |
| OLP | Oral lichen planus |
| OLL | Oral lichenoid lesion |
| OED | Oral epithelial dysplasia |
| Interleukin-6 | Interleukin-6 |
| CRP | C-reactive protein |
| TME | Tumor microenvironment |
| ECM | Extracellular matrix |
| CAF | Cancer-associated fibroblasts |
| GRα | Glucocorticoid receptor α |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Haefner, M.F.; Lang, K.; Krug, D.; Koerber, S.A.; Uhlmann, L.; Kieser, M.; Debus, J.; Sterzing, F. Prognostic factors, patterns of recurrence and toxicity for patients with esophageal cancer undergoing definitive radiotherapy or chemo-radiotherapy. J. Radiat. Res. 2015, 56, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; Gonzalez-Moles, M.A.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Rangel, R.; Pickering, C.R.; Sikora, A.G.; Spiotto, M.T. Genetic Changes Driving Immunosuppressive Microenvironments in Oral Premalignancy. Front. Immunol. 2022, 13, 840923. [Google Scholar] [CrossRef]
- Sathasivam, H.P.; Casement, J.; Bates, T.; Sloan, P.; Thomson, P.; Robinson, M.; Kist, R. Gene expression changes associated with malignant transformation of oral potentially malignant disorders. J. Oral Pathol. Med. 2021, 50, 60–67. [Google Scholar] [CrossRef]
- Deng, S.; Wang, S.; Shi, X.; Zhou, H. Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 8940. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Udaltsova, N.; Engels, E.A.; Katzel, J.A.; Yanik, E.L.; Katki, H.A.; Lingen, M.W.; Silverberg, M.J. Oral Leukoplakia and Risk of Progression to Oral Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2020, 112, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Datta, S.; Ray, J.G.; Chaudhuri, K.; Chatterjee, R. Liquid biopsy: miRNA as a potential biomarker in oral cancer. Cancer Epidemiol. 2019, 58, 137–145. [Google Scholar] [CrossRef]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Dis. Mon. 2020, 66, 100988. [Google Scholar] [CrossRef]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef]
- Osan, C.; Chira, S.; Nutu, A.M.; Braicu, C.; Baciut, M.; Korban, S.S.; Berindan-Neagoe, I. The Connection between MicroRNAs and Oral Cancer Pathogenesis: Emerging Biomarkers in Oral Cancer Management. Genes 2021, 12, 1989. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiang, Y.; Singh, A.P.; Takeshita, F. MicroRNAs: Emerging novel targets of cancer therapies. BioMed Res. Int. 2015, 2015, 506323. [Google Scholar] [CrossRef]
- Elewaily, M.I.; Elsergany, A.R. Emerging role of exosomes and exosomal microRNA in cancer: Pathophysiology and clinical potential. J. Cancer Res. Clin. Oncol. 2021, 147, 637–648. [Google Scholar] [CrossRef]
- Tastan, B.; Tarakcioglu, E.; Birinci, Y.; Park, Y.; Genc, S. Role of Exosomal MicroRNAs in Cell-to-Cell Communication. Methods Mol. Biol. 2022, 2257, 269–292. [Google Scholar]
- Mariner, P.D.; Korst, A.; Karimpour-Fard, A.; Stauffer, B.L.; Miyamoto, S.D.; Sucharov, C.C. Improved Detection of Circulating miRNAs in Serum and Plasma Following Rapid Heat/Freeze Cycling. Microrna 2018, 7, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, S.; Liu, X. MicroRNA profiling of plasma exosomes from patients with ovarian cancer using high-throughput sequencing. Oncol. Lett. 2019, 17, 5601–5607. [Google Scholar] [CrossRef]
- Hagiwara, K.; Katsuda, T.; Gailhouste, L.; Kosaka, N.; Ochiya, T. Commitment of Annexin A2 in recruitment of microRNAs into extracellular vesicles. FEBS Lett. 2015, 589, 4071–4078. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef]
