Complications of Percutaneous Tracheostomy-Assisting Techniques in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Screening, Selection and Data Extraction
- (1)
- Minor complications:
- (a)
- Minor bleeding: bleeding controlled with digital compression, without hemodynamic instability and without the need for surgical revision or transfusion.
- (b)
- Transient hypoxia: oxygen desaturation during the procedure, defined as SpO2 < 90% but ≥85%.
- (c)
- Transient hypotension: a decrease in blood pressure requiring fluid resuscitation with <1000 mL, without initiation or escalation of inotropic support.
- (d)
- Barotrauma: occurrence of subcutaneous emphysema.
- (e)
- Tracheal ring rupture: disruption of a tracheal cartilage ring at any stage of the procedure, recorded only in cases performed under endoscopic guidance.
- (f)
- Technical complications without clinical repercussions: isolated events such as endotracheal tube cuff puncture, difficulty in cannula insertion, or inability to complete the procedure, provided these events do not result in desaturation or airway loss.
- (2)
- Major complications:
- (a)
- Major bleeding: bleeding leading to hemodynamic instability and/or requiring surgical revision and/or blood transfusion.
- (b)
- False passage: creation of a tract resulting in tracheal injury, mediastinal emphysema, or oxygen desaturation (SpO2 < 85%).
- (c)
- Barotrauma: occurrence of pneumothorax or mediastinal emphysema.
- (d)
- Technical complications with clinical repercussions: events such as endotracheal tube cuff puncture, difficulty in cannula insertion, or inability to complete the procedure, when associated with adverse outcomes, including desaturation, airway loss, or severe complications necessitating a change in management strategy.
2.6. Risk of Bias Assessment and Quality of Evidence
2.7. Statistical Synthesis
2.8. Ethical Approval
3. Results
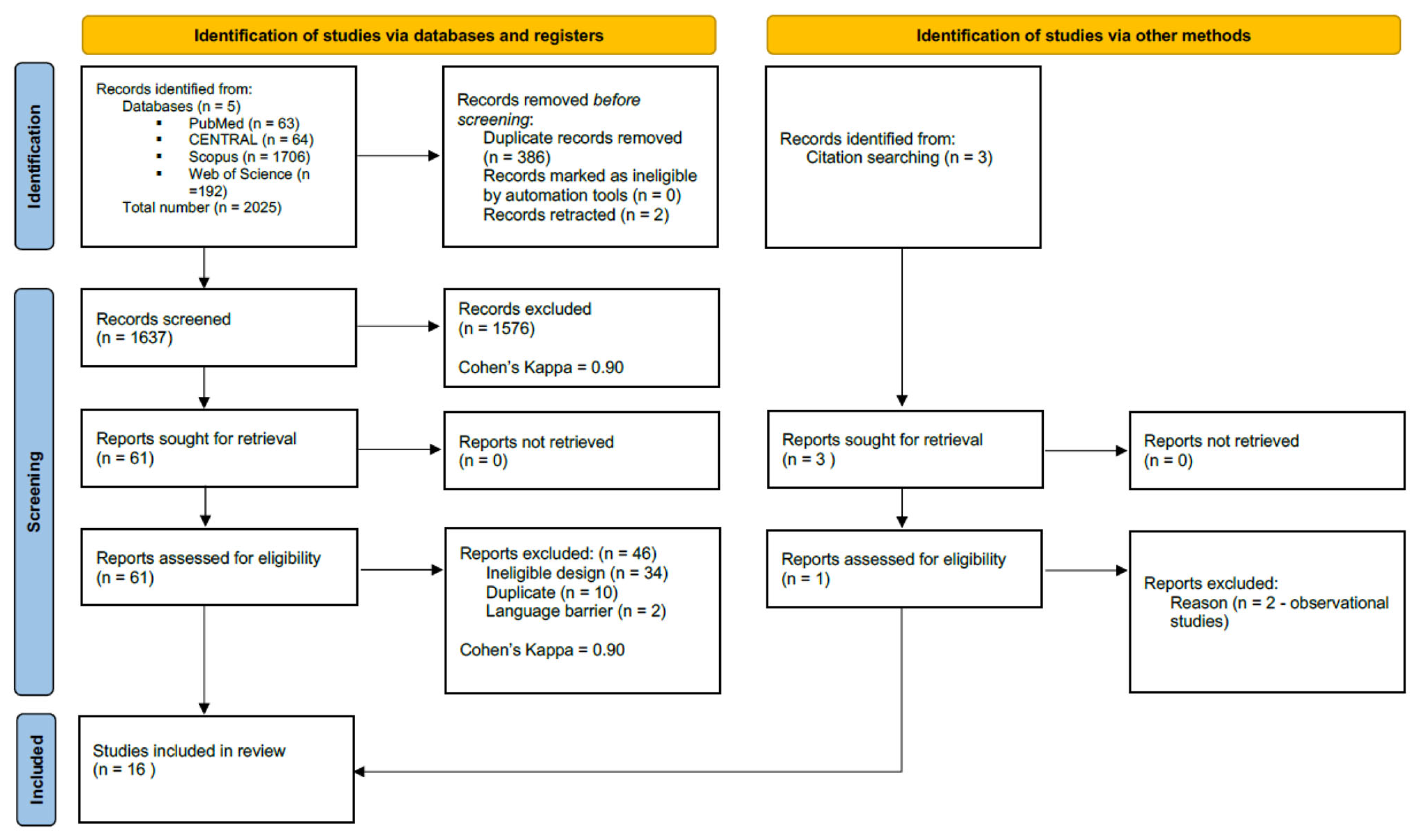
3.1. Search and Selection
3.2. Basic Characteristics of Studies Included
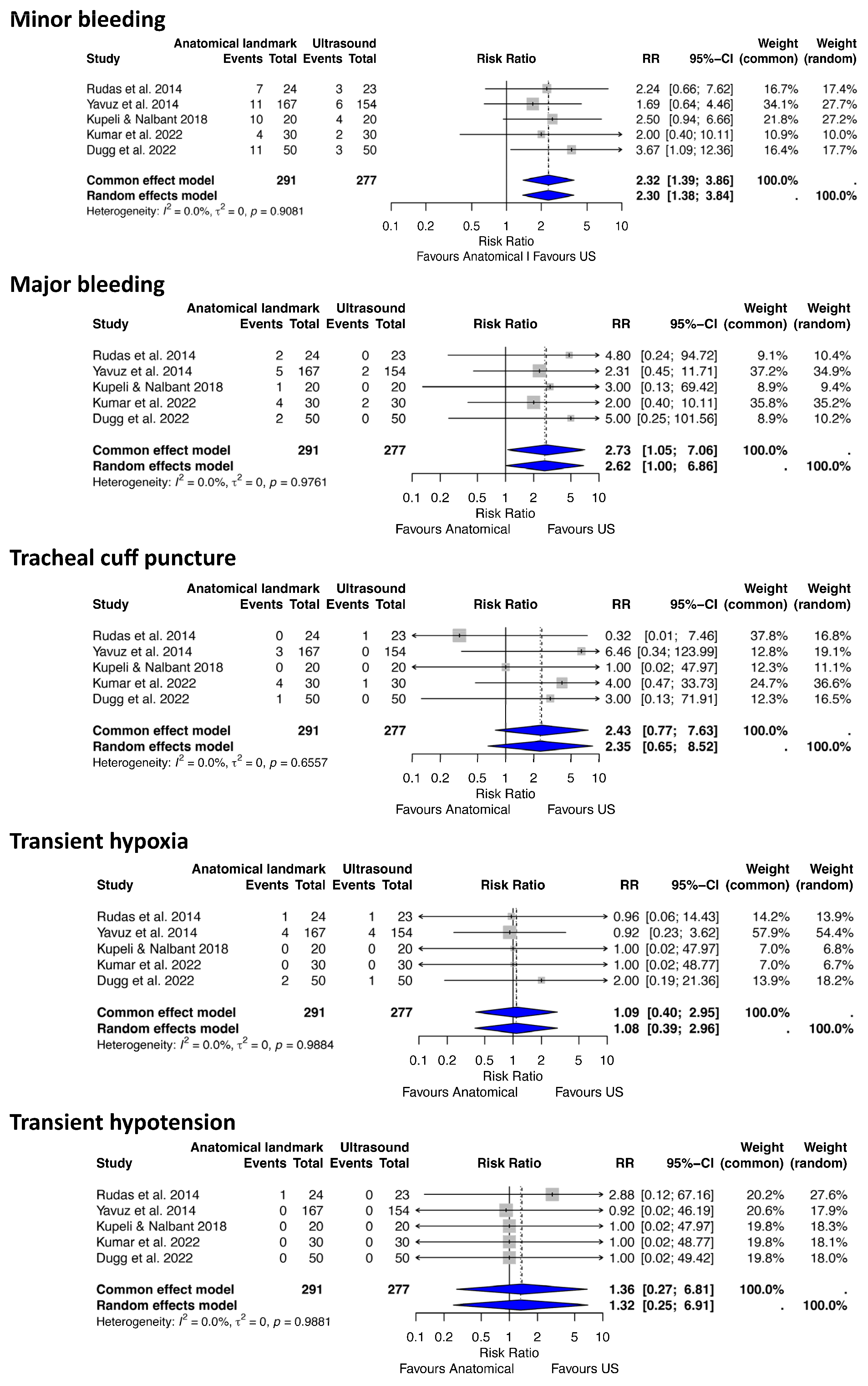
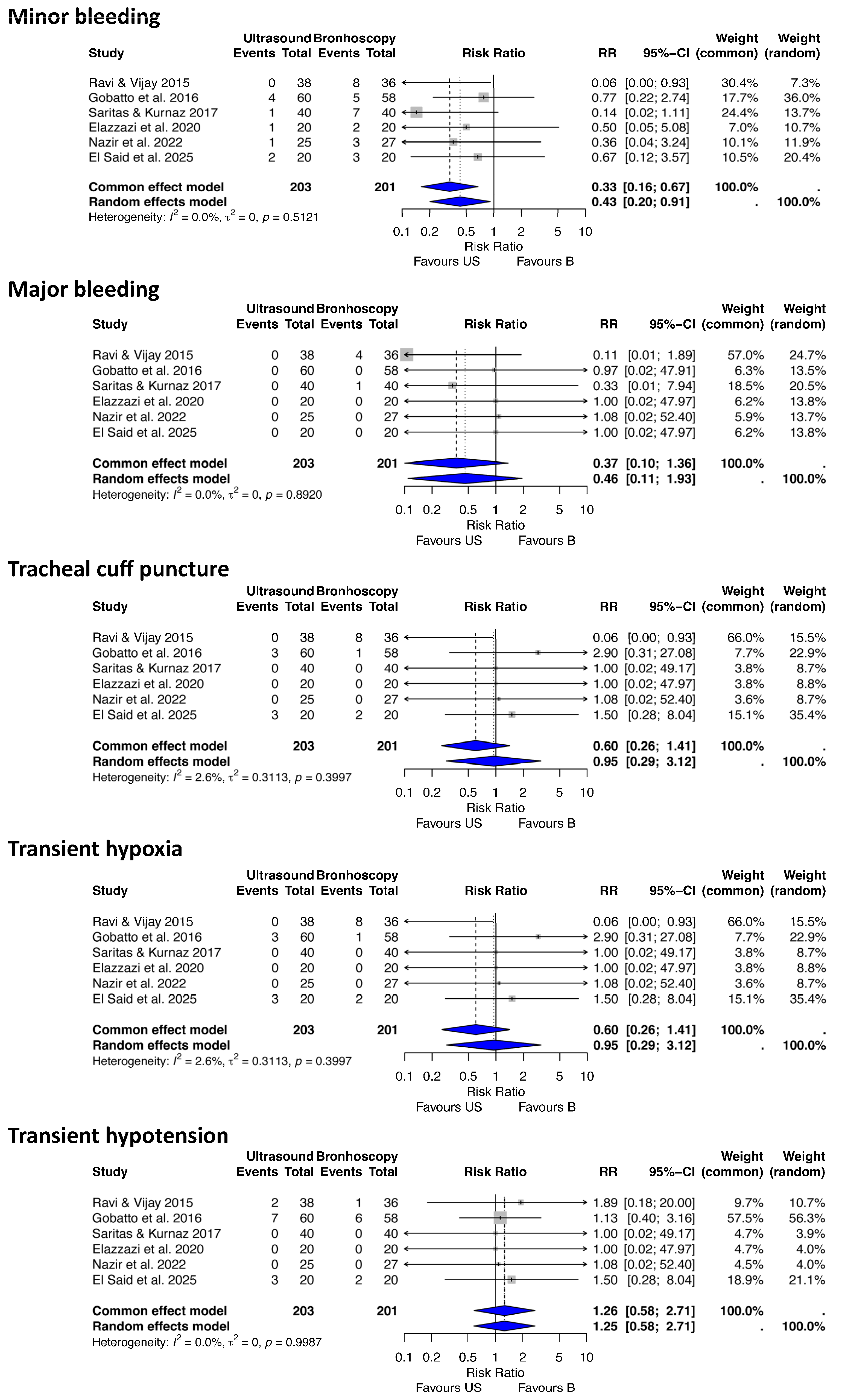
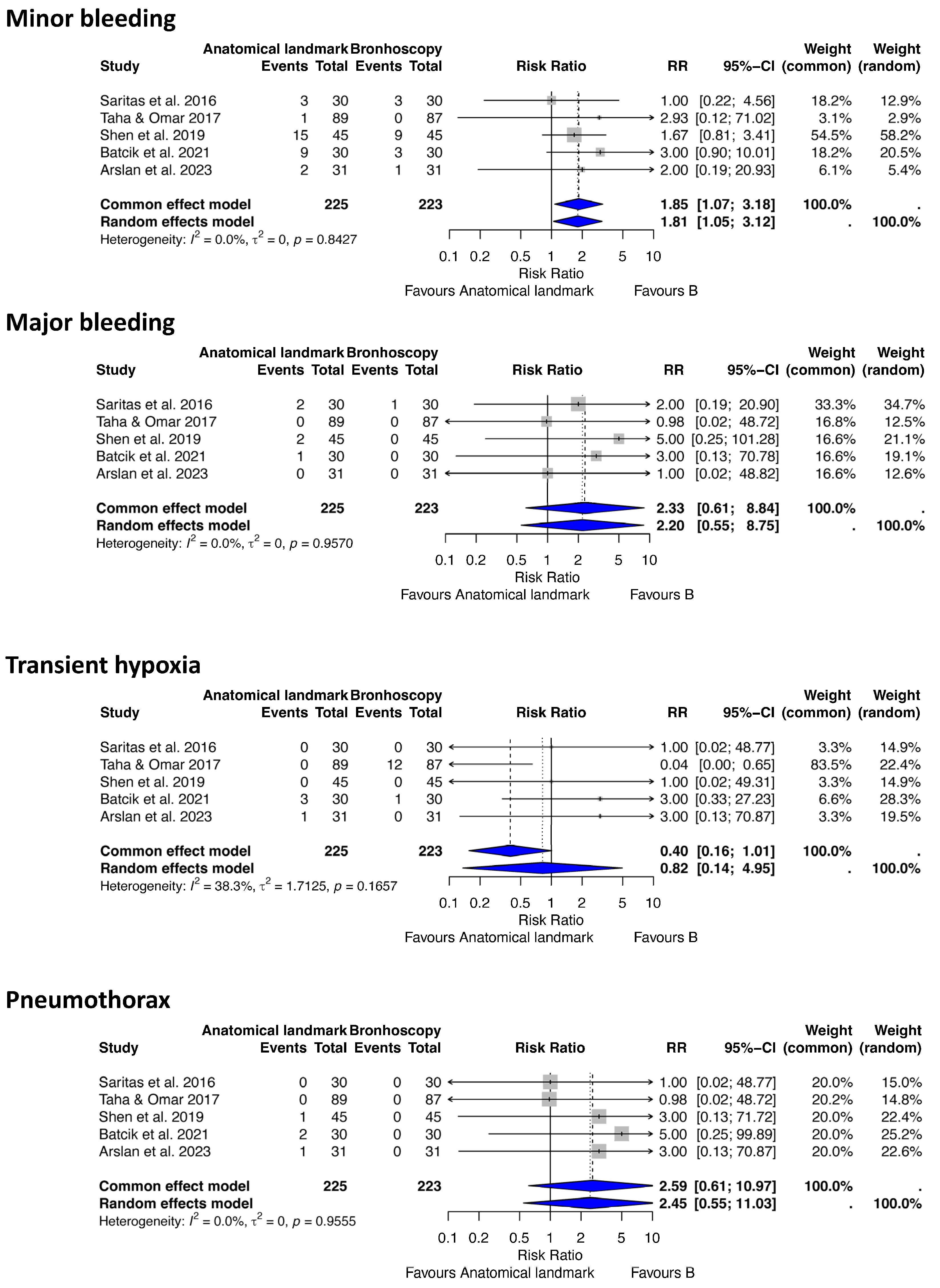
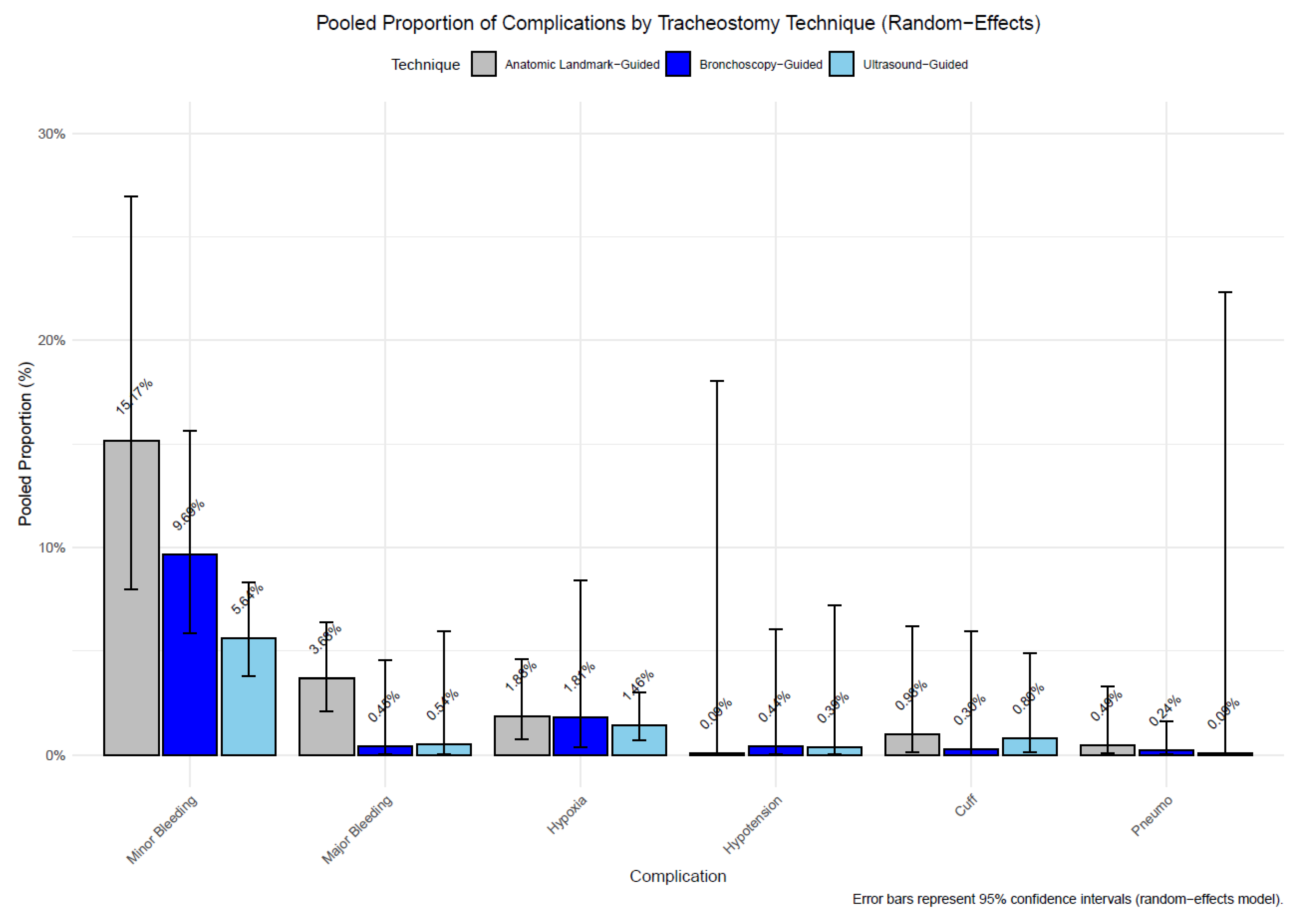
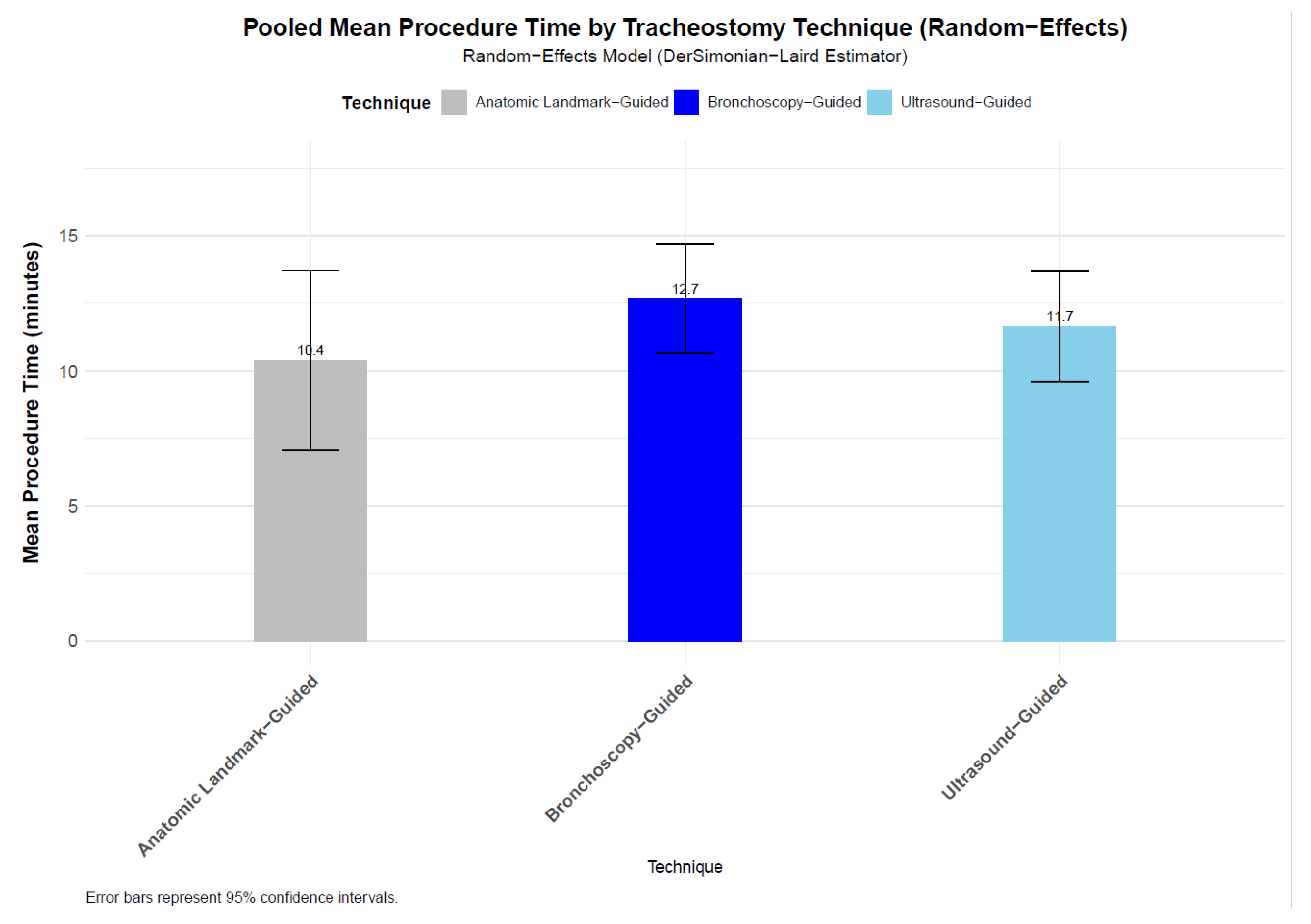
3.3. Statistical Results
- (a)
- Anatomical landmark vs. Ultrasound-Guided PDT
- (b) Ultrasound vs. Bronchoscopy-Guided PDT
- (c) Anatomical landmark vs. Bronchoscopy-Guided Tracheostomy
3.4. Risk of Bias Assessment and Quality of Evidence
3.5. Heterogeneity and Publication Bias
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ciaglia, P.; Firsching, R.; Syniec, C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. CHEST 1985, 87, 715–719. [Google Scholar] [CrossRef]
