The Role of the Greater Omentum Flap in the Prevention of Low Anterior Resection Syndrome (LARS): A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Endpoints
2.4. Eligibility and Exclusion Criteria
2.5. Quality Assessment
2.6. Study Selection and Data Collection
2.7. Statistical Analysis
3. Results
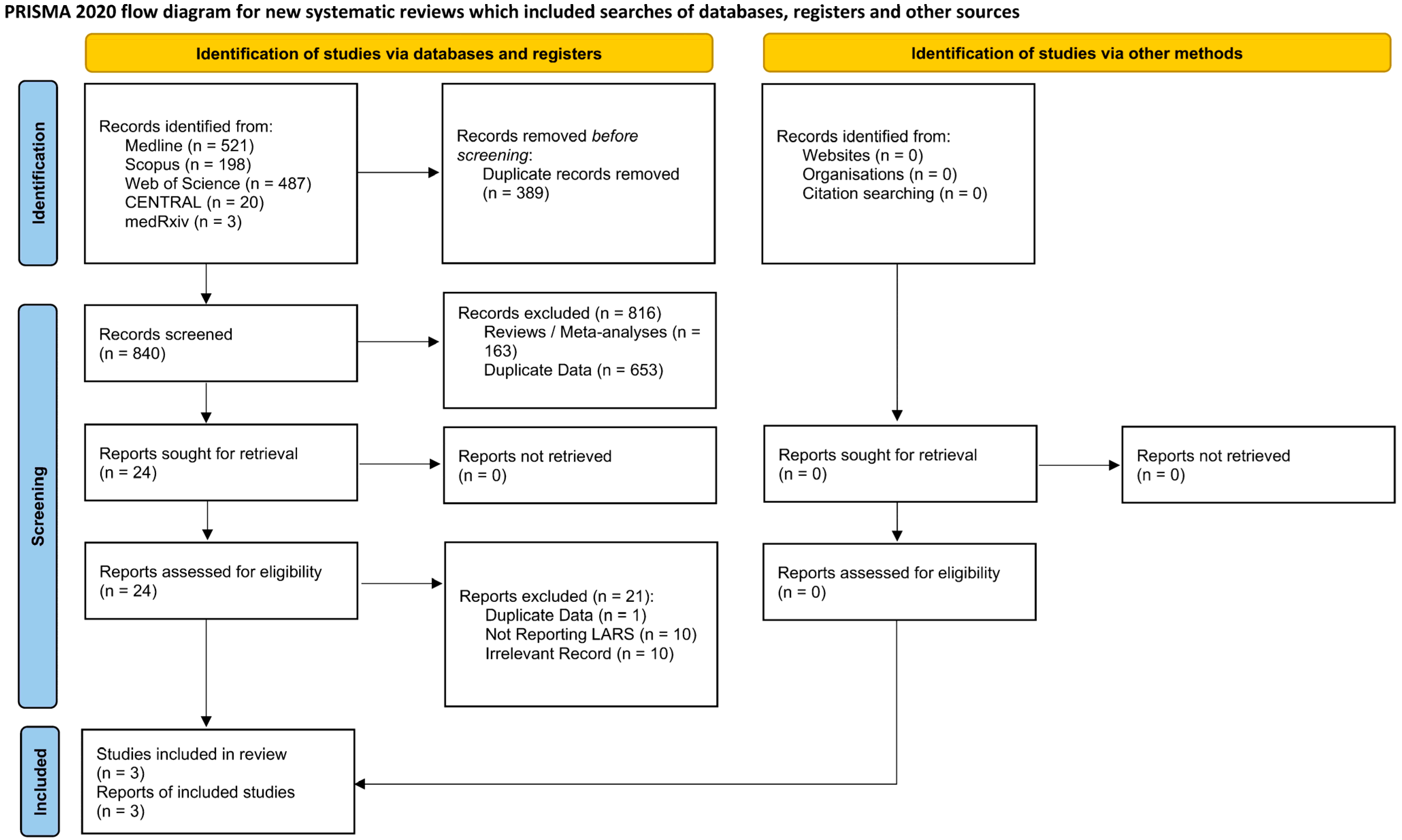
3.1. Search Results
3.2. Study Characteristics
3.3. Quality Assessment
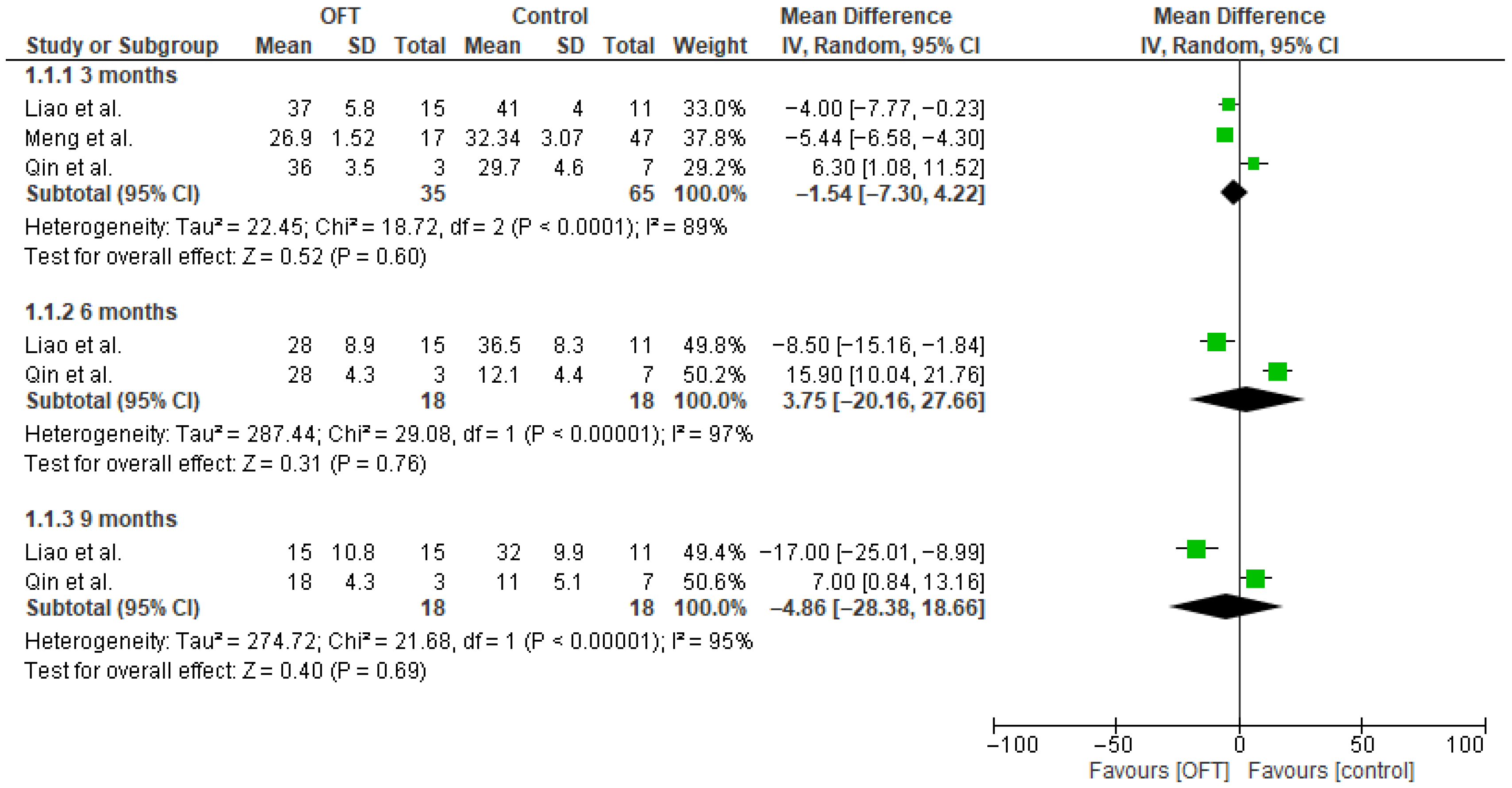
3.4. Primary Outcome
3.5. Secondary Outcomes
3.6. Publication Bias
4. Discussion
4.1. Evidence Appraisal
4.2. Strengths
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items For Systematic Reviews And Meta-Analyses |
| ROBINS-I | Risk of Bias in Non-Randomized Studies of Interventions |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| 95% CI | 95% Confidence Interval |
| MD | Mean Difference |
| OR | Odds Ratio |
| IV | Inverse Variance |
| MH | Mantel–Haenszel |
| RE | Random Effects |
| FE | Fixed-Effect |
| n/a | Not Applicable |
| BMI | Body Mass Index |
| ASA | American Society of Anesthesiologists |
| TNM | Tumor Node Metastasis |
| TME | Total Mesorectal Excision |
| FAP | Familial Adenomatous Polyposis |
| OFT | Omental Flap Transposition |
| LARS | Low Anterior Resection Syndrome |
References
- Rouleau-Fournier, F.; Brown, C.J. Can Less Be More? Organ Preservation Strategies in the Management of Rectal Cancer. Curr. Oncol. 2019, 26, S16–S23. [Google Scholar] [CrossRef]
- Schlechter, B.L. Management of Rectal Cancer. Hematol. Oncol. Clin. N. Am. 2022, 36, 521–537. [Google Scholar] [CrossRef]
- Plummer, J.M.; Leake, P.-A.; Albert, M.R. Recent Advances in the Management of Rectal Cancer: No Surgery, Minimal Surgery or Minimally Invasive Surgery. World J. Gastrointest. Surg. 2017, 9, 139. [Google Scholar] [CrossRef]
- Migliore, M.; Giuffrida, M.C.; Marano, A.; Pellegrino, L.; Giraudo, G.; Barili, F.; Borghi, F. Robotic versus Laparoscopic Right Colectomy within a Systematic ERAS Protocol: A Propensity-Weighted Analysis. Updates Surg. 2021, 73, 1057–1064. [Google Scholar] [CrossRef]
- Knol, J.; Keller, D.S. Total Mesorectal Excision Technique—Past, Present, and Future. Clin. Colon Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Q.; Du, J.; Tian, Z.; Li, Y.; Yu, B.; Niu, W. Risk Factors of the Low Anterior Resection Syndrome (LARS) after Ileostomy Reversal in Rectal Cancer Patient. Sci. Rep. 2024, 14, 28281. [Google Scholar] [CrossRef]
