Eight-Year Retrospective Analysis of Mortality in Patients with Moderate to Severe Hyponatremia: A Comprehensive Study
Abstract
1. Introduction
2. Methods
2.1. Power Analysis
2.2. Statistical Analysis
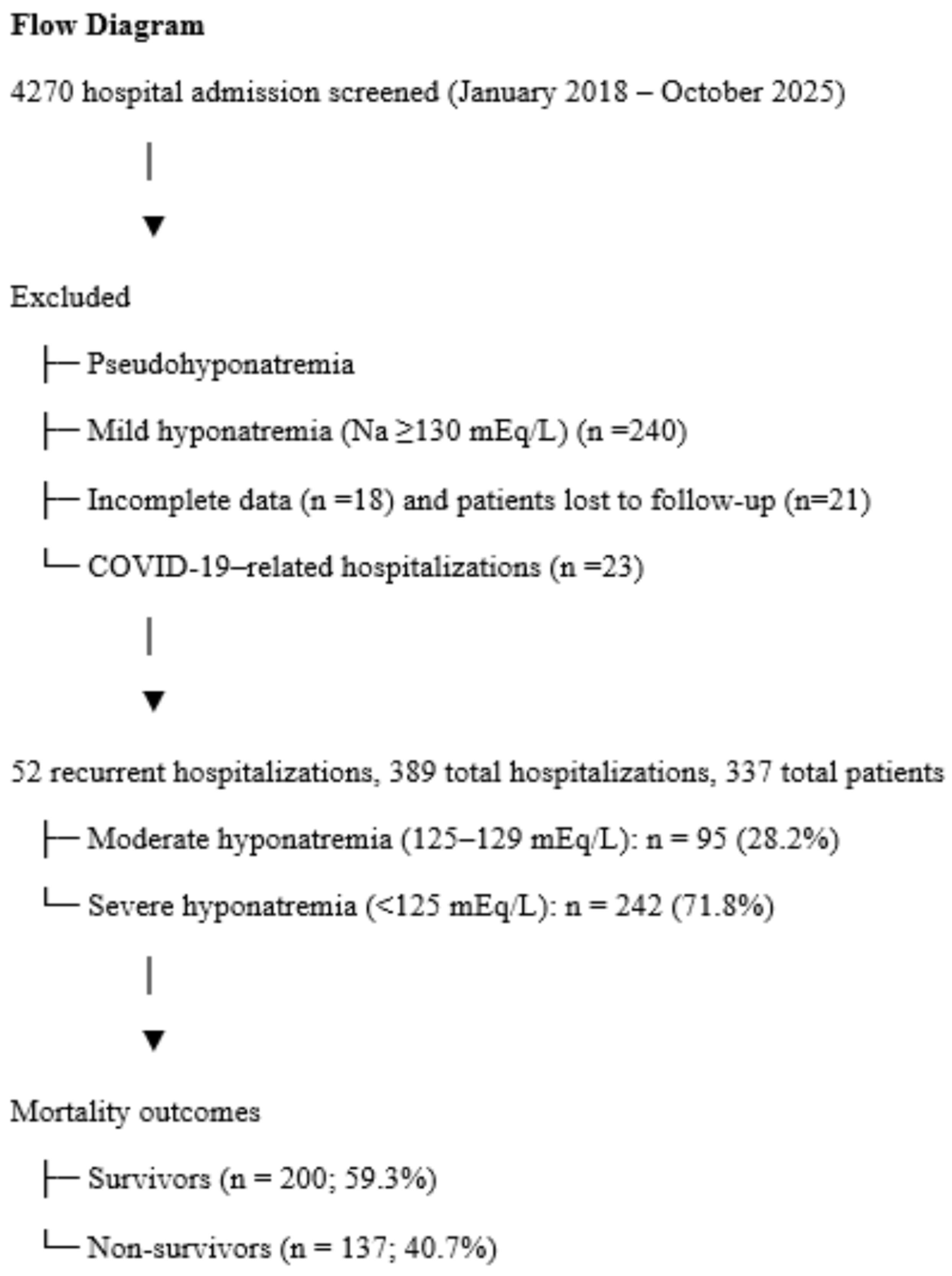
3. Results
3.1. Mortality Comparison (Table 1)
Demographic and Clinical Characteristics
| Mortality | |||
|---|---|---|---|
| Survivors (n = 200) | Non-Survivors (n = 137) | p-Value | |
| Demographical | |||
| Age (years) | 65.78 ± 15.64 | 71.66 ± 14.09 | <0.001 1 |
| Sex (F/M) | 127/73 | 76/61 | 0.139 2 |
| Admission season | 0.999 2 | ||
| Autumn | 47 (23.5) | 33 824.1) | |
| Spring | 40 (20) | 27 (19.7) | |
| Summer | 70 (35) | 48 (35) | |
| Winter | 43 (21.5) | 29 (21.2) | |
| Comorbidities | |||
| Diabetes mellitus | 80 (40) | 58 (42.3) | 0.668 2 |
| Hypertension | 150 (75) | 97 (70.8) | 0.392 2 |
| CKD | 44 (22) | 36 (26.3) | 0.365 2 |
| CAD | 77 (38.5) | 68 (49.6) | 0.043 2 |
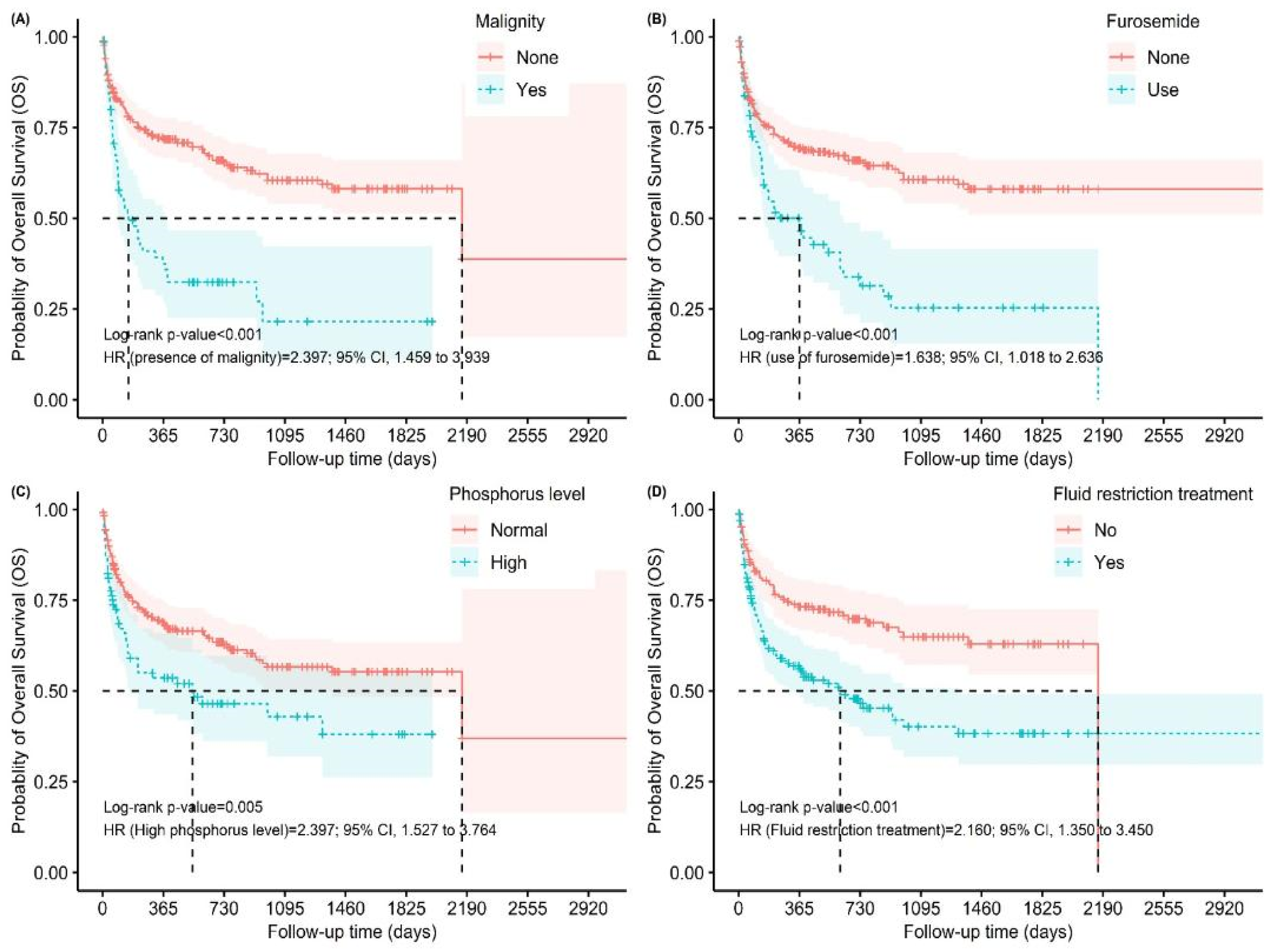
| Malignity | 22 (11) | 44 (32.4) | <0.001 2 |
| COPD | 49 (24.5) | 33 (24.1) | 0.931 2 |
| Pneumonia | 11 (5.5) | 15 (10.9) | 0.102 3 |
| No. of hospitalization | 1.96 (0 to 18) | 1.18 (1 to 3) | 0.005 4 |
| Length of stay (days) | 6.67 (1 to 25) | 9.73 (1 to 43) | <0.001 4 |
| EF | 58 (18 to 68) | 55 (10 to 65) | <0.001 4 |
| PAP | 32.5 (18 to 72) | 40 (20 to 73) | 0.086 4 |
| Drugs | |||
| Thiazide diuretic use | 82 (41) | 46 (33.6) | 0.168 2 |
| Antidepressive-antiepileptic | 59 (29.5) | 36 (26.3) | 0.518 2 |
| ACEi/ARB use | 97 (48.5) | 62 (45.3) | 0.558 2 |
| Spiranolactone | 27 (13.5) | 41 (29.9) | <0.001 2 |
| Furosemide | 27 (13.6) | 47 (34.3) | <0.001 2 |
| Chemotherapy | 16 (8) | 30 (21.9) | <0.001 2 |
| Other drugs | 171 (85.5) | 118 (86.1) | 0.871 2 |
| Glucose (72–106 mg/dL) | 118 [97–141.25] | 121 [100–158] | 0.282 4 |
| High glucose | 143 (71.5) | 102 (74.5) | 0.550 2 |
| Urea nitrogen (16.6–48.5 mg/dL) | 42 [24.5–84] | 60 [37–130] | <0.001 4 |
| High urea | 86 (43) | 81 (59.1) | 0.004 2 |
| Creatinine (0.5–0.9 mg/dL) | 1.02 [0.70–1.87] | 1.25 [0.84–2.32] | 0.015 4 |
| High creatinine | 98 (49) | 85 (62) | 0.018 2 |
| eGFR (ml/min/1.73 m2) | 72.55 [32.35–97.40] | 55 [28–87.5] | 0.011 4 |