- Lais, G.; Piquilloud, L. Tracheostomy: Update on why, when and how. Curr. Opin. Crit. Care 2025, 31, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Sutherasan, Y.; Antonelli, M.; Brunetti, I.; Corcione, A.; Laffey, J.G.; Putensen, C.; Servillo, G.; Pelosi, P. Tracheostomy procedures in the intensive care unit: An international survey. Crit. Care 2015, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Sustić, A.; Kovac, D.; Zgaljardić, Z.; Zupan, Z.; Krstulović, B. Ultrasound-guided percutaneous dilatational tracheostomy: A safe method to avoid cranial misplacement of the tracheostomy tube. Intensive Care Med. 2000, 26, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Tomsic, J.P.; Connolly, M.C.; Joe, V.C.; Wong, D.T. Evaluation of bronchoscopic-assisted percutaneous tracheostomy. Am. Surg. 2006, 72, 970–972. [Google Scholar] [CrossRef]
- Sustić, A. Role of ultrasound in the airway management of critically ill patients. Crit. Care Med. 2007, 35, S173–S177. [Google Scholar] [CrossRef]
- Rudas, M.; Seppelt, I.; Herkes, R.; Hislop, R.; Rajbhandari, D.; Weisbrodt, L. Traditional landmark versus ultrasound guided tracheal puncture during percutaneous dilatational tracheostomy in adult intensive care patients: A randomised controlled trial. Crit. Care 2014, 18, 514. [Google Scholar] [CrossRef]
- Dinh, V.A.; Farshidpanah, S.; Lu, S.; Stokes, P.; Chrissian, A.; Shah, H.; Giri, P.; Hecht, D.; Nguyen, H.B. Real-time sonographically guided percutaneous dilatational tracheostomy using a long-axis approach compared to the landmark technique. J. Ultrasound Med. 2014, 33, 1407–1415. [Google Scholar] [CrossRef]
- Sarıtaş, A.; Kurnaz, M.M. Comparison of Bronchoscopy-Guided and Real-Time Ultrasound-Guided Percutaneous Dilatational Tracheostomy: Safety, Complications, and Effectiveness in Critically Ill Patients. J. Intensive Care Med. 2019, 34, 191–196. [Google Scholar] [CrossRef]
- Gobatto, A.L.N.; Besen, B.A.M.P.; Tierno, P.F.G.M.M.; Mendes, P.V.; Cadamuro, F.; Joelsons, D.; Melro, L.; Park, M.; Malbouisson, L.M.S. Comparison between ultrasound- and bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients: A retrospective cohort study. J. Crit. Care 2015, 30, 220.e13–220.e17. [Google Scholar] [CrossRef]
- Guinot, P.G.; Zogheib, E.; Petiot, S.; Marienne, J.P.; Guerin, A.M.; Monet, P.; Zaatar, R.; Dupont, H. Ultrasound-guided percutaneous tracheostomy in critically ill obese patients. Crit. Care 2012, 16, R40. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.W.; Peters, J.I.; Shelledy, D.C. Effects of fiberoptic bronchoscopy during mechanical ventilation in a lung model. CHEST 2000, 118, 824–831. [Google Scholar] [CrossRef]
- Reilly, P.M.; Sing, R.F.; Giberson, F.A.; Anderson, H.L.; Rotondo, M.F.; Tinkoff, G.H.; Schwab, C.W. Hypercarbia during tracheostomy: A comparison of percutaneous endoscopic, percutaneous Doppler, and standard surgical tracheostomy. Intensive Care Med. 1997, 23, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Añón, J.M.; Arellano, M.S.; Pérez-Márquez, M.; Díaz-Alvariño, C.; Márquez-Alonso, J.A.; Rodríguez-Peláez, J.; Nanwani-Nanwani, K.; Martín-Pellicer, A.; Civantos, B.; López-Fernández, A.; et al. The role of routine FIBERoptic bronchoscopy monitoring during percutaneous dilatational TRACHeostomy (FIBERTRACH): A study protocol for a randomized, controlled clinical trial. Trials 2021, 22, 423. [Google Scholar] [CrossRef]
