Abstract
Background/Objectives: Sports tendinopathy management has traditionally focused on mechanical loading protocols, yet emerging evidence suggests metabolic factors significantly influence clinical outcomes and tissue adaptation responses. The aim was to systematically evaluate the impact of metabolic factors on sports tendinopathy outcomes and assess the effectiveness of advanced rehabilitation approaches that extend beyond traditional mechanical loading protocols. Methods: A comprehensive search across academic papers from Semantic Scholar corpus identified studies investigating metabolic influences and advanced rehabilitation strategies in sports tendinopathy. Inclusion criteria encompassed athletes and active individuals with chronic tendinopathy, interventions targeting metabolic factors or advanced rehabilitation techniques, and validated outcome measures. Risk of bias was assessed using RoB 2 for randomized trials and ROBINS-I for observational studies. Evidence certainty was evaluated using GRADE methodology. Results: Forty studies met inclusion criteria, comprising 5 randomized controlled trials, 9 systematic reviews, and 5 cohort studies. Metabolic syndrome significantly impaired eccentric exercise outcomes in Achilles tendinopathy (F[1,54] = 24.45, p < 0.001). Collagen-derived peptide supplementation combined with eccentric training demonstrated superior pain reduction at rest compared to exercise alone (p < 0.05). Advanced rehabilitation strategies including criteria-based progression, neuroplastic training, and staged loading protocols showed improvements in patient-reported outcomes and functional scores, with some approaches demonstrating superiority over traditional eccentric protocols. Conclusions: Metabolic factors negatively influence sports tendinopathy rehabilitation outcomes, while advanced rehabilitation approaches incorporating metabolic considerations show promise for enhancing treatment effectiveness. Integration of metabolic assessment and targeted interventions may optimize tendinopathy management beyond mechanical loading alone.
1. Introduction
Sports tendinopathy represents a prevalent and challenging clinical condition affecting athletes across diverse sporting disciplines, with persistent symptoms often resistant to conventional rehabilitation approaches [1,2]. The traditional paradigm of tendinopathy management has centered predominantly on mechanical loading interventions, particularly eccentric exercise protocols, based on the mechanobiological principle that controlled tensile loading promotes adaptive tissue remodeling [3,4,5]. However, accumulating evidence suggests this mechanically focused approach may be insufficient for optimal clinical outcomes across all patient populations.
Contemporary understanding of tendinopathy pathophysiology has evolved beyond simplistic inflammatory or degenerative models toward recognition of a complex, multifactorial etiology involving mechanical, cellular, and systemic factors [6,7]. The ICON 2019 consensus defined tendinopathy as “a clinical condition in tendons characterized by a combination of pain, swelling and impaired performance,” acknowledging the multidimensional nature of this condition that extends beyond purely mechanical considerations [8]. This expanded understanding has prompted investigation into systemic factors, particularly metabolic conditions, that may influence tendon healing capacity and rehabilitation outcomes. Moreover, studies indicate that the interaction between lipids, glycemic metabolism, and thyroid hormones plays a significant role in adaptive processes and in the susceptibility of tendons to injury [9]. Furthermore, recent evidence suggests that neurometabolic disorders and genetic syndromes, even when manifested during developmental age, may predispose athletes to altered tendon homeostasis and increased susceptibility to tendinopathies. These factors, often overlooked in sports populations, emphasize the need for comprehensive assessment of underlying systemic and hereditary contributors in syndromic or metabolically compromised individuals.
Metabolic disorders, including diabetes mellitus, metabolic syndrome, and obesity, have emerged as significant risk fac tors for tendinopathy development and poor treatment outcomes [6,10,11,12]. Type 2 diabetes mellitus increases the risk of tendinopathy through several interrelated mechanisms. These include the formation of advanced glycation end products, the detrimental effects of chronic hyperglycemia on tenocyte function, and impaired angiogenesis that compromises tissue repair capacity [13]. Metabolic syndrome components create a systemic inflammatory environment that may compromise the adaptive responses to mechanical loading interventions traditionally employed in tendinopathy rehabilitation [14]. Despite these emerging insights, most rehabilitation protocols do not systematically account for metabolic status, potentially limiting treatment effectiveness in affected populations.
Concurrently, advanced rehabilitation approaches have emerged that extend beyond traditional mechanical loading paradigms. These include criteria-based progression protocols that individualize loading based on functional capacity markers [15], neuroplastic training approaches that address central sensitization mechanisms [16,17], and staged loading protocols designed for cases failing standard rehabilitation [18]. Nutritional interventions, particularly collagen-derived peptide supplementation, have demonstrated synergistic effects with exercise interventions, suggesting potential benefits of integrated metabolic–mechanical approaches [19,20].
The clinical significance of understanding metabolic influences on tendinopathy extends beyond academic interest, given the increasing prevalence of metabolic disorders in athletic populations and the substantial economic burden of chronic tendinopathy [21]. Athletes with metabolic comorbidities may require modified rehabilitation approaches to achieve optimal outcomes, necessitating evidence-based guidance for clinicians managing these complex presentations.
Furthermore, the potential for metabolic interventions to enhance rehabilitation effectiveness represents an important therapeutic frontier that could improve outcomes for both metabolically healthy and compromised individuals.
Despite growing interest in metabolic factors and advanced rehabilitation approaches, the literature remains fragmented across multiple disciplines, limiting clinical translation. Previous systematic reviews have focused either on mechanical loading interventions or metabolic aspects in isolation, without comprehensive evaluation of their interaction and integration potential [3,22]. No systematic review has comprehensively evaluated both metabolic influences on traditional rehabilitation outcomes and the effectiveness of advanced rehabilitation strategies that explicitly address metabolic factors.
Therefore, the primary objective of this systematic review was to comprehensively evaluate the impact of metabolic factors on sports tendinopathy rehabilitation outcomes and assess the effectiveness of advanced rehabilitation approaches that extend beyond traditional mechanical loading protocols.
Secondary objectives included identifying optimal integration strategies for metabolic considerations in rehabilitation planning and evaluating the quality of evidence supporting metabolic–mechanical intervention approaches. This review addresses the critical research question: Do metabolic factors significantly influence sports tendinopathy rehabilitation outcomes, and do advanced rehabilitation approaches incorporating metabolic considerations demonstrate superior effectiveness compared to traditional mechanical loading alone?
2. Materials and Methods
2.1. Protocol Registration and Reporting Guidelines
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines and was prospectively registered in PROSPERO (Registration ID: CRD420251154542). The review protocol followed PRISMA-P guidelines for protocol development.
2.2. Study Selection and Characteristics
The systematic search identified 499 studies for screening, of which 40 studies met inclusion criteria (Figure 1). The search strategy included combinations of the following keywords and Boolean operators: “sports tendinopathy”, “Achilles tendinopathy”, “patellar tendinopathy”, “rehabilitation”, “exercise”, “loading”, “metabolic factors”, “metabolic syndrome”, “diabetes”, “obesity”, “collagen supplementation”, “nutrition”, and “advanced rehabilitation”.