- Keane, C.; Fearnhead, N.S.; Bordeianou, L.G.; Christensen, P.; Basany, E.E.; Laurberg, S.; Mellgren, A.; Messick, C.; Orangio, G.R.; Verjee, A.; et al. International Consensus Definition of Low Anterior Resection Syndrome. Dis. Colon Rectum 2020, 63, 274–284. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumoto, K.; Amano, S.; Fujioka, M.; Honzumi, M. Anorectal Pressure and Rectal Compliance after Low Anterior Resection. Br. J. Surg. 1980, 67, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, Y.S. Serial Evaluation of Anorectal Function Following Low Anterior Resection of the Rectum. Int. J. Colorectal Dis. 1998, 13, 241–246. [Google Scholar] [CrossRef]
- Meng, L.; Qin, H.; Huang, Z.; Liao, J.; Cai, J.; Feng, Y.; Luo, S.; Lai, H.; Tang, W.; Mo, X. Analysis of Presacral Tissue Structure in LARS and the Prevention of LARS by Reconstruction of Presacral Mesorectum with Pedicled Greater Omentum Flap Graft. Tech. Coloproctol. 2021, 25, 1291–1300. [Google Scholar] [CrossRef]
- Liao, J.; Qin, H.; Wang, Z.; Meng, L.; Wang, W.; Liu, J.; Mo, X. Mesorectal Reconstruction with Pedicled Greater Omental Transplantation to Relieve Low Anterior Resection Syndrome Following Total Intersphincteric Resection in Patients with Ultra-Low Rectal Cancer. BMC Surg. 2023, 23, 236. [Google Scholar] [CrossRef]
- Qin, T.; Liao, J.; Qin, H.; Meng, L.; Wang, W.; Huang, Z.; Liu, J.; Mo, X. Advantages of Total Proctocolectomy with Straight Ileoanal Anastomosis plus Pedicled Omental Transposition for Familial Adenomatous Polyposis: A Preliminary Study. World J. Surg. Oncol. 2022, 20, 20. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.5 (Updated August 2024); Cochrane: Alberta, ON, Canada, 2024. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Ntellas, P.; Dadouli, K.; Perivoliotis, K.; Sogka, E.; Pentheroudakis, G.; Ioannou, M.; Hadjichristodoulou, C.; Tepetes, K.; Mauri, D. Microvessel Density and Impact of Angiogenesis on Survival of Resected Pancreatic Cancer Patients: A Systematic Review and Meta-Analysis. Pancreas 2019, 48, 233–241. [Google Scholar] [CrossRef]
- Qin, H.; Meng, L.; Huang, Z.; Liao, J.; Feng, Y.; Luo, S.; Lai, H.; Tang, W.; Mo, X. A Study on the Clinical Application of Greater Omental Pedicle Flap Transplantation to Correct Anterior Resection Syndrome in Patients with Low Rectal Cancer. Regen. Ther. 2021, 18, 146–151. [Google Scholar] [CrossRef]
- Bülow, S.; Christensen, I.J.; Harling, H.; Kronborg, O.; Fenger, C. Recurrence and Survival after Mesorectal Excision for Rectal Cancer. Br. J. Surg. 2003, 90, 974–980. [Google Scholar] [CrossRef]
- Rosen, H.; Sebesta, C.G.; Sebesta, C. Management of Low Anterior Resection Syndrome (LARS) Following Resection for Rectal Cancer. Cancers 2023, 15, 778. [Google Scholar] [CrossRef]
- Kawano, F.; Lim, M.A.; Kemprecos, H.J.; Tsai, K.; Cheah, D.; Tigranyan, A.; Kaviamuthan, K.; Pillai, A.; Chen, J.C.R.; Polites, G.; et al. Robotic Central Pancreatectomy with Omental Pedicle Flap: Tactics and Tips. Ann. Surg. Oncol. 2025, 32, 5421–5422. [Google Scholar] [CrossRef]
- Slaman, A.E.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Improved Anastomotic Leakage Rates after the “Flap and Wrap” Reconstruction in Ivor Lewis Esophagectomy for Cancer. Dis. Esophagus 2022, 36, doac036. [Google Scholar] [CrossRef]
- van Campenhout, I.; van Rees, J.M.M.; Ceelen, W.; Tanis, P.J.J.; Rothbarth, J.; Verhoef, C. Omentoplasty in Patients Undergoing Abdominoperineal Resection After Long-Course Chemoradiation for Locally Advanced and Locally Recurrent Rectal Cancer: A Comparative Single-Institution Cohort Study. Dis. Colon Rectum 2023, 66, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Ozben, V.; Aytac, E.; Liu, X.; Ozuner, G. Does Omental Pedicle Flap Reduce Anastomotic Leak and Septic Complications after Rectal Cancer Surgery? Int. J. Surg. 2016, 27, 53–57. [Google Scholar] [CrossRef]
- Merad, F.; Hay, J.M.; Fingerhut, A.; Flamant, Y.; Molkhou, J.M.; Laborde, Y. Omentoplasty in the Prevention of Anastomotic Leakage after Colonic or Rectal Resection: A Prospective Randomized Study in 712 Patients. French Associations for Surgical Research. Ann. Surg. 1998, 227, 179–186. [Google Scholar] [CrossRef]
- Killeen, S.; Devaney, A.; Mannion, M.; Martin, S.T.; Winter, D.C. Omental Pedicle Flaps Following Proctectomy: A Systematic Review. Color. Dis. 2013, 15, e634–e645. [Google Scholar] [CrossRef]
- Agnifili, A.; Schietroma, M.; Carloni, A.; Mattucci, S.; Caterino, G.; Lygidakis, N.J.; Carlei, F. The Value of Omentoplasty in Protecting Colorectal Anastomosis from Leakage. A Prospective Randomized Study in 126 Patients. Hepatogastroenterology 2004, 51, 1694–1697. [Google Scholar]
- Degiuli, M.; Elmore, U.; De Luca, R.; De Nardi, P.; Tomatis, M.; Biondi, A.; Persiani, R.; Solaini, L.; Rizzo, G.; Soriero, D.; et al. Risk Factors for Anastomotic Leakage after Anterior Resection for Rectal Cancer (RALAR Study): A Nationwide Retrospective Study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Colorectal Dis. 2022, 24, 264–276. [Google Scholar] [CrossRef]
- Tsalikidis, C.; Mitsala, A.; Mentonis, V.I.; Romanidis, K.; Pappas-Gogos, G.; Tsaroucha, A.K.; Pitiakoudis, M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Curr. Oncol. 2023, 30, 3111–3137. [Google Scholar] [CrossRef]
- Tonini, V.; Zanni, M. Impact of Anastomotic Leakage on Long-Term Prognosis after Colorectal Cancer Surgery. World J. Gastrointest. Surg. 2023, 15, 745–756. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and Grading of Anastomotic Leakage Following Anterior Resection of the Rectum: A Proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Wiggins, T.; Markar, S.R.; Arya, S.; Hanna, G.B. Anastomotic Reinforcement with Omentoplasty Following Gastrointestinal Anastomosis: A Systematic Review and Meta-Analysis. Surg. Oncol. 2015, 24, 181–186. [Google Scholar] [CrossRef] [PubMed]
- van Garderen, J.A.; Wiggers, T.; van Geel, A.N. Complications of the Pedicled Omentoplasty. Neth. J. Surg. 1991, 43, 171–174. [Google Scholar]
- Klaver, Y.L.B.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T.; de Hingh, I.H.J.T. Omentoplasty in Rectal Cancer Surgery Prolongs Post-Operative Ileus. Int. J. Colorectal Dis. 2008, 23, 165–169. [Google Scholar] [CrossRef]
- Bednarski, B.K.; Nickerson, T.P.; You, Y.N.; Messick, C.A.; Speer, B.; Gottumukkala, V.; Manandhar, M.; Weldon, M.; Dean, E.M.; Qiao, W.; et al. Randomized Clinical Trial of Accelerated Enhanced Recovery after Minimally Invasive Colorectal Cancer Surgery (RecoverMI Trial). J. Br. Surg. 2019, 106, 1311–1318. [Google Scholar] [CrossRef]
- Gray, M.; Marland, J.R.K.; Murray, A.F.; Argyle, D.J.; Potter, M.A. Predictive and Diagnostic Biomarkers of Anastomotic Leakage: A Precision Medicine Approach for Colorectal Cancer Patients. J. Pers. Med. 2021, 11, 471. [Google Scholar] [CrossRef]
- Kumar, S.; Tandon, V.; Govil, D. Low Anterior Resection Syndrome (LARS): A Contemporary Surgical Review of Incidence, Pathophysiology, Risk Stratification and Functional Outcomes. Apollo Med. 2025, 09760016251368678. [Google Scholar] [CrossRef]
- Varghese, C.; Wells, C.I.; Bissett, I.P.; O’Grady, G.; Keane, C. The Role of Colonic Motility in Low Anterior Resection Syndrome. Front. Oncol. 2022, 12, 975386. [Google Scholar] [CrossRef]
- Verras, G.I.; Mulita, F. Butyrylcholinesterase Levels Correlate with Surgical Site Infection Risk and Severity after Colorectal Surgery: A Prospective Single-Center Study. Front. Surg. 2024, 11, 1379410. [Google Scholar] [CrossRef]
| First Author | Publication Date | Type of Study | Country | Single/Multi-Center | Study Period | Group | Number | Male | BMI | Age | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meng et al. [10] | 2021 | Retrospective | China | single | 2018–2020 | OFT | 17 | 10 | 20.58 (3.7) | 49.82 (14.74) | 3 |
| Control | 47 | 31 | 21.9 (2.9) | 60.6 (9.2) | |||||||
| Liao et al. [11] | 2023 | Retrospective | China | single | 2015–2022 | OFT | 15 | 4 | 21.23 (3.3) | 59 (9.25) | 12 |
| Control | 11 | 5 | 24.02 (3.7) | 57 (10.25) | |||||||
| Qin et al. [12] | 2022 | Retrospective | China | single | 2015–2021 | OFT | 3 | 5 | 20.83 (2.7) | 33 (11.5) | 9 |
| Control | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perivoliotis, K.; Baloyiannis, I.; Sarakatsianou, C.; Tzovaras, G. The Role of the Greater Omentum Flap in the Prevention of Low Anterior Resection Syndrome (LARS): A Systematic Review of the Literature. J. Clin. Med. 2025, 14, 7937. https://doi.org/10.3390/jcm14227937
Perivoliotis K, Baloyiannis I, Sarakatsianou C, Tzovaras G. The Role of the Greater Omentum Flap in the Prevention of Low Anterior Resection Syndrome (LARS): A Systematic Review of the Literature. Journal of Clinical Medicine. 2025; 14(22):7937. https://doi.org/10.3390/jcm14227937
Chicago/Turabian StylePerivoliotis, Konstantinos, Ioannis Baloyiannis, Chamaidi Sarakatsianou, and George Tzovaras. 2025. "The Role of the Greater Omentum Flap in the Prevention of Low Anterior Resection Syndrome (LARS): A Systematic Review of the Literature" Journal of Clinical Medicine 14, no. 22: 7937. https://doi.org/10.3390/jcm14227937
APA StylePerivoliotis, K., Baloyiannis, I., Sarakatsianou, C., & Tzovaras, G. (2025). The Role of the Greater Omentum Flap in the Prevention of Low Anterior Resection Syndrome (LARS): A Systematic Review of the Literature. Journal of Clinical Medicine, 14(22), 7937. https://doi.org/10.3390/jcm14227937