Abstract
Background: A decreased level of adiponectin is known as a predictor of adverse left ventricular remodeling and major adverse cardiac events (MACEs). We evaluated long-term MACEs following ST-segment elevation myocardial infarction (STEMI) in relation to adiponectin levels. Methods: This prospective study included a total of 73 consecutive STEMI patients. Adiponectin, CK, CK-MB, cTnI, CRP, HDL cholesterol, LDL cholesterol, triglycerides, and other routine laboratory parameters were considered, and myocardial revascularization and two-dimensional echocardiography were performed. Subjects were divided into two groups according to their serum adiponectin concentrations. Results: In total, 24 (32.87%) patients suffered from MACEs, 19 (26.02%) with adiponectin value ≤ 1.8 ng/mL (group 1) and 5 (6.84%) with adiponectin value > 1.8 ng/mL (group 2) (p < 0.013). Heart failure (Killip >1) was present in 14 cases (19.17%) in group 1 and in 3 cases (4.1%) in group 2 (p < 0.001). Kaplan–Meier analysis was used to depict the occurrence of MACEs according to the adiponectin threshold identified during hospitalization (1.8 ng/mL). The log-rank test revealed a statistically significant difference in survival between groups (p = 0.013), and the AUC value for adiponectin was 0.77 (95% CI, 0.66–0.89), p = 0.01. Based on univariate logistic regression analysis, adiponectin and BMI were significantly associated with MACEs (p = 0.018, p = 0.034). Multivariate logistic regression analysis shows that serum adiponectin predicts MACEs after STEMI (p = 0.011). Conclusions: We found significant associations between adiponectin levels and MACEs in patients who survived STEMI. The established cut-off value of 1.8 ng/mL for adiponectin during hospitalization identified patients at risk for MACEs.
1. Introduction
Adiponectin, a 244-amino acid protein hormone secreted by adipose tissue, is encoded by the ADIPOQ gene localized to human chromosome 3q27. This novel adipokine was first identified in mice by Scherer et al., who termed it the “Adipocyte complement-related protein of 30 kDa” (Arcp30).
Matsuzawa and colleagues identified the human homologue in adipose tissue, referring to it as “adipose most 2 abundant gene transcript 1” (apM1). Tomita and colleagues identified adiponectin as a “gelatin-binding protein”, reporting its molecular mass to be nearly 28 kDa (GBP28) [1,2,3,4].
Recent studies have extended the sources of adiponectin beyond adipose tissue, demonstrating its synthesis and secretion by human cardiomyocytes [5]. Circulating adiponectin represents about 0.02% of the plasma protein and modulates a number of metabolic functions via receptors ADIPOR1, ADIPOR 2, and T-Cad [6,7,8]. Remarkably, Denzel et al. provided evidence that T-cadherin is necessary for adiponectin binding to cardiomyocytes [9,10].
Adiponectin, in association with other adipokines, has been shown to induce AMPK-Akt-phosphorylation and to potentiate anti-inflammatory peroxisome-proliferator-activated-receptor (PPAR)-a-effect [11,12]. Furthermore, adiponectin enhances nitric oxide (NO) both directly, via AMPK activation, and indirectly, by reducing the glucose level, contributing to vasodilatory cardioprotective effects and angiogenesis. Evidence suggests that PPAR agonists may serve as valuable therapeutic targets in the reduction of cardiovascular risk [13,14,15].
Ouchi et al. were the first to describe the vascular effects of adiponectin on endothelial vascular cells, reporting its ability to inhibit the expression of adhesion molecules, including intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E selectin [16,17,18] (Figure 1). ICAM-1, as a proinflammatory mediator, is associated with elevated soluble suppression of tumorigenicity-2 (sST2) levels, facilitating the recruitment and activation of inflammatory cells within the injured vascular wall. Through inhibition of the interleukin-33/suppression of tumorigenicity-2 (IL-33/ST2) axis, which mediates group 2 innate lymphoid cells (ILC2) activation, adiponectin exerts anti-inflammatory effects counteracting the proinflammatory milieu reflected in elevated sST2 concentrations [19,20].
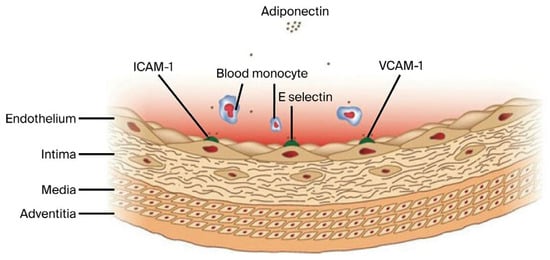
Figure 1.
Inhibitory effects of adiponectin on the expression of adhesion molecules ICAM-1, VCAM-1, and E-Selectin.
The anti-atherosclerotic effects of adiponectin are mediated through its anti-inflammatory actions and anti-atherogenic mechanisms [21,22,23,24] (Figure 2). Oxidative modification of low-density lipoprotein (LDL), involving both lipid and protein components, produces oxidized LDL (OxLDL). Elevated levels of OxLDL in the circulation and vascular wall contribute to endothelial dysfunction by downregulating eNOS expression, promoting HIF-1a accumulation, and inducing HIF-1-dependent gene activation in macrophages via the redox-mediated pathway [25,26,27,28,29,30].