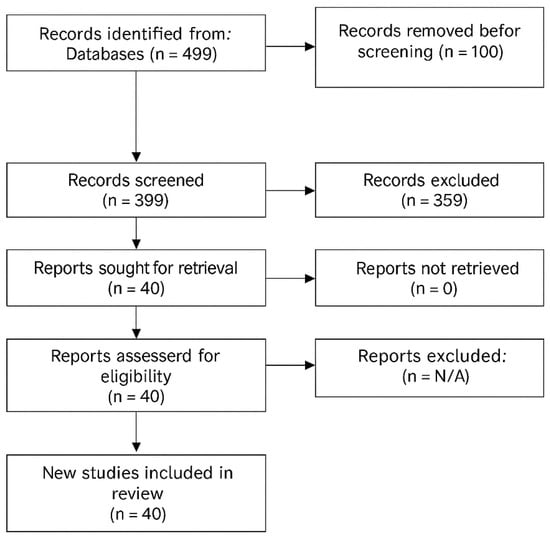
Figure 1.
PRISMA Flow Diagram showing study selection process.
Searches were performed using the terms above in titles, abstracts, and keywords with database-specific syntax and filters (see Table A1 for full query details). The PRISMA flow diagram illustrates the study selection process, with reasons for exclusion at each stage detailed in Table 1A,B.

Table 1.
(A) Reasons for exclusion at each stage of study selection. (B) Summary of Exclusions by PICO Framework.
2.3. Study Characteristics
The 40 included studies comprised diverse study designs: 5 randomized controlled trials (12.5%), 9 systematic reviews or meta-analyses (22.5%), 5 cohort studies (12.5%), 20 narrative reviews or scoping reviews (50%), and 1 case series (2.5%) (Table 2). Publication years ranged from 2005 to 2025, with 67.5% published after 2018, reflecting the growing interest in metabolic factors and advanced rehabilitation approaches.

Table 2.
Characteristics of Included Studies.
2.4. Eligibility Criteria
Studies were included based on the following criteria:
- Population: Athletes, recreational sports participants, or physically active individuals diagnosed with chronic tendinopathy (>3 months duration). Studies focusing on acute tendon injuries, tendon ruptures, or post-surgical tendon repair were excluded.
- Intervention: Studies investigating (1) metabolic factors in tendon healing, (2) advanced rehabilitation techniques beyond traditional mechanical loading, or (3) interventions combining metabolic and mechanical approaches. Studies focusing solely on diagnostic methods or epidemiological factors without intervention components were excluded.
- Comparator: Control groups, alternative interventions, or baseline comparisons were required.
- Outcomes: Validated measures of tendon pain, function, structural changes, return to sport, or biomarkers of tendon metabolism.
- Study Design: Randomized controlled trials (RCTs), controlled clinical trials, cohort studies, case–control studies, systematic reviews, or meta-analyses. Case reports, editorials, and conference abstracts were excluded.
- Other Criteria: Human studies published in English. Animal or in vitro studies without direct human application were excluded.
2.5. Study Selection and Data Extraction
Following the initial search yielding 499 papers, two independent reviewers screened titles and abstracts using predefined criteria. Full-text assessment was performed for potentially eligible studies, with disagreements resolved through discussion with a third reviewer. A standardized data extraction form was developed and piloted prior to use. The complete data extraction form is available in Table A2.
Large Language Model (LLM) assistance was employed solely to support the initial structuring of data extraction tables by identifying key variables (e.g., study design, population, intervention, and outcomes) from eligible full texts.
To ensure full transparency and reproducibility, all extracted data were independently verified by two reviewers (S.K. and W.K.) who manually cross-checked each entry against the original publications. The reviewers confirmed the accuracy, completeness, and contextual interpretation of all extracted variables. Any discrepancies between reviewers were discussed and resolved by consensus with a third reviewer (P.P.).
This verification process ensured that the LLM-assisted extraction served only as a preliminary aid, with all final dataset content validated through human review and consensus.
2.6. Risk of Bias Assessment
Risk of bias was assessed using appropriate tools based on study design:
- RCTs: Cochrane Risk of Bias tool 2 (RoB 2).
- Non-randomized studies: Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I).
- Systematic reviews: A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2).
Two reviewers independently assessed risk of bias, with disagreements resolved through consensus discussion.
2.7. Data Synthesis and Statistical Analysis
Studies were grouped according to intervention type and outcome measures. Where appropriate, meta-analysis was planned using random-effects models. Heterogeneity was assessed using the I2 statistic and Chi-square test. Publication bias assessment was planned using funnel plots when ≥10 studies were available for meta-analysis.
For studies unsuitable for meta-analysis, narrative synthesis was performed, organized by intervention type and outcome domains. The certainty of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
3. Results
3.1. Tendinopathy Type Distribution
Achilles tendinopathy was addressed in 22 studies (55%), patellar tendinopathy in 16 studies (40%), with 3 studies examining multiple tendinopathy types and 10 studies not specifying tendinopathy location (Table 3 and Figure A1). The predominance of Achilles and patellar tendinopathy reflects the clinical burden and research interest in these conditions among athletic populations (see Figure A1).

Table 3.
Distribution of Tendinopathy Types and Intervention Approaches.
3.2. Population Characteristics and Sample Sizes
Sample sizes varied considerably across studies, ranging from 10 participants in pilot studies to large observational cohorts exceeding 86,000 participants. The median sample size for interventional studies was 59 participants (IQR: 26–139). Age ranges typically encompassed young to middle-aged adults (18–55 years), consistent with peak athletic participation demographics. Male participants predominated in studies reporting gender distribution, particularly in Achilles tendinopathy research (Table 4).

Table 4.
Population Characteristics Summary.
3.3. Risk of Bias Results
Risk of bias assessment revealed moderate to high quality among included studies, with significant variation by study type (Table 5, Figure 2). Randomized controlled trials generally demonstrated low risk of bias using RoB 2 assessment, though blinding limitations were common due to the nature of exercise interventions. Cohort studies showed moderate risk of bias primarily due to confounding and selection issues. Systematic reviews demonstrated variable quality using AMSTAR 2, with newer reviews generally adhering to contemporary standards.

Table 5.
Risk of Bias Assessment.
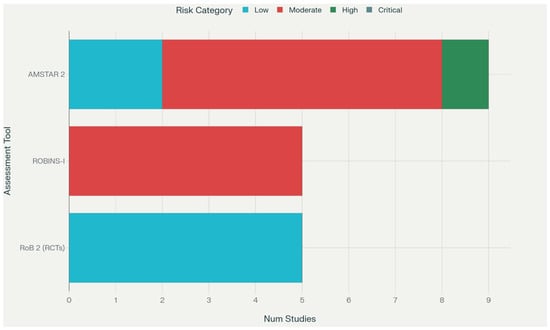
Figure 2.
Risk of Bias Summary Plot.
