Effectiveness of Photobiomodulation to Treat Motor and Non-Motor Symptoms of Parkinson’s Disease: A Randomised Clinical Trial with Extended Treatment
Abstract
1. Introduction
Objective
2. Materials and Methods
2.1. Regulatory Approval
2.2. Recruitment
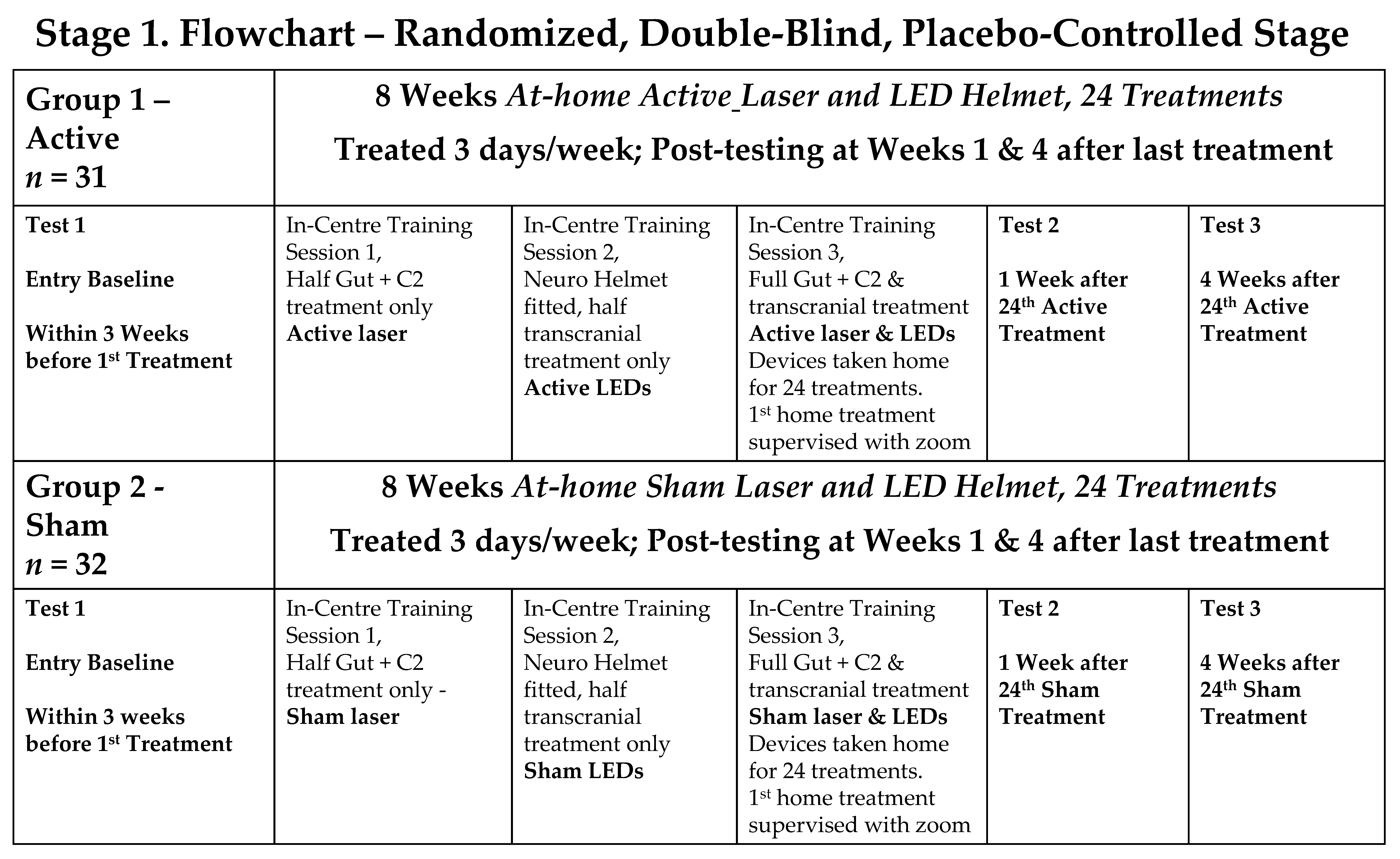
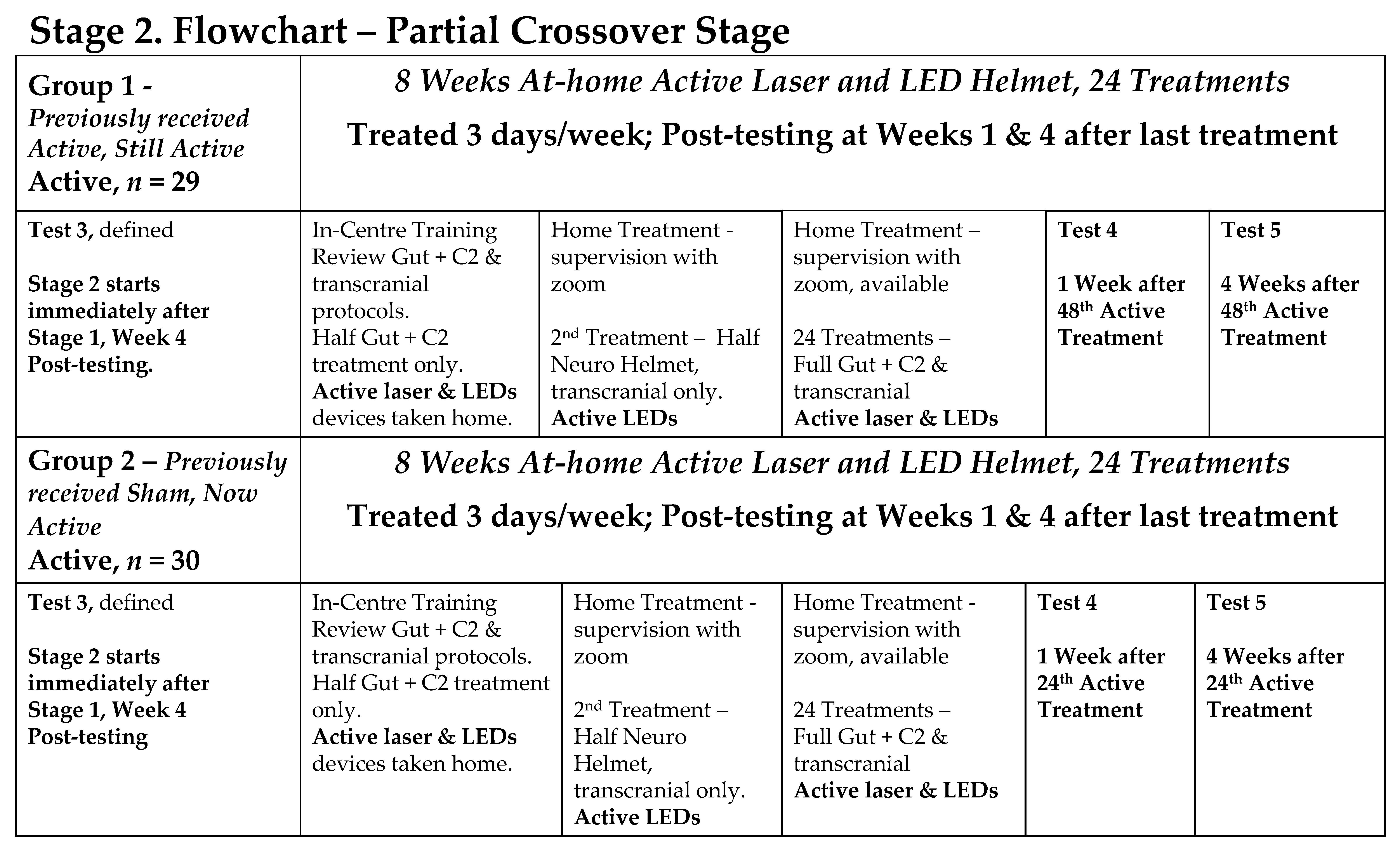
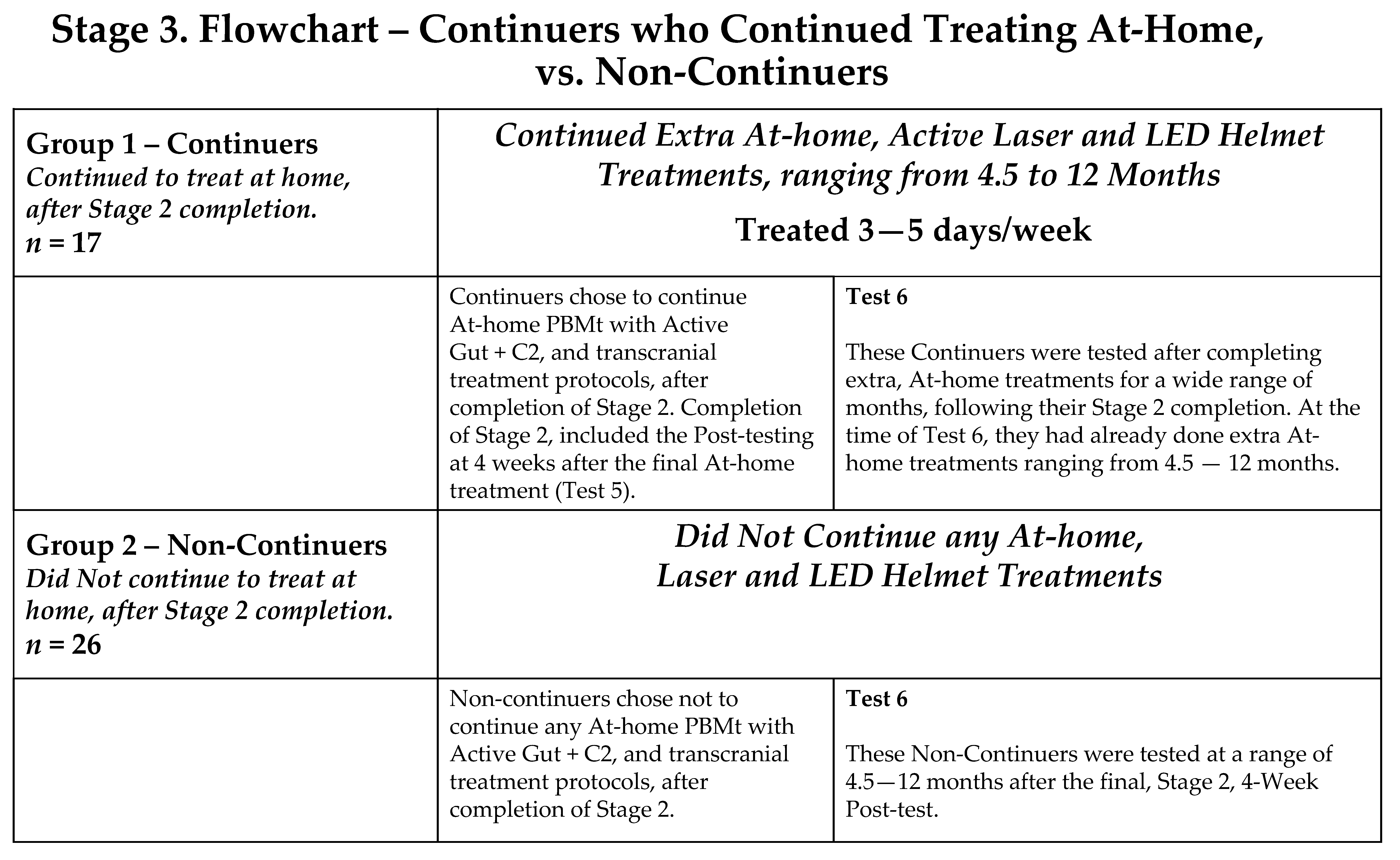
2.3. Study Design
- Stage 1: a double-blind, randomised, placebo-controlled trial (RCT), with participants randomly divided into an Active PBMt group and a Sham PBMt group. Participants in the Sham Group were informed that the LED helmet device only produced near-infrared light, invisible to the naked eye. The only researchers who knew group allocation were a statistician (SR) and researchers who trained the participants in at-home use of PBMt (AS, JS, KT). Outcome assessments were conducted by the neurophysiotherapist (OH), who was blinded to the treatment groups.