| Admission Sodium (135–145 mEq/L) | 122 [117.75–125] | 122 [118–126] | 0.603 4 |
| Discharge Sodium (135–145 mEq/L) | 134 [132–136] | 134 [132–137] | 0.775 4 |
| Low discharge sodium | 115 (57.5) | 74 (54) | 0.527 2 |
| Potassium (3.5–5.1 mmol/L) | 4.27 [3.79–4.88] | 4.49 [3.94–5.30] | 0.007 4 |
| High potassium | 54 (27) | 57 (41.6) | 0.005 2 |
| Calcium (8.6–10.5 mg/dL) | 8.80 [8.38–9.30] | 8.80 [8.20–9.20] | 0.310 5 |
| Low calcium | 67 (33.5) | 59 (43.1) | 0.075 2 |
| Magnesium (1.62.6 mg/dL) | 1.74 [1.57–1.95] | 1.84 [1.61–2.11] | 0.027 4 |
| Low magnesium | 83 (41.5) | 57 (41.6) | 0.985 2 |
| Phosphorus (2.5–4.5 mg/dL) | 3.30 [2.90–4.30] | 3.50 [2.90–3.80] | 0.090 4 |
| High phosphorus | 42 (21) | 43 (31.4) | 0.031 2 |
| Albumin (3.5–5.2 gr/dL) | 3.76 ± 0.55 | 3.40 ± 0.63 | <0.001 1 |
| Low albumin | 81 (40.5) | 75 (54.7) | 0.010 2 |
| Uric acid (2.4–5.7 mg/dL) | 5.25 [3.38–7.23] | 6.30 [4.10–8.20] | 0.005 4 |
| High uric acid | 90 (45) | 72 (52.6) | 0.173 2 |
| TSH (0.27–4.2 µIU/mL) | 1.36 [0.81–2.28] | 1.48 [0.80–2.33] | 0.870 4 |
| Cortisol (6.2–19.4 µg(dL) | 15.10 [11.40–19.70] | 15.45 [12–20.08] | 0.471 4 |
| ACTH (7.2–63.3 pg/mL) | 25.50 [14.07–45.88] | 24.80 [15–43.60] | 0.968 4 |
| Urine osmolality (50–1200 mosm/kgH2O) | 382.75 ± 150.82 | 394.20 ± 164.96 | 0.721 1 |
| Urine sodium (mEq/L) | 49.50 [30–70.25] | 49 [29–88] | 0.443 4 |
| High urine sodium (>20 mEq/L) | 43 (26.2) | 25 (26.9) | 0.908 2 |
| Serum osmolality (275–285 mosm/kgH2O) | 270 [265–278] | 273 [259–290] | 0.362 4 |
| Treatment | |||
| High hypertonic sodium | 161 (80.5) | 100 (73) | 0.105 2 |
| Fluid restriction | 84 (42) | 84 (61.3) | <0.001 2 |
| Tolvaptan | 14 (7) | 9 (6.6) | >0.999 3 |
3.2. Comorbidities
3.3. Hospitalization Parameters
3.4. Medication Use
3.5. Laboratory Findings
3.6. Treatment Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adrogué, H.J.; Madias, N.E. Hyponatremia. N. Engl. J. Med. 2000, 342, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Incidence and prevalence of hyponatremia. Am. J. Med. 2006, 119 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef]
