Pre-Hospital Artificial Intelligence-Guided, Focused Echocardiography in Patients with Acute Chest Pain for Diagnosis of Acute Coronary Syndrome
Abstract
1. Introduction
2. Methods
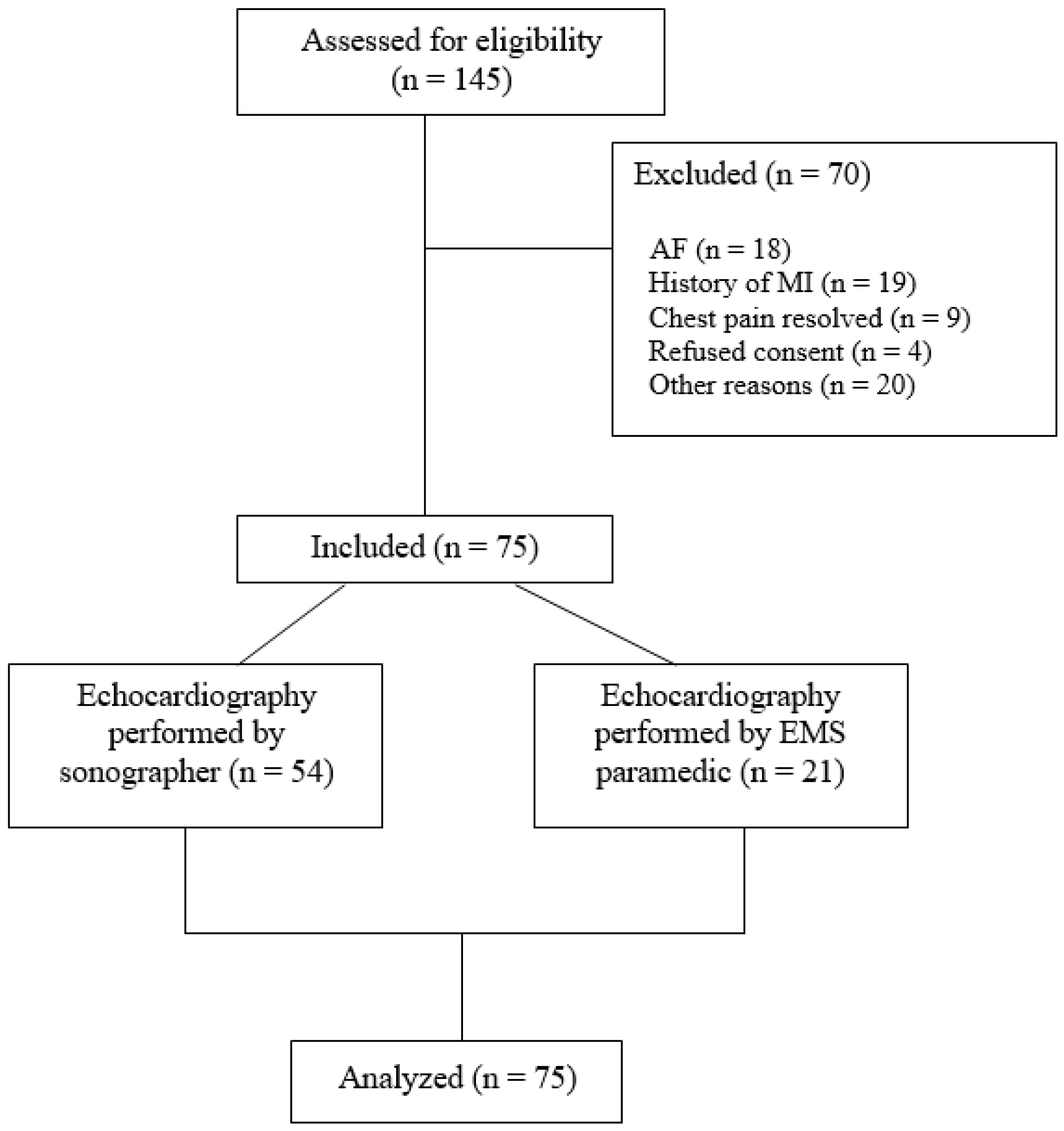
2.1. Patient Enrollment
2.2. Standard Care
2.3. Equipment and Training
2.4. Image Analysis
2.5. Statistical Analysis
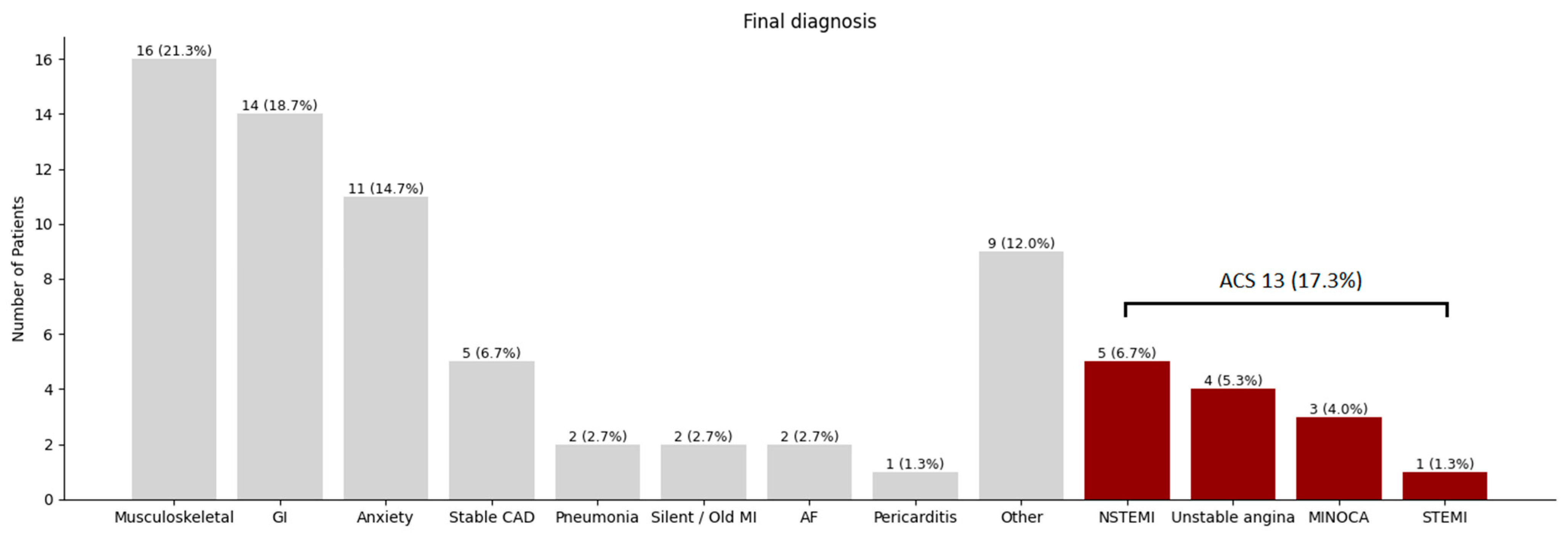
3. Results
3.1. Image Acquisition
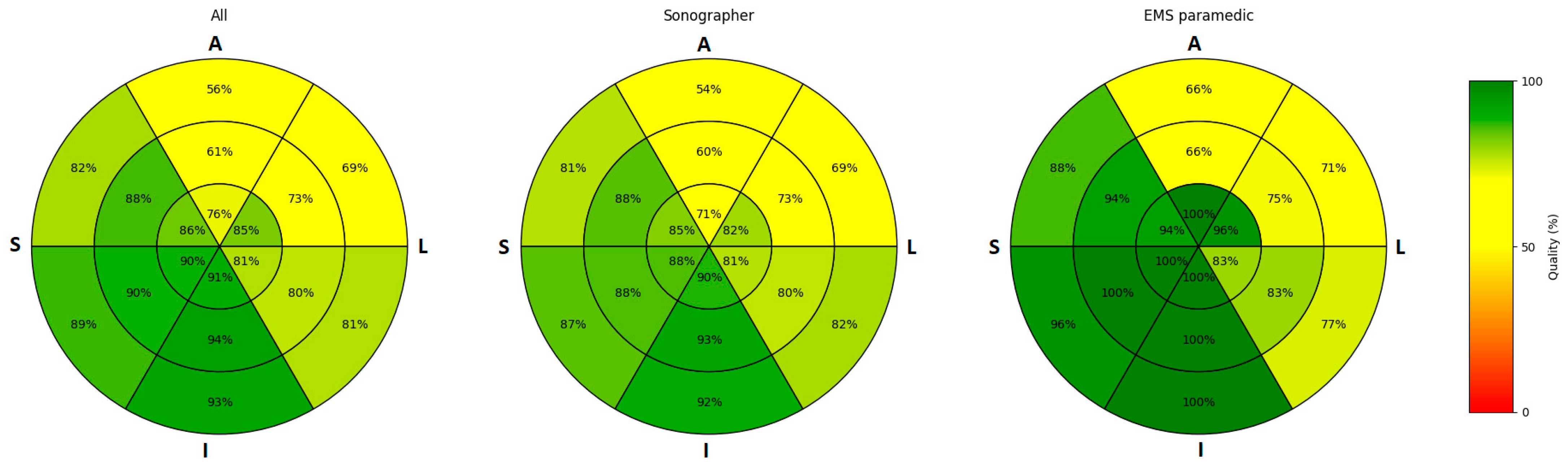
3.2. Image Quality
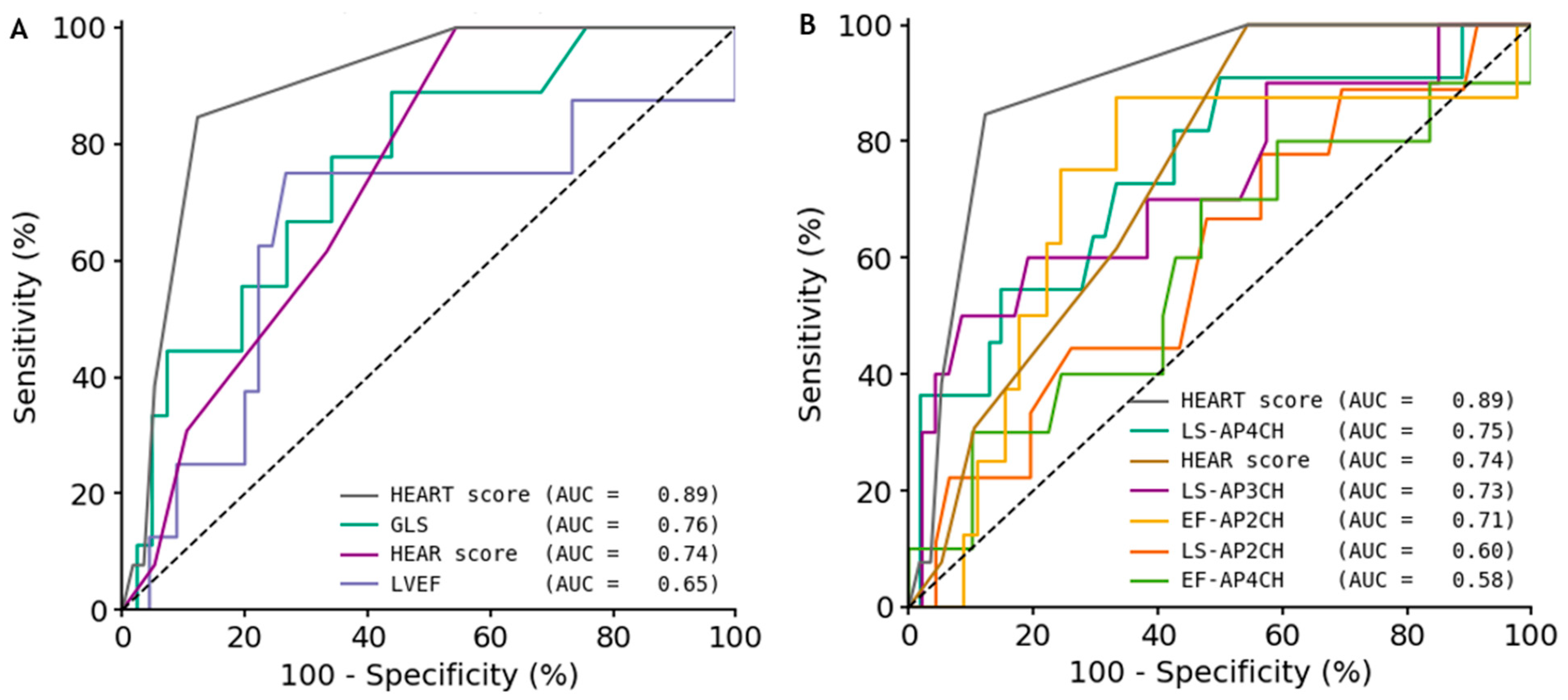
3.3. Diagnostic Performance
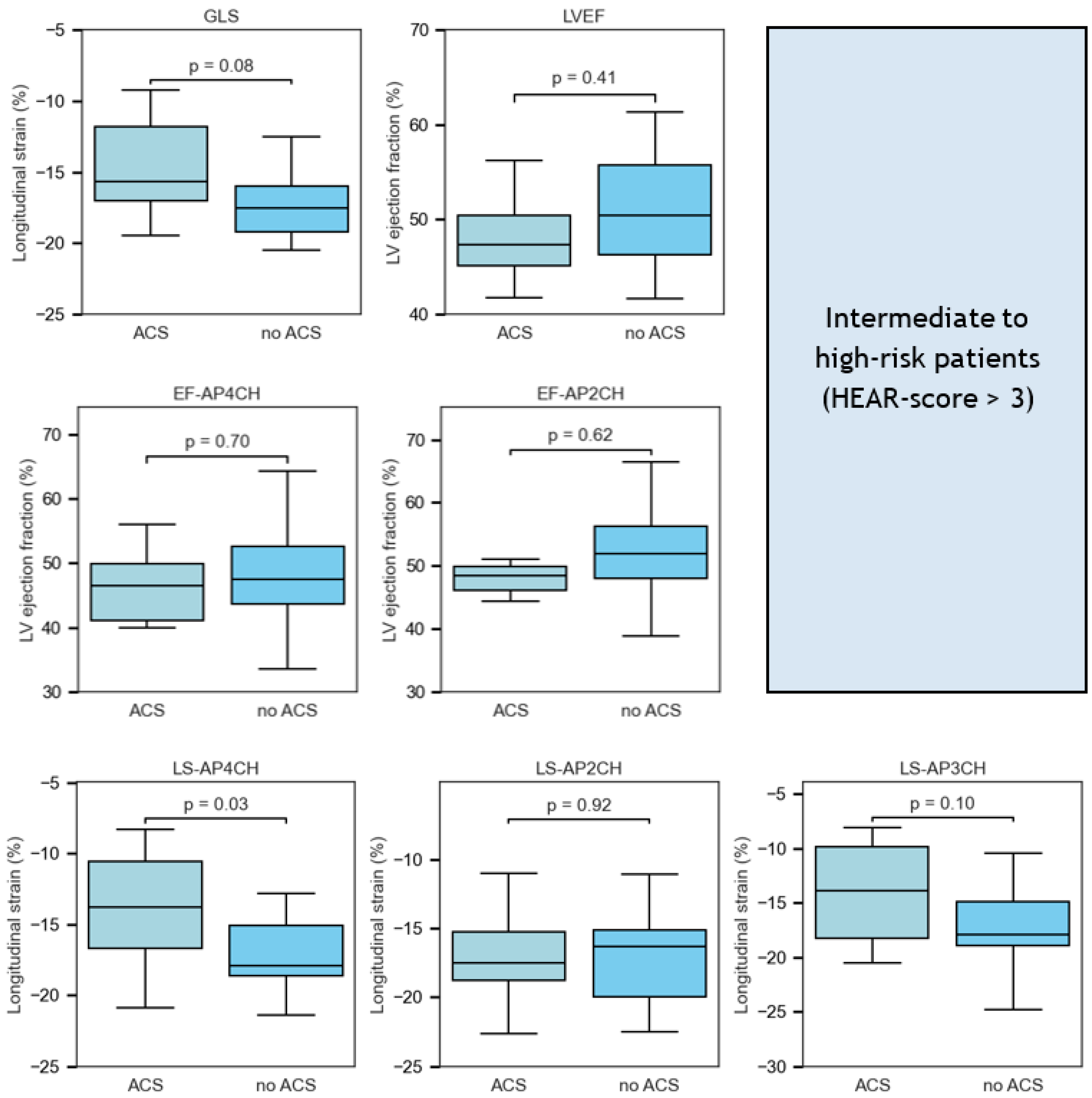
3.4. Intermediate–High Risk
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| AI | artificial intelligence |
| AP4CH | apical 4-chamber |
| AP2CH | apical 2-chamber |
| AP3CH | apical 3-chamber |
| AUC | area under the curve |
| CAG | coronary angiography |
| ECG | electrocardiogram |
| EF | ejection fraction |
| FoCUS | focus cardiac ultrasound |
| GLS | global longitudinal strain |
| HEAR(T) | history, ECG, age, risk factors (troponin) |
| LV | left ventricle |
| LS | longitudinal strain |
| LVEF | left ventricular ejection fraction |
| NSTEMI | non ST-elevation myocardial infarction |
| PCI | percutaneous coronary intervention |
| STEMI | ST-elevation myocardial infarction |
References
- Goodacre, S.; Cross, E.; Arnold, J.; Angelini, K.; Capewell, S.; Nicholl, J. The health care burden of acute chest pain. Heart 2005, 91, 229. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.P.; Andrew, E.; Nehme, Z.; Bloom, J.; Okyere, D.; Cox, S.; Anderson, D.; Stephenson, M.; Lefkovits, J.; Taylor, A.J.; et al. Incidence, diagnoses and outcomes of ambulance attendances for chest pain: A population-based cohort study. Ann. Epidemiol. 2022, 72, 32–39. [Google Scholar] [CrossRef]
- Dawson, L.P.; Smith, K.; Cullen, L.; Nehme, Z.; Lefkovits, J.; Taylor, A.J.; Stub, D. Care Models for Acute Chest Pain That Improve Outcomes and Efficiency: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2333–2348. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Golwala, H.; Tripathi, A.; Bin Abdulhak, A.A.; Bavishi, C.; Riaz, H.; Mallipedi, V.; Pandey, A.; Bhatt, D.L. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: A systematic review and meta-analysis. Eur. Heart J. 2017, 38, 3082–3089. [Google Scholar] [CrossRef]
- Spencer, K.T.; Flachskampf, F.A. Focused Cardiac Ultrasonography. JACC Cardiovasc. Imaging 2019, 12, 1243–1253. [Google Scholar] [CrossRef]
- Narang, A.; Bae, R.; Hong, H.; Thomas, Y.; Surette, S.; Cadieu, C.; Chaudhry, A.; Martin, R.P.; McCarthy, P.M.; Rubenson, D.S.; et al. Utility of a Deep-Learning Algorithm to Guide Novices to Acquire Echocardiograms for Limited Diagnostic Use. JAMA Cardiol. 2021, 6, 624–632. [Google Scholar] [CrossRef]
- Schneider, M.; Bartko, P.; Geller, W.; Dannenberg, V.; König, A.; Binder, C.; Goliasch, G.; Hengstenberg, C.; Binder, T. A machine learning algorithm supports ultrasound-naïve novices in the acquisition of diagnostic echocardiography loops and provides accurate estimation of LVEF. Int. J. Cardiovasc. Imaging 2021, 37, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Culp, B.C.; Mock, J.D.; Chiles, C.D.; Culp, W.C., Jr. The pocket echocardiograph: Validation and feasibility. Echocardiography 2010, 27, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Aldaas, O.M.; Igata, S.; Raisinghani, A.; Kraushaar, M.; DeMaria, A.N. Accuracy of left ventricular ejection fraction determined by automated analysis of handheld echocardiograms: A comparison of experienced and novice examiners. Echocardiography 2019, 36, 2145–2151. [Google Scholar] [CrossRef]
- Backus, B.E.; Six, A.J.; Kelder, J.C.; Mast, T.P.; van den Akker, F.; Mast, E.G.; Monnink, S.H.; van Tooren, R.M.; Doevendans, P.A. Chest pain in the emergency room: A multicenter validation of the HEART Score. Crit. Pathw. Cardiol. 2010, 9, 164–169. [Google Scholar] [CrossRef]
- Six, A.J.; Backus, B.E.; Kelder, J.C. Chest pain in the emergency room: Value of the HEART score. Neth. Heart J. 2008, 16, 191–196. [Google Scholar] [CrossRef]
- O’Rielly, C.M.; Andruchow, J.E.; McRae, A.D. External validation of a low HEAR score to identify emergency department chest pain patients at very low risk of major adverse cardiac events without troponin testing. Cjem 2022, 24, 68–74. [Google Scholar] [CrossRef]
- Todd, F.; Duff, J.; Carlton, E. Identifying low-risk chest pain in the emergency department without troponin testing: A validation study of the HE-MACS and HEAR risk scores. Emerg. Med. J. 2022, 39, 515–518. [Google Scholar] [CrossRef]