- Stage 2: a partial cross-over where all participants received active PBMt.
- Stage 3: participants had the choice of continuing at-home PBMt (“continuers”) or not continuing PBMt (“non-continuers”).
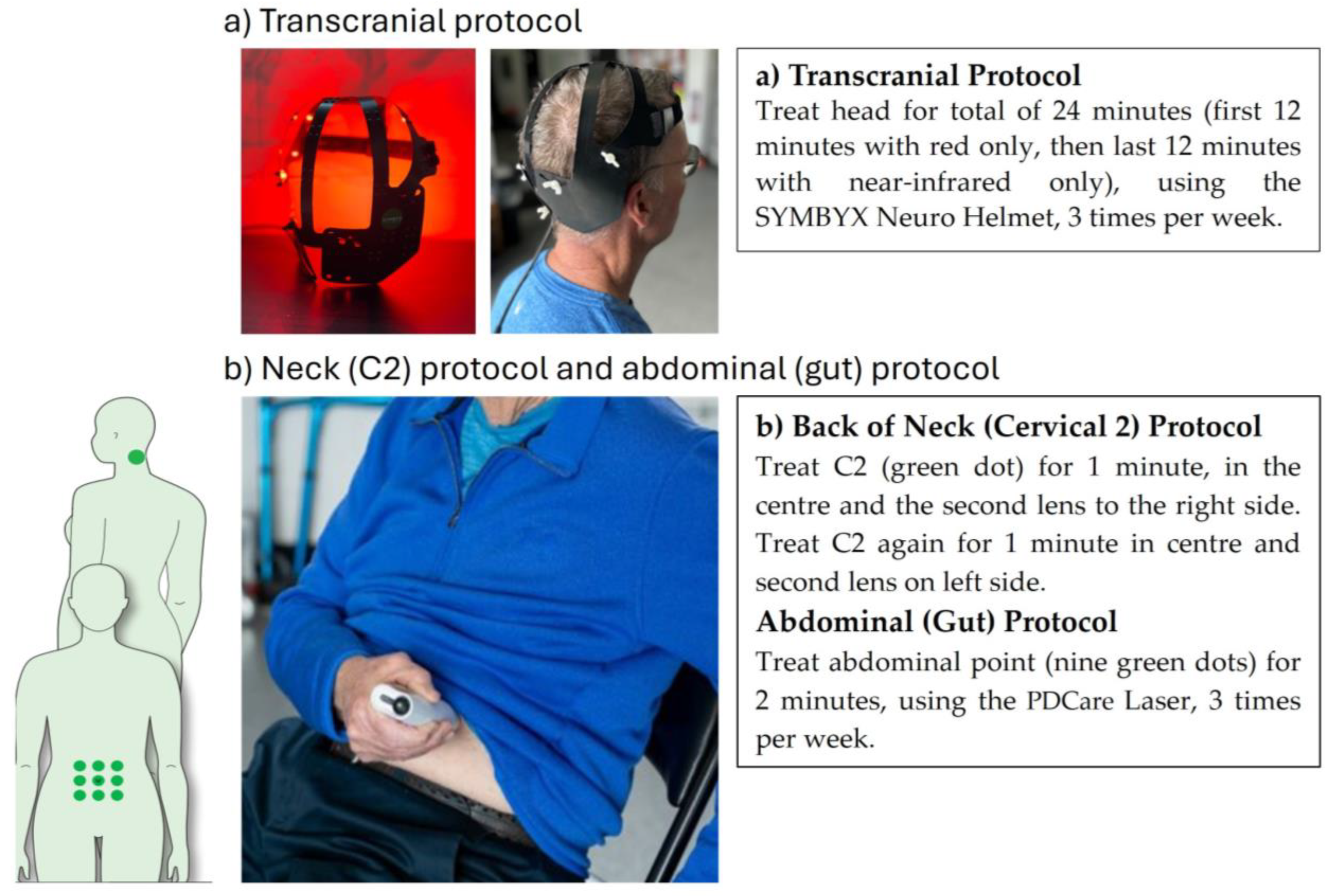
2.4. Photobiomodulation Therapy Intervention
2.5. Participant Contact, Support and Safety
2.6. Outcome Measurement Assessments
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBMt | Photobiomodulation treatment |
| PwP | People Living with Parkinson’s |
| HRQoL | Health Related Quality of Life |
| PD | Parkinson’s Disease |
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Holden, S.K.; Finseth, T.; Sillau, S.H.; Berman, B.D. Progression of MDS-UPDRS Scores Over Five Years in De Novo Parkinson Disease from the Parkinson’s Progression Markers Initiative Cohort. Mov. Disord. Clin. Pract. 2018, 5, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Severson, K.A.; Chahine, L.M.; Smolensky, L.A.; Dhuliawala, M.; Frasier, M.; Ng, K.; Ghosh, S.; Hu, J. Discovery of Parkinson’s disease states and disease progression modelling: A longitudinal data study using machine learning. Lancet Digit. Health 2021, 3, e555–e564. [Google Scholar] [CrossRef]
- Heimrich, K.G.; Schönenberg, A.; Santos-García, D.; Mir, P.; Coppadis Study Group; Prell, T. The Impact of Nonmotor Symptoms on Health-Related Quality of Life in Parkinson’s Disease: A Network Analysis Approach. J. Clin. Med. 2023, 12, 2573. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Barton, B.; de Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez Lloret, S.; Weintraub, D.; Sampaio, C.; The collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee; Chahine, L.; et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—An evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef]
- Sivanandy, P.; Leey, T.C.; Xiang, T.C.; Ling, T.C.; Wey Han, S.A.; Semilan, S.L.A.; Hong, P.K. Systematic review on Parkinson’s disease medications, emphasizing on three recently approved drugs to control Parkinson’s symptoms. Int. J. Environ. Res. Public Health 2021, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Foltynie, T.; Limousin, P.; Poewe, W. How Does Deep Brain Stimulation Change the Course of Parkinson’s Disease? Mov. Disord. 2022, 37, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Serva, S.N.; Bernstein, J.; Thompson, J.A.; Kern, D.S.; Ojemann, S.G. An update on advanced therapies for Parkinson’s disease: From gene therapy to neuromodulation. Front. Surg. 2022, 9, 863921. [Google Scholar] [CrossRef]
- Karu, T.; Pyatibrat, L.; Kalendo, G. Irradiation with He Ne laser increases ATP level in cells cultivated in vitro. J. Photochem. Photobiol. B Biol. 1995, 27, 219–223. [Google Scholar] [CrossRef]
- Quirk, B.J.; Whelan, H.T. What lies at the heart of photobiomodulation: Light, cytochrome c oxidase, and nitric oxide—Review of the evidence. Photobiomodul. Photomed. Laser Surg. 2020, 38, 527–530. [Google Scholar] [CrossRef]
- El Massri, N.; Lemgruber, A.P.; Rowe, I.J.; Moro, C.; Torres, N.; Reinhart, F.; Chabrol, C.; Benabid, A.-L.; Mitrofanis, J. Photobiomodulation-induced changes in a monkey model of Parkinson’s disease: Changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp. Brain Res. 2017, 235, 1861–1874. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Kannarkat, G.T.; Boss, J.M.; Tansey, M.G. The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Swanepoel, A.C.; Buys, A.V.; Vermeulen, N.; Duim, W.; Kell, D.B. Eryptosis as a marker of Parkinson’s disease. Aging 2014, 6, 788. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Parkinsons Dis. 2023, 9, 18. [Google Scholar] [CrossRef]
- Deleidi, M.; Gasser, T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell. Mol. Life Sci. 2013, 70, 4259–4273. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Kumar, H.; Kim, I.S.; Song, S.-Y.; Choi, D.-K. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediat. Inflamm. 2013, 2013, 952375. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef]