- Barba, C.A.; Angood, P.B.; Kauder, D.R.; Latenser, B.; Martin, K.; McGonigal, M.D.; Phillips, G.R.; Rotondo, M.F.; Schwab, C.W. Bronchoscopic guidance makes percutaneous tracheostomy a safe, cost-effective, and easy-to-teach procedure. Surgery 1995, 118, 879–883. [Google Scholar] [CrossRef]
- Hinerman, R.; Alvarez, F.; Keller, C.A. Outcome of bedside percutaneous tracheostomy with bronchoscopic guidance. Intensive Care Med. 2000, 26, 1850–1856. [Google Scholar] [CrossRef]
- Kost, K.M. Endoscopic percutaneous dilatational tracheotomy: A prospective evaluation of 500 consecutive cases. Laryngoscope 2005, 115 Pt 2, 1–30. [Google Scholar] [CrossRef]
- Gadkaree, S.K.; Schwartz, D.; Gerold, K.; Kim, Y. Use of Bronchoscopy in Percutaneous Dilational Tracheostomy. JAMA Otolaryngol.—Head Neck Surg. 2016, 142, 143–149. [Google Scholar] [CrossRef]
- Easterday, T.S.; Moore, J.W.; Redden, M.H.; Feliciano, D.V.; Henderson, V.J.; Humphries, T.; Kohler, K.E.; Ramsay, P.T.; Spence, S.D.; Walker, M.; et al. Percutaneous Tracheostomy under Bronchoscopic Visualization Does Not Affect Short-Term or Long-Term Complications. Am. Surg. 2017, 83, 696–698. [Google Scholar] [CrossRef]
- Jackson, L.S.M.; Davis, J.W.; Kaups, K.L.; Sue, L.P.; Wolfe, M.M.; Bilello, J.F.; Lemaster, D. Percutaneous tracheostomy: To bronch or not to bronch--that is the question. J. Trauma. 2011, 71, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Dennis, B.M.; Eckert, M.J.; Gunter, O.L.; Morris, J.A.; May, A.K. Safety of bedside percutaneous tracheostomy in the critically ill: Evaluation of more than 3,000 procedures. J. Am. Coll. Surg. 2013, 216, 858–865, Discussion 865–867. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Omar, A.S. Percutaneous dilatational tracheostomy. Is bronchoscopy necessary? A randomized clinical trial. Trends Anaesth. Crit. Care 2017, 15, 20–24. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Chandler, J.; Hopewell, S. Cochrane methods—Twenty years experience in developing systematic review methods. Syst. Rev. 2013, 2, 76. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 4 September 2022).
- Munn, Z.; Stern, C.; Aromataris, E.; Lockwood, C.; Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 2018, 18, 5. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Gobatto, A.L.N.; Besen, B.A.M.P.; Tierno, P.F.G.M.M.; Mendes, P.V.; Cadamuro, F.; Joelsons, D.; Melro, L.; Carmona, M.J.C.; Santori, G.; Pelosi, P.; et al. Ultrasound-guided percutaneous dilational tracheostomy versus bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients (TRACHUS): A randomized noninferiority controlled trial. Intensive Care Med. 2016, 42, 342–351. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Harbord, R.M.; Harris, R.J.; Sterne, J.A.C. Updated Tests for Small-study Effects in Meta-analyses. Stata J. 2009, 9, 197–210. [Google Scholar] [CrossRef]
