Downstream Occlusion During Mechanical Thrombectomy: Clinical Implications and Endovascular Trajectory
Abstract
1. Introduction
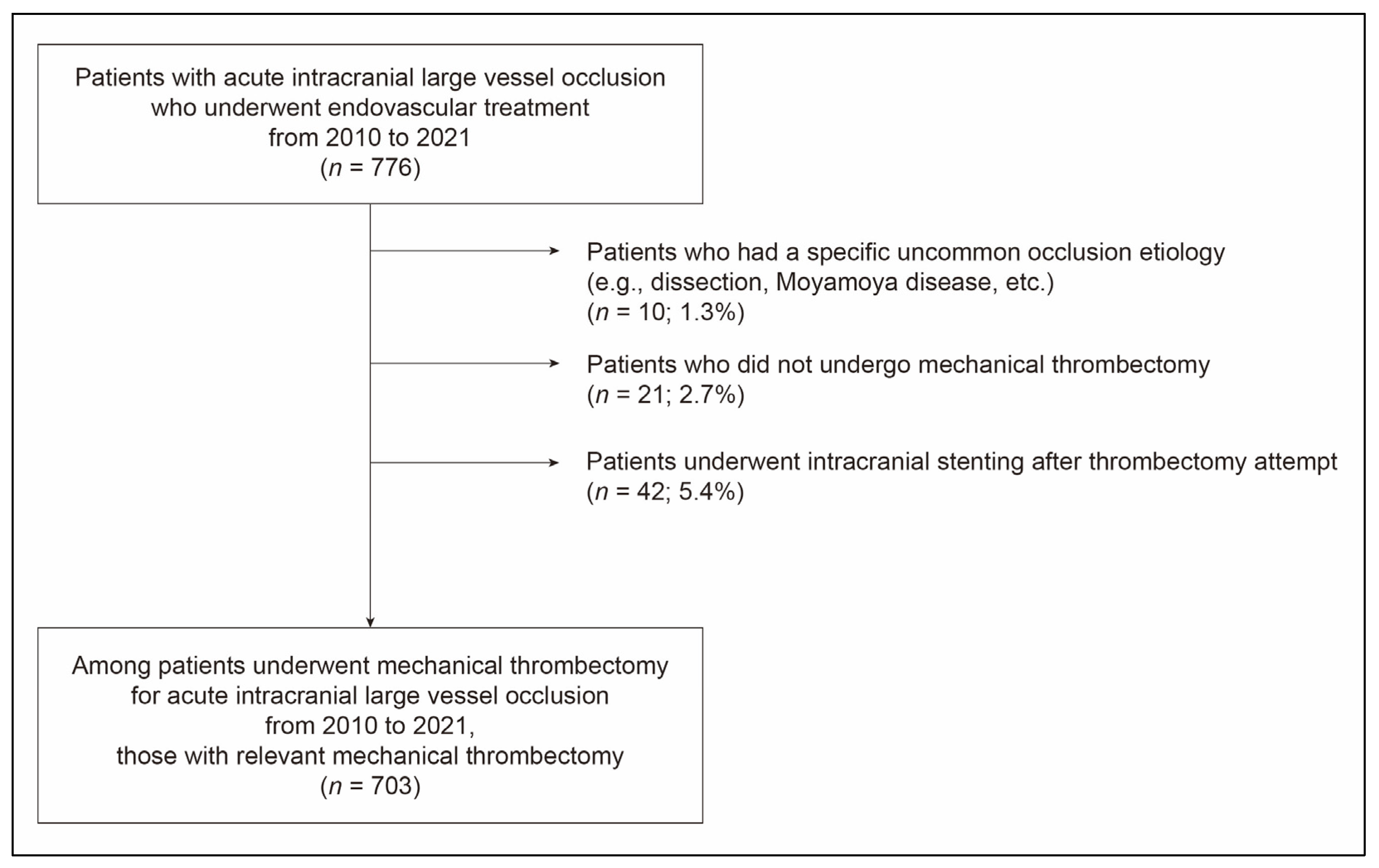
2. Materials and Methods
2.1. Study Population
2.2. Mechanical Thrombectomy Procedure
2.3. Study Variables
2.4. Statistical Analysis
3. Results
3.1. Downstream Occlusion and Associated Factors
3.2. Endovascular and Clinical Outcomes in Downstream Occlusion
3.3. Endovascular Trajectory of Downstream Occlusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Zaidat, O.O.; Yoo, A.J.; Khatri, P.; Tomsick, T.A.; von Kummer, R.; Saver, J.L.; Marks, M.P.; Prabhakaran, S.; Kallmes, D.F.; Fitzsimmons, B.F.; et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013, 44, 2650–2663. [Google Scholar] [CrossRef]
- Kaesmacher, J.; Dobrocky, T.; Heldner, M.R.; Bellwald, S.; Mosimann, P.J.; Mordasini, P.; Bigi, S.; Arnold, M.; Gralla, J.; Fischer, U. Systematic review and meta-analysis on outcome differences among patients with TICI 2b versus TICI 3 reperfusions: Success revisited. J. Neurol. Neurosurg. Psychiatry 2018, 89, 910–917. [Google Scholar] [CrossRef] [PubMed]
- LeCouffe, N.E.; Kappelhof, M.; Treurniet, K.M.; Lingsma, H.F.; Zhang, G.; van den Wijngaard, I.R.; van Es, A.; Emmer, B.J.; Majoie, C.; Roos, Y.; et al. 2B, 2C, or 3: What Should Be the Angiographic Target for Endovascular Treatment in Ischemic Stroke? Stroke 2020, 51, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Zaidat, O.O.; Castonguay, A.C.; Linfante, I.; Gupta, R.; Martin, C.O.; Holloway, W.E.; Mueller-Kronast, N.; English, J.D.; Dabus, G.; Malisch, T.W.; et al. First Pass Effect: A New Measure for Stroke Thrombectomy Devices. Stroke 2018, 49, 660–666. [Google Scholar] [CrossRef]
- Kaesmacher, J.; Boeckh-Behrens, T.; Simon, S.; Maegerlein, C.; Kleine, J.F.; Zimmer, C.; Schirmer, L.; Poppert, H.; Huber, T. Risk of Thrombus Fragmentation during Endovascular Stroke Treatment. AJNR Am. J. Neuroradiol. 2017, 38, 991–998. [Google Scholar]
- Ye, G.; Qi, P.; Chen, K.; Tan, T.; Cao, R.; Chen, J.; Lu, J.; Wang, D. Risk of secondary embolism events during mechanical thrombectomy for acute ischemic stroke: A single-center study based on histological analysis. Clin. Neurol. Neurosurg. 2020, 193, 105749. [Google Scholar] [CrossRef]