- El Massri, N.; Mitrofanis, J. The experimental evidence for photobiomodulation-induced cellular and behavioral changes in animal models of Parkinson’s disease: A template for translation to patients. In Photobiomodulation in the Brain; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–231. [Google Scholar]
- Johnstone, D.M.; Moro, C.; Stone, J.; Benabid, A.-L.; Mitrofanis, J. Turning on lights to stop neurodegeneration: The potential of near infrared light therapy in Alzheimer’s and Parkinson’s disease. Front. Neurosci. 2016, 9, 500. [Google Scholar] [CrossRef]
- Reinhart, F.; Massri, N.E.; Torres, N.; Chabrol, C.; Molet, J.; Johnstone, D.M.; Stone, J.; Benabid, A.L.; Mitrofanis, J.; Moro, C. The behavioural and neuroprotective outcomes when 670 nm and 810 nm near infrared light are applied together in MPTP-treated mice. Neurosci. Res. 2017, 117, 42–47. [Google Scholar] [CrossRef]
- Moro, C.; El Massri, N.; Darlot, F.; Torres, N.; Chabrol, C.; Agay, D.; Auboiroux, V.; Johnstone, D.M.; Stone, J.; Mitrofanis, J.; et al. Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson’s disease. Brain Res. 2016, 1648, 19–26. [Google Scholar] [CrossRef]
- Gordon, L.C.; Martin, K.L.; Torres, N.; Benabid, A.L.; Mitrofanis, J.; Stone, J.; Moro, C.; Johnstone, D.M. Remote photobiomodulation targeted at the abdomen or legs provides effective neuroprotection against parkinsonian MPTP insult. Eur. J. Neurosci. 2023, 57, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Liebert, A.; Herkes, G. Parkinson’s Disease and Photobiomodulation: Potential for Treatment. J. Pers. Med. 2024, 14, 112. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Laakso, E.-L.; Jalilitabaei, P.; Tilley, S.; Kiat, H.; Mitrofanis, J. Remote Photobiomodulation Treatment for the Clinical Signs of Parkinson’s Disease: A Case Series Conducted During COVID-19. Photobiomodul. Photomed. Laser Surg. 2022, 40, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Bicknell, B.; Laakso, E.L.; Heller, G.; Jalilitabaei, P.; Tilley, S.; Mitrofanis, J.; Kiat, H. Improvements in clinical signs of Parkinson’s disease using photobiomodulation: A prospective proof-of-concept study. BMC Neurol. 2021, 21, 256. [Google Scholar] [CrossRef]
- Bicknell, B.; Saltmarche, A.; Hares, O.; Herkes, G.; Liebert, A. Parkinson’s disease and the Interaction of Photobiomodulation, the Microbiome, and Antibiotics: A Case Series. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Liebert, A.; Saltmarche, A.; McConaghy, M.; Hares, O.; Bicknell, B.; Herkes, G. Photobiomodulation as part of a multi-disciplinary approach for the treatment of Parkinson’s disease symptoms. Med. Res. Arch. 2024, 12. [Google Scholar] [CrossRef]
- Herkes, G.; McGee, C.; Liebert, A.; Bicknell, B.; Isaac, A.; Kiat, H.; McLachlan, C. A novel transcranial photobiomodulation device to address motor signs of Parkinson’s disease: A parallel randomised feasibility study. EClinicalMedicine 2023, 66, 102338. [Google Scholar] [CrossRef]
- McGee, C.; Liebert, A.; Bicknell, B.; Pang, V.; Isaac, V.; McLachlan, C.S.; Kiat, H.; Herkes, G. A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes. J. Clin. Med. 2023, 12, 2846. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Bicknell, B.; Laakso, E.-L.; Tilley, S.; Heller, G.; Kiat, H.; Herkes, G. Improvements in clinical signs and symptoms of Parkinson’s disease using photobiomodulation: A five-year follow-up. BMC Neurol. 2024, 24, 381. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-Y.; Cho, K.-H.; Jin, C.; Lee, J.; Kim, T.-H.; Jung, W.-S.; Moon, S.-K.; Ko, C.-N.; Cho, S.-Y.; Jeon, C.-Y.; et al. Exercise Therapies for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsons Dis. 2020, 2020, 2565320. [Google Scholar] [CrossRef] [PubMed]
- Zhen, K.; Zhang, S.; Tao, X.; Li, G.; Lv, Y.; Yu, L. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 146. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef]