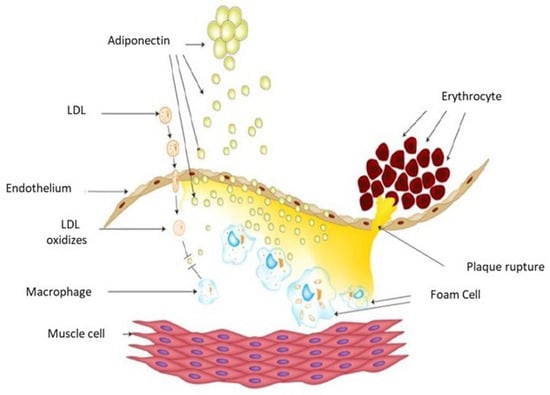
Figure 2.
Schematic drawing of the atherosclerosis process. Adiponectin inhibits OxLDL and reduces macrophage transformation into foam cells, leading to its consumption in circulating plasma.
Experimental studies demonstrate that adiponectin inhibits OxLDL-induced cell proliferation by suppressing cellular superoxide generation, thereby reducing the transformation of macrophages into foam cells [31,32]. Fibroblast growth factor 21 (FGF21) further protects against atherosclerosis by stimulating adiponectin expression in adipose tissue and inhibiting cholesterol synthesis in the liver [33,34,35].
Adiponectin protects against cardiac injury and failure by activating AMPK to prevent apoptosis and, via COX-2, suppressing TNF-ɑ production to reduce post-infarction systolic dysfunction [36,37,38,39,40,41].
Hemodynamic overload arising from increased vascular stiffness and peripheral resistance elevates left ventricular filling pressures in diastolic dysfunction and limits systolic reverse following myocardial infarction, which may contribute to heart failure and poor outcomes. Clinical evidence indicates that plasma adiponectin is an independent predictor of left ventricular systolic dysfunction, while reduced adiponectin levels are associated with diastolic dysfunction [42,43,44]. In an animal model of myocardial infarction, FGF21 gene transfer via intramuscular adenoviral injection improved systolic function after two weeks, an effect that was mediated through adiponectin signaling [45].
To our knowledge, no prior study has investigated the predictive value of adiponectin on long-term MACEs in STEMI patients.
The aim of this study is to evaluate the impact of hypoadiponectinemia as an independent predictor of major adverse cardiac events following STEMI.
2. Methods
2.1. Study Design
In this prospective study, 73 consecutive patients with ST-segment elevation myocardial infarction (STEMI) were included and divided into two groups according to their serum adiponectin concentrations (group 1 (adiponectin value ≤ 1.8 ng/mL) and group 2 (adiponectin value > 1.8 ng/mL)). Inclusion criteria for STEMI were as follows: (a) detection of cardiac biomarkers (cardiac troponin (cTn)) (ESC 0 h/1 h and 0 h/2 h algorithms), (b) symptoms of ischemia, and (c) ECG changes including new ST elevation at the J-point in at least two contiguous leads (≥2.5 mm in men <40 years, ≥2 mm in men ≥40 years, or ≥1.5 mm in women regardless of age in leads V2-V3 or ≥1 mm in the other leads), provided that left ventricular hypertrophy (LVH) or left bundle branch block (LBBB) were absent [46]. The exclusion criteria were previous myocardial infarction, previous diabetes mellitus, renal insufficiency, inflammatory disease, and acute infectious disease.
Clinical history was evaluated with regard to established coronary risk factors (dyslipidemia, arterial hypertension, and smoking), previous medications, and time from onset to admission. During the follow-up period, the risk of reinfarction appears to be especially driven by baseline characteristics and treatment patterns, rather than the infarct type itself (STEMI or NSTEMI) [47].
Laboratory parameters included adiponectin, creatine kinase (CK), the MB fraction of creatine kinase (CK-MB), cTnI, CRP, HDL cholesterol, LDL cholesterol, triglycerides, and routine parameters. Blood samples were obtained approximately 30 min after admission. Serum intended for adiponectin measurement was stored at −70 °C until biochemical analyses were performed. Serum adiponectin concentrations were measured with the ELISA method at room temperature (20–23 °C) in accordance with the manufacturer’s instructions using Phoenix Pharmaceuticals ELISA kits (Awareness Technologies Inc: UCCK, Prishtina), Kosovo; and a Siemens BEP 2000 (Dubrava University Hospital, Zagreb, Croatia).
Two-dimensional echocardiography was performed for all patients. The LV ejection fraction was assessed using the modified Simpson’s biplane method from apical 4- and 2-chamber views. Diastolic function was evaluated using mitral inflow PW Doppler (E/A, E wave deceleration time), tissue Doppler (E/e′), and pulmonary inflow PW Doppler (S, D) measurements and graded 0–3. Adverse LV remodeling was defined as a > 20% increase in LV end-diastolic or end-systolic volumes, while adverse diastolic remodeling was defined as ≥ 1 grade or persistence of a restrictive pattern at follow-up.
All patients underwent revascularization with periprocedural pharmacotherapy according to current guidelines. Post-PCI standard therapy included aspirin (100 mg), clopidogrel (75 mg), or prasugrel (10 mg); beta-blockers; lipid-lowering agents; and angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB), in line with international recommendations [46,48].