- Yavuz, A.; Yılmaz, M.; Göya, C.; Alimoglu, E.; Kabaalioglu, A. Advantages of US in percutaneous dilatational tracheostomy: Randomized controlled trial and review of the literature. Radiology 2014, 273, 927–936. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, S.; Hussain, M.; Singh, R.; Ahmed, W.; Anand, R. Comparison of percutaneous tracheostomy methods in ICU patients: Conventional anatomical landmark method versus ultrasonography method—A randomised controlled trial. Indian J. Anaesth. 2022, 66, S207–S212. [Google Scholar] [CrossRef] [PubMed]
- Dugg, K.; Kathuria, S.; Gupta, S.; Gautam, P.L.; Singh, T.; Bansal, H. Comparison of landmark guided and ultrasound guided percutaneous dilatational tracheostomy: Efficiency, efficacy and accuracy in critically ill patients. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 281–287. [Google Scholar] [CrossRef]
- Kupeli, I.; Nalbant, R.A. Comparison of 3 techniques in percutaneous tracheostomy: Traditional landmark technique; ultrasonography-guided long-axis approach; and short-axis approach—Randomised controlled study. Anaesth. Crit. Care Pain Med. 2018, 37, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.R.; Vijay, M.N. Real time ultrasound-guided percutaneous tracheostomy: Is it a better option than bronchoscopic guided percutaneous tracheostomy? Med. J. Armed Forces India 2015, 71, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Elazzazi, H.; Aboseif, E.; Abdelrazik, R.; Elbardan, A. Bronchoscopy guided v.s ultrasound guided percutaneous tracheostomy. QJM Int. J. Med. 2020, 113 (Suppl. S1), hcaa039.043. [Google Scholar] [CrossRef]
- Nazir, H.; Naqvi, S.M.A.; Ahmad, A.; Zartash, S.; Khan, A.R.; Aslam, W. Ultrasound Versus Bronchoscopy Guided Percutaneous Dilatational Tracheostomy: A Comparative Analysis. Pak. J. Med. Res. 2022, 61, 173–178. [Google Scholar]
- El Said, A.M.; Fathi, Y.I.; Salama, A.E.; Alshafei, H.A. Comparative Study between Ultrasound-guided Percutaneous Dilatational Tracheostomy Versus Bronchoscopy-guided Percutaneous Dilatational Tracheostomy in Mechanically Ventilated Critically Ill Patients. Zagazig Univ. Med. J. 2025, 31, 2832–2844. [Google Scholar] [CrossRef]
- Saritas, A.; Saritas, P.U.; Kurnaz, M.M.; Beyaz, S.G.; Ergonenc, T. The role of fiberoptic bronchoscopy monitoring during percutaneous dilatational tracheostomy and its routine use into tracheotomy practice. J. Pak. Med. Assoc. 2016, 66, 83–89. [Google Scholar]