- Bergmann, I.; Buttner, B.; Teut, E.; Jacobshagen, C.; Hinz, J.; Quintel, M.; Mansur, A.; Roessler, M. Pre-hospital transthoracic echocardiography for early identification of non-ST-elevation myocardial infarction in patients with acute coronary syndrome. Crit. Care 2018, 22, 29. [Google Scholar] [CrossRef]
- Jacobsen, L.; Grenne, B.; Olsen, R.B.; Jortveit, J. Feasibility of prehospital identification of non-ST-elevation myocardial infarction by ECG, troponin and echocardiography. Emerg. Med. J. 2022, 39, 679–684. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Kebed, K.; Laffin, L.; Stone, J.; Addetia, K.; Lang, R.M.; Mor-Avi, V. Reproducibility and experience dependence of echocardiographic indices of left ventricular function: Side-by-side comparison of global longitudinal strain and ejection fraction. Echocardiography 2017, 34, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Myhre, P.L.; Gaibazzi, N.; Tuttolomondo, D.; Sartorio, D.; Ugolotti, P.T.; Covani, M.; Bettella, A.; Suma, S. Concordance of left ventricular volumes and function measurements between two human readers, a fully automated AI algorithm, and the 3D heart model. Front. Cardiovasc. Med. 2024, 11, 1400333. [Google Scholar] [CrossRef]
- Camaro, C.; Aarts, G.W.A.; Adang, E.M.M.; van Hout, R.; Brok, G.; Hoare, A.; Rodwell, L.; de Pooter, F.; de Wit, W.; Cramer, G.E.; et al. Rule-out of non-ST-segment elevation acute coronary syndrome by a single, pre-hospital troponin measurement: A randomized trial. Eur. Heart J. 2023, 44, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, A.; Zito, E.; Pierucci, N.; Matteucci, A.; La Fazia, V.M. A Talk with ChatGPT: The Role of Artificial Intelligence in Shaping the Future of Cardiology and Electrophysiology. J. Pers. Med. 2025, 15, 205. [Google Scholar] [CrossRef]