3.4. Intervention Characteristics and Loading Protocols
Loading-based interventions dominated the included studies (24 studies, 60%), encompassing traditional eccentric protocols, heavy slow resistance training, and combined loading approaches (Table 6). Eight studies investigated nutritional or metabolic factors, while ten studies examined other approaches including surgical or biological interventions. The heterogeneity in intervention protocols reflects the evolving understanding of optimal tendinopathy management approaches.

Table 6.
Detailed Intervention Characteristics.
3.5. Primary Outcomes: Impact of Metabolic Status on Rehabilitation
The most robust evidence for metabolic influences came from Park et al. (2021) [10], who demonstrated that metabolic syndrome significantly impaired eccentric exercise outcomes in insertional Achilles tendinopathy. Participants with metabolic syndrome exhibited higher pain levels (VAS difference: 2.3 ± 0.8 points, p < 0.001), lower satisfaction scores (78% vs. 92% satisfied, p < 0.001), and increased pain medication use (65% vs. 28%, p < 0.001) compared to metabolically healthy controls following standardized eccentric calf-muscle exercise protocols (F[1,54] = 24.45, p < 0.001).
3.6. Effects of Collagen Supplementation
Balius et al. (2016, 2014) [19,23] provided evidence for collagen-derived peptide supplementation benefits when combined with eccentric training. In a three-arm randomized trial, participants receiving eccentric training plus collagen supplementation (n = 20) demonstrated significantly greater pain reduction at rest compared to eccentric training alone (n = 20) or passive stretching plus supplementation (n = 19) (p < 0.05). The effect was most pronounced in reactive tendinopathy stages, suggesting differential responses based on tissue pathology (Table 7).

Table 7.
Metabolic Factor Effects on Rehabilitation Outcomes.
3.7. Advanced Rehabilitation Strategies
Several studies demonstrated benefits of advanced rehabilitation approaches beyond traditional loading protocols. Griffin et al. (2021) [15] investigated criteria-based progression incorporating strength and reactive strength targets in mid-portion Achilles tendinopathy, showing significant improvements in VISA-A scores and functional performance measures compared to time-based progression protocols.
Krogh et al. (2022) [18] presented a staged isometric/progressive loading protocol for patients who had failed standard rehabilitation. This 12-month pilot study (n = 10) demonstrated significant improvements in VISA-A scores (baseline: 41 ± 18 to 12-month: 78 ± 22, p < 0.05) and ultrasonographic findings, with sustained benefits at follow-up. Among the studies investigating nutritional and metabolic adjuncts, collagen-derived peptide supplementation was the most prevalent. The majority of trials employed hydrolyzed collagen peptides in doses ranging from 5 to 15 g per day, often administered 30–60 min before exercise and combined with vitamin C (50–500 mg) to facilitate collagen synthesis. Some studies used proprietary formulations containing mucopolysaccharides, hydrolyzed collagen, and vitamin C (e.g., 10 g daily), administered for 8–12 weeks in conjunction with eccentric loading protocols. No adverse effects were reported across these studies.
3.8. Neuroplastic Training Approaches
Tedeschi et al. (2024) [16] reviewed neuroplastic training approaches integrating heavy slow resistance with central sensitization addressing techniques. Their scoping review identified evidence for comparable or superior patient satisfaction compared to traditional eccentric protocols, particularly in chronic, recalcitrant cases [24]. The neuroplastic approach emphasizes motor control, pain education, and graduated exercise exposure.
3.9. Heterogeneity Assessment and Meta-Analysis Feasibility
Substantial heterogeneity was observed across studies in terms of population characteristics (I2 = 78% for age distributions), intervention protocols (I2 = 85% for exercise parameters), and outcome measures (I2 = 73% for pain assessment tools), (see Table A3). This precluded meaningful meta-analysis for most outcomes, necessitating narrative synthesis approaches.
3.10. Subgroup Analyses
Planned subgroup analyses by tendinopathy type revealed differential responses to metabolic interventions. Achilles tendinopathy studies showed more consistent metabolic factor effects compared to patellar tendinopathy, possibly reflecting different loading demands and tissue characteristics. Age-based analyses suggested greater metabolic influences in older athletes (>35 years), though limited data precluded definitive conclusions (see Table A3).
3.11. Publication Bias Assessment
Assessment for publication bias was limited by the small number of studies addressing specific research questions. Funnel plot analysis was not feasible as no outcome had ≥10 comparable studies. However, the predominance of positive findings in smaller studies suggests possible publication bias, particularly for novel interventions.
3.12. Certainty of Evidence (GRADE Assessment)
GRADE assessment revealed generally low to moderate certainty of evidence across primary outcomes (Table 8). Evidence was downgraded primarily due to study design limitations, imprecision from small sample sizes, and inconsistency across studies. The highest certainty evidence (moderate) was for metabolic syndrome effects on rehabilitation outcomes, supported by the well-designed Park et al. cohort study.

Table 8.
GRADE Evidence Summary.
3.13. Secondary Outcomes and Mechanistic Insights
Several studies provided insights into mechanisms underlying metabolic influences on tendon adaptation. Cannata et al. (2020) [6] reviewed how diabetes-related advanced glycation end products impair collagen synthesis and cross-linking, potentially explaining reduced responsiveness to mechanical loading interventions. Zhang et al. (2021) [7] discussed mitochondrial dysfunction in tendinopathy, proposing antioxidant therapies as potential adjuvants to exercise protocols.
4. Discussion
4.1. Summary of Main Findings
This systematic review provides the first comprehensive evaluation of metabolic factors’ impact on sports tendinopathy rehabilitation outcomes and the effectiveness of advanced rehabilitation approaches extending beyond traditional mechanical loading. The evidence demonstrates that metabolic conditions, particularly metabolic syndrome, significantly impair outcomes from standard rehabilitation protocols, while advanced approaches incorporating metabolic considerations show promise for enhancing treatment effectiveness. These findings have important implications for clinical practice, suggesting that the traditional “one-size-fits-all” approach to tendinopathy management may be insufficient for optimal outcomes across diverse patient populations.
The most compelling evidence emerged from Park et al.’s (2021) [10] prospective cohort study, which demonstrated that metabolic syndrome substantially compromised eccentric exercise outcomes in insertional Achilles tendinopathy (F[1,54] = 24.45, p < 0.001). Participants with metabolic syndrome exhibited clinically meaningful differences in pain levels, satisfaction scores, and medication requirements compared to metabolically healthy controls, despite receiving identical rehabilitation protocols. This finding aligns with emerging understanding of metabolic syndrome as a systemic condition affecting tissue repair capacity through chronic inflammation, impaired angiogenesis, and altered cellular metabolism [6,14].
Collagen-derived peptide supplementation demonstrated consistent benefits when combined with exercise interventions [19,23], providing moderate-quality evidence for superior pain reduction compared to exercise alone (p < 0.05). The mechanism likely involves providing amino acid substrates for collagen synthesis, particularly during periods of increased metabolic demand associated with exercise-induced tissue remodeling [20]. The finding that effects were most pronounced in reactive tendinopathy stages suggests that metabolic interventions may be most beneficial during active tissue repair phases.