- Cascio Rizzo, A.; Schwarz, G.; Cervo, A.; Giussani, G.; Ceresa, C.; Gatti, A.; De Angeli, F.; Motto, C.; Guccione, A.; Tortorella, R.; et al. Safety and efficacy of endovascular thrombectomy for primary and secondary MeVO. J. Stroke Cerebrovasc. Dis. 2024, 33, 107492. [Google Scholar] [CrossRef]
- Rodriguez-Calienes, A.; Vivanco-Suarez, J.; Sequeiros, J.M.; Galecio-Castillo, M.; Zevallos, C.B.; Farooqui, M.; Ortega-Gutierrez, S. Mechanical thrombectomy for the treatment of primary and secondary distal medium-vessel occlusion stroke: Systematic review and meta-analysis. J. Neurointerv. Surg. 2023, 15, e460–e467. [Google Scholar] [CrossRef]
- Ospel, J.M.; Nguyen, T.N.; Jadhav, A.P.; Psychogios, M.N.; Clarencon, F.; Yan, B.; Goyal, M. Endovascular Treatment of Medium Vessel Occlusion Stroke. Stroke 2024, 55, 769–778. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 h after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M. Causes and Solutions of Endovascular Treatment Failure. J. Stroke 2017, 19, 131–142. [Google Scholar] [CrossRef]
- Kang, D.H.; Hwang, Y.H. Frontline Contact Aspiration Treatment for Emergent Large Vessel Occlusion: A Review Focused on Practical Techniques. J. Stroke 2019, 21, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Jabre, R.; Orenday-Barraza, J.M.; Eldin, M.S.; Chen, C.J.; Al-Saiegh, F.; Abbas, R.; El Naamani, K.; Gooch, M.R.; Jabbour, P.M.; et al. A review of mechanical thrombectomy techniques for acute ischemic stroke. Interv. Neuroradiol. 2023, 29, 450–458. [Google Scholar] [CrossRef]
- Medrano-Martorell, S.; Pumar-Perez, M.; Gonzalez-Ortiz, S.; Capellades-Font, J. A review of the anatomy of the middle cerebral artery for the era of thrombectomy: A radiologic tool based on CT angiography and perfusion CT. Radiologia (Engl. Ed.) 2021, 63, 505–511. [Google Scholar] [CrossRef]
- Barbato, F.; Allocca, R.; Bosso, G.; Numis, F.G. Anatomy of Cerebral Arteries with Clinical Aspects in Patients with Ischemic Stroke. Anatomia 2022, 1, 152–169. [Google Scholar] [CrossRef]
- Bilgin, C.; Bolsegui, M.L.; Ghozy, S.; Hassankhani, A.; Kobeissi, H.; Jabal, M.S.; Gupta, R.; De Rubeis, G.; Kadirvel, R.; Brinjikji, W.; et al. Common design and data elements reported in active mechanical thrombectomy trials focusing on distal medium vessel occlusions and minor strokes: A systematic review. J. Neurointerv. Surg. 2024, 17, 530–538. [Google Scholar] [CrossRef]