| n = 75 | |
|---|---|
| Age, years | 61 (±12) |
| Male sex, n (%) | 36 (48) |
| Body mass index, kg/m2 | 29 (25–31) |
| Diabetes mellitus, n (%) | 10 (13) |
| Hypertension, n (%) | 37 (49) |
| Dyslipidemia, n (%) | 26 (35) |
| Smoking, n (%) | 20 (27) |
| Positive family history, n (%) | 20 (27) |
| History of atherosclerotic disease, n (%) | 10 (13) |
| History of obstructive CAD, n (%) | 4 (5) |
| History of PCI, n (%) | 3 (4) |
| Heart rate, min−1 | 77 (71–98) |
| Systolic blood pressure, mmHg | 159 (145–183) |
| Diastolic blood pressure, mmHg | 95 (83–100) |
| Oxygen saturation, % | 98 (97–99) |
| HEAR-score | 4 (3–5) |
| HEART-score | 4 (3–6) |
| Imaging performed, n (%) | 31 (41) |
| Chest X-ray, n (%) | 20 (27) |
| TTE, n (%) | 14 (19) |
| CT-a scan, n (%) | 2 (3) |
| CAG in non PCI-capable center | 4 (5) |
| CAG in PCI-capable center | 11 (15) |
| ACS | 13 (17) |
| PCI performed | 8 (11) |
| Total (n = 75) | Sonographer (n = 54) | Paramedic (n = 21) | |
|---|---|---|---|
| Acquisition | |||
| One apical view acquired, n (%) | 68 (91) | 52 (96) | 16 (76) |
| Two apical views acquired, n (%) | 62 (83) | 51 (94) | 11 (52) |
| All apical views acquired, n (%) | 50 (67) | 45 (83) | 5 (24) |
| AP4CH view acquired, n (%) | 66 (88) | 52 (96) | 14 (67) |
| AP2CH view acquired, n (%) | 55 (73) | 46 (85) | 9 (43) |