- Braun, M.M.; Barstow, C.H.; Pyzocha, N.J. Diagnosis and management of sodium disorders: Hyponatremia and hypernatremia. Am. Fam. Physician 2015, 91, 299–307. [Google Scholar]
- Peri, A. Morbidity and mortality of hyponatremia. Front. Horm. Res. 2019, 52, 36–48. [Google Scholar]
- Corona, G.; Giuliani, C.; Verbalis, J.G.; Forti, G.; Maggi, M.; Peri, A. Hyponatremia improvement is associated with a reduced risk of mortality: Evidence from a meta-analysis. PLoS ONE 2015, 10, e0124105. [Google Scholar] [CrossRef]
- Verbalis, J.G.; Goldsmith, S.R.; Greenberg, A.; Korzelius, C.; Schrier, R.W.; Sterns, R.H.; Thompson, C.J. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am. J. Med. 2013, 126 (Suppl. S1), S1–S42. [Google Scholar] [CrossRef]
- Hao, J.; Li, Y.; Zhang, X.; Pang, C.; Wang, Y.; Nigwekar, S.U.; Qiu, L.; Chen, L. The prevalence and mortality of hyponatremia is seriously underestimated in Chinese general medical patients: An observational retrospective study. BMC Nephrol. 2017, 18, 328. [Google Scholar] [CrossRef]
- Holland-Bill, L.; Christiansen, C.F.; Heide-Jørgensen, U.; Ulrichsen, S.P.; Ring, T.; Jørgensen, J.O.; Sørensen, H.T. Hyponatremia and mortality risk: A Danish cohort study of 279,508 acutely hospitalized patients. Eur. J. Endocrinol. 2015, 173, 71–81. [Google Scholar] [CrossRef]
- Wald, R.; Jaber, B.L.; Price, L.L.; Upadhyay, A.; Madias, N.E. Impact of hospital-associated hyponatremia on selected outcomes. Arch. Intern. Med. 2010, 170, 294–302. [Google Scholar] [CrossRef]
- Ghosal, A.; Qadeer, H.A.; Nekkanti, S.K.; Pradhan, P.; Okoye, C.; Waqar, D. A conspectus of euvolemic hyponatremia, its various etiologies, and treatment modalities: A comprehensive review of the literature. Cureus 2023, 15, e43390. [Google Scholar] [CrossRef]
- Voets, P. Examining the significance of arginine vasopressin release to elucidate the often multifactorial etiology of hypotonic hyponatremia: A novel criterion. Physiol. Rep. 2024, 12, e15967. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur. J. Endocrinol. 2014, 170, G1–G47. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, E.J.; Zietse, R. Diagnosis and treatment of hyponatremia: Compilation of the guidelines. J. Am. Soc. Nephrol. 2017, 28, 1340–1349. [Google Scholar] [CrossRef]