- Shen, G.; Yin, H.; Cao, Y.; Zhang, M.; Wu, J.; Jiang, X.; Yu, T.; Lu, W. Percutaneous dilatational tracheostomy versus fibre optic bronchoscopy-guided percutaneous dilatational tracheostomy in critically ill patients: A randomised controlled trial. Ir. J. Med. Sci. 2019, 188, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Batcik, S.; Suren, M.; Comlekci, M.; Ozdamar, M.; Ozdemir, H.; Fadillioglu, S.; Aldemir, T. Comparison of patients undergoing tracheostomy with and without fiberoptic bronchoscopy. Ann. Clin. Anal. Med. 2021, 12, 333–337. [Google Scholar] [CrossRef]
- Arslan, K.; Kaya, E.; Sahin, A.S. Classical Blind Percutaneous Dilatational Tracheostomy vs Fiberoptic Bronchoscopy Guided Percutaneous Dilatational Tracheostomy in the Intensive Care Unit: Complications, Mortality, and Outcomes. Duzce Med. J. 2023, 25, 273–278. [Google Scholar] [CrossRef]
- Plata, P.; Gaszynski, T. Ultrasound-guided percutaneous tracheostomy. Anaesthesiol. Intensive Ther. 2019, 51, 126–132. [Google Scholar] [CrossRef]
- Alansari, M.; Alotair, H.; Al Aseri, Z.; Elhoseny, M.A. Use of ultrasound guidance to improve the safety of percutaneous dilatational tracheostomy: A literature review. Crit. Care 2015, 19, 229. [Google Scholar] [CrossRef]
- Fernandez, L.; Norwood, S.; Roettger, R.; Gass, D.; Wilkins, H.I.I.I. Bedside Percutaneous Tracheostomy With Bronchoscopic Guidance in Critically Ill Patients. Arch. Surg. 1996, 131, 129–132. [Google Scholar] [CrossRef]
- Roy, C.F.; Silver, J.A.; Turkdogan, S.; Siafa, L.; Correa, J.A.; Kost, K. Complication Rate of Percutaneous Dilatational Tracheostomy in Critically Ill Adults with Obesity: A Systematic Review and Meta-analysis. JAMA Otolaryngol.—Head Neck Surg. 2023, 149, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xuan, L.; Wu, W.; Zhu, D.; Zheng, Y. Comparison of Percutaneous Dilatational Tracheostomy Guided by Ultrasound and Bronchoscopy in Critically Ill Obese Patients. J. Ultrasound Med. 2018, 37, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.H.; Teng, S.; Schimmel, M.; Duran, C.; Sardi, A.; Islam, S. A Network Comparative Meta-analysis of Percutaneous Dilatational Tracheostomies Using Anatomic Landmarks, Bronchoscopic, and Ultrasound Guidance Versus Open Surgical Tracheostomy. Lung 2019, 197, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Gobatto, A.L.N.; Besen, B.A.M.P.; Cestari, M.; Pelosi, P.; Malbouisson, L.M.S. Ultrasound-Guided Percutaneous Dilational Tracheostomy: A Systematic Review of Randomized Controlled Trials and Meta-Analysis. J. Intensive Care Med. 2020, 35, 445–452. [Google Scholar] [CrossRef]
- Lin, K.T.; Kao, Y.S.; Chiu, C.W.; Lin, C.H.; Chou, C.C.; Hsieh, P.Y.; Lin, Y.R. Comparative effectiveness of ultrasound-guided and anatomic landmark percutaneous dilatational tracheostomy: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0258972. [Google Scholar] [CrossRef]
- Wen, D.; Yang, X.; Liang, Z.; Hu, Y.; Wang, S.; Zhang, D.; Wang, Y.; Shen, Y.; Yan, F. Effectiveness of ultrasound-guided versus anatomical landmark-guided percutaneous dilatational tracheostomy: A systematic review and meta-analysis. BMC Anesthesiol. 2025, 25, 211. [Google Scholar] [CrossRef]