Over a follow-up period of one year, patients were prospectively evaluated for major adverse cardiac events (MACEs), comprising angina pectoris, reinfarction, congestive heart failure, stroke, and cardiac death.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional Ethics Committees of Dubrava University Hospital, Zagreb, and the University Clinical Center of Kosovo, Prishtina.
2.2. Statistical Analysis
Descriptive data are presented as means ± standard deviation (SD) for normally distributed variables and as median with interquartile range (IQR) for non-normally distributed variables. Comparisons between the groups were performed using Student’s t test, the Kruskal–Wallis test, the Mann–Whitney test, and the chi-square test, depending on the distribution and types of variables. Receiver operating characteristic (ROC) curve analysis provided a graphical representation of the true positive rate versus the false positive rate across different cut-off points, facilitating the selection of the optimal threshold for a given context. The area under the curve (AUC) quantified the predictive accuracy for binary outcomes. Univariate and multivariate logistic regression analyses were subsequently performed to identify predictors of major adverse cardiac events (MACEs). Kaplan–Meier estimates were used to assess the association between adiponectin levels and MACEs over a 12-month follow-up period. Kaplan–Meier survival analysis was applied since only one predictor was used (adiponectin).
MACEs were categorized as MACE type 1 (angina pectoris), 2 (myocardial reinfarction), 3 (heart failure), 4 (stroke), or 5 (death) for the purpose of statistical analysis. The cut-off of adiponectin was determined using the value of adiponectin as a test variable, and MACE type 3 was used as a state variable, resulting in an adiponectin value of 1.8 ng/mL. The log-rank test was used to compare the survival distributions of two samples. A two-tailed p-value of <0.05 was considered statistically significant. Data were analyzed using SPSS statistical software, version 26.
3. Results
3.1. Patient Characteristics
The baseline characteristics of the study population are detailed in Table 1. The mean age was 59.91 ±11.21 years, and 55 (75.34%) participants were male. A total of 43 (58.9%), 11 (15.06%), and 37 (50.68%) patients had a medical history of hypertension, prediabetes mellitus, and smoking, respectively. In terms of the coronary angiographic findings, the number of patients with multivessel coronary artery disease was 22 (30.13%). An inverse correlation between adiponectin and BMI was observed using Spearman’s correlation analysis (Figure 3, p = 0.02) and HbA1C (Figure 4, p < 0.001) in all study subjects. The comparison of body mass index (BMI) and HbA1C between groups with different levels of adiponectin (≤1.8 ng/mL and >1.8 ng/L) was statistically significant (the p-value was 0.003 for BMI and 0.023 for HbA1C).

Table 1.
Baseline characteristics of patients.
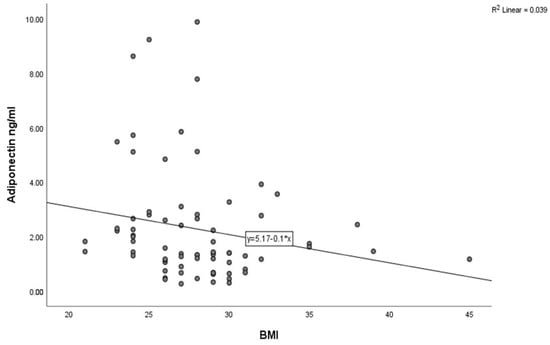
Figure 3.
Correlation between adiponectin levels and BMI in the study population (Spearman’s correlation coefficient r = −0.269, p < 0.02).
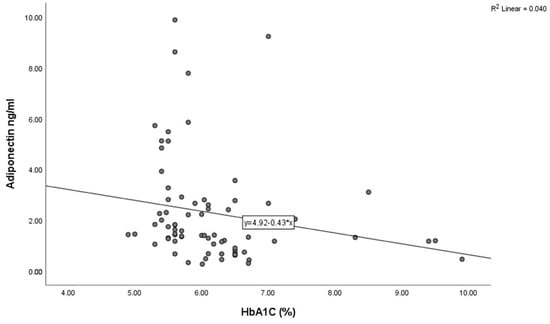
Figure 4.
Correlation between adiponectin levels and HbA1C in the study population (Spearman’s correlation coefficient r = −0.384, p < 0.01).
3.2. Risk Factors and MACEs
To identify factors associated with the occurrence of MACEs, we performed univariate logistic regression analysis including age, gender, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, adiponectin, LDL cholesterol, HDL cholesterol, triglycerides, fasting glucose, creatinine, and CRP levels. Adiponectin and BMI were significantly associated with MACEs (p = 0.018, p = 0.034) (Table 2). In multivariate logistic regression analysis, serum adiponectin independently predicted the occurrence of MACEs after STEMI (p = 0.011) (Table 3).

Table 2.
Predictors of MACEs in univariate regression analysis.

Table 3.
Multivariate regression analysis for MACEs.
3.3. Adiponectin and MACEs
In total, 24 patients (32.87%) experienced MACEs, 19 (26.02%) with adiponectin values ≤ 1.8 ng/mL and 5 (6.84%) with values > 1.8 ng/mL (p < 0.013) (Table 1). Heart failure (Killip > 1) occurred in 14 cases (19.17%) in group 1 and in 3 cases (4.1%) in group 2 (p < 0.001). Kaplan–Meier curves were used to show the number of MACEs and the proportion of patients that survived at each even time point based on the cut-off value of adiponectin during hospitalization (1.8 ng/mL) (Figure 5) [49]. The log-rank test demonstrated a significant difference in survival between groups (p = 0.013).