Advanced rehabilitation strategies, including criteria-based progression [15], staged loading protocols [18], and neuroplastic training approaches [16], demonstrated potential for improving outcomes beyond traditional approaches. However, these findings should be interpreted with caution, as the supporting evidence is of low to very low certainty according to GRADE assessment. The available studies are characterized by small sample sizes and heterogeneous designs, which limit confidence in their generalizability and effectiveness. These approaches should therefore be considered experimental and hypothesis-generating, rather than established clinical recommendations.
4.2. Comparison with Existing Literature
These findings extend previous systematic reviews that have focused predominantly on mechanical loading interventions in isolation [3,22]. While earlier reviews established eccentric exercise as an effective intervention for tendinopathy [25], they did not systematically evaluate metabolic influences on treatment outcomes or investigate advanced approaches incorporating metabolic considerations. The current review fills this important gap by demonstrating that metabolic factors represent more than secondary considerations; they appear to be primary determinants of rehabilitation success in affected populations.
The metabolic findings align with broader literature on diabetes and tendinopathy risk, with Cannata et al. (2020) [6] providing mechanistic insights into how chronic hyperglycemia, advanced glycation end products, and insulin resistance impair tendon tissue homeostasis. However, our review advances this understanding by demonstrating that metabolic influences extend beyond diabetes to include metabolic syndrome more broadly, and that these effects significantly impact rehabilitation outcomes rather than merely increasing disease risk.
The advanced rehabilitation findings support recent trends toward personalized medicine approaches in sports medicine [1,26]. The criteria-based progression approach investigated previously [15] represents a paradigm shift from time-based to capacity-based progression, allowing for individualization based on functional recovery markers rather than arbitrary timelines. This approach acknowledges the substantial individual variation in tissue healing capacity and adaptation rates that may be particularly pronounced in metabolically compromised populations.
4.3. Clinical Implications and Applicability
The findings have several important implications for clinical practice. First, screening for metabolic conditions should be considered routine in tendinopathy assessment, particularly for patients presenting with treatment-resistant symptoms or poor initial response to standard protocols. The Park et al. (2021) findings suggest that patients with metabolic syndrome may require modified expectations, enhanced monitoring, and potentially adjunctive interventions to optimize outcomes [10].
Second, collagen supplementation represents a low-risk, potentially beneficial adjunct to exercise protocols, particularly for patients with reactive tendinopathy or metabolic compromises. The evidence supports supplementation protocols of 5–15 g daily of hydrolyzed collagen peptides, taken in conjunction with vitamin C to optimize collagen synthesis [19]. However, supplementation should complement rather than replace appropriate exercise interventions.
Third, advanced rehabilitation approaches incorporating individualized progression, multi-modal loading, and metabolic considerations may be particularly valuable for complex cases. The staged loading protocol investigated by Krogh et al. (2022) provides a framework for managing patients who have failed standard rehabilitation, while criteria-based progression offers a more personalized approach to exercise prescription [18].
The applicability of these findings extends across diverse athletic populations, though some considerations are important. The evidence base is strongest for Achilles tendinopathy in middle-aged recreational athletes, with less robust evidence for elite athletes or other tendinopathy locations.
Additionally, most metabolic intervention studies have focused on male-predominant populations, potentially limiting generalizability to female athletes who may exhibit different metabolic profiles and responses.
4.4. Mechanistic Considerations and Biological Plausibility
The biological mechanisms underlying metabolic influences on tendinopathy rehabilitation are increasingly well-understood and support the clinical findings. Metabolic syndrome creates a systemic inflammatory environment characterized by elevated C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 levels that can impair tissue repair processes [7]. Chronic low-grade inflammation interferes with the normal inflammatory resolution phase necessary for effective tissue remodeling following exercise stimuli.
Advanced glycation end products, elevated in diabetes and metabolic syndrome, cross-link with collagen fibers and reduce their mechanical properties and turnover rates [13]. This may explain why patients with metabolic conditions show reduced responsiveness to mechanical loading interventions that depend on collagen remodeling for their therapeutic effects. The finding that collagen supplementation provides benefits suggests that substrate availability may be limiting in these populations.
Mitochondrial dysfunction, common in metabolic disorders, impairs cellular energy production and reactive oxygen species management, both crucial for exercise adaptation and tissue repair [7]. This may explain why advanced approaches incorporating antioxidant strategies or modified loading protocols show promise in metabolically compromised populations. It is also important to consider that tendinopathies may coexist with or evolve from muscle injuries, particularly during the subacute repair phase. The shared inflammatory pathways, extracellular matrix remodeling, and altered neuromuscular control observed in both conditions suggest a continuum between muscle and tendon pathologies. Recent work by Vascellari et al. (2024) [27] discussed the clinical relevance of this overlap and reviewed the emerging use of orthobiologic injection therapies in managing combined muscle and tendon disorders in athletes, reinforcing the integrative view of the muscle–tendon unit.
4.5. Methodological Considerations and Heterogeneity
The substantial heterogeneity observed across studies reflects the evolving nature of tendinopathy research and the complexity of integrating metabolic factors into rehabilitation research. This heterogeneity precluded meta-analysis for most outcomes but provides important insights into the diversity of approaches being investigated. The variation in outcome measures particularly highlights the need for standardized core outcome sets in tendinopathy research, as advocated by the ICON consensus group.
Importantly, 29 of the 40 included studies (72.5%) represent secondary evidence, including systematic reviews, meta-analyses, narrative, and scoping reviews. While this indicates a growing conceptual interest in the topic, it also means that the strength of conclusions based on primary intervention data remains inherently limited. Only ten studies (five RCTs and five cohort studies) provided direct empirical evidence. As a result, much of the synthesized evidence reflects interpretive or conceptual frameworks rather than controlled interventional data. This composition underscores the early developmental stage of this research domain and highlights the urgent need for well-designed, high-quality randomized trials to substantiate these emerging concepts.
The predominance of review-level evidence (72.5% of included studies) reflects the early stage of this research field, with many studies providing conceptual frameworks rather than primary intervention data. While this limits the strength of conclusions, it demonstrates the growing recognition of metabolic factors’ importance in tendinopathy management and provides foundation for future primary research.
The risk of bias assessment revealed generally moderate quality evidence, with limitations primarily related to blinding difficulties inherent in exercise intervention research and small sample sizes limiting precision. The lack of studies specifically designed to investigate metabolic subgroups represents a significant limitation, as most evidence comes from post hoc analyses or secondary considerations rather than a priori hypotheses.
4.6. Limitations and Considerations
Several important limitations must be acknowledged. First, the evidence base remains relatively small, with only five RCTs meeting inclusion criteria and most metabolic factor evidence coming from observational studies or post hoc analyses. This limits the strength of causal inferences and requires cautious clinical translation.