| First Author (Year) | Design | Population | Intervention | Comparator | Outcome | Study Period and Country | Sample Size | Conclusion | Quality Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical Landmark vs. Ultrasound-Guided Percutaneous Tracheostomy | ||||||||||
| 1 | Kumar (2022) [38] | RCT | Critically ill patients aged >20 years requiring tracheostomy after prolonged mechanical ventilation | USG | ALG | Deviation from midline, number of passes/trials, duration of procedure, immediate peri-procedural complications | April 2019 to December 2020, India | 60 (30 each group) | Ultrasound-guided PDT showed superiority over landmark PDT in terms of less number of trials, midline puncture and fewer complications. However, it took a little longer to perform USG-guided PDT. | Some concerns (primarily lack of reported allocation concealment and unblinded outcome assessment for some measures). |
| 2 | Dugg (2022) [39] | RCT | Critically ill ICU patients aged ≥18 years on prolonged mechanical ventilation requiring tracheostomy | USG | ALG | Efficiency (assessment time, procedure time), efficacy (number of attempts, complications), accuracy (deviation from midline, puncture site) | 2020–2021 (approximate based on submission dates), India | 100 (50 each group) | Ultrasound-guided PDT is associated with reduction in periprocedural complications as compared to landmark technique, although it takes slightly longer time. | Some concerns (mainly concealment not detailed; partial blinding may affect some outcomes, though primary outcome measurement was fairly objective). |
| 3 | Kupeli (2018) [40] | RCT | Critically ill adults, mean age 68, mean APACHE II 27.4 | USG | ALG | Puncture success, complications | December 2017, Turkey | 40 (20 each group) | Out-of-plane US had higher first-entry success, fewer complications | Some concerns RCT with randomization and complete follow-up; minor limitation from unspecified allocation concealment. |
| 4 | Rudas (2014) [7] | RCT | Long-term ventilated ICU adults | USG | ALG | Puncture accuracy, complications | March–December 2011, Australia | 47 (23 US, 24 ALG) | Higher midline accuracy in US (87% vs. 50%, p = 0.006), fewer complications | Low RoB RCT with blinded assessors and complete follow-up; allocation concealment not detailed. |
| 5 | Yavuz (2014) [37] | RCT | Critically ill adults | USG | ALG | Complications, procedure time | December 2007–January 2011, Turkey | 341 (166 US, 175 ALG) | Lower complications in US (7.8% vs. 15.0%, p = 0.054), longer time in US (p = 0.001) | Some concerns Randomization, institutional review board approval, complete follow-up, though blinding and allocation concealment details are not specified. |
| Ultrasound vs. Bronchoscopy-Guided Percutaneous Tracheostomy | ||||||||||