- Dineen, R.; Thompson, C.J.; Sherlock, M. Hyponatraemia—Presentations and management. Clin. Med. 2017, 17, 263–269. [Google Scholar] [CrossRef]
- Warren, A.M.; Grossmann, M.; Christ-Crain, M.; Russell, N. Syndrome of inappropriate antidiuresis: From pathophysiology to management. Endocr. Rev. 2023, 44, 819–861. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Lee, J.; Shin, J.; Kim, S.H.; Hwang, J.H. Thiazide-associated hyponatremia in arterial hypertension patients: A nationwide population-based cohort study. J. Hypertens. 2024, 42, 123–133. [Google Scholar] [CrossRef]
- Mustajoki, S. Severe hyponatraemia (P-Na < 116 mmol/L) in the emergency department: A series of 394 cases. Intern. Emerg. Med. 2023, 18, 781–789. [Google Scholar]
- Ramírez, E.; Rodríguez, A.; Queiruga, J.; García, I.; Díaz, L.; Martínez, L.; Muñoz, R.; Muñoz, M.; Tong, H.Y.; Martínez, J.C.; et al. Severe hyponatremia is often drug induced: 10-year results of a prospective pharmacovigilance program. Clin. Pharmacol. Ther. 2019, 106, 1362–1379. [Google Scholar] [CrossRef]
- Becerra Añez, K.M.; Sánchez Juan, C.; Artero Fullana, A.; Jiménez Portilla, A.; Ferrer García, J.C. Sodium evolution in hyponatremia: A retrospective analysis in a tertiary care center. Endocrinol. Diabetes Nutr. 2025, 72, 501563. [Google Scholar] [CrossRef]
- Rondon, H.; Badireddy, M. Hyponatremia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470386/ (accessed on 14 June 2023).
- Ternero-Vega, J.E.; Jiménez-de-Juan, C.; Castilla-Yelamo, J.; Cantón-Habas, V.; Sánchez-Ruiz-Granados, E.; Barón-Ramos, M.; Ropero-Luis, G.; Gómez-Salgado, J.; Bernabeu-Wittel, M. Impact of hyponatremia in patients hospitalized in internal medicine units. Medicine 2024, 103, e38312. [Google Scholar] [CrossRef]
- Waikar, S.S.; Mount, D.B.; Curhan, G.C. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am. J. Med. 2009, 122, 857–865. [Google Scholar] [CrossRef]
- Zhao, W.; Qin, J.; Lu, G.; Wang, Y.; Qiao, L.; Li, Y. Association between hyponatremia and adverse clinical outcomes of heart failure: Current evidence based on a systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1339203. [Google Scholar] [CrossRef]