| AP3CH view acquired, n (%) | 59 (79) | 50 (93) | 9 (43) |
| Quality | |||
| AP4CH view %, median (IQR) | 96 (83–100) | 96 (75–100) | 92 (83–100) |
| AP2CH view %, median (IQR) | 83 (71–100) | 83 (69–100) | 100 (83–100) |
| AP3CH view %, median (IQR) | 100 (75–100) | 100 (75–100) | 92 (75–100) |
| Successful measurements | |||
| GLS, n (%) | 50 (67) | 45 (83) | 5 (24) |
| LVEF, n (%) | 53 (71) | 44 (82) | 9 (43) |
| Predictor | AUC (95% CI) | Threshold | Sens (95% CI) | Spec (95% CI) | PPV (95% CI) | NPV (95% CI) | Acc (95% CI) |
|---|---|---|---|---|---|---|---|
| HEART-score | 0.89 (0.80–0.97) | 6.0 | 0.85 (0.60–1.00) | 0.88 (0.78–0.95) | 0.61 (0.38–0.84) | 0.96 (0.90–1.00) | 0.87 (0.79–0.94) |
| GLS | 0.76 (0.58–0.92) | −17.5 | 0.89 (0.64–1.00) | 0.56 (0.40–0.71) | 0.31 (0.14–0.48) | 0.96 (0.86–1.00) | 0.62 (0.50–0.76) |
| LS-AP4CH | 0.75 (0.55–0.90) | −17.5 | 0.91 (0.69–1.00) | 0.50 (0.37–0.64) | 0.27 (0.14–0.41) | 0.96 (0.89–1.00) | 0.57 (0.45–0.69) |
| HEAR-score | 0.74 (0.62–0.86) | 4.0 | 1.00 (1.00–1.00) | 0.46 (0.33–0.59) | 0.30 (0.15–0.45) | 1.00 (1.00–1.00) | 0.56 (0.44–0.67) |
| LS-AP3CH | 0.73 (0.52–0.91) | −13.0 | 0.50 (0.17–0.83) | 0.91 (0.83–0.98) | 0.56 (0.22–0.88) | 0.90 (0.80–0.98) | 0.84 (0.74–0.93) |
| EF-AP2CH | 0.71 (0.47–0.90) | 51.0 | 0.88 (0.57–1.00) | 0.67 (0.52–0.80) | 0.32 (0.14–0.53) | 0.97 (0.89–1.00) | 0.70 (0.58–0.81) |
| LVEF | 0.65 (0.37–0.89) | 48.5 | 0.75 (0.40–1.00) | 0.73 (0.59–0.85) | 0.33 (0.12–0.56) | 0.94 (0.86–1.00) | 0.74 (0.62–0.85) |