- Axmann, M.; Meier, S.M.; Karner, A.; Strobl, W.; Stangl, H.; Plochberger, B. Serum and Lipoprotein Particle miRNA Profile in Uremia Patients. Genes 2018, 9, 533. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Roles of circulating microRNA(s) in human breast cancer. Arch. Biochem. Biophys. 2020, 695, 108583. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; Group, Q. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Trachtenberg, A.J.; Kuo, W.P.; Cheng, Y.S. Genomewide Study of Salivary MicroRNAs for Detection of Oral Cancer. J. Dent. Res. 2014, 93 (Suppl 7), 86S–93S. [Google Scholar] [CrossRef]
- Lundegard, M.; Nylander, K.; Danielsson, K. Difficulties detecting miRNA-203 in human whole saliva by the use of PCR. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e130–e134. [Google Scholar] [CrossRef] [PubMed]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary microRNAs in oral cancer. Oral Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Byun, J.S.; Hong, S.H.; Choi, J.K.; Jung, J.K.; Lee, H.J. Diagnostic profiling of salivary exosomal microRNAs in oral lichen planus patients. Oral Dis. 2015, 21, 987–993. [Google Scholar] [CrossRef]
- Hung, K.F.; Liu, C.J.; Chiu, P.C.; Lin, J.S.; Chang, K.W.; Shih, W.Y.; Kao, S.Y.; Tu, H.F. MicroRNA-31 upregulation predicts increased risk of progression of oral potentially malignant disorder. Oral Oncol. 2016, 53, 42–47. [Google Scholar] [CrossRef]
- Duz, M.B.; Karatas, O.F.; Guzel, E.; Turgut, N.F.; Yilmaz, M.; Creighton, C.J.; Ozen, M. Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: A pilot study. Cell. Oncol. 2016, 39, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M.; Jafari, S.; Barati, M.; Mahdipour, M.; Gholami, M.S. Predictive value of salivary microRNA-320a, vascular endothelial growth factor receptor 2, CRP and IL-6 in Oral lichen planus progression. Inflammopharmacology 2017, 25, 577–583. [Google Scholar] [CrossRef]
- Maheswari, T.N.U.; Nivedhitha, M.S. Study to explore the significance of saliva as a diagnostic tool to detect microRNA in oral potentially malignant disorders. J. Adv. Pharm. Educ. Res. 2017, 7, 278–282. [Google Scholar]
- Aghbari, S.M.H.; Gaafar, S.M.; Shaker, O.G.; Ashiry, S.E.; Zayed, S.O. Evaluating the accuracy of microRNA27b and microRNA137 as biomarkers of activity and potential malignant transformation in oral lichen planus patients. Arch. Dermatol. Res. 2018, 310, 209–220. [Google Scholar] [CrossRef]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18, 439. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Patel, S.; Modi, B.; Shah, F.; Rawal, R. Uncovering the potential of CD44v/SYNE1/miR34a axis in salivary fluids of oral cancer patients. J. Oral Pathol. Med. 2018, 47, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, M.; Shahidi, M.; Manifar, S.; Jafari, S.; Mashhadi Abbas, F.; Barati, M.; Mortazavi, H.; Shirkhoda, M.; Farzanegan, A.; Elmi Rankohi, Z. Diagnostic and prognostic relevance of salivary microRNA-21, -125a, -31 and -200a levels in patients with oral lichen planus—A short report. Cell. Oncol. 2018, 41, 329–334. [Google Scholar] [CrossRef]
- Yap, T.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; Seers, C.; McCullough, M. Predicting the Presence of Oral Squamous Cell Carcinoma Using Commonly Dysregulated MicroRNA in Oral Swirls. Cancer Prev. Res. 2018, 11, 491–502. [Google Scholar] [CrossRef]
- Yap, T.; Seers, C.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; McCullough, M. Non-invasive screening of a microRNA-based dysregulation signature in oral cancer and oral potentially malignant disorders. Oral Oncol. 2019, 96, 113–120. [Google Scholar] [CrossRef]