- Akirov, A.; Diker-Cohen, T.; Steinmetz, T.; Amitai, O.; Shimon, I. Sodium levels on admission are associated with mortality risk in hospitalized patients. Eur. J. Intern. Med. 2017, 46, 25–29. [Google Scholar] [CrossRef]
- Ryoo, J.; Choi, A.; Cho, H.; Bae, W. Relationship of severity of hyponatremia and adverse outcomes in children visiting the emergency department. Front. Pediatr. 2024, 12, 1379727. [Google Scholar] [CrossRef]
- Al Yaqoubi, I.H.; Al-Maqbali, J.S.; Al Farsi, A.A.; Al Jabri, R.K.; Khan, S.A.; Al Alawi, A.M. Prevalence of hyponatremia among medically hospitalized patients and associated outcomes: A retrospective cohort study. Ann. Saudi Med. 2024, 44, 339–348. [Google Scholar] [CrossRef]
- Kutz, A.; Ebrahimi, F.; Sailer, C.O.; Wagner, U.; Schuetz, P.; Mueller, B.; Christ-Crain, M. Seasonality of hypoosmolar hyponatremia in medical inpatients: Data from a nationwide cohort study. J. Clin. Endocrinol. Metab. 2020, 105, dgz320. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, T.; Hoermann, R.; Wootton, E.; Mansouri, N.; Stevens, M.; Vo, H.; Grossmann, M.; Warren, A.M. Increased ambient temperature and hyponatremia presentations: A 10-year retrospective study at an Australian hospital. J. Clin. Endocrinol. Metab. 2025, 110, e2666–e2673. [Google Scholar] [CrossRef] [PubMed]
- Sterns, R.H. Disorders of plasma sodium—Causes, consequences, and correction. N. Engl. J. Med. 2015, 372, 55–65. [Google Scholar] [CrossRef]
- Rusinaru, D.; Tribouilloy, C.; Berry, C.; Richards, A.M.; Whalley, G.A.; Earle, N.; Poppe, K.K.; Guazzi, M.; Macin, S.M.; Komajda, M.; et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: An individual patient data meta-analysis. Eur. J. Heart Fail. 2012, 14, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Rusinaru, D.; Mahjoub, H.; Soulière, V.; Lévy, F.; Peltier, M.; Slama, M.; Massy, Z. Prognosis of heart failure with preserved ejection fraction: A five-year prospective population-based study. Eur. Heart J. 2008, 29, 339–347. [Google Scholar] [CrossRef]
- Singh, P.; Arora, S.; Singh, D.; Kalra, S.; Singh, A.; Arora, U.; Mittal, N.; Goyal, M.K.; Kaur, S.; Kalra, E. Hyponatremia and outcome: Is severity more important than etiology? Cureus 2023, 15, e42808. [Google Scholar] [CrossRef]
- Yoon, J.; Ahn, S.H.; Lee, Y.J.; Kim, C.M. Hyponatremia as an independent prognostic factor in patients with terminal cancer. Support. Care Cancer 2015, 23, 1735–1740. [Google Scholar] [CrossRef]
- Shuto, E.; Taketani, Y.; Tanaka, R.; Harada, N.; Isshiki, M.; Sato, M.; Nashiki, K.; Amo, K.; Yamamoto, H.; Higashi, Y.; et al. Dietary phosphorus acutely impairs endothelial function. J. Am. Soc. Nephrol. 2009, 20, 1504–1512. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Xu, J.; He, M. Association between serum phosphate level and mortality of patients with aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2024, 47, 891. [Google Scholar] [CrossRef]
- Steck, D.T.; Mostofi, N.; Togashi, K.; Li, R.; Wu, D.; Wells, L.; Fong, C.T.; Tillinghast, K.; O’Reilly-Shah, V.N.; Jelacic, S. Clinical outcomes in patients with phosphate abnormalities after cardiac surgery: A retrospective cohort study. Anesth. Analg. 2025, 140, 938–946. [Google Scholar] [CrossRef]
| Univariate Model | Multiple Model | |||
|---|---|---|---|---|
| HR [95% CI] | p-Value | HR [95% CI] | p-Value | |
| Demographical | ||||
| Age (years) | 1.021 [1.008 to 1.034] | <0.001 | 1.027 [1.006 to 1.049] | 0.009 |
| Comorbidities | ||||
| CAD | 1.461 [1.045 to 2.044] | 0.026 | 1.171 [0.644 to 2.129] | 0.603 |
| Malignity | 2.685 [1.865 to 3.864] | <0.001 | 2.183 [1.238 to 3.848] | 0.007 |
| Volume status (ref:euovolemic) | ||||
| Hypervolemic | 2.121 [1.454 to 3.092] | <0.001 | 1.027 [0.559 to 1.887] | 0.930 |
| Hypovolemic | 1.154 [0.728 to 1.829] | 0.542 | 1.315 [0.695 to 2.488] | 0.398 |
| No. of hospitalization | 0.642 [0.472 to 0.8739 | 0.004 | 0.334 [0.191 to 0.582] | <0.001 |
| Length of stay (days) | 1.070 [1.044 to 1.096] | <0.001 | 1.021 [0.989 to 1.054] | 0.189 |
| EF | 0.99 [0.955 to 0.983] | <0.001 | 0.978 [0.959 to 0.997] | 0.024 |
| Drugs | ||||
| Aldactone | 1.937 [1.342 to 2.794] | <0.001 | 1.216 [0.694 to 2.129] | 0.493 |
| Furosemide | 2.228 [1.562 to 3.179] | <0.001 | 1.789 [1.039 to 3.082] | 0.035 |