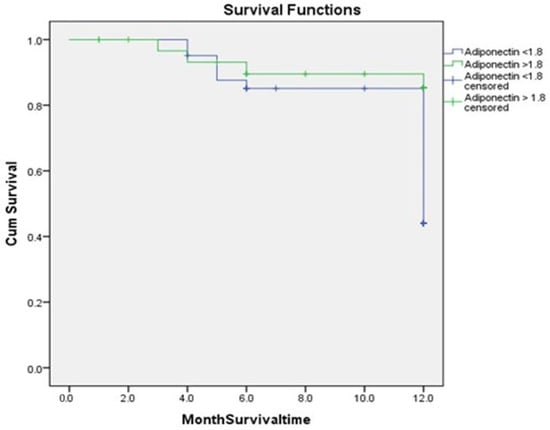
Figure 5.
Kaplan–Meier estimates showing higher recurrence rates of MACEs among patients with adiponectin levels ≤ 1.8 compared to higher adiponectin levels > 1.8 (p = 0.013).
A receiver operating characteristic (ROC) curve, which plots the true positive rate against the false positive rate across varying cut-off points, showed an AUC of 0.77 (95% CI, 0.66–0.89), p = 0.01 (Figure 6) [49].
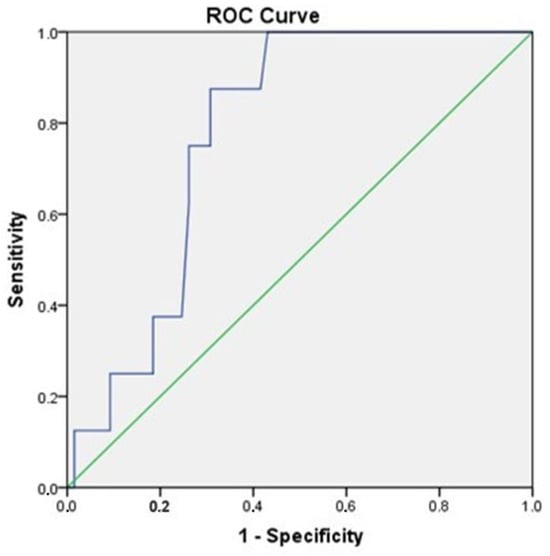
Figure 6.
ROC curve analysis of adiponectin for the prediction of MACEs (95% CI, 0.66–0.89), p = 0.01.
Table 4 presents the area under the curve (AUC) values for the biomarkers (troponin I, creatine kinase, creatine kinase-MB, adiponectin, and C-reactive protein).

Table 4.
Area under the curve values for biomarkers.
4. Discussion
In this study, we investigated the influence of adiponectin on the prediction of long-term MACEs in non-diabetic STEMI patients.
Collateral blood flow plays a crucial role after acute coronary ischemia. In our study, 30.12% of patients presented with multiple-vessel disease, with no significant difference between groups. Furthermore, low adiponectin levels were found in all patients, independent of anatomical localization and the number of diseased arteries. These findings imply that adiponectin may be involved in the pathophysiological response during the acute phase of coronary artery disease. One possible explanation is that the process of new microvessel formation after acute myocardial infarction may be associated with decreased circulating adiponectin concentrations. Consistent with this, recent evidence shows that adiponectin facilitates the migratory activity of endothelial progenitor cells [50,51]. Therefore, adiponectin may play a contributory role in revascularization processes, leading to its utilization and subsequent reduction in circulating plasma levels. Low plasma adiponectin levels may also relate to microvascular myocardial ischemia or impaired myocardial energy utilization [52,53]. We speculate that cellular-level consumption of endogenous adiponectin during neovascularization after acute coronary syndrome may lead to hyperglycemia. In our study, HbA1c values differed significantly between the two adiponectin groups (p = 0.023). These findings suggest that the degree of hyperglycemia reflects the severity of hypoadiponectinemia, which in turn may activate counter-protective mechanisms responsible for major adverse cardiac events.
In our study, the participants were not within the normal or healthy weight range, and a significant difference was observed between the two groups. Previous research reported that weight reduction therapy increased the plasma adiponectin concentration, and an inverse correlation of adiponectin levels with body weight and obesity has been well established [54,55,56]. The present findings confirm this association in non-diabetic patients.
Our findings confirmed that adiponectin inversely correlated with HbA1C and BMI (Figure 3 and Figure 4), while all of these parameters were associated with MACEs, supporting the idea that adiponectin alone or together with HbA1C level and not just HbA1C alone predicts the cardiovascular risk. In non-diabetic STEMI patients, adiponectin appears to reflect not only chronic metabolic risk but also acute insulin-resistance states and endothelial dysfunction that influence reperfusion efficacy and post-infarction remodeling. A lower preprocedural adiponectin level is strongly associated with increased insulin resistance, as reflected by higher HOMA-IR scores, and an increased risk of restenosis and adverse ischemic events, suggesting that hypoadiponectinemia identifies a metabolically vulnerable phenotype even in the absence of diagnosed diabetes [57]. Recent reports further indicate that adiponectin, induced via fibroblast growth factor 21, contributes to the regulation of energy and vascular homeostasis and provides protection against cardiometabolic disorders [34,35].