- Uma Maheswari, T.N.; Nivedhitha, M.S.; Ramani, P. Expression profile of salivary micro RNA-21 and 31 in oral potentially malignant disorders. Braz. Oral Res. 2020, 34, e002. [Google Scholar] [CrossRef]
- Prasad, S.R.; Pai, A.; Shyamala, K.; Yaji, A. Expression of Salivary miRNA 21 in Oral Submucous Fibrosis (OSMF): An Observational Study. Microrna 2020, 9, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; You, G.R.; Lee, C.J.; Lu, Y.C.; Tang, S.J.; Huang, Y.F.; Huang, Y.C.; Lee, L.Y.; Fan, K.H.; Chen, Y.C.; et al. Systemic Investigation Identifying Salivary miR-196b as a Promising Biomarker for Early Detection of Head-Neck Cancer and Oral Precancer Lesions. Diagnostics 2021, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Romani, C.; Salviato, E.; Paderno, A.; Zanotti, L.; Ravaggi, A.; Deganello, A.; Berretti, G.; Gualtieri, T.; Marchini, S.; D’Incalci, M.; et al. Genome-wide study of salivary miRNAs identifies miR-423-5p as promising diagnostic and prognostic biomarker in oral squamous cell carcinoma. Theranostics 2021, 11, 2987–2999. [Google Scholar] [CrossRef]
- Mehterov, N.; Vladimirov, B.; Sacconi, A.; Pulito, C.; Rucinski, M.; Blandino, G.; Sarafian, V. Salivary miR-30c-5p as Potential Biomarker for Detection of Oral Squamous Cell Carcinoma. Biomedicines 2021, 9, 1079. [Google Scholar] [CrossRef]
- Kumari, P.; Syed, S.A.; Wahid, M.; Qureshi, M.A.; Kumar, R. Expression of miR-31 in saliva-liquid biopsy in patients with oral squamous cell carcinoma. J. Taibah Univ. Med. Sci. 2021, 16, 733–739. [Google Scholar] [CrossRef]
- Khan, S.; Butt, S.A.; Hassan, S.; Khan, R. Expression of Salivary miRNA-31 in Oral Submucous fibrosis. J. Adv. Med. Med. Res. 2021, 33, 137–144. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, H.; Li, L.; Wang, K. Clinical significance of miR-142-3p in oral lichen planus and its regulatory role in keratinocyte proliferation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 441–447. [Google Scholar] [CrossRef]
- Tu, H.F.; Lin, L.H.; Chang, K.W.; Cheng, H.W.; Liu, C.J. Exploiting salivary miR-375 as a clinical biomarker of oral potentially malignant disorder. J. Dent. Sci. 2022, 17, 659–665. [Google Scholar] [CrossRef]
- Di Stasio, D.; Romano, A.; Boschetti, C.E.; Montella, M.; Mosca, L.; Lucchese, A. Salivary miRNAs Expression in Potentially Malignant Disorders of the Oral Mucosa and Oral Squamous Cell Carcinoma: A Pilot Study on miR-21, miR-27b, and miR-181b. Cancers 2022, 15, 291. [Google Scholar] [CrossRef]
- Scholtz, B.; Horvath, J.; Tar, I.; Kiss, C.; Marton, I.J. Salivary miR-31-5p, miR-345-3p, and miR-424-3p Are Reliable Biomarkers in Patients with Oral Squamous Cell Carcinoma. Pathogens 2022, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, S.; Patel, P.; Mandlik, D.; Patel, K.; Tanavde, V. Salivary Exosomal miRNA-1307-5p Predicts Disease Aggressiveness and Poor Prognosis in Oral Squamous Cell Carcinoma Patients. Int. J. Mol. Sci. 2022, 23, 10639. [Google Scholar] [CrossRef]
- Faur, C.I.; Roman, R.C.; Jurj, A.; Raduly, L.; Almasan, O.; Rotaru, H.; Chirila, M.; Moldovan, M.A.; Hedesiu, M.; Dinu, C. Salivary Exosomal MicroRNA-486-5p and MicroRNA-10b-5p in Oral and Oropharyngeal Squamous Cell Carcinoma. Medicina 2022, 58, 1478. [Google Scholar] [CrossRef]
- Mehdipour, M.; Shahidi, M.; Anbari, F.; Mirzaei, H.; Jafari, S.; Kholghi, A.; Lotfi, E.; Manifar, S.; Mashhadiabbas, F. Salivary level of microRNA-146a and microRNA-155 biomarkers in patients with oral lichen planus versus oral squamous cell carcinoma. BMC Oral Health 2023, 23, 433. [Google Scholar] [CrossRef]
- Garg, A.; Urs, A.B.; Koner, B.C.; Augustine, J.; Guru, S.A. Evaluation of Diagnostic Significance of Salivary miRNA-184 and miRNA-21 in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. Head Neck Pathol. 2023, 17, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Tarrad, N.A.F.; Hassan, S.; Shaker, O.G.; AbdelKawy, M. Salivary LINC00657 and miRNA-106a as diagnostic biomarkers for oral squamous cell carcinoma, an observational diagnostic study. BMC Oral Health 2023, 23, 994. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, A.; Mohajertehran, F.; Aghaee-Bakhtiari, S.H.; Ayatollahi, H.; Douzandeh, K.; Pakfetrat, A.; Mohtasham, N. Downregulation of salivary miR-3928 as a potential biomarker in patients with oral squamous cell carcinoma and oral lichen planus. Clin. Exp. Dent. Res. 2024, 10, e877. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, F.; Tenore, G.; Macali, F.; Vicidomini, T.; Podda, G.M.; Fantozzi, P.J.; Silvestri, V.; Porzio, V.; Valentini, V.; Ottini, L.; et al. Expression Analysis of Circulating microRNAs in Saliva and Plasma for the Identification of Clinically Relevant Biomarkers for Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. Cancers 2024, 16, 2990. [Google Scholar] [CrossRef]
- MM, E.L.; Korien, I.A.; Rashwan, W.A.; Shaker, O.G. The oncogenic potential of salivary microRNA-93 and microRNA-412-3p in oral lichen planus: A case-control study. BDJ Open 2024, 10, 98. [Google Scholar]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Chiamulera, M.M.A.; Zancan, C.B.; Remor, A.P.; Cordeiro, M.F.; Gleber-Netto, F.O.; Baptistella, A.R. Salivary cytokines as biomarkers of oral cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 205. [Google Scholar] [CrossRef]
- Aziz, S.; Ahmed, S.S.; Ali, A.; Khan, F.A.; Zulfiqar, G.; Iqbal, J.; Khan, A.A.; Shoaib, M. Salivary Immunosuppressive Cytokines IL-10 and IL-13 Are Significantly Elevated in Oral Squamous Cell Carcinoma Patients. Cancer Invest 2015, 33, 318–328. [Google Scholar] [CrossRef]
- Coon, J.; Kingsley, K.; Howard, K.M. miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 5317. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Costela-Ruiz, V.J.; Garcia-Recio, E.; Olmedo-Gaya, M.V.; Ruiz, C.; Reyes-Botella, C. Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 12215. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Gomar-Vercher, S.; Simon-Soro, A.; Montiel-Company, J.M.; Almerich-Silla, J.M.; Mira, A. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLoS ONE 2018, 13, e0198021. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.; Vella, L.J.; Seers, C.; Nastri, A.; Reynolds, E.; Cirillo, N.; McCullough, M. Oral swirl samples—A robust source of microRNA protected by extracellular vesicles. Oral Dis. 2017, 23, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, Characterization, and Clinical Utility for Oral Cancer Detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wei, Y.Y.; Yang, C.C.; Liu, C.J.; Yeh, L.Y.; Chou, C.H.; Chang, K.W.; Lin, S.C. miR-125b suppresses oral oncogenicity by targeting the anti-oxidative gene PRXL2A. Redox Biol. 2019, 22, 101140. [Google Scholar] [CrossRef]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardinas Lopez, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Bugshan, A.; Farooq, I. Oral squamous cell carcinoma: Metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. F1000Research 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kim, J.W.; Paeng, J.Y. Clinical analysis of neck node metastasis in oral cavity cancer. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 282–288. [Google Scholar] [CrossRef]
- Mallinger, W.D.; Quick, C.M. Benign and Premalignant Lesions of the Endometrium. Surg. Pathol. Clin. 2019, 12, 315–328. [Google Scholar] [CrossRef]