- Larson, D.; Yeh, C.; Rafferty, M.; Bega, D. High satisfaction and improved quality of life with Rock Steady Boxing in Parkinson’s disease: Results of a large-scale survey. Disabil. Rehabil. 2022, 44, 6034–6041. [Google Scholar] [CrossRef]
- Tucak, C.; Chih, H.; Mastaglia, F.; Rodrigues, J. The ‘PD Warrior’ exercise program improves motor outcomes and quality of life in patients with early Parkinson’s disease: Results of a pilot study. Intern. Med. J. 2023, 54, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Furlong, T.M.; Watson, C. Human Brainstem: Cytoarchitecture, Chemoarchitecture, Myeloarchitecture; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef]
- Lang, J.T.; Kassan, T.O.; Devaney, L.L.; Colon-Semenza, C.; Joseph, M.F. Test-Retest Reliability and Minimal Detectable Change for the 10-Meter Walk Test in Older Adults With Parkinson’s disease. J. Geriatr. Phys. Ther. 2016, 39, 165–170. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Soke, F.; Guclu-Gunduz, A.; Ozkan, T.; Ozkul, C.; Gulsen, C.; Kocer, B. Reliability and validity of the timed 360° turn test in people with Parkinson’s disease. Eur. Geriatr. Med. 2020, 11, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Earhart, G.M.; Cavanaugh, J.T.; Ellis, T.; Ford, M.P.; Foreman, K.B.; Dibble, L. The 9-hole PEG test of upper extremity function: Average values, test-retest reliability, and factors contributing to performance in people with Parkinson disease. J. Neurol. Phys. Ther. 2011, 35, 157–163. [Google Scholar] [CrossRef]
- Gill, D.J.; Freshman, A.; Blender, J.A.; Ravina, B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov. Disord. 2008, 23, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory. In STOP, THAT and One Hundred Other Sleep Scales; Springer: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Edelstein, B.A.; Drozdick, L.W.; Ciliberti, C.M. Chapter 1—Assessment of Depression and Bereavement in Older Adults. In Handbook of Assessment in Clinical Gerontology, 2nd ed.; Lichtenberg, P.A., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 3–43. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893. [Google Scholar] [CrossRef]
- Carney, C.E.; Moss, T.G.; Harris, A.L.; Edinger, J.D.; Krystal, A.D. Should we be anxious when assessing anxiety using the Beck Anxiety Inventory in clinical insomnia patients? J. Psychiatr. Res. 2011, 45, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Hagell, P.; Nygren, C. The 39 item Parkinson’s disease questionnaire (PDQ-39) revisited: Implications for evidence based medicine. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1191–1198. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Kohnen, R.; Högl, B.; Metta, V.; Sixel-Döring, F.; Frauscher, B.; Hülsmann, J.; Martinez-Martin, P.; Chaudhuri, K.R. Parkinson’s disease sleep scale—Validation of the revised version PDSS-2. Mov. Disord. 2011, 26, 644–652. [Google Scholar] [CrossRef]
- Muntean, M.-L.; Benes, H.; Sixel-Döring, F.; Chaudhuri, K.R.; Suzuki, K.; Hirata, K.; Zimmermann, J.; Trenkwalder, C. Clinically relevant cut-off values for the Parkinson’s Disease Sleep Scale-2 (PDSS-2): A validation study. Sleep Med. 2016, 24, 87–92. [Google Scholar] [CrossRef]
- Cassano, P.; Caldieraro, M.A.; Norton, R.; Mischoulon, D.; Trinh, N.-H.; Nyer, M.; Dording, C.; Hamblin, M.R.; Campbell, B.; Iosifescu, D.V. Reported Side Effects, Weight and Blood Pressure, After Repeated Sessions of Transcranial Photobiomodulation. Photobiomodul. Photomed. Laser Surg. 2019, 37, 651–656. [Google Scholar] [CrossRef]
- Cassano, P.; Norton, R.; Caldieraro, M.A.; Vahedifard, F.; Vizcaino, F.; McEachern, K.M.; Iosifescu, D. Tolerability and safety of transcranial photobiomodulation for mood and anxiety disorders. Photonics 2022, 9, 507. [Google Scholar] [CrossRef]
- Witek, N.; Stebbins, G.T.; Goetz, C.G. What influences placebo and nocebo responses in Parkinson’s disease? Mov. Disord. 2018, 33, 1204–1212. [Google Scholar] [CrossRef]
- Quattrone, A.; Barbagallo, G.; Cerasa, A.; Stoessl, A.J. Neurobiology of placebo effect in Parkinson’s disease: What we have learned and where we are going. Mov. Disord. 2018, 33, 1213–1227. [Google Scholar] [CrossRef]
- Goetz, C.G.; Wuu, J.; McDermott, M.P.; Adler, C.H.; Fahn, S.; Freed, C.R.; Hauser, R.A.; Olanow, W.C.; Shoulson, I.; Tandon, P.K.; et al. Placebo response in Parkinson’s disease: Comparisons among 11 trials covering medical and surgical interventions. Mov. Disord. 2008, 23, 690–699. [Google Scholar] [CrossRef]
- Gamborg, M.; Hvid, L.G.; Dalgas, U.; Langeskov-Christensen, M. Parkinson’s disease and intensive exercise therapy—An updated systematic review and meta-analysis. Acta Neurol. Scand. 2022, 145, 504–528. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Eccles, F.J.R.; Garner, I.W.; Murray, C.D.; Doyle, C.; Simpson, J. The joint impact of symptom deterioration and social factors on wellbeing for people with Parkinson’s during the COVID-19 pandemic in the UK. J. Neurol. Sci. 2023, 452, 120768. [Google Scholar] [CrossRef]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 (Suppl. 1), 14–20. [Google Scholar] [CrossRef]
- Gökçal, E.; Gür, V.E.; Selvitop, R.; Babacan Yildiz, G.; Asil, T. Motor and Non-Motor Symptoms in Parkinson’s Disease: Effects on Quality of Life. Noro-Psikyatri Ars. 2017, 54, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Ledesma, S.; Carroll, J.D.; González-Muñoz, A.; Burton, P. Outcomes of whole-body photobiomodulation on pain, quality of life, leisure physical activity, pain catastrophizing, kinesiophobia, and self-efficacy: A prospective randomized triple-blinded clinical trial with 6 months of follow-up. Front. Neurosci. 2024, 18, 1264821. [Google Scholar] [CrossRef]
- Abuhasira, R.; Zlotnik, Y.; Horev, A.; Ifergane, G. Fibromyalgia-Like Syndrome Associated with Parkinson’s Disease—A Cohort Study. J. Clin. Med. 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]
- Gordon, L.; Kim, B.; Petrucco, C.; Kim, J.Y.; Benson, P.; Stone, J.; Johnstone, D.M. Remote photobiomodulation as a neuroprotective intervention—Harnessing the indirect effects of photobiomodulation. In Photobiomodulation in the Brain; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–154. [Google Scholar]
- Blivet, G.; Meunier, J.; Roman, F.J.; Touchon, J. Neuroprotective effect of a new photobiomodulation technique against Aβ25–35 peptide–induced toxicity in mice: Novel hypothesis for therapeutic approach of Alzheimer’s disease suggested. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Liebert, A.; McLachlan, C.S.; Kiat, H. Microbiome Changes in Humans with Parkinson’s Disease after Photobiomodulation Therapy: A Retrospective Study. J. Pers. Med. 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Saltmarche, A.; Bicknell, B.; Herkes, G. Five-year changes in MDS-UPDRS-III and the gut microbiome in Parkinson’s disease with photobiomodulation treatment. Park. Relat. Disord. 2025, 134, 107756. [Google Scholar] [CrossRef]
- Su, S.; Veeravagu, A.; Grant, G. Neuroplasticity after Traumatic Brain Injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Popescu, B.O.; Batzu, L.; Ruiz, P.J.G.; Tulbă, D.; Moro, E.; Santens, P. Neuroplasticity in Parkinson’s disease. J. Neural Transm. 2024, 131, 1329–1339. [Google Scholar] [CrossRef]
- Xuan, W.; Vatansever, F.; Huang, L.; Hamblin, M.R. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt. 2014, 19, 108003. [Google Scholar] [CrossRef] [PubMed]
- Horsager, J.; Knudsen, K.; Sommerauer, M. Clinical and imaging evidence of brain-first and body-first Parkinson’s disease. Neurobiol. Dis. 2022, 164, 105626. [Google Scholar] [CrossRef]


| Outcome Measure | Test | Description | Interpretation |
|---|---|---|---|
| Primary Outcome Measure | |||
| Functional mobility | Timed Up and Go (TUG) | Assessors measured the time taken for a participant to stand from a chair, walk 3 m, turn around a marker, return and sit down [39] | A quicker time is better. ≥14 s is indicative of falls risk [39] |
| Secondary Outcome Measures | |||