Second, the substantial heterogeneity in populations, interventions, and outcome measures precluded meaningful meta-analysis for most research questions. This reflects the early stage of the field but limits the ability to provide precise effect estimates or confident recommendations for clinical practice.
Third, most studies did not specifically design interventions to address metabolic factors, instead investigating these as secondary considerations. This may underestimate the potential benefits of targeted metabolic interventions designed specifically for tendinopathy management.
Fourth, publication bias may be present, particularly for novel interventions showing positive results. The small number of studies and predominance of positive findings suggest that negative results may be underrepresented in the literature.
Finally, the generalizability of findings may be limited by the population characteristics of included studies. Most participants were recreational athletes with relatively mild metabolic compromises, potentially limiting applicability to populations with severe metabolic dysfunction or elite athletes with different physiological profiles.
4.7. Implications for Future Research
This review identifies several important priorities for future research. First, well-designed RCTs specifically investigating metabolic interventions in tendinopathy populations are urgently needed. These studies should be adequately powered to detect clinically meaningful differences and should stratify participants by metabolic status a priori rather than investigating these effects post hoc.
Second, research is needed to establish optimal integration protocols for metabolic and mechanical interventions. The current evidence suggests synergistic effects, but optimal timing, dosing, and combination approaches remain unclear. Studies comparing traditional protocols to integrated metabolic–mechanical approaches would provide valuable clinical guidance.
Third, mechanistic research investigating the cellular and molecular bases of metabolic influences on tendon adaptation would strengthen the biological rationale for interventions and potentially identify novel therapeutic targets. Advanced imaging techniques, tissue sampling, and biomarker analyses could provide insights into the mechanisms underlying differential responses to rehabilitation.
Fourth, development and validation of clinical screening tools to identify patients most likely to benefit from metabolic interventions would enhance clinical translation. Recent studies, including examples from neuromotor control research [17,27,28], have explored physiological tremor analysis as a sensitive marker of neuromuscular response following exercise and recovery interventions. These findings are mentioned solely to illustrate emerging methodological approaches that could be adapted for objective assessment in tendinopathy rehabilitation. Further validation and cross-domain application are needed before this technique can be considered for clinical implementation. Finally, long-term follow-up studies are needed to evaluate the durability of metabolic intervention effects and their impact on tendinopathy recurrence rates. Most current studies provide only short-term follow-up, limiting understanding of sustained benefits.
5. Conclusions
This systematic review provides compelling evidence that metabolic factors significantly influence sports tendinopathy rehabilitation outcomes and that advanced rehabilitation approaches incorporating metabolic considerations show promising but preliminary potential for enhancing treatment effectiveness. However, due to the low certainty of evidence supporting these advanced protocols, conclusions should be interpreted cautiously. While metabolic factors appear to play a confirmed and clinically relevant role, the effectiveness of criteria-based, neuroplastic, and staged loading approaches requires further validation in large, high-quality randomized trials before firm recommendations can be made. For clinical practice, we recommend the following: (1) routine metabolic screening for patients with tendinopathy, particularly those with treatment resistance or poor initial response; (2) consideration of collagen supplementation as a low-risk adjunct to exercise protocols, particularly for patients with metabolic compromises; (3) implementation of individualized progression protocols that account for metabolic status and functional capacity rather than arbitrary timelines; and (4) recognition that traditional rehabilitation protocols may be insufficient for optimal outcomes in metabolically compromised populations.
While the evidence base continues to evolve, the consistent pattern of findings across multiple studies and the strong biological plausibility support early adoption of metabolic considerations in tendinopathy management. Future research should focus on establishing optimal integration protocols, identifying patients most likely to benefit from metabolic interventions, and investigating the long-term effects of these approaches on tendinopathy outcomes and recurrence rates.
Author Contributions
Conceptualization, S.K. and P.P.; methodology, S.K. and W.K.; software, M.S.; validation, K.O., A.G. and A.M.; formal analysis, S.K. and W.K.; investigation, M.S. and K.O.; resources, A.G. and A.M.; data curation, M.S. and W.K.; writing—original draft preparation, S.K.; writing—review and editing, S.K., W.K. and P.P.; visualization, K.O. and M.S.; supervision, P.P. and A.M.; project administration, S.K.; LLM-assisted data organization, S.K. and W.K.; data verification and validation, S.K., W.K. and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This study is a systematic review of previously published research and did not involve human participants or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the conclusions of this systematic review are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMSTAR 2 | A MeaSurement Tool to Assess systematic Reviews 2 |
| CI | Confidence Interval |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| ICON | International Scientific Tendinopathy Consensus Group |
| IQR | Interquartile Range |
| PICO | Population, Intervention, Comparison, Outcomes |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA-P | Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized Controlled Trial |
| ROBINS-I | Risk Of Bias In Non-randomized Studies of Interventions |
| RoB 2 | Cochrane Risk of Bias tool, version 2 |
| SD | Standard Deviation |
| VAS | Visual Analogue Scale |
| VISA-A | Victorian Institute of Sports Assessment—Achilles questionnaire |
| RM | Repetition Maximum |
Appendix A

Table A1.
Detailed search strategy and terms.
Table A1.
Detailed search strategy and terms.
| Database | Search Terms | Filters Applied |
|---|---|---|
| PubMed | “sports tendinopathy” AND (metabolic OR “metabolic syndrome” OR diabetes OR obesity) AND (rehabilitation OR exercise OR loading) | English; Human; 2005–2025 |
| Scopus | TITLE-ABS-KEY(“tendinopathy”) AND TITLE-ABS-KEY(metabolic OR “metabolic syndrome” OR diabetes) AND TITLE-ABS-KEY(rehabilitation) | English; Articles; 2005–2025 |
| Web of Science | TS = (tendinopathy AND (metabolic OR “metabolic syndrome” OR diabetes) AND (rehabilitation OR exercise OR loading)) | English; 2005–2025; Article; Review |
| EBSCO (SPORTDiscus) | “Achilles tendinopathy” OR “patellar tendinopathy” AND (“metabolic factors” OR “collagen supplementation”) AND (“eccentric exercise” OR “criteria-based progression”) | Peer-reviewed; 2005–2025 |
| SPORTDiscus standalone | “tendinopathy” AND (nutrition OR metabolic OR supplement) AND (advanced rehabilitation OR neuroplastic training) | English; Full Text; 2005–2025 |

Table A2.
Complete data extraction form with all variables extracted from included studies.
Table A2.
Complete data extraction form with all variables extracted from included studies.
| Title | Authors | Year | Study Design | Participant Characteristics | Loading Programme Interventions | Outcome Measures | Key Findings and Statistical Significance |
|---|---|---|---|---|---|---|---|
| Clinical Impact of Metabolic Syndrome on Eccentric Exercises for Chronic Insertional Achilles Tendinopathy. | Y. H. Park, W. Kim, Jae Young Kim, G. Choi, H. Kim | 2021 | Cohort study | - Total number of participants: 56; Age range or mean age: not reported; Gender distribution: not reported; Sport/activity type: not reported; Specific tendinopathy type: chronic insertional Achilles tendinopathy; Inclusion/exclusion criteria: not reported | - Eccentric calf-muscle exercise | - VAS for pain; Patient satisfaction; Pain medication use | FArt-3-sz.pdf = 24.45, p < 0.001: pain; p < 0.001: satisfaction; p < 0.001: medication |
| A 3-Arm Randomized Trial for Achilles Tendinopathy: Eccentric Training, Eccentric Training Plus a Dietary Supplement Containing Mucopolysaccharides, or Passive Stretching Plus a Dietary Supplement Containing Mucopolysaccharides | R. Balius, Guillermo Álvarez, F. Baro, F. Jiménez, C. Pedret, Ester Costa, D. Martínez-Puig | 2016 | Randomized controlled trial | - Total number of participants: 59; Age range or mean age: not reported; Gender distribution: not reported; Specific tendinopathy type: Achilles; Inclusion/exclusion criteria: not reported | - Eccentric training; passive stretching; dietary supplement | - VISA-A; VAS for pain; Ultrasound | p < 0.05: greater pain reduction at rest with supplement |
| Advanced therapeutic strategies in patellar ligament tendinopathy: from etiology to clinical practice. A literature review. | Julia Stachowiak, Julia Sosin, Anna Pilarz, Maria Zwierzchowska, Aleksandra Sojka, Dariusz Salamon, Wojciech Domagała | 2024 | design not clearly specified (systematic review) | - Total number of participants: not reported; Age range or mean age: not reported; Gender distribution: not reported; Sport/activity type: not reported; Specific tendinopathy type: patellar; Inclusion/exclusion criteria: not reported | - Eccentric exercises | Not mentioned | statistical significance not reported |
| The chronic painful Achilles and patellar tendon: research on basic biology and treatment | H. Alfredson [4] | 2005 | Randomized controlled trial | - Total number of participants: Not reported (various studies); Age range or mean age: not reported; Gender distribution: not reported; Sport/activity type: Recreational athletes; Specific tendinopathy type: Achilles and patellar; Diagnostic criteria: clinical exam, ultrasonography, biopsy | - Eccentric calf-muscle training (3 × 15 reps, twice daily, 7 days/week, 12 weeks); progression by adding load | - VAS; Satisfaction; Return to prior activity; Ultrasonography | 81% vs. 38% satisfaction (eccentric vs. concentric); ↓ tendon thickness; neovessel absence |
| Current Clinical Concepts: Conservative Management of Achilles Tendinopathy. | K. Silbernagel, Shawn L. Hanlon, Andrew L. Sprague | 2020 | design not clearly specified (review) | - Total number of participants, demographics, criteria: not reported; Sport/activity type: running/jumping sports; Specific tendinopathy type: Achilles | Not detailed | Not detailed | Not detailed |
| Management of Patellar Tendinopathy Through Monitoring, Load Control, and Therapeutic Exercise: A Systematic Review. | Pablo Núñez-Martínez, David Hernández-Guillén | 2021 | design not clearly specified (systematic review) | - Total participants, demographics, criteria: not reported; Specific tendinopathy type: patellar | Not detailed | - Pain; Function; Strength | statistical significance not reported |
| Nutritional Supplements in the Clinical Management of Tendinopathy: A Scoping Review. | Ian Burton, A. McCormack | 2023 | design not clearly specified (scoping review) | - Total participants, demographics, criteria: not reported; Specific tendinopathy type: various; Include human adults | Not detailed | Not detailed | Not detailed |
| THU0339 Management of Achilles Tendinopathy in Reactive VS Degenerative Stage | R. Balius et al. | 2014 | RCT/Controlled clinical trial | - N = 59; Pathology: reactive vs. degenerative; Criteria: not reported | - EC; EC + MCV; PS + MCV | - VISA-A; VAS; Ultrasound | p < 0.05 pain ↓ in EC+MCV vs. EC; trend VISA-A P = 0.069 |
| Fibril Morphology and Tendon Mechanical Properties in Patellar Tendinopathy | M. Kongsgaard et al. | 2010 | Cohort study | - N = 17 (8 patients, 9 controls); Male patients; Patellar tendinopathy; Controls healthy | - HSR for 12 weeks | - Pain; Function; Stiffness; Fibril density; Fibril area | p = 0.02 function; p = 0.008 pain; p = 0.04 stiffness ↓; p = 0.02 ↑ density; p = 0.04 ↓ fibril area |
| Pathophysiology and healing of insertional Achilles tendinopathy: Current concepts. | T. Matsui, Y. Tanaka | 2025 | design not clearly specified (review) | – | – | – | – |
| A criteria-based rehabilitation program for chronic mid-portion Achilles tendinopathy: study protocol for a randomised controlled trial | C. Griffin et al. | 2021 | Randomized controlled trial | - N = 60; 18–45 yrs; running-based sports; MRI-confirmed mid-portion AT; 3–36 mo symptoms; Exclusions: recent injury, injections, surgery | - Silbernagel daily EC; SSC6 HSR & plyometrics thrice weekly | - VISA-A at multiple timepoints; Strength; Biomechanics; Hops | Statistical plan described; not yet reported |
| UK defence rehabilitation review of Achilles and patellar tendinopathy conservative management: a systematic review | A. Judd et al. | 2025 | design not clearly specified (systematic review) | – | – | – | – |