- Berger, M.C.; Simgen, A.; Dietrich, P.; Naziri, W. Safety and Efficacy of Thrombectomy for Distal Medium Vessel Occlusions of the Middle Cerebral Artery. Neurointervention 2025, 20, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Maegerlein, C.; Prothmann, S.; Lucia, K.E.; Zimmer, C.; Friedrich, B.; Kaesmacher, J. Intraprocedural Thrombus Fragmentation During Interventional Stroke Treatment: A Comparison of Direct Thrombus Aspiration and Stent Retriever Thrombectomy. Cardiovasc. Intervent. Radiol. 2017, 40, 987–993. [Google Scholar] [CrossRef]
- Pilato, F.; Valente, I.; Calandrelli, R.; Alexandre, A.; Arena, V.; Dell’Aquila, M.; Broccolini, A.; Della Marca, G.; Morosetti, R.; Frisullo, G.; et al. Clot evaluation and distal embolization risk during mechanical thrombectomy in anterior circulation stroke. J. Neurol. Sci. 2022, 432, 120087. [Google Scholar] [CrossRef] [PubMed]
- Sporns, P.B.; Hanning, U.; Schwindt, W.; Velasco, A.; Buerke, B.; Cnyrim, C.; Minnerup, J.; Heindel, W.; Jeibmann, A.; Niederstadt, T. Ischemic Stroke: Histological Thrombus Composition and Pre-Interventional CT Attenuation Are Associated with Intervention Time and Rate of Secondary Embolism. Cerebrovasc. Dis. 2017, 44, 344–350. [Google Scholar] [CrossRef]
- Wong, G.J.; Yoo, B.; Liebeskind, D.; Baharvahdat, H.; Gornbein, J.; Jahan, R.; Szeder, V.; Duckwiler, G.; Tateshima, S.; Colby, G.; et al. Frequency, Determinants, and Outcomes of Emboli to Distal and New Territories Related to Mechanical Thrombectomy for Acute Ischemic Stroke. Stroke 2021, 52, 2241–2249. [Google Scholar] [CrossRef]
- Yi, H.J.; Sung, J.H.; Lee, D.H. Bridging Intravenous Thrombolysis Before Mechanical Thrombectomy for Large Artery Occlusion May be Detrimental with Thrombus Fragmentation. Curr. Neurovasc. Res. 2020, 17, 18–26. [Google Scholar] [CrossRef]
- Suh, H.I.; Hong, J.M.; Lee, K.S.; Han, M.; Choi, J.W.; Kim, J.S.; Demchuk, A.M.; Lee, J.S. Imaging Predictors for Atherosclerosis-Related Intracranial Large Artery Occlusions in Acute Anterior Circulation Stroke. J. Stroke 2016, 18, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, J.M.; Kim, J.S. Diagnostic and Therapeutic Strategies for Acute Intracranial Atherosclerosis-related Occlusions. J. Stroke 2017, 19, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, J.; Wang, J.; Li, J.; Gu, S.; Yao, Y.; Xiong, H.; Li, Y. Imaging features of cardioembolic stroke on 4-dimensional computed tomography angiography. Quant. Imaging Med. Surg. 2023, 13, 6026–6036. [Google Scholar] [CrossRef]
- Weafer, F.M.; Duffy, S.; Machado, I.; Gunning, G.; Mordasini, P.; Roche, E.; McHugh, P.E.; Gilvarry, M. Characterization of strut indentation during mechanical thrombectomy in acute ischemic stroke clot analogs. J. Neurointerv. Surg. 2019, 11, 891–897. [Google Scholar] [CrossRef]
- Yoo, A.J.; Andersson, T. Thrombectomy in Acute Ischemic Stroke: Challenges to Procedural Success. J. Stroke 2017, 19, 121–130. [Google Scholar] [CrossRef]
- Baek, J.H.; Kwon, I.; Kim, S.; Nam, H.S.; Kim, Y.D.; Kim, B.M.; Kim, D.J.; Song, T.J.; Heo, J.H. Thrombi with a Higher Erythrocyte Composition Are More Fragile in Acute Stroke. J. Stroke 2024, 26, 454–457. [Google Scholar] [CrossRef]
- Baek, J.H. Traditional Thrombus Composition and Related Endovascular Outcomes: Catching up with the Recent Evidence. Neurointervention 2024, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Yoon, W.; Kim, T.S.; Kim, H.S.; Heo, T.W.; Park, M.S. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. AJNR Am. J. Neuroradiol. 2015, 36, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.; Dai, D.; Wang, S.; Douglas, A.; Kadirvel, R.; Layton, K.F.; Thacker, I.C.; Gounis, M.J.; Chueh, J.Y.; Puri, A.S.; et al. Platelet-Rich Emboli in Cerebral Large Vessel Occlusion Are Associated with a Large Artery Atherosclerosis Source. Stroke 2019, 50, 1907–1910. [Google Scholar] [CrossRef] [PubMed]


| Downstream Occlusion (+) (n = 254) | Downstream Occlusion (−) (n = 449) | p-Value | |
|---|---|---|---|
| Demographic and stroke risk factors | |||
| Age (years) | 70.1 (±12.0) | 70.9 (±12.4) | 0.384 |
| Men | 127 (50.0) | 229 (51.0) | 0.798 |
| Hypertension | 169 (66.5) | 336 (74.8) | 0.019 |
| Diabetes | 72 (28.3) | 154 (34.3) | 0.105 |
| Dyslipidemia | 49 (19.3) | 121 (26.9) | 0.023 |
| Current smoking | 35 (13.8) | 62 (13.8) | 0.991 |
| Coronary artery occlusive disease | 53 (20.9) | 101 (22.5) | 0.616 |
| Atrial fibrillation | 168 (66.1) | 246 (54.8) | 0.003 |
| Stroke conditions | |||
| Initial NIHSS score | 15.0 [11.0–19.0] | 14.0 [9.0–19.0] | 0.006 |
| Intravenous tPA administration | 95 (37.4) | 157 (35.0) | 0.518 |
| Location of initial occlusion | <0.001 a | ||
| Anterior circulation | 232 (91.3) | 386 (86.0) | 0.036 b |
| Internal carotid artery | 93 (36.6) | 90 (20.0) | |
| Middle cerebral artery | |||
| M1 segment | 107 (42.1) | 186 (41.4) | |
| M2 segment | 32 (12.6) | 110 (24.5) | |
| Posterior circulation | 22 (8.7) | 63 (14.0) | |
| Vertebral artery | 2 (0.8) | 3 (0.7) | |
| Basilar artery | 20 (7.9) | 60 (13.4) | |
| Tandem occlusion | 21 (8.3) | 42 (9.4) | 0.628 |
| Procedural conditions | |||
| Onset-to-puncture time (minutes) | 268.0 [160.0–591.0] | 280.0 [167.0–571.0] | 0.661 |
| Use of balloon guiding catheter | 189 (74.4) | 325 (72.4) | 0.560 |
| Relevant thrombectomy modality | 0.024 | ||
| Stent retriever | 201 (79.1) | 385 (85.7) | |
| Contact aspiration | 53 (20.9) | 64 (14.3) |
| OR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
|---|---|---|---|---|
| Hypertension | 0.67 (0.48–0.94) | 0.019 | 0.63 (0.45–0.90) | 0.012 |
| Dyslipidemia | 0.65 (0.45–0.94) | 0.023 | 0.66 (0.45–0.90) | 0.037 |
| Atrial fibrillation | 1.61 (1.17–2.22) | 0.003 | 1.54 (1.10–2.16) | 0.012 |
| Initial NIHSS score | 1.03 (1.01–1.06) | 0.010 | 1.02 (0.99–1.05) | 0.174 |
| Intravenous tPA administration | 1.11 (0.81–1.53) | 0.518 | 1.12 (0.80–1.56) | 0.516 |
| Location of initial occlusion | ||||
| Most proximal artery | 3.55 (2.18–5.79) | <0.001 | 3.01 (1.78–5.09) | <0.001 |
| Proximal artery | 1.78 (1.14–2.79) | 0.012 | 1.76 (1.10–2.81) | 0.019 |
| Distal artery | Reference | Reference | ||
| Use of balloon guiding catheter | 1.11 (0.78–1.57) | 0.560 | 1.21 (0.83–1.76) | 0.312 |
| Relevant thrombectomy modality | ||||
| Stent retriever | Reference | Reference | ||
| Contact aspiration | 1.59 (1.06–2.37) | 0.024 | 1.25 (0.80–1.93) | 0.329 |
| Location of Downstream Occlusion | Location of Initial Occlusion | |||||
|---|---|---|---|---|---|---|
| ICA (n = 183) | M1 (n = 293) | M2 (n = 142) | VA (n = 5) | BA (n = 80) | ||
| MCA | M1 | 48 (51.5) | ||||
| M2 | 33 (35.4) | 80 (74.8) | 7 (21.9) | |||
| M3 | 2 (2.2) | 8 (7.4) | 11 (34.3) | |||
| M4 | 3 (3.2) | 19 (17.8) | 14 (43.8) | |||
| ACA | A1 | 2 (2.2) | ||||
| A2 | 2 (2.2) | |||||
| A3 | 2 (2.2) | |||||
| BA | 2 (100.0) | |||||
| PCA | P1 | 5 (25.0) | ||||
| P2 | 1 (1.1) a | 10 (50.0) | ||||
| P3 | 1 (5.0) | |||||
| SCA | 4 (20.0) | |||||
| Total | 93 (50.8) | 107 (36.5) | 32 (22.5) | 2 (40.0) | 20 (25.0) | |
| Downstream Occlusion (+) (n = 254) | Downstream Occlusion (−) (n = 449) | p-Value | |
|---|---|---|---|
| Endovascular outcomes | |||
| Recanalization status | |||
| Final successful recanalization | 235 (92.5) | 410 (91.3) | 0.577 |
| Final mTICI grade | <0.001 | ||
| 0 | 2 (0.8) | 18 (4.0) | |
| 1 | 1 (0.4) | 4 (0.9) | |
| 2a | 16 (6.3) | 17 (3.8) | |
| 2b | 153 (60.2) | 39 (8.7) | |
| 3 | 82 (32.3) | 371 (82.6) | <0.001 a |
| First-pass effect | 28 (11.0) | 227 (50.6) | <0.001 |
| Number of thrombectomy device pass | 3.2 (±1.9) | 2.1 (±1.9) | <0.001 |
| Puncture-to-recanalization time (min) | 41.0 [27.0–67.5] | 32.0 [20.0–53.8] | <0.001 |
| Clinical outcomes | |||
| Functional independence | 102 (40.2) | 208 (46.3) | 0.114 |
| Any intracranial hemorrhage | 155 (61.0) | 202 (45.0) | <0.001 |
| Symptomatic intracranial hemorrhage | 42 (16.5) | 40 (8.9) | 0.002 |
| Subarachnoid hemorrhage | 6 (2.4) | 18 (4.0) | 0.248 |
| Mortality | 41 (16.1) | 61 (13.6) | 0.355 |
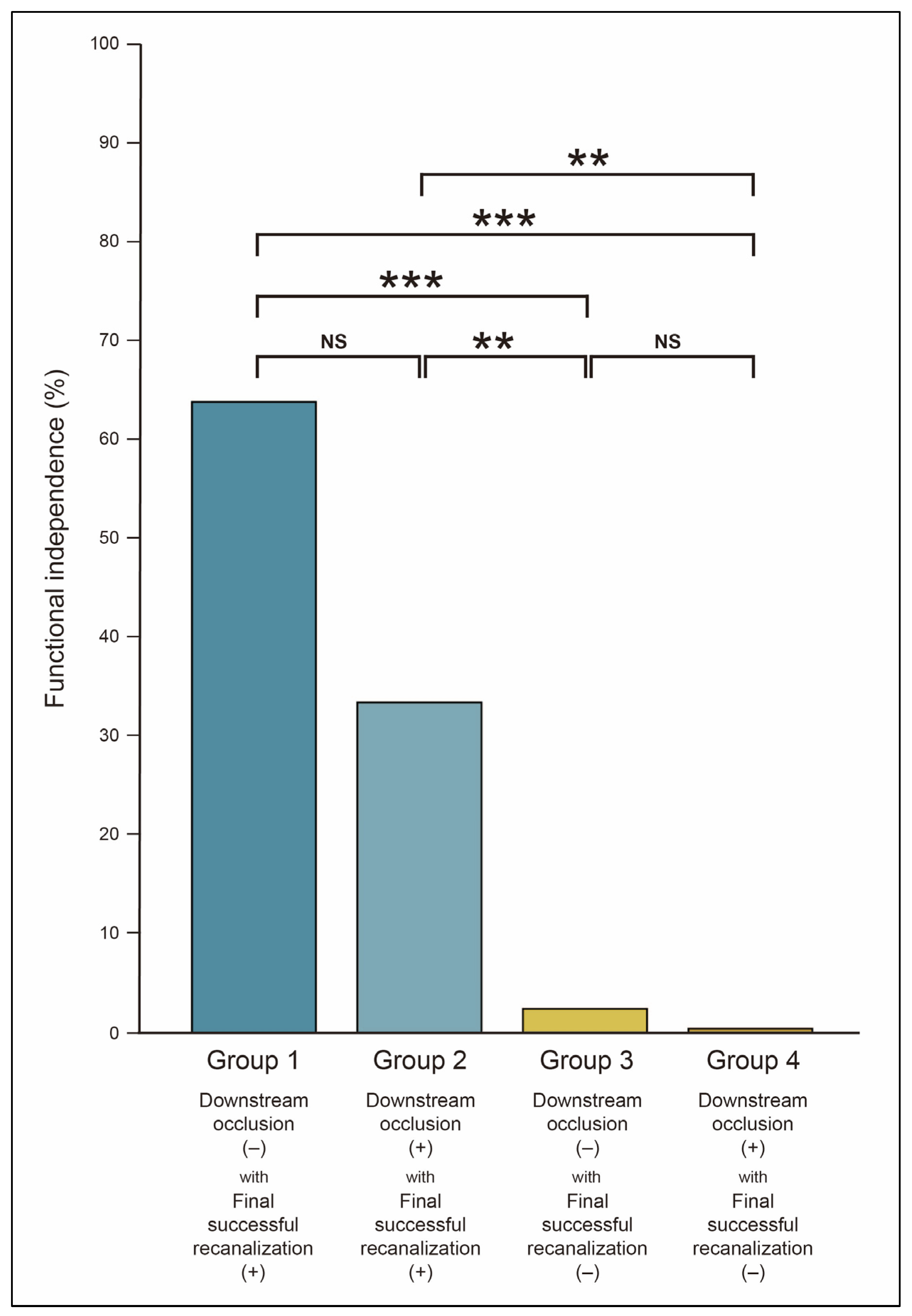
| DOC | Final SR | mRS 0–2 (n = 310) | mRS 3–6 (n = 393) | aOR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Group 1 (n = 410) | (−) | (+) | 201 (64.8) | 209 (53.2) | Reference | |
| Group 2 (n = 235) | (+) | (+) | 101 (32.6) | 134 (34.1) | 0.86 (0.58–1.26) | 0.435 |
| Group 3 (n = 39) | (−) | (−) | 7 (2.3) | 32 (8.1) | 0.15 (0.05–0.40) | <0.001 |
| Group 4 (n = 19) | (+) | (−) | 1 (0.3) | 18 (4.6) | 0.09 (0.01–0.76) | 0.026 |
| Total | Further Recanalization Attempt | No Further Recanalization Attempt | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Final mTICI Grade | Total | Final mTICI Grade | Total | ||||||||||
| 3 | 2b | 2a | 1 | 2b | 2a | 1 | 0 | ||||||
| MCA | M1 a | 2b b | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 2a | 42 | 20 | 17 | 3 | 0 | 40 | 0 | 2 | 0 | 0 | 2 | ||
| 1 | 4 | 0 | 2 | 0 | 1 | 3 | 0 | 0 | 0 | 1 | 1 | ||
| M2 | 2b | 62 | 22 | 18 | 0 | 0 | 40 | 21 | 0 | 0 | 1 | 22 | |
| 2a | 57 | 23 | 27 | 4 | 0 | 54 | 0 | 3 | 0 | 0 | 3 | ||
| 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| M3 | 2b | 19 | 2 | 6 | 0 | 0 | 8 | 11 | 0 | 0 | 0 | 11 | |
| 2a | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | ||
| M4 | 2b | 35 | 0 | 2 | 0 | 0 | 2 | 32 | 1 | 0 | 0 | 33 | |
| 2a | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | ||
| ACA | A1 | 2b | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 2a | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| A2 | 2b | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| A3 | 2b | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | |
| BA | 2a | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| PCA | P1 | 2b | 3 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 2a | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| P2 | 2b | 10 | 3 | 2 | 0 | 0 | 5 | 5 | 0 | 0 | 0 | 5 | |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | ||
| P3 | 2b | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| SCA | 2b | 4 | 3 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | |
| Total | 254 | 82 | 80 | 9 | 1 | 172 | 73 | 7 | 0 | 2 | 82 | ||
| Clinical Outcomes | Further Recanalization Attempt | p-Value | |
|---|---|---|---|
| (+) (n = 172) | (−) (n = 82) | ||
| Functional independence | 69 (40.1) | 33 (40.2) | 0.985 |
| Hemorrhagic complications | |||
| Any intracranial hemorrhage | 115 (66.9) | 40 (48.8) | 0.006 |
| Symptomatic intracranial hemorrhage | 32 (18.6) | 10 (12.2) | 0.199 |
| Subarachnoid hemorrhage | 5 (2.9) | 1 (1.2) | 0.667 |
| Mortality | 28 (16.3) | 13 (15.9) | 0.931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.-H.; Nam, H.S.; Kim, Y.D.; Kim, B.M.; Kim, D.J.; Song, T.-J.; Chung, Y.; Heo, J.H. Downstream Occlusion During Mechanical Thrombectomy: Clinical Implications and Endovascular Trajectory. J. Clin. Med. 2025, 14, 7797. https://doi.org/10.3390/jcm14217797
Baek J-H, Nam HS, Kim YD, Kim BM, Kim DJ, Song T-J, Chung Y, Heo JH. Downstream Occlusion During Mechanical Thrombectomy: Clinical Implications and Endovascular Trajectory. Journal of Clinical Medicine. 2025; 14(21):7797. https://doi.org/10.3390/jcm14217797
Chicago/Turabian StyleBaek, Jang-Hyun, Hyo Suk Nam, Young Dae Kim, Byung Moon Kim, Dong Joon Kim, Tae-Jin Song, Yeongu Chung, and Ji Hoe Heo. 2025. "Downstream Occlusion During Mechanical Thrombectomy: Clinical Implications and Endovascular Trajectory" Journal of Clinical Medicine 14, no. 21: 7797. https://doi.org/10.3390/jcm14217797
APA StyleBaek, J.-H., Nam, H. S., Kim, Y. D., Kim, B. M., Kim, D. J., Song, T.-J., Chung, Y., & Heo, J. H. (2025). Downstream Occlusion During Mechanical Thrombectomy: Clinical Implications and Endovascular Trajectory. Journal of Clinical Medicine, 14(21), 7797. https://doi.org/10.3390/jcm14217797







