Anatomical and Functional Outcomes of Sutureless Scleral-Fixated Carlevale Intraocular Lens Implantation: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
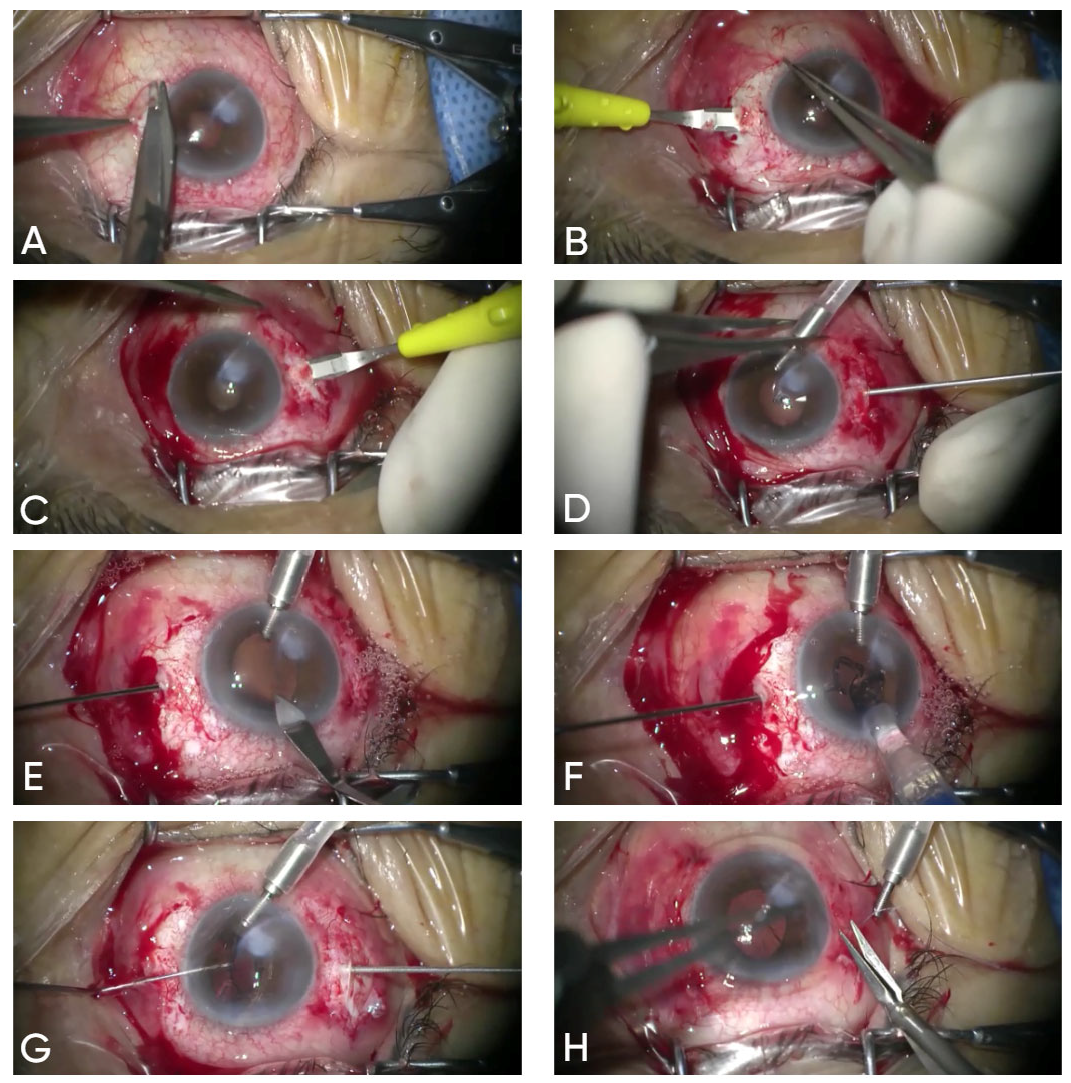
2.1. Surgical Technique
2.2. Statistical Analysis
3. Results
3.1. Visual Acuity
3.2. Refraction
3.3. Intra- and Postoperative Abnormalities and Complications
3.4. IOL Position
3.5. Intraocular Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSF-IOL | Sutureless scleral-fixated intraocular lens |
| VA | Visual acuity |
| RE | Refractive error |
| IOP | Intraocular pressure |
| logMAR | Logarithm of the Minimum Angle of Resolution |
| D | Diopter |
| CME | Cystoid macular edema |
| ERM | Epiretinal membrane |
| PEX | Pseudoexfoliation syndrome |
| BCVA | Best corrected visual acuity |
| OCT | Optical coherence tomography |
| PCIOL | Posterior chamber intraocular lens |
| ACIOL | Anterior chamber intraocular lens |
References
- Lundström, M.; Brege, K.G.; Florén, I.; Lundh, B.; Stenevi, U.; Thorburn, W. Postoperative Aphakia in Modern Cataract Surgery—Part 1: Analysis of Incidence and Risks Based on 5-Year Data from the Swedish National Cataract Register. J. Cataract. Refract. Surg. 2004, 30, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Lundström, M.; Brege, K.G.; Florén, I.; Lundh, B.; Stenevi, U.; Thorburn, W. Postoperative Aphakia in Modern Cataract Surgery —Part 2: Detailed Analysis of the Cause of Aphakia and the Visual Outcome. J. Cataract. Refract. Surg. 2004, 30, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Aref, A.A. Intraocular Lens Implantation In The Ciliary Sulcus: Challenges And Risks. Clin. Ophthalmol. 2019, 13, 2317. [Google Scholar] [CrossRef] [PubMed]
- Por, Y.M.; Lavin, M.J. Techniques of Intraocular Lens Suspension in the Absence of Capsular/Zonular Support. Surv. Ophthalmol. 2005, 50, 429–462. [Google Scholar] [CrossRef]