| 1 | Gobatto (2016) [31] | RCT (Non-inferiority) | Critically ill, mechanically ventilated adults, mean age 48.4, 68.6% male | USG | BG | Procedure failure (1.7% both groups, 90% CI −5.57 to 5.85) | March 2014–May 2015, Brazil | 118 (60 US, 58 B) | Non-inferiority met, minor complications 33.3% vs. 20.7% (p = 0.122) | Low RoB RCT with clear randomization, complete follow-up, and defined non-inferiority margin; minor limitation due to lack of blinding. |
| 2 | Sarıtas (2019) [9] | RCT | Critically ill adults, mean APACHE II 17.9 | USG | BG | Hemorrhage, procedure duration | February–March 2017, Turkey | 80 (40 US, 40 B) | Lower hemorrhage in US-PDT (p < 0.05), shorter duration in US-PDT (p < 0.05) | Low RoB RCT with randomization and complete data; lack of blinding of participants is a minor issue. |
| 3 | Ravi (2015) [41] | RCT | Critically ill obese adults | USG | BG | Complications, procedure time | February 2014–January 2015, India | 74 (38 US, 36 B) | Lower complication rate in USPCT (32.1% vs. 75%, p < 0.05), shorter time | Some concerns RCT with randomization and complete data; lack of blinding is a minor limitation. |
| 4 | Elazzazi (2020) [42] | RCT | Critically ill patients in ICU with factors increasing procedural difficulty (e.g., morbid obesity, difficult anatomy, cervical spine precautions) | USG | BG | Value of US in assisting PDT (e.g., identifying cervical anatomy, vasculature, thyroid; preventing vascular puncture or other complications) | Not specified, Egypt (Ain Shams University) | 40 (20/20) | Ultrasound has emerged as a potentially useful tool in assisting PDT, especially when factors increase technical difficulty; several studies demonstrate its value, but no specific comparative results provided in excerpt. | Some concerns (abstract-only; lacks full methods, results, or sample details) |
| 5 | Nazir (2022) [43] | RCT | Obese ICU patients requiring PDT | USG | BG | Procedure efficacy and postoperative complications (e.g., intra-procedural complications like bleeding, hypoxemia; operation time; no postoperative complications noted) | April 2020 to April 2021, Pakistan (Services Hospital, Lahore) | 52 (25 US, 27 B) | Ultrasound-guided procedure is superior to bronchoscopy-guided PDT among obese ICU patients with a low percentage of intra- and post-operative complications; shorter operation time in ultrasound group. | Some concerns (RCT with randomization and clear outcomes) |
| 6 | El Said (2025) [44] | RCT | Mechanically ventilated critically ill patients in ICU requiring prolonged ventilation | USG | BG | Safety and effectiveness (e.g., procedural duration, complications, outcomes like P/F ratio, ICU/hospital stay, mortality; no significant differences except shorter duration in ultrasound) | Not specified, Egypt (Menoufia University Hospital) | 40 (20 US, 20 B) | Ultrasound- or bronchoscopy-guided PDT showed comparable results in terms of complications and outcomes in critically ill patients; however, a significant difference was noted in procedural duration (shorter with ultrasound). | Low RoB (RCT with randomization, ethical approval, and CONSORT compliance) |