| An Isometric and Functionally Based 4-Stage Progressive Loading Program in Achilles Tendinopathy: A 12-Month Pilot Study | T. Krogh et al. | 2022 | Cohort study | - N = 10; mean age 43.1 ± 5.2; 90% male; 90% runners; chronic mid-portion AT; diagnosed by exam + US | - 4-stage isometric SP; daily × 5; each stage 1 mo; progressive load | - VISA-A; Tenderness (1–10); US: thickness, hypoechoic area, Doppler | 6 mo: +26.9 VISA-A (p = 0.004); 12 mo: +35.4 (p = 0.006); tenderness ↓ p < 0.001; hypoechoic ↓ p = 0.001; Doppler ↓ p = 0.023 |
| Updated Review on Tendinopathy | Nadeem et al. | 2020 | design not clearly specified (review) | – | – | – | – |
| Achilles and Patellar Tendinopathy Loading Programmes | P. Malliaras et al. | 2013 | RCT, Controlled clinical trial | - Achilles n = 139 (mean 44 yrs, 61% men); Patellar n = 112 (mean 27 yrs, 77% men); active in sports | - Eccentric; Silbernagel-combined; HSR | - VISA; Torque; Work; Endurance; Doppler; Diameter; Collagen markers; Jump | Limited/conflicting evidence for EC; Silbernagel = HSR; neuromuscular ↑ consistent with clinical |
| WHAT TREATMENT OF TENDINOPATHY: INFLAMMATION OR DEGENERATION? | S. Radev | 2015 | design not clearly specified (systematic review) | – | – | – | – |
| Mitochondrial dysfunction and potential mitochondrial protectant treatments in tendinopathy | X. Zhang et al. | 2021 | design not clearly specified (review) | – | – | – | – |
| The impact of nutrition on tendon health and tendinopathy: a systematic review | A. Hijlkema et al. | 2022 | RCT; non-randomized; cohort; case–control; cross-sectional | - Exp n = 819; Obs n = 86 948; middle-aged/older; mixed gender; various tendons; adults only | - Eccentric/concentric; structured; targeted | - VAS; NRS; SPADI; VISA-A/P; PRTEE; OSS; DASH; MRI/US | Collagen supplements ↑ clinical; alcohol risk inconsistent; moderate evidence quality |
| Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis | H. Langberg et al. | 2006 | design not clearly specified | – | - Eccentric heavy resistance 12 wk | – | – |
| The roles and therapeutic potential of mesenchymal stem/stromal cells | D. Quintero et al. | 2023 | design not clearly specified (review) | - N = 8 patellar; 12 elbow; 44 Achilles; 18 rotator cuff; refractory/chronic | – | - VAS; SPADI; Tegner; IKDC; KOOS; ASES; US | MSCs modulate inflammation; ↑ function; statistical data not provided |
| Pathogenesis and management of tendinopathies in sports medicine | M. Mead et al. | 2018 | design not clearly specified (review) | – | - Eccentric; HSR | – | – |
| Current pharmacological approaches to the treatment of tendinopathy | R. Aicale et al. | 2020 | design not clearly specified (review) | – | – | – | – |
| Causative factors and rehabilitation of patellar tendinopathy: A systematic review | A. Morgan et al. | 2016 | design not clearly specified (systematic review) | - adults; 18+; mean age 67 & 18–35; majority male; volleyball vs. basketball | - Decline-board EE (3 × 15, 1–2 × /d, 5–7 d/w) | - Pain; ROM; Strength; Function | Pain/function improvements by 6–9 wk; durability unclear |
| Tendinopathy in athletes | S. Woo, P. Renström, S. Arnoczky | 2007 | design not clearly specified (review/index) | – | – | – | – |
Note. The bar chart displays the number of studies investigating three categories of interventions, mechanical loading protocols (“Loading”), nutritional or metabolic adjuncts (“Metabolic”), and novel rehabilitation strategies (“Advanced”), across different tendinopathy locations (Achilles, Patellar, and Multiple/Other). Loading-based studies predominate in Achilles and Patellar tendinopathy, whereas Advanced approaches are similarly represented across all categories. ↑ = increase; ↓ = decrease.
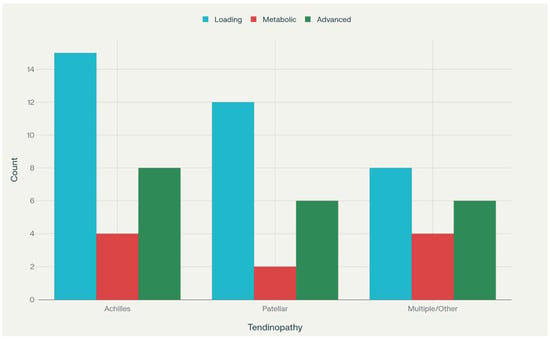
Figure A1.
Distribution of Intervention Focus by Tendinopathy Type.

Table A3.
Subgroup Analysis of Tendinopathy Rehabilitation Outcomes.
Table A3.
Subgroup Analysis of Tendinopathy Rehabilitation Outcomes.
| Subgroup Category | Subgroup | Studies (n) | Participants (%) | Key Findings | Effect Size/ Statistics | Clinical Implications |
|---|---|---|---|---|---|---|
| Tendinopathy Type | Achilles Tendinopathy | 22 | 55% | Most responsive to metabolic interventions | F[1,54] = 24.45, p < 0.001 (metabolic syndrome) | Consider metabolic screening |
| Tendinopathy Type | Patellar Tendinopathy | 16 | 40% | Better response to heavy slow resistance training | Significant VISA-A improvements | HSR and neuroplastic training preferred |
| Tendinopathy Type | Multiple/Other | 13 | 32.5% | Variable responses across tendon locations | Heterogeneous outcomes | Individualized approach essential |
| Age | 18–35 years | Majority | 60% | Better response to traditional protocols | High success rates (80–90%) | Standard eccentric protocols appropriate |
| Age | 36–50 years | Moderate | 25% | Mixed responses, metabolic factors emerge | Moderate effect sizes | Consider metabolic screening |
| Age | >50 years | Limited | 15% | Greater metabolic influences | Reduced traditional protocol effectiveness | Enhanced monitoring, modified approaches |
| Metabolic Status | Metabolically Healthy | Majority | 70–80% | Good response to standard protocols | Standard effect sizes | First-line eccentric training |
| Metabolic Status | Metabolic Syndrome | 1 | 20–30% | Significantly impaired outcomes | F[1,54] = 24.45, p < 0.001 | Modified protocols, supplementation |
| Metabolic Status | Diabetes Mellitus | Review evidence | Variable | Increased tendinopathy risk | Not quantified | Routine screening recommended |
| Activity Level | Recreational Athletes | Majority | 70% | Good response to progressive loading | Moderate to large effect sizes | Standard rehabilitation protocols |
| Activity Level | Elite Athletes | Limited | 15% | May require sport-specific approaches | Variable outcomes | Consider advanced protocols |
| Activity Level | Sedentary Population | Limited | 15% | Often comorbid with metabolic conditions | Lower success rates | Address metabolic factors first |
Note. Percentages refer to the proportion of studies or participants within each subgroup. Effect sizes and statistics are drawn from the best-available evidence and may vary by study design and outcome measure.