- Hoffman, R.S.; Fine, I.H.; Packer, M. Scleral Fixation without Conjunctival Dissection. J. Cataract. Refract. Surg. 2006, 32, 1907–1912. [Google Scholar] [CrossRef]
- Khan, M.A.; Gupta, O.P.; Smith, R.G.; Ayres, B.D.; Raber, I.M.; Bailey, R.S.; Hsu, J.; Spirn, M.J. Scleral Fixation of Intraocular Lenses Using Gore-Tex Suture: Clinical Outcomes and Safety Profile. Br. J. Ophthalmol. 2016, 100, 638–643. [Google Scholar] [CrossRef]
- Lewis, J.S. Ab Externo Sulcus Fixation. Ophthalmic Surg. 1991, 22, 692–695. [Google Scholar] [CrossRef]
- Malbran, E.S.; Malbran, E.; Negri, I. Lens Guide Suture for Transport and Fixation in Secondary IOL Implantation after Intracapsular Extraction. Int. Ophthalmol. 1986, 9, 151–160. [Google Scholar] [CrossRef]
- Mittelviefhaus, H.; Wiek, J. A Refined Technique of Transscleral Suture Fixation of Posterior Chamber Lenses Developed for Cases of Complicated Cataract Surgery with Vitreous Loss. Ophthalmic Surg. 1993, 24, 698–701. [Google Scholar] [CrossRef]
- Szurman, P.; Petermeier, K.; Aisenbrey, S.; Spitzer, M.S.; Jaissle, G.B. Z-Suture: A New Knotless Technique for Transscleral Suture Fixation of Intraocular Implants. Br. J. Ophthalmol. 2010, 94, 167–169. [Google Scholar] [CrossRef]
- Abbey, A.M.; Hussain, R.M.; Shah, A.R.; Faia, L.J.; Wolfe, J.D.; Williams, G.A. Sutureless Scleral Fixation of Intraocular Lenses: Outcomes of Two Approaches. The 2014 Yasuo Tano Memorial Lecture. Graefe Arch. Clin. Exp. Ophthalmol. 2015, 253, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kumar, D.A.; Jacob, S.; Baid, C.; Agarwal, A.; Srinivasan, S. Fibrin Glue-Assisted Sutureless Posterior Chamber Intraocular Lens Implantation in Eyes with Deficient Posterior Capsules. J. Cataract. Refract. Surg. 2008, 34, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Prenner, J.L.; Feiner, L.; Wheatley, H.M.; Connors, D. A Novel Approach for Posterior Chamber Intraocular Lