Diagnostic Utility of Monocyte Distribution Width for Early Sepsis Detection in Cancer-Enriched Emergency Cohort
Abstract
1. Introduction
2. Materials and Methods
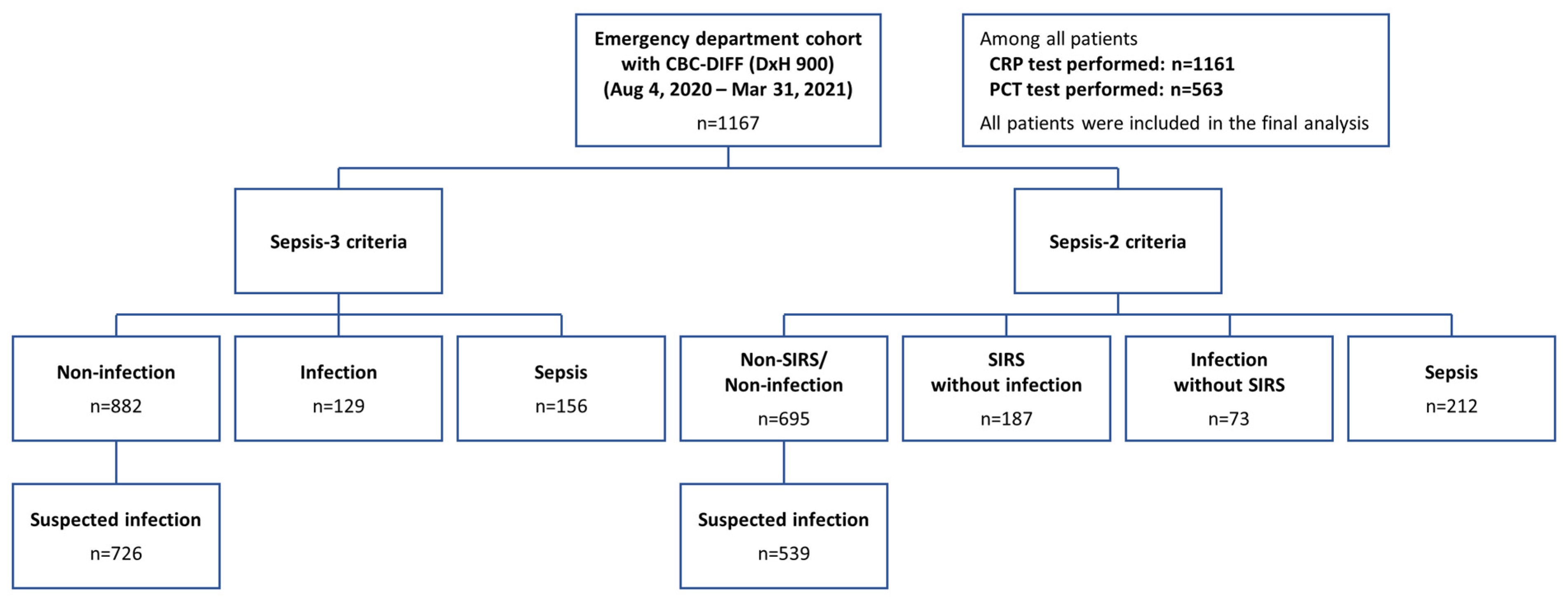
2.1. Study Population
2.2. Clinical Classification
2.3. Laboratory Measurement
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
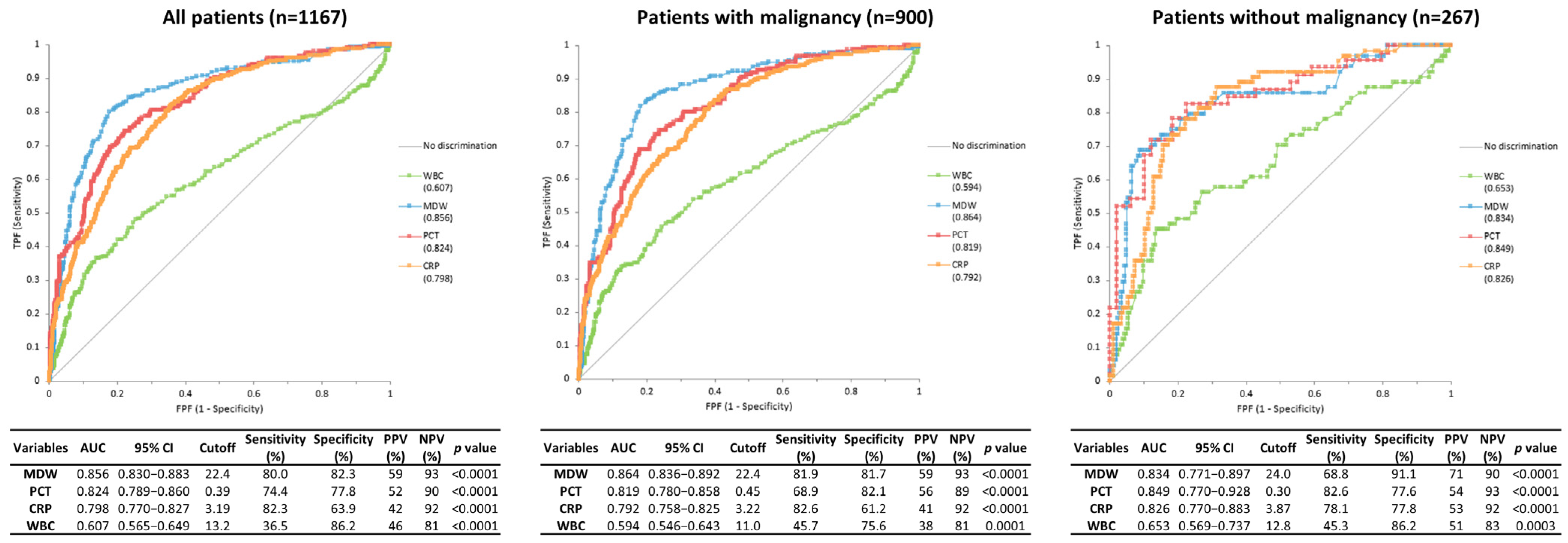
3.2. Diagnostic Performance of Biomarkers for Identification of Sepsis According to the Presence or Absence of Malignancy
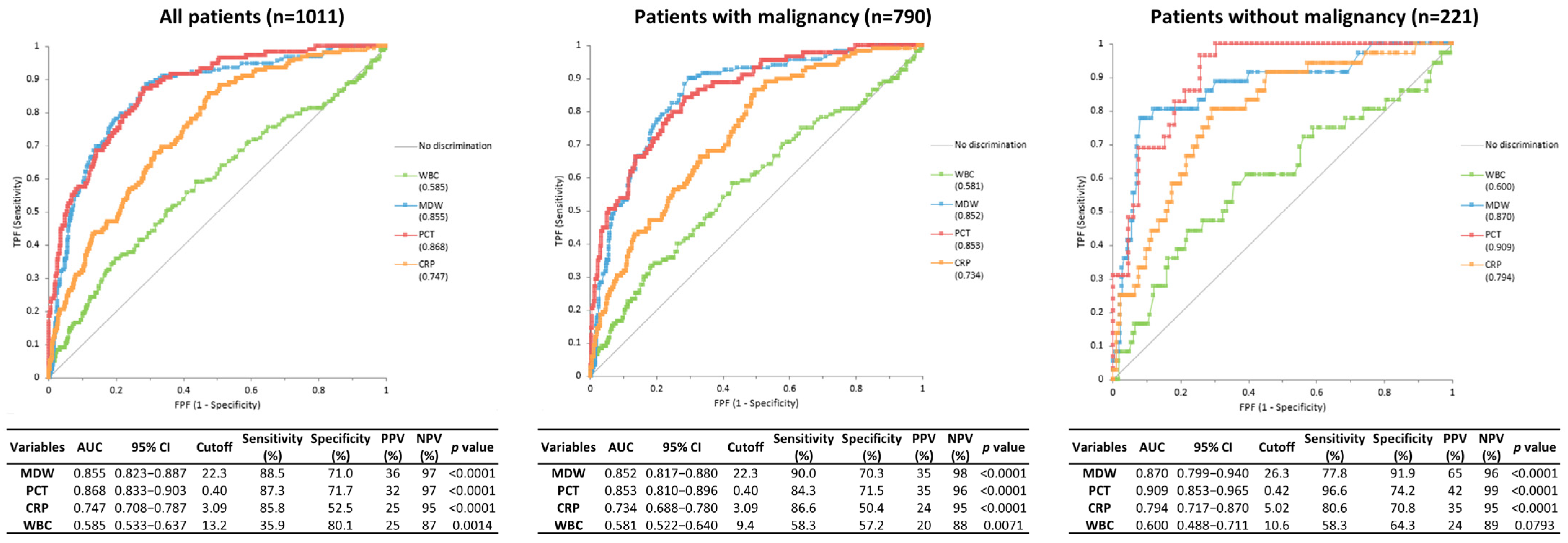
3.3. Diagnostic Performance of Biomarkers for Discrimination Between Infection and Non-Infection
3.4. Diagnostic Performance of Biomarkers for Identification of Sepsis in Patients with Leukopenia
3.5. Diagnostic Performance of Biomarkers for Identification of Sepsis and Infection in Patients with Initial Signs of Infection
3.6. Screening Performance of Biomarkers with a Cutoff Value Recommended by the Manufacturer
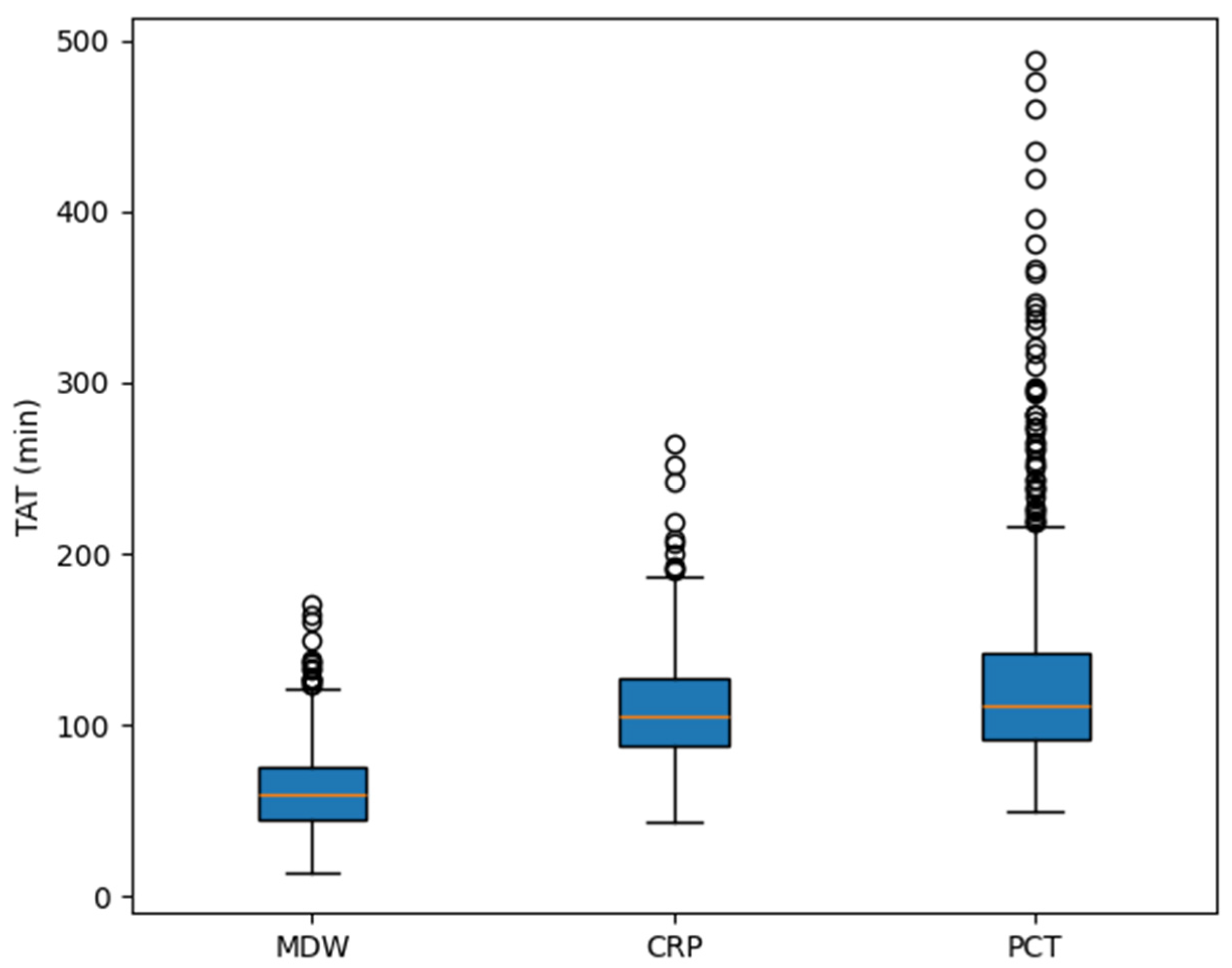
3.7. Comparison of TAT Among the Biomarkers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef]
- Peltan, I.D.; Brown, S.M.; Bledsoe, J.R.; Sorensen, J.; Samore, M.H.; Allen, T.L.; Hough, C.L. ED Door-to-Antibiotic Time and Long-term Mortality in Sepsis. Chest 2019, 155, 938–946. [Google Scholar] [CrossRef]
- Mirouse, A.; Vigneron, C.; Llitjos, J.F.; Chiche, J.D.; Mira, J.P.; Mokart, D.; Azoulay, E.; Pène, F. Sepsis and Cancer: An Interplay of Friends and Foes. Am. J. Respir. Crit. Care Med. 2020, 202, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Danai, P.A.; Moss, M.; Mannino, D.M.; Martin, G.S. The epidemiology of sepsis in patients with malignancy. Chest 2006, 129, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Parrillo, J.E.; Seymour, C.; Angus, D.C.; Bicking, K.; Tejidor, L.; Magari, R.; Careaga, D.; Williams, J.; Closser, D.R.; et al. Improved Early Detection of Sepsis in the ED with a Novel Monocyte Distribution Width Biomarker. Chest 2017, 152, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Parrillo, J.E.; Seymour, C.W.; Angus, D.C.; Bicking, K.; Esguerra, V.G.; Peck-Palmer, O.M.; Magari, R.T.; Julian, M.W.; Kleven, J.M.; et al. Monocyte Distribution Width: A Novel Indicator of Sepsis-2 and Sepsis-3 in High-Risk Emergency Department Patients. Crit. Care Med. 2019, 47, 1018–1025. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Tak, T.; van Groenendael, R.; Pickkers, P.; Koenderman, L. Monocyte Subsets Are Differentially Lost from the Circulation during Acute Inflammation Induced by Human Experimental Endotoxemia. J. Innate Immun. 2017, 9, 464–474. [Google Scholar] [CrossRef]
- Nates, J.L.; Pène, F.; Darmon, M.; Mokart, D.; Castro, P.; David, S.; Povoa, P.; Russell, L.; Nielsen, N.D.; Gorecki, G.P.; et al. Septic shock in the immunocompromised cancer patient: A narrative review. Crit. Care 2024, 28, 285. [Google Scholar] [CrossRef]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874. [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Ford, M.L.; Coopersmith, C.M. Cancer and sepsis. Clin. Sci. 2023, 137, 881–893. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Crouser, E.D.; Parrillo, J.E.; Martin, G.S.; Huang, D.T.; Hausfater, P.; Grigorov, I.; Careaga, D.; Osborn, T.; Hasan, M.; Tejidor, L. Monocyte distribution width enhances early sepsis detection in the emergency department beyond SIRS and qSOFA. J. Intensive Care 2020, 8, 33. [Google Scholar] [CrossRef]
- Agnello, L.; Bivona, G.; Vidali, M.; Scazzone, C.; Giglio, R.V.; Iacolino, G.; Iacona, A.; Mancuso, S.; Ciaccio, A.M.; Lo Sasso, B.; et al. Monocyte distribution width (MDW) as a screening tool for sepsis in the Emergency Department. Clin. Chem. Lab. Med. 2020, 58, 1951–1957. [Google Scholar] [CrossRef]
- Agnello, L.; Iacona, A.; Maestri, S.; Lo Sasso, B.; Giglio, R.V.; Mancuso, S.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Independent Validation of Sepsis Index for Sepsis Screening in the Emergency Department. Diagnostics 2021, 11, 1292. [Google Scholar] [CrossRef]
- Poz, D.; Crobu, D.; Sukhacheva, E.; Rocchi, M.B.L.; Anelli, M.C.; Curcio, F. Monocyte distribution width (MDW): A useful biomarker to improve sepsis management in Emergency Department. Clin. Chem. Lab. Med. 2022, 60, 433–440. [Google Scholar] [CrossRef]
- Agnello, L.; Giglio, R.V.; Bivona, G.; Scazzone, C.; Gambino, C.M.; Iacona, A.; Ciaccio, A.M.; Lo Sasso, B.; Ciaccio, M. The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis. Diagnostics 2021, 11, 1881. [Google Scholar] [CrossRef]
- Polilli, E.; Sozio, F.; Frattari, A.; Persichitti, L.; Sensi, M.; Posata, R.; Di Gregorio, M.; Sciacca, A.; Flacco, M.E.; Manzoli, L.; et al. Comparison of Monocyte Distribution Width (MDW) and Procalcitonin for early recognition of sepsis. PLoS ONE 2020, 15, e0227300. [Google Scholar] [CrossRef]
- Woo, A.; Oh, D.K.; Park, C.J.; Hong, S.B. Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS ONE 2021, 16, e0250101. [Google Scholar] [CrossRef]
- Hausfater, P.; Robert Boter, N.; Morales Indiano, C.; Cancella de Abreu, M.; Marin, A.M.; Pernet, J.; Quesada, D.; Castro, I.; Careaga, D.; Arock, M.; et al. Monocyte distribution width (MDW) performance as an early sepsis indicator in the emergency department: Comparison with CRP and procalcitonin in a multicenter international European prospective study. Crit. Care 2021, 25, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Seak, C.J.; Chaou, C.H.; Su, T.H.; Gao, S.Y.; Chien, C.Y.; Ng, C.J. Comparison of the diagnostic accuracy of monocyte distribution width and procalcitonin in sepsis cases in the emergency department: A prospective cohort study. BMC Infect. Dis. 2022, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Motawea, K.R.; Rozan, S.S.; Elsayed Talat, N.; Elhalag, R.H.; Mohammed Reyad, S.; Chebl, P.; Swed, S.; Sawaf, B.; Hadeel Alfar, H.; Farwati, A.; et al. Comparison of monocyte distribution width and Procalcitonin as diagnostic markers for sepsis: Meta-analysis of diagnostic test accuracy studies. PLoS ONE 2023, 18, e0288203. [Google Scholar] [CrossRef] [PubMed]
- Eisinger, G.J.; Hosler, Q.; Crouser, E.D.; Herman, D.D. Diagnostic Performance of Monocyte Distribution Width for the Detection of Sepsis: A Systematic Review and Meta-Analysis. J. Am. Coll. Emerg. Physicians Open 2025, 6, 100073. [Google Scholar] [CrossRef]
- Encabo, M.; Hernández-Álvarez, E.; Oteo, D.; García-Álvarez, A.; Martínez-Novillo González, M.; Sanz-Casla, M.T.; González-Del Castillo, J. Monocyte distribution width (MDW) as an infection indicator in severe patients attending in the Emergency Department: A pilot study. Rev. Esp. Quimioter. 2023, 36, 267–274. [Google Scholar] [CrossRef]
- Esposito, J.E.; D’Amato, M.; Parruti, G.; Polilli, E. Monocyte Distribution Width for Sepsis Diagnosis in the Emergency Department and Intensive Care Unit: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 7444. [Google Scholar] [CrossRef]
- Levin, S.; Sarani, N.; Hinson, J.; Naiman, M.; Cannon, C.; Smith, A.; Steinhart, B.; DeBraine, A.; Kehoe, S.; Immhoff, B.; et al. The Complete Blood Count Sepsis Index Using Monocyte Distribution Width for Early Detection of Sepsis in Patients Without Obvious Signs. Crit. Care Explor. 2025, 7, e1194. [Google Scholar] [CrossRef]
- Wang, J.; Hu, M.; Wang, N.; Huang, T.; Wu, H.; Li, H. Combined detection of monocyte distribution width and procalcitonin for diagnosing and prognosing neonatal sepsis. BMC Infect. Dis. 2025, 25, 64. [Google Scholar] [CrossRef]

| Variables | Sepsis-3 Criteria | p Value | ||
|---|---|---|---|---|
| Non-Infection (n = 882) | Infection (n = 129) | Sepsis (n = 156) | ||
| Age, years | 65.0 (55.9–72.0) | 68.0 (58.0–74.0) | 69.5 (61.4–75.6) | <0.0001 |
| Sex | ||||
| Male, n (%) | 574 (65.1%) | 80 (62.0%) | 93 (59.6%) | 0.3737 |
| Female, n (%) | 308 (34.9%) | 49 (38.0%) | 63 (40.4%) | |
| Malignancy | ||||
| No, n (%) | 203 (23.0%) | 28 (21.7%) | 36 (23.1%) | 0.9448 |
| Yes, n (%) | 679 (77.0%) | 101 (78.3%) | 120 (76.9%) | |
| qSOFA score | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0002 |
| SOFA score | 0.0 (0.0–2.0) | 1.0 (0.0–1.0) | 4.0 (2.4–5.0) | <0.0001 |
| Culture | ||||
| Positive, n (%) | 0 (0) | 80 (62.0) | 73 (46.8) | <0.0001 |
| Negative, n (%) | 409 (46.4) | 37 (28.7) | 68 (43.6) | |
| Not performed, n (%) | 473 (53.6) | 12 (9.3) | 15 (9.6) | |
| WBC count (×109/L) | 8.20 (5.90–11.00) | 10.40 (6.03–17.30) | 10.55 (7.10–15.10) | <0.0001 |
| CRP (mg/L) | 1.30 (0.29–5.71) | 9.35 (4.20–16.26) | 9.54 (4.57–18.89) | <0.0001 |
| PCT (ng/mL) | 0.15 (0.07–0.34) | 0.49 (0.18–0.98) | 2.31 (0.58–9.39) | <0.0001 |
| MDW | 19.30 (17.60–21.30) | 24.40 (21.60–26.93) | 27.60 (24.14–32.10) | <0.0001 |
| Biomarkers | Cutoff | Non-Infection | Infection | Sepsis | |||
|---|---|---|---|---|---|---|---|
| Above | Less | Above | Less | Above | Less | ||
| MDW | 21.5 | 23.4% (206/882) | 76.6% (676/882) | 76.0% (98/129) | 24.0% (31/129) | 91.0% (142/156) | 9.0% (14/156) |
| CRP | 0.30 mg/L | 74.8% (657/878) | 25.2% (221/878) | 94.5% (121/128) | 5.5% (7/128) | 97.4% (151/155) | 2.6% (4/155) |
| PCT | 0.50 ng/mL | 16.0% (57/356) | 84.0% (299/356) | 48.3% (43/89) | 51.7% (46/89) | 78.8% (93/118) | 21.2% (25/118) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Park, J.; Lim, H.J.; Kwon, Y.J.; Choi, H.-W.; Kee, S.-J.; Kim, S.H.; Shin, M.G.; Nah, E.-H.; Shin, J.H. Diagnostic Utility of Monocyte Distribution Width for Early Sepsis Detection in Cancer-Enriched Emergency Cohort. J. Clin. Med. 2025, 14, 8089. https://doi.org/10.3390/jcm14228089
Choi YJ, Park J, Lim HJ, Kwon YJ, Choi H-W, Kee S-J, Kim SH, Shin MG, Nah E-H, Shin JH. Diagnostic Utility of Monocyte Distribution Width for Early Sepsis Detection in Cancer-Enriched Emergency Cohort. Journal of Clinical Medicine. 2025; 14(22):8089. https://doi.org/10.3390/jcm14228089
Chicago/Turabian StyleChoi, Yong Jun, Jooheon Park, Ha Jin Lim, Yong Jun Kwon, Hyun-Woo Choi, Seung-Jung Kee, Soo Hyun Kim, Myung Geun Shin, Eun-Hee Nah, and Jong Hee Shin. 2025. "Diagnostic Utility of Monocyte Distribution Width for Early Sepsis Detection in Cancer-Enriched Emergency Cohort" Journal of Clinical Medicine 14, no. 22: 8089. https://doi.org/10.3390/jcm14228089
APA StyleChoi, Y. J., Park, J., Lim, H. J., Kwon, Y. J., Choi, H.-W., Kee, S.-J., Kim, S. H., Shin, M. G., Nah, E.-H., & Shin, J. H. (2025). Diagnostic Utility of Monocyte Distribution Width for Early Sepsis Detection in Cancer-Enriched Emergency Cohort. Journal of Clinical Medicine, 14(22), 8089. https://doi.org/10.3390/jcm14228089