| LS-AP2CH | 0.60 (0.40–0.79) | −18.8 | 0.78 (0.50–1.00) | 0.43 (0.29–0.58) | 0.21 (0.09–0.35) | 0.91 (0.77–1.00) | 0.49 (0.36–0.62) |
| EF-AP4CH | 0.58 (0.37–0.79) | 47.7 | 0.70 (0.40–1.00) | 0.53 (0.39–0.67) | 0.23 (0.09–0.40) | 0.90 (0.78–1.00) | 0.56 (0.42–0.68) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kadi, S.; Zanstra, M.; Siegers, A.; Bouma, B.J.; van Rossum, A.C.; Kamp, O. Pre-Hospital Artificial Intelligence-Guided, Focused Echocardiography in Patients with Acute Chest Pain for Diagnosis of Acute Coronary Syndrome. J. Clin. Med. 2025, 14, 7938. https://doi.org/10.3390/jcm14227938
El Kadi S, Zanstra M, Siegers A, Bouma BJ, van Rossum AC, Kamp O. Pre-Hospital Artificial Intelligence-Guided, Focused Echocardiography in Patients with Acute Chest Pain for Diagnosis of Acute Coronary Syndrome. Journal of Clinical Medicine. 2025; 14(22):7938. https://doi.org/10.3390/jcm14227938
Chicago/Turabian StyleEl Kadi, Soufiane, Mark Zanstra, Arjen Siegers, Berto J. Bouma, Albert C. van Rossum, and Otto Kamp. 2025. "Pre-Hospital Artificial Intelligence-Guided, Focused Echocardiography in Patients with Acute Chest Pain for Diagnosis of Acute Coronary Syndrome" Journal of Clinical Medicine 14, no. 22: 7938. https://doi.org/10.3390/jcm14227938
APA StyleEl Kadi, S., Zanstra, M., Siegers, A., Bouma, B. J., van Rossum, A. C., & Kamp, O. (2025). Pre-Hospital Artificial Intelligence-Guided, Focused Echocardiography in Patients with Acute Chest Pain for Diagnosis of Acute Coronary Syndrome. Journal of Clinical Medicine, 14(22), 7938. https://doi.org/10.3390/jcm14227938