| Functional mobility | TUG-Dual | As for TUG, except that the participant was carrying a cup of water [39] | A quicker time is better. ≥14 s is indicative of falls risk [39] |
| TUG-Cognitive | As for TUG, except that the participant was asked to count backwards from 40 by twos [39] | A quicker time is better. ≥4 s is indicative of falls risk [39] | |
| Gait | 10 m walk Test (10MWT) | Participants walked a straight 10 m track. After walking 2 m, assessors measured the time taken to walk a further 6 m and the number of strides taken [40] | A faster speed is better. <1.1 m/s is indicative of falls risk Fewer strides is better |
| Neurological assessment | MDS-UPDRS | Evaluation of various aspects of Parkinson’s disease by a trained MDS-UPDRS assessor (OH), including non-motor and motor experiences of daily living, motor complications, and burden of disease [41]. MDS-UPDRS Parts I, II, III and IV were also separately reported | A lower score is better. Part I 10 and below is mild Part II 12 and below is mild Part III 32 and lower is mild Part IV 32 and below is mild |
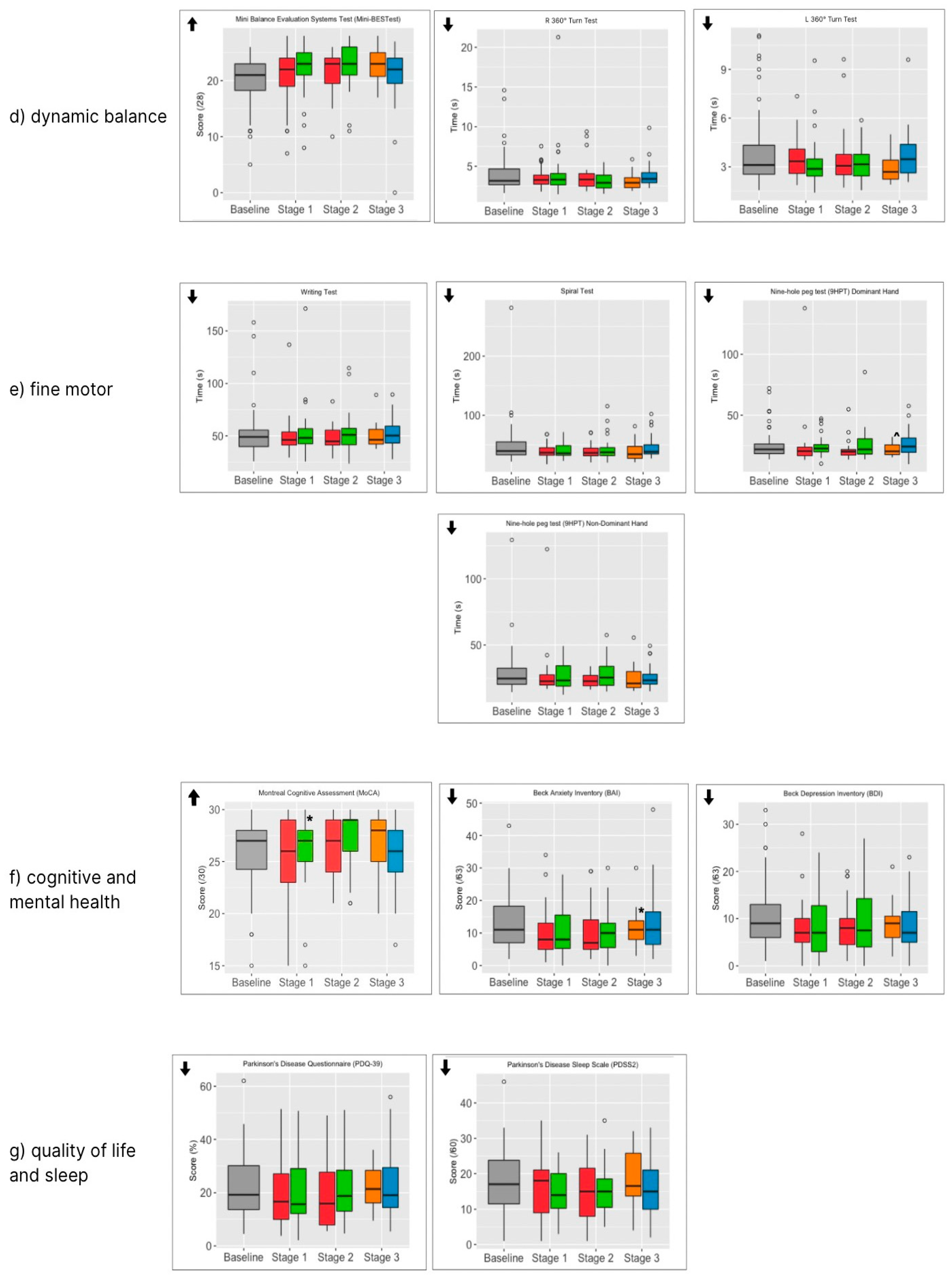
| Dynamic balance | Mini-BESTest | A balance test consisting of 14 items, including tasks divided into four subcomponents: anticipatory postural adjustments, postural responses, sensory orientation, and dynamic gait [42] | A higher score is better. |
| 360° Turn test | Participants turn 360 degrees, and assessors measure the time taken and number of steps to complete the turn [43] | A faster speed is better Fewer steps is better | |
| Fine motor skills | Nine-hole peg test (9HPT) | Assessors recorded the time taken for participants to place 9 pegs in holes and then return the pegs to the reservoir. Both hands were tested [44] | A faster time is better |
| Writing Test | Participants wrote the same sentence at each assessment. The time taken to complete the sentence was recorded | A faster time is better | |
| Spiral Test | Participants drew a spiral within a template on a sheet of paper, and assessors recorded the time taken to complete the task | A faster time is better | |
| Cognition | Montreal Cognitive Assessment (MoCA) | Participants completed the MoCA test version 8.1 (www.mocatest.org (accessed on 15 October 2022)), which was scored by a trained assessor [45] | A higher score is better (max 30). ≤25 may indicate mild cognitive decline |
| Mental health | Beck Depression Inventory (BDI-II) | Participants completed a self-report questionnaire of 21 items, rating characteristic attitudes and symptoms of depression [46] | A lower score is better ≥13 is indicative of minimal depression 14–19 is indicative of mild depression [47] |
| Beck Anxiety Inventory (BAI) | Participants completed a self-report questionnaire of 21 items, rating common somatic and cognitive symptoms of anxiety [48] | A lower score is better (max 63) ≤7 is indicative of no anxiety 8–15 is indicative of mild anxiety [49] | |
| Quality of Life and activities of daily living | Parkinson’s disease questionnaire (PDQ39) | Participants completed a 39-item self-report questionnaire that assesses how often they experience difficulties across eight dimensions of daily living, including relationships, social situations, and communication. It also assesses the impact of Parkinson’s on specific dimensions of functioning and wellbeing [50] | A lower score indicates a better QoL |
| Sleep quality | Parkinson’s disease sleep scale (PDSS2) | Participants completed a self-report questionnaire of 10 questions that assesses the level of sleep disruption being experienced [51] | A lower score is better. A score ≥ 18 may indicate clinically relevant PD-specific sleep disturbances [52] |
| Entry Baseline | Stage 1 | Stage 2 | Stage 3 | ||||
|---|---|---|---|---|---|---|---|
| Outcome Measures | (n = 63) | Active (n = 29) | Sham (n = 30) | Active Then Active (n = 24) | Sham Then Active (n = 27) | Continuers (n = 17) | Non- Continuers (n = 26) |
| Primary Outcome Measure | |||||||
| TUG time | 11.4 (3.2) | 12.3 (4.2) | 11.0 (4.0) | 12.0 (4.0) | 11.6 (4.8) | 9.8 (3.8) | 12.0 (5.6) |
| p = 0.016 * | |||||||
| Secondary Outcome Measures | |||||||
| TUG-dual time | 12.1 (3.2) | 12.7 (4.3) | 12.0 (5.1) | 12.3 (3.9) | 13.1 (11.6) | 10.2 (3.4) | 13.5 (10.3) |
| TUG-cognitive time | 15.4 (9.2) | 16.6 (8.9) | 13.6 (6.1) | 15.6 (7.7) | 13.6 (6.6) | 12.3 (5.7) | 14.6 (6.5) |
| p = 0.058 ^ | |||||||
| MDS UPDRS total score | 59.8 (22.1) | 51.9 (21.5) | 55.9 (21.0) | 49.3 (20.6) | 54.4 (19.8) | 47.8 (19.4) | 53.1 (19.6) |
| p = 0.062 ^ | |||||||
| MDS-UPDRS-I | 12.1 (7.3) | 11.5 (6.0) | 10.3 (5.3) | 10.1 (5.9) | 10.2 (4.8) | 10.0 (5.4) | 11.7 (5.4) |
| MDS-UPDRS-II | 16.4 (6.9) | 13.8 (7.7) | 14.5 (6.4) | 10.7 (4.6) | 12.7 (5.7) | 11.2 (5.0) | 13.0 (5.3) |
| p = 0.048 * | |||||||
| MDS-UPDRS-III | 28.1 (11.2) | 24.4 (9.3) | 27.7 (12.8) | 24.1 (12.7) | 24.4 (10.2) | 22.9 (11.3) | 24.9 (12.3) |
| MDS-UPDRS-IV | 2.7 (3.5) | 3.9 (4.8) | 3.7 (4.4) | 3.3 (2.8) | 3.6 (2.6) | 4.3 (2.4) | 3.4 (2.3) |
| 10MWT time | 7.9 (3.0) | 8.4 (3.4) | 7.4 (2.0) | 8.1 (2.8) | 7.0 (1.8) | 7.1 (0.2) | 8.4 (3.5) |
| Right 360° turn test time | 4.1 (2.4) | 3.7 (1.4) | 3.5 (1.5) | 3.9 (2.1) | 3.1 (1.1) | 3.2 (1.1) | 3.8 (1.6) |
| Left 360° turn test time | 3.9 (2.2) | 3.6 (1.3) | 3.1 (1.1) | 3.7 (1.9) | 3.2 (1.1) | 3.0 (0.9) | 3.7 (1.6) |
| Sentence write time | 51.3 (18.7) | 50.7 (19.6) | 49.8 (13.3) | 47.9 (12.9) | 52.9 (20.2) | 50.7 (12.7) | 51.4 (14.8) |
| Spiral draw time | 49.4 (35.5) | 39.4 (12.6) | 41.0 (13.5) | 39.6 (13.4) | 44.2 (20.9) | 31.0 (17.5) | 46.7 (19.3) |
| 9HPT dominant hand | 24.8 (11.5) | 24.6 (22.4) | 24.5 (8.6) | 21.7 (8.7) | 26.0 (14.0) | 21.4 (5.0) | 26.9 (10.7) |
| p = 0.087 ^ | |||||||
| 9HPT non-dominant hand | 28.9 (16.1) | 27.2 (19.2) | 27.0 (10.0) | 23.2 (5.1) | 27.6 (11.0) | 25.5 (10.9) | 25.9 (8.9) |
| MoCA | 26.0 (3.4) | 25.6 (3.7) | 26.4 (2.8) | 26.4 (2.9) | 27.5 (2.7) | 27.1 (2.8) | 25.6 (3.2) |
| p = 0.015 * | |||||||
| Beck depression | 10.8 (7.0) | 8.0 (5.5) | 8.7 (6.4) | 8.4 (5.8) | 9.3 (7.0) | 9.3 (4.6) | 8.7 (5.8) |
| Beck anxiety | 13.2 (8.4) | 10.5 (7.8) | 9.9 (6.7) | 10.6 (8.1) | 11.2 (7.8) | 11.8 (6.5) | 14.0 (10.4) |
| p = 0.050 * | |||||||
| PDQ39 | 22.2 (12.2) | 20.0 (13.5) | 21.4 (13.4) | 18.3 (11.9) | 21.6 (12.3) | 22.0 (8.5) | 24.1 (13.8) |
| PDSS2 | 18.1 (8.9) | 16.1 (8.1) | 14.6 (6.5) | 14.7 (8.3) | 15.2 (7.2) | 18.8 (8.7) | 15.7 (7.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saltmarche, A.E.; Hares, O.; Bicknell, B.; Liebert, A.; Naeser, M.; Ramachandran, S.; Sykes, J.; Togeretz, K.; Namini, A.; Heller, G.Z.; et al. Effectiveness of Photobiomodulation to Treat Motor and Non-Motor Symptoms of Parkinson’s Disease: A Randomised Clinical Trial with Extended Treatment. J. Clin. Med. 2025, 14, 7463. https://doi.org/10.3390/jcm14217463
Saltmarche AE, Hares O, Bicknell B, Liebert A, Naeser M, Ramachandran S, Sykes J, Togeretz K, Namini A, Heller GZ, et al. Effectiveness of Photobiomodulation to Treat Motor and Non-Motor Symptoms of Parkinson’s Disease: A Randomised Clinical Trial with Extended Treatment. Journal of Clinical Medicine. 2025; 14(21):7463. https://doi.org/10.3390/jcm14217463
Chicago/Turabian StyleSaltmarche, Anita E., Orla Hares, Brian Bicknell, Ann Liebert, Margaret Naeser, Sujith Ramachandran, Jenna Sykes, Kaley Togeretz, Ashley Namini, Gillian Z. Heller, and et al. 2025. "Effectiveness of Photobiomodulation to Treat Motor and Non-Motor Symptoms of Parkinson’s Disease: A Randomised Clinical Trial with Extended Treatment" Journal of Clinical Medicine 14, no. 21: 7463. https://doi.org/10.3390/jcm14217463
APA StyleSaltmarche, A. E., Hares, O., Bicknell, B., Liebert, A., Naeser, M., Ramachandran, S., Sykes, J., Togeretz, K., Namini, A., Heller, G. Z., & Herkes, G. (2025). Effectiveness of Photobiomodulation to Treat Motor and Non-Motor Symptoms of Parkinson’s Disease: A Randomised Clinical Trial with Extended Treatment. Journal of Clinical Medicine, 14(21), 7463. https://doi.org/10.3390/jcm14217463