The present study demonstrated that serum adiponectin levels were indirectly associated with plasma HDL cholesterol concentrations, suggesting that HDL may serve as an intermediate factor in the relationship between serum adiponectin and coronary artery disease. Adiponectin was shown to increase the level of HDL through the inhibition of hepatic lipase expression. Both adiponectin and HDL have also been reported to increase nitric oxide production via activation of the AMPK-Akt-eNOS phosphorylation pathway, thereby contributing to vasodilatory cardioprotective effects [58,59,60,61,62]. Also, the combined action of statins (HMG-CoA reductase inhibitors) and adiponectin has been reported to upregulate eNOS expression [63,64]. Thus, the observed association between adiponectin and HDL may indirectly support the hypothesis that impaired angiogenesis and altered vascular tone regulation in non-diabetic patients following acute myocardial infarction play a pivotal role in the pathophysiology of major adverse cardiac events.
Adiponectin is closely related to metabolic regulation and is considered to exert both anti-atherogenic and anti-inflammatory effects. Previous studies have demonstrated an association between adiponectin and subclinical atherosclerosis [65,66]. In patients with significant coronary stenosis, coronary flow reserve has been correlated with vulnerable plaques and elevated CRP levels. However, in our study, no association was found between CRP levels and the prediction of MACEs, suggesting that CRP predominantly reflects the acute phase of coronary artery disease. We therefore hypothesize that, following acute coronary syndrome, patients may lose the protective effects of adiponectin, potentially due to its involvement in OxLDL inhibition and the consequent reduction in macrophage transformation into foam cells (Figure 2). This results in adiponectin consumption in the circulating plasma and a high level of CRP. Such a mechanism may explain both the consumption of adiponectin in the circulation and the concomitant elevation in CRP.
In STEMI patients, adiponectin and NT-proBNP have emerged as valuable biomarkers for predicting major adverse cardiac events (MACEs). Low adiponectin levels in the acute phase of myocardial infarction are associated with elevated left ventricular filling pressures due to diastolic dysfunction, adverse left ventricular remodeling, and increased MACE incidence. Conversely, elevated NT-proBNP reflects myocardial wall stress, neurohormonal activation, and impaired ventricular function, serving as an independent predictor of heart failure and mortality [67,68,69,70,71]. In our study, low levels of adiponectin in the acute phase were associated with high rates of MACEs after STEMI, with significant variability in admission-day adiponectin values observed among patients stratified by MACE occurrence [72]. A cut-off value of 1.8 ng/mL for adiponectin levels on the day of admission was set to perform survival analysis and examine the number of MACEs and corresponding survival rates.
Establishing biomarkers and their respective cut-offs for forecasting LV remodeling and long-term MACEs post-myocardial infarction provides valuable insights into clinical management [73]. This approach enables early initiation of appropriate treatment for these patients.
One limitation is that this observational study had a relatively limited number of patients.
5. Conclusions
We found significant associations between adiponectin levels and MACEs in patients who survived acute ST-elevation myocardial infarction. The established cut-off value of 1.8 ng/mL for adiponectin during hospitalization identified patients at risk for MACEs.
Author Contributions
Conceptualization, X.K. and J.V.; methodology, X.K. and B.B.; software, I.B., A.B. and B.B.; validation, A.B., I.B. and J.V.; investigation, X.K. and B.B.; writing—original draft preparation, X.K. and A.B.; writing—review and editing: I.B. and A.B.; supervision, J.V. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional Ethics Committee of Dubrava University Hospital-Zagreb and the University Clinical Center of Kosovo, Prishtina (413, 21 January 2011).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in this study in accordance with ethical guidelines.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request due to privacy and ethical considerations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nguyen, T.M. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matzusawa, Y. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef]
- Nakano, Y.; Tobe, T.; Choi-Miura, N.H.; Mazda, T.; Tomita, M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 1996, 120, 803–812. [Google Scholar] [CrossRef]
- Pineiro, R.; Iglesias, M.J.; Gallego, R.; Raghay, K.; Eliras, S.; Rubio, J.; Diéguez, C.; Gualillo, O.; Gonzalez-Juanatey, J.R.; Lago, F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005, 579, 5163–5169. [Google Scholar] [CrossRef] [PubMed]