- Denisov, E.V.; Schegoleva, A.A.; Gervas, P.A.; Ponomaryova, A.A.; Tashireva, L.A.; Boyarko, V.V.; Bukreeva, E.B.; Pankova, O.V.; Perelmuter, V.M. Premalignant lesions of squamous cell carcinoma of the lung: The molecular make-up and factors affecting their progression. Lung Cancer 2019, 135, 21–28. [Google Scholar] [CrossRef]
- Ali, M.; Gupta, G.; Silu, M.; Chand, D.; Samor, V. Narrow band imaging in early diagnosis of laryngopharyngeal malignant and premalignant lesions. Auris Nasus Larynx 2022, 49, 676–679. [Google Scholar] [CrossRef]
- Woo, S.B. Oral Epithelial Dysplasia and Premalignancy. Head Neck Pathol. 2019, 13, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, D.; Huber, M.A.; Kerr, A.R. Oral Potentially Malignant Disorders and Oral Cavity Cancer. Dermatol. Clin. 2020, 38, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Matse, J.H.; Yoshizawa, J.; Wang, X.; Elashoff, D.; Bolscher, J.G.; Veerman, E.C.; Leemans, C.R.; Pegtel, D.M.; Wong, D.T.; Bloemena, E. Human Salivary Micro-RNA in Patients with Parotid Salivary Gland Neoplasms. PLoS ONE 2015, 10, e0142264, Correction in PLoS ONE 2016, 11, e0146701. [Google Scholar] [CrossRef]
- Humeau, M.; Vignolle-Vidoni, A.; Sicard, F.; Martins, F.; Bournet, B.; Buscail, L.; Torrisani, J.; Cordelier, P. Salivary MicroRNA in Pancreatic Cancer Patients. PLoS ONE 2015, 10, e0130996. [Google Scholar] [CrossRef]
- Machida, T.; Tomofuji, T.; Maruyama, T.; Yoneda, T.; Ekuni, D.; Azuma, T.; Miyai, H.; Mizuno, H.; Kato, H.; Tsutsumi, K.; et al. miR-1246 and miR-4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol. Rep. 2016, 36, 2375–2381. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, S.C.; Yang, C.C.; Cheng, H.W.; Chang, K.W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck 2012, 34, 219–224. [Google Scholar] [CrossRef]
- Brito, J.A.; Gomes, C.C.; Guimaraes, A.L.; Campos, K.; Gomez, R.S. Relationship between microRNA expression levels and histopathological features of dysplasia in oral leukoplakia. J. Oral Pathol. Med. 2014, 43, 211–216. [Google Scholar] [CrossRef]
- Yap, T.; Celentano, A.; Seers, C.; McCullough, M.J.; Farah, C.S. Molecular diagnostics in oral cancer and oral potentially malignant disorders—A clinician’s guide. J. Oral Pathol. Med. 2020, 49, 1–8. [Google Scholar] [CrossRef]
- Sheedy, F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Peacock, O.; Lee, A.C.; Cameron, F.; Tarbox, R.; Vafadar-Isfahani, N.; Tufarelli, C.; Lund, J.N. Inflammation and MiR-21 pathways functionally interact to downregulate PDCD4 in colorectal cancer. PLoS ONE 2014, 9, e110267. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Jaeger, S.A.; Hirsch, H.A.; Bulyk, M.L.; Struhl, K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol. Cell 2010, 39, 493–506. [Google Scholar] [CrossRef]
- Okayama, H.; Schetter, A.J.; Harris, C.C. MicroRNAs and inflammation in the pathogenesis and progression of colon cancer. Dig. Dis. 2012, 30 (Suppl. 2), 9–15. [Google Scholar] [CrossRef]
- Spannuth, W.A.; Nick, A.M.; Jennings, N.B.; Armaiz-Pena, G.N.; Mangala, L.S.; Danes, C.G.; Lin, Y.G.; Merritt, W.M.; Thaker, P.H.; Kamat, A.A.; et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int. J. Cancer 2009, 124, 1045–1053. [Google Scholar] [CrossRef]
- Silva, S.R.; Bowen, K.A.; Rychahou, P.G.; Jackson, L.N.; Weiss, H.L.; Lee, E.Y.; Townsend, C.M., Jr.; Evers, B.M. VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int. J. Cancer 2011, 128, 1045–1056. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Rezaei, F.; Mohammadi, H.; Heydari, M.; Sadeghi, M.; Mozaffari, H.R.; Khavid, A.; Godiny, M.; Brand, S.; M. Dürsteler, K.; Beatrix Brühl, A.; et al. Association between IL-8 (-251T/A) and IL-6 (-174G/C) Polymorphisms and Oral Cancer Susceptibility: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 405. [Google Scholar] [CrossRef]
- Karakasheva, T.A.; Lin, E.W.; Tang, Q.; Qiao, E.; Waldron, T.J.; Soni, M.; Klein-Szanto, A.J.; Sahu, V.; Basu, D.; Ohashi, S.; et al. IL-6 Mediates Cross-Talk. between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res. 2018, 78, 4957–4970. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Xing, R.D.; Zhang, F.M.; Duan, Y.Q. Serum soluble CD44v6 levels in patients with oral and maxillofacial malignancy. Oral Dis. 2009, 15, 570–572. [Google Scholar] [CrossRef]
- Ma, L.; Dong, L.; Chang, P. CD44v6 engages in colorectal cancer progression. Cell Death Dis. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jian, X.; Guo, F.; Li, N.; Jiang, C.; Yin, P.; Min, A.J.; Huang, L. miR-203 inhibits arecoline-induced epithelial-mesenchymal transition by regulating secreted frizzled-related protein 4 and transmembrane-4 L six family member 1 in oral submucous fibrosis. Oncol. Rep. 2015, 33, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ai, R.; Hao, Y.; Jiang, L.; Dan, H.; Ji, N.; Zeng, X.; Zhou, Y.; Chen, Q. Role of miR-155 in immune regulation and its relevance in oral lichen planus. Exp. Ther. Med. 2019, 17, 575–586. [Google Scholar] [CrossRef]
- Farshbaf, A.; Mohajertehran, F.; Sahebkar, A.; Garmei, Y.; Sabbagh, P.; Mohtasham, N. The role of altered microRNA expression in premalignant and malignant head and neck lesions with epithelial origin. Health Sci. Rep. 2022, 5, e921. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Wang, Y.H.; Quan, H.Z.; Liu, O.S.; Li, Y.P.; Li, Y.; Zhu, W.; Munnee, K.; Tang, Z.G. Investigation of the association between miR-181b, Bcl-2 and LRIG1 in oral verrucous carcinoma. Mol. Med. Rep. 2016, 14, 2991–2996. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H. miR-451 elevation relieves inflammatory pain by suppressing microglial activation-evoked inflammatory response via targeting TLR4. Cell Tissue Res. 2018, 374, 487–495. [Google Scholar] [CrossRef]
- Ge, X.; Xie, H.; Wang, L.; Li, R.; Zhang, F.; Xu, J.; Zhao, B.; Du, J. MicroRNA-122 promotes apoptosis of keratinocytes in oral lichen planus through suppressing VDR expression. J. Cell. Mol. Med. 2021, 25, 3400–3407. [Google Scholar] [CrossRef]
- Aghbari, S.M.H.; Abushouk, A.I.; Shakir, O.G.; Zayed, S.O.; Attia, A. Correlation between tissue expression of microRNA-137 and CD8 in oral lichen planus. Clin. Oral Investig. 2018, 22, 1463–1467. [Google Scholar] [CrossRef]
- Yakirevich, E.; Matoso, A.; Sabo, E.; Wang, L.J.; Tavares, R.; Meitner, P.; Morris, D.J.; Pareek, G.; Delellis, R.A.; Resnick, M.B. Expression of the glucocorticoid receptor in renal cell neoplasms: An immunohistochemical and quantitative reverse transcriptase polymerase chain reaction study. Hum. Pathol. 2011, 42, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Meijer, O.C.; Koorneef, L.L.; Kroon, J. Glucocorticoid receptor modulators. Ann. Endocrinol. 2018, 79, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.C.; Sun, H.Y.; Zhen, Y.X.; Zhang, H.; Shi, H.; Wang, X.X. Low expression of glucocorticoid receptor a in oral lichen planus correlates with activation of nuclear factor kappaB: A preliminary study. J. Oral Pathol. Med. 2014, 43, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.X.; Yang, X.; Jiang, L.; Zhou, Z.J.; Zhu, Y.Q. Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer 2013, 13, 129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).