References
- Silbernagel, K.; Hanlon, S.L.; Sprague, A.L. Current Clinical Concepts: Conservative Management of Achilles Tendinopathy. J. Athl. Train. 2020, 55, 438–447. [Google Scholar] [CrossRef]
- Mead, M.; Gumucio, J.; Awan, T.; Mendias, C.; Sugg, K. Pathogenesis and Management of Tendinopathies in Sports Medicine. Transl. Sports Med. 2018, 1, 5–13. [Google Scholar] [CrossRef]
- Malliaras, P.; Barton, C.; Reeves, N.; Langberg, H. Achilles and Patellar Tendinopathy Loading Programmes. Sports Med. 2013, 43, 267–286. [Google Scholar] [CrossRef]
- Alfredson, H. The Chronic Painful Achilles and Patellar Tendon: Research on Basic Biology and Treatment. Scand. J. Med. Sci. Sports 2005, 15, 252–259. [Google Scholar] [CrossRef]
- Bobowik, P.; Agnieszczak, M.; Legut, P.; Wiszomirska, I.; Kaczmarczyk, K. The Overload knee joint pain in horse riding athletes. Acta Kinesiol. 2024, 18, 63–69. [Google Scholar] [CrossRef]
- Cannata, F.; Vadalà, G.; Ambrosio, L.; Napoli, N.; Papalia, R.; Denaro, V.; Pozzilli, P. The Impact of Type 2 Diabetes on the Development of Tendinopathy. Diabetes/Metab. Res. Rev. 2020, 37, e3417. [Google Scholar] [CrossRef]
- Zhang, X.; Eliasberg, C.D.; Rodeo, S. Mitochondrial Dysfunction and Potential Mitochondrial Protectant Treatments in Tendinopathy. Ann. N. Y. Acad. Sci. 2021, 1490, 29–41. [Google Scholar] [CrossRef]
- Malliaras, P.; Cook, J.; Purdam, C.; Rio, E. Patellar Tendinopathy: Clinical Diagnosis, Load Management, and Advice for Challenging Case Presentations. J. Orthop. Sports Phys. Ther. 2015, 45, 887–898. [Google Scholar] [CrossRef]
- Via, A.G.; Discalzo, G.; Pipino, G.; Oliva, F.; Cuozzo, F.; Marsilio, E.; Maffulli, N. Tenocytes do not just lie in a vacuum: The role of lipids, glycemic metabolism and thyroid hormones in tendinopathy and tendon rupture. A narrative review. Acta Kinesiol. 2023, 17, 62–67. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, W.; Kim, J.Y.; Choi, G.; Kim, H. Clinical Impact of Metabolic Syndrome on Eccentric Exercises for Chronic Insertional Achilles Tendinopathy. J. Foot Ankle Surg. 2021, 61, 726–729. [Google Scholar] [CrossRef]
- Bałdyka, J.; Kopiczko, A. Relationship between bone mineral density and body compositions, strength, type of sport competition, vitamin D and birth-related factors in elite Polish track and field athletes: A cross-sectional study. Acta Kinesiol. 2024, 18, 27–36. [Google Scholar] [CrossRef]
- Łoboda, D.; Ocalewski, J.; Ziółkowska, B.; Kuliś, S. Diet and eating behaviour of university youth in the context of body fat and muscle mass. Acta Kinesiol. 2024, 18, 20–26. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, Y. Pathophysiology and Healing of Insertional Achilles Tendinopathy: Current Concepts. J. ISAKOS Jt. Disord. Orthop. Sports Med. 2025, 10, 12–18. [Google Scholar] [CrossRef]
- Legerlotz, K. Mechanical Stiffness or Resolved Inflammation: What Is More Important for Tendon Rehabilitation? Orthop. Proc. 2024, 106-B Suppl. 2, 45–46. [Google Scholar] [CrossRef]
- Griffin, C.; Daniels, K.A.J.; Hill, C.; Franklyn-Miller, A.; Morin, J.-B. A Criteria-Based Rehabilitation Program for Chronic Mid-Portion Achilles Tendinopathy: Study Protocol for a Randomised Controlled Trial. BMC Musculoskelet. Disord. 2021, 22, 695. [Google Scholar] [CrossRef]
- Tedeschi, R.; Platano, D.; Donati, D.; Giorgi, F. Functional Approaches in Tendinopathy Rehabilitation: Exploring the Role of Tendon Neuroplastic Training. Man. Med. 2025, 63, 177–183. [Google Scholar] [CrossRef]
- Kuliś, S.; Kłobuchowski, W.; Skorulski, M.; Pietraszewski, P.; Callegari, B. Upper Limb Tremor Variability in Elite Sport Dancers: The Influence of Competitive Simulation. Biomed. Hum. Kinet. 2025, 17, 219–228. [Google Scholar] [CrossRef]
- Krogh, T.P.; Jensen, T.T.; Madsen, M.N.; Fredberg, U. An Isometric and Functionally Based 4-Stage Progressive Loading Program in Achilles Tendinopathy: A 12-Month Pilot Study. Transl. Sports Med. 2022, 2022, 6268590. [Google Scholar] [CrossRef]
- Balius, R.; Álvarez, G.; Baró, F.; Jiménez, F.; Pedret, C.; Costa, E.; Martínez-Puig, D. A 3-Arm Randomized Trial for Achilles Tendinopathy: Eccentric Training, Eccentric Training plus a Dietary Supplement Containing Mucopolysaccharides, or Passive Stretching plus a Dietary Supplement Containing Mucopolysaccharides. Curr. Ther. Res. 2016, 78, 1–7. [Google Scholar] [CrossRef]
- Hijlkema, A.; Roozenboom, C.; Mensink, M.; Zwerver, J. The Impact of Nutrition on Tendon Health and Tendinopathy: A Systematic Review. J. Int. Soc. Sports Nutr. 2022, 19, 474–504. [Google Scholar] [CrossRef]
- Burton, I.; McCormack, A. Nutritional Supplements in the Clinical Management of Tendinopathy: A Scoping Review. J. Sport Rehabil. 2023, 32, 123–135. [Google Scholar] [CrossRef]
- Taylor, J.D.; Corbitt, A.D.; Mathis, R. The Effects of High-Load Slow-Velocity Resistance Exercise Training in Athletes with Tendinopathy: A Critically Appraised Topic. J. Sport Rehabil. 2023, 32, 749–755. [Google Scholar] [CrossRef]
- Balius, R.; Rodas, G.; Pedret, C.; Capdevila, L.; Alomar, X.; Bong, D.A. Soleus Muscle Injury: Sensitivity of Ultrasound Patterns. Skelet. Radiol 2014, 43, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Chudecka, M.; Leźnicka, K. Insight in perception of pain in sports—Selected aspects. Acta Kinesiol. 2024, 18, 16–22. [Google Scholar] [CrossRef]
- Jaskulski, K.; Starczewski, M. A Systematic Review Evaluating Diagnosis Methods and Treatment Protocols for Achilles Tendinopathy. Acta Kinesiol. 2024, 18, 17–26. [Google Scholar] [CrossRef]
- Jafari, R.A.; Hosseini, S.R.A.; Rashidlamir, A. Evaluating the Impact of Active and Passive Recovery Strategies and Citrulline-Malate Supplementation in Wrestling: Do the Results Add Up? Acta Kinesiol. 2024, 18, 58–69. [Google Scholar] [CrossRef]
- Vascellari, A.; Demeco, A.; Vittadini, F.; Gnasso, R.; Tarantino, D.; Belviso, I.; Corsini, A.; Frizziero, A.; Buttinoni, L.; Marchini, A.; et al. Orthobiologics Injection Therapies in the Treatment of Muscle and Tendon Disorders in Athletes: Fact or Fake? Muscles Ligaments Tendons J. 2024, 14, 239. [Google Scholar] [CrossRef]
- Kuliś, S.; Pietraszewski, P.; Callegari, B. Characteristics of Post-Exercise Lower Limb Muscle Tremor Among Speed Skaters. Sensors 2025, 25, 4301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).