- Crosson, S.M.; Marques, A.; Dib, P.; Dotson, C.D.; Munger, S.D.; Zolotukhin, S. Taste receptor cells in mice express receptors for the hormone adiponectin. Chem. Senses 2019, 44, 409–422. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Hug, C.H.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313. [Google Scholar] [CrossRef]
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 2018, 3, e99680. [Google Scholar] [CrossRef]
- Denzel, M.S.; Scimia, M.; Zumstein, P.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-catherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef]
- Jung, H.N.; Jung, C.H. The role of anti-inflammatory adipokines in cardiometabolic disorders: Moving beyond adiponectin. Int. J. Mol. Sci. 2021, 22, 13529. [Google Scholar] [CrossRef]
- Krasniqi, X.; Vincelj, J.; Kocinaj, D.; Bakalli, A. Evaluative Value of Apelin-12 in Acute Myocardial Infarction. Int. Cardiovasc. Res. J. 2023, 17, e139796. [Google Scholar]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I PPAR-α. Future Cardiol. 2017, 13, 259–278. [Google Scholar] [CrossRef]
- Marfella, R.; D’Amico, M.; Di Filippo, C.; Piegari, E.; Nappo, F.; Esposito, K.; Berrino, L.; Rossi, F.; Giugliano, D. Myocardial infarction in diabetic rats: Role of hyperglycemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia 2002, 45, 1172–1181. [Google Scholar]
- Nicholls, S.J.; Uno, K. Peroxisome proliferator-activated receptor (PPARα/γ) agonists as a potential target to reduce cardiovascular risk in diabetes. Diab Vasc. Dis. Res. 2012, 9, 89–94. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, L.; Zhong, N.; Wen, D.; Liu, L. Multifaced roles of adipokines in endothelial cell function. Front. Endocrinol. 2024, 15, 1490143. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuryama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura Tet, a.l. Novel modulator for endothelial adhesion molecules adipocyte- derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M.; et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar] [CrossRef]
- Roy, I.; Jover, E.; Matilla, L.; Alvarez, V.; Fernández-Celis, A.; Beunza, M.; Escribano, E.; Gainza, A.; Sádaba, R.; López-Andrés, N. Soluble ST2 as a new oxidative stress and inflammation marker in metabolic syndrome. Int. J. Environ. Res. Public Health 2023, 20, 2579. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Luo, L.; Wu, D.; Ding, X.; Zheng, H.; Wu, H.; Liu, B.; Yang, X.; Silva, F.; et al. Adiponectin restrains ILC2 activation by AMPK-mediated feedback inhibi-tion of IL-33 signaling. J. Exp. Med. 2021, 218, e20191054. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; Xu, X.; Terasawa, T.; Dong, L.Q. Anti-inflammatory and anti-proliferative action of adiponectin mediated by insulin signaling cascade in human vascular smooth muscle cells. Mol. Biol. Rep. 2020, 47, 6561–6572. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Qiu, S.; Yang, G.; Wu, Q. Adiponectin and metabolic cardiovascular diseases: Therapeutic opportunities and challenges. Genes. Dis. 2023, 10, 1525–1536. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002, 10, 2767–2770. [Google Scholar] [CrossRef]
- Diez, J.J.; Iglesias, P. The role of the novel adipocite-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Kakino, A.; Fujita, Y.; Ke, L.Y.; Chan, H.C.; Tsai, M.H.; Dai, C.Y.; Chen, C.-H.; Sawamura, T. Adiponectin forms a complex with atherogenic LDL and inhibits its downstream effects. J. Lipid Res. 2021, 62, 100001. [Google Scholar] [CrossRef]
- Steinberg, D.; Witztum, J.L. Lipoproteins and atherogenesis: Current concepts. JAMA 1990, 264, 3047–3052. [Google Scholar] [CrossRef]
- Tsimikas, S. Oxidized low-density lipoprotein. Curr. Atheroscler. Rep. 2006, 8, 55–61. [Google Scholar] [CrossRef]
- Penny, W.F.; Ben-Yehuda, O.; Kuroe, K.; Long, J.; Bond, A.; Bhargava, V.; Peterson, J.F.; McDaniel, M.; Juliano, J.; Witztum, J.L.; et al. Improvement of coronary artery endothelial dysfunction with lipid-lowering therapy: Heterogeneity of segmental response and correlation with plasma-oxidized low density lipoprotein. J. Am. Coll. Cardiol. 2001, 37, 766–774. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Romeo, F.; Sawamura, T.; Saldeen, T.; Mehta, J. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: Role of LOX-1. J. Pharmacol. Exp. Ther. 2002, 302, 601–605. [Google Scholar] [CrossRef]
- Shatrov, V.A.; Sumbayev, V.V.; Zhou, J.; Brüne, B. Oxidized low-density lipoprotein (oxLDL) triggers hypoxia-inducible factor-1α (HIF-1α) accumulation via redox-dependent mechanisms. Blood J. 2003, 101, 4847–4849. [Google Scholar] [CrossRef]
- Sargolzaei, J.; Chamani, E.; Kazemi, T.; Fallah, S.; Soori, H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clin. Biochem. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Nishida, M.; Matsuyama, A.; Okamoto, Y.; Ishigami, M.; Kuriyama, H.; Kishida, K.; Nishizawa, H. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 2001, 103, 1057–1063. [Google Scholar] [CrossRef]
- Tabari, F.S.; Karimian, A.; Parsian, H.; Rameshknia, V.; Mahmoodpour, A.; Majidinia, M.; Maniati, M.; Yousefi, B. The roles of FGF21 in atherosclerosis pathogenesis. Rev. Endocr. Metab. Disord. 2019, 20, 103–114. [Google Scholar] [CrossRef]
- Hui, X.; Feng, T.; Liu, Q.; Gao, Y.; Xu, A. The FGF21–adiponectin axis in controlling energy and vascular homeostasis. J. Mol. Cell Biol. 2016, 8, 110–119. [Google Scholar] [CrossRef]
- Lin, Z.; Pan, X.; Wu, F.; Ye, D.; Zhang, Y.; Wang, Y.; Jin, L.; Lian, Q.; Huang, Y.; Ding, H.; et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 2015, 131, 1861–1871. [Google Scholar] [CrossRef]
- Shibata, R.; Sato, K.; Pimentel, D.R.; Takemura, Y.; Kihara, S.; Ohashi, K.; Funahashi, T.; Ouchi, N.; Walsh, K. Adiponectin protects against myocardial ischemia–reperfusion injury through AMPK- and COX-2 dependent mechanism. Nat. Med. 2005, 11, 1096–1103. [Google Scholar] [CrossRef]
- Kalisz, M.; Baranowska, B.; Wolińska-Witort, E.; Mączewski, M.; Mackiewicz, U.; Tułacz, D.; Gora, M.; Martyńska, L.; Bik, W. Total and high molecular weight adiponectin levels in the rat model of post-myocardial infarction heart failure. J. Physiol. Pharmacol. 2015, 66, 673–680. [Google Scholar]
- Zaidi, H.; Aksnes, T.; Åkra, S.; Eggesbø, H.B.; Byrkjeland, R.; Seljeflot, I.; Opstad, T.B. Abdominal adipose tissue associates with adiponectin and TNFα in middle-aged healthy men. Front. Endocrinol. 2022, 13, 874977. [Google Scholar] [CrossRef]
- Bryant, D.; Becker, L.; Richardson, J.; Shelton, J.; Franco, F.; Peschock, R.; Thompson, M.; Giroir, B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation 1998, 97, 1375–1381. [Google Scholar] [CrossRef]
- Sugano, M.; Hata, T.; Tsuchida, K.; Suematsu, N.; Oyama, J.; Satoh, S.; Makino, N. Local delivery of soluble TNF-α receptor 1gene reduces infarct size following ischemia/reperfusion injury in rats. Mol. Cell Biochem. 2004, 266, 127–132. [Google Scholar] [CrossRef]
- Shibata, R.; Izumiya, Y.; Sato, K.; Papanicolaou, K.; Kihara, S.; Colluci, W.; Sam, F.; Ouchi, N.; Walsh, K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J. Mol. Cell Cardiol. 2007, 42, 1065–1074. [Google Scholar] [CrossRef]
- Norvik, J.V.; Schirmer, H.; Ytrehus, K.; Jenssen, T.G.; Zykova, S.N.; Eggen, A.E.; Eriksen, B.O.; Solbu, M.D. Low adiponectin is associated with diastolic dysfunction in women: A cross-sectional study from the Tromsø Study. BMC Cardiovasc. Disord. 2017, 17, 79. [Google Scholar] [CrossRef]
- Cavusoglu, E.; Chobra, V.; Battala, V.; Ruwende, C.; Yanamadala, S.; Eng, C.; Pinsky, D.J.; Marmur, J.D. Baseline plasma adiponectin levels as a predictor of left ventricular dysfunction in patient referred for coronary angiography. Am. J. Cardiol. 2008, 101, 1073–1078. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Varughese, G.I.; Sriraman, R.; Arunagirinathan, G. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J. Diabetes 2013, 4, 177–189. [Google Scholar] [CrossRef]
- Joki, Y.; Ohashi, K.; Yuasa, D.; Shibata, R.; Ito, M.; Matsuo, K.; Kambara, T.; Uemura, Y.; Hayakawa, S.; Hiramatsu-Ito, M.; et al. FGF21 attenuates pathological myo-cardial remodeling following myocardial infarction through the adiponectin-dependent mecha-nism. Biochem. Biophys. Res. Commun. 2015, 459, 124–130. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Spadafora, L.; Pastena, P.; Cacciatore, S.; Betti, M.; Biondi-Zoccai, G.; D’Ascenzo, F.; De Ferrari, G.M.; De Filippo, O.; Versaci, F.; Sciarretta, S.; et al. One-Year Prognostic Differences and Management Strategies between ST-Elevation and Non-ST-Elevation Myocardial Infarction: Insights from the PRAISE Registry. Am. J. Cardiovasc. Drugs 2025, 25, 681–691. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardiothorac. Surg. 2019, 55, 4–90. [Google Scholar] [CrossRef]
- Krasniqi, X.; Bakalli, A.; Vincelj, J.; Berisha, B.; Sejdiu Basri Zijabeg, D. Predictive value of adiponectin on long term MACE in STEMI patients. Anatol. J. Cardiol. 2024, 28 (Suppl. 1), S1–S185. [Google Scholar]
- Nakamura, N.; Naruse, K.; Matsuki, T.; Hamada, Y.; Nakashima, E.; Kamiya, H.; Matsubara, T.; Enomoto, A.; Takahashi, M.; Oiso, Y.; et al. Adiponectin promotes migration activities of endothelial progenitor cells via Cdc42/Rac1. FEBS Lett. 2009, 583, 2457–2463. [Google Scholar] [CrossRef]
- Wang, S.; Miao, J.; Qu, M.; Yang, G.Y.; Shen, L. Adiponectin modulates the function of endothelial progenitor cells via AMPK/eNOS signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 64–70. [Google Scholar] [CrossRef]
- Refaat, H.; Tantawy, A. Low plasma adiponectin levels are associated with vulnerable plaque features in patients with acute coronary syndrome: An optical coherence tomography study. Cardiovasc. Revasc Med. 2021, 25, 63–71. [Google Scholar] [CrossRef]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Zuriaga, M.A.; MacLauchlan, S.; Aprahamian, T.R.; Walsh, K. Divergent roles for adiponectin receptor 1 (AdipoR1) and AdipoR2 in mediating revascularization and metabolic dysfunction in vivo. J. Biol. Chem. 2014, 289, 16200–16213. [Google Scholar] [CrossRef]
- Lim, S.; Quon, M.J.; Koh, K.K. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis 2014, 233, 721–728. [Google Scholar] [CrossRef]
- Puglisi, M.J.; Fernandez, M.L. Modulation of C-reactive protein, tumor necrosis factor-α, and adiponectin by diet, exercise, and weight loss. J. Nutr. 2008, 138, 2293–2296. [Google Scholar] [CrossRef]
- Coughlin, C.C.; Finck, B.N.; Eagon, J.C.; Halpin, V.J.; Magkos, F.; Mohammed, B.S.; Klein, S. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity 2007, 15, 640–645. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Marfella, R.; Calabrò, P.; Piscione, F.; Furbatto, F.; Esposito, G.; Galiero, R.; Gragnano, F.; Rinaldi, L.; et al. Adiponectin and insulin resistance are related to restenosis and overall new PCI in subjects with normal glucose tolerance: The prospective AIRE Study. Cardiovasc. Diabetol. 2019, 18, 24. [Google Scholar] [CrossRef]
- Ouchi, N.; Kobayashi, H.; Kihara, S.; Kumada, M.; Sato, K.; Inoue, T.; Funahashi, T.; Walsh, K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004, 279, 1304–1309. [Google Scholar] [CrossRef]
- Chen, H.; Montagnani, M.; Funahashi, T.; Shimomura, I.; Quon, M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef]
- Nofer, J.R.; van der Giet, M.; Tolle, M.; Wolinska, I.; von Wnuck Lipinski, K.; Baba, H.; Tietge, U.J.; Gödecke, A.; Ishii, I.; Kleuser, B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Investig. 2004, 113, 569–581. [Google Scholar] [CrossRef]
- Drew, B.G.; Fidge, N.H.; Gallon-Beaumier, G.; Kemp, B.; Kingwell, B. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc. Natl. Acad. Sci. USA 2004, 101, 6999–7004. [Google Scholar] [CrossRef]
- Katsiki, N.; Mantzoros, C.S. Statins in relation to adiponectin: A significant association with clinical implications. Atherosclerosis 2016, 253, 270–272. [Google Scholar] [CrossRef][Green Version]
- Celermajer, D.; Chow, C.; Marijon, E.; Anstey, N.; Woo, K. Cardiovascular Disease in the Developing World: Prevalences, Patterns, and the potential of Early Disease Detection. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Omelchenko, E.; Gavish, D.; Shargorodsky, M. Adiponectin is better predictor of subclinical atherosclerosis than liver function tests in patients with nonalcoholic fatty liver disease. J. Am. Soc. Hypertens. 2014, 8, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Zusi, C.; Csermely, A.; Rinaldi, E.; Bertoldo, K.; Bonetti, S.; Boselli, M.L.; Travia, D.; Bonora, E.; Bonadonna, R.C.; Trombetta, M. Crosstalk between genetic variability of adiponectin and leptin, glucose--insulin system and subclinical atherosclerosis in patients with newly diagnosed type 2 diabetes. The Verona Newly Diagnosed Type 2 Diabetes Study 14. Diabetes Obes. Metab. 2023, 25, 2650–2658. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Adverse Cardiac Remodelling after Acute Myocardial Infarction: Old and New Biomarkers. Dis. Markers 2020, 2020, 1215802. [Google Scholar] [CrossRef]
- Kercheva, M.; Ryabova, T.; Gusakova, A.; Suslova, T.E.; Ryabov, V.; Karpov, R.S. Serum Soluble ST2 and Adverse Left Ventricular Remodeling in Patients with ST-Segment Elevation Myocardial Infarction. Clin. Med. Insights Cardiol. 2019, 13, 1179546819842804. [Google Scholar] [CrossRef]
- Barbarash, O.; Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Belik, E.; Akbasheva, O.; Karetnikova, V.; Shilov, A. Prognostic Value of Soluble ST2 During Hospitalization for ST-Segment Elevation Myocardial Infarction. Ann. Lab. Med. 2016, 36, 313–319. [Google Scholar] [CrossRef]
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. JACC Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef]
- Mehran, R.; Steg, P.G.; Pfeffer, M.A.; Jering, K.; Claggett, B.; Lewis, E.F.; Granger, C.; Køber, L.; Maggioni, A.; Mann, D.L.; et al. The Effects of Angiotensin Receptor-Neprilysin Inhibition on Major Coronary Events in Patients with Acute Myocardial Infarction: Insights from the PARADISE-MI Trial. Circulation 2022, 146, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Berisha, B. Influence of Adiponectin and Glycated Hemoglobin in Prediction of Major Adverse Cardiac Events in Non-Diabetic Patients After ST-Segment Elevation Myocardial Infarction. Ph.D. Thesis, University of Zagreb, School of Medicine, Zagreb, Croatia, 2022. [Google Scholar]
- Krasniqi, X.; Bakalli, A.; Kocinaj, D.; Krasniqi, F.; Vincelj, J. Identifying biomarkers and establishing their threshold values concordantly with LV remodeling for predicting long-term MACE following STEMI. Eur. Heart J. Cardiovasc. Imaging 2025, 26 (Suppl. 1), jeae333.088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).