| Anatomical Landmark vs. Bronchoscopy-Guided Percutaneous Tracheostomy | ||||||||||
| 1 | Saritas (2016) [45] | RCT | Critically ill ventilated ICU patients | ALG | BG | Complications, number of needle passes, procedure duration | 2013–2014, Turkey | 60 (30 vs. 30) | FOB significantly reduced complications and needle passes; longer procedure time | Low RoB (well-described RCT, prospective, balanced groups) |
| 2 | Taha (2017) [22] | RCT | Critically ill adults, mean age 55.6 | ALG | BG | Procedure time, complications | January–May 2017, UAE | 176 (89 AL, 87 B) | Procedure time 5 vs. 12 min, no major complications in either group | Moderate RoB (randomization simple, but some methodological limitations) |
| 3 | Shen (2019) [46] | RCT | Critically ill adults, mean SOFA 7.5 | ALG | BG | First-time success, complications | May–December 2018, China | 90 (45 each group) | Higher first-time success in FOB-PDT (93.3% vs. 64.4%, p < 0.05) | Low RoB RCT with randomization and complete data; minor limitation due to lack of blinding. |
| 4 | Batcik (2021) [47] | RCT | ICU patients with prolonged ventilation | ALG | BG | Procedure time, blood gases, complications, ICU stay | Not specified (study ~2020–2021), Turkey | 60 (30 vs. 30) | FOB prolonged procedure time; complication rates similar | Some concerns (small sample, randomization but limited outcome detail) |
| 5 | Arslan (2023) [48] | RCT | ICU patients on ventilation | ALG | BG | Complications, mortality, procedure time, ICU stay | 2022–2023, Turkey | 62 (31 vs. 31) | FOB reduced complications, ICU stay, and procedure time; no mortality difference | Low RoB (prospective, randomized, clear reporting) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grajdieru, O.; Bodolea, C.; Moisoiu, V.; Petrișor, C.; Constantinescu, C. Complications of Percutaneous Tracheostomy-Assisting Techniques in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2025, 14, 8050. https://doi.org/10.3390/jcm14228050
Grajdieru O, Bodolea C, Moisoiu V, Petrișor C, Constantinescu C. Complications of Percutaneous Tracheostomy-Assisting Techniques in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2025; 14(22):8050. https://doi.org/10.3390/jcm14228050
Chicago/Turabian StyleGrajdieru, Olga, Constantin Bodolea, Vlad Moisoiu, Cristina Petrișor, and Catalin Constantinescu. 2025. "Complications of Percutaneous Tracheostomy-Assisting Techniques in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 14, no. 22: 8050. https://doi.org/10.3390/jcm14228050
APA StyleGrajdieru, O., Bodolea, C., Moisoiu, V., Petrișor, C., & Constantinescu, C. (2025). Complications of Percutaneous Tracheostomy-Assisting Techniques in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 14(22), 8050. https://doi.org/10.3390/jcm14228050