| Laboratory | ||||
| Urea | 1.005 [1.002 to 1.007] | <0.001 | ||
| High urea | 1.732 [1.230 to 2.437] | 0.001 | 0.508 [0.274 to 0.942] | 0.031 |
| Creatinine | 1.089 [1.023 to 1.159] | 0.007 | ||
| High creatinine | 0.647 [0.458 to 0.915] | 0.013 | 0.886 [0.359 to 2.186] | 0.793 |
| eGFR | 0.993 [0.989 to 0.998] | 0.008 | 2.506 [1.118 to 5.613] | 0.025 |
| Potassium | 1.267 [1.101 to 1.458] | <0.001 | ||
| High potassium | 1.583 [1.126 to 2.224] | 0.008 | 1.241 [0.773 to 1.992] | 0.371 |
| Magnesium | 1.129 [0.899 to 1.417] | 0.296 | ||
| High phosphorus | 1.675 [1.166 to 2.408] | 0.005 | 2.242 [1.317 to 3.815] | 0.003 |
| Albumin | 0.462 [0.652 to 0.607] | <0.001 | ||
| Low albumin | 1.532 [1.094 to 2.145] | 0.013 | 1.381 [0.857 to 2.226] | 0.185 |
| Uric acid | 1.073 [1.018 to 1.130] | 0.008 | 0.704 [0.430 to 1.153] | 0.163 |
| Treatment | ||||
| Fluid restriction | 1.960 [1.387 to 2.768] | <0.001 | 0.404 [0.209 to 0.777] | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coşkun Yavuz, Y.; Biyik, Z.; Korez, M.K.; Kaya, M.Z.; Altintepe, L. Eight-Year Retrospective Analysis of Mortality in Patients with Moderate to Severe Hyponatremia: A Comprehensive Study. J. Clin. Med. 2025, 14, 7834. https://doi.org/10.3390/jcm14217834
Coşkun Yavuz Y, Biyik Z, Korez MK, Kaya MZ, Altintepe L. Eight-Year Retrospective Analysis of Mortality in Patients with Moderate to Severe Hyponatremia: A Comprehensive Study. Journal of Clinical Medicine. 2025; 14(21):7834. https://doi.org/10.3390/jcm14217834
Chicago/Turabian StyleCoşkun Yavuz, Yasemin, Zeynep Biyik, Muslu Kazım Korez, Mustafa Zahid Kaya, and Lutfullah Altintepe. 2025. "Eight-Year Retrospective Analysis of Mortality in Patients with Moderate to Severe Hyponatremia: A Comprehensive Study" Journal of Clinical Medicine 14, no. 21: 7834. https://doi.org/10.3390/jcm14217834
APA StyleCoşkun Yavuz, Y., Biyik, Z., Korez, M. K., Kaya, M. Z., & Altintepe, L. (2025). Eight-Year Retrospective Analysis of Mortality in Patients with Moderate to Severe Hyponatremia: A Comprehensive Study. Journal of Clinical Medicine, 14(21), 7834. https://doi.org/10.3390/jcm14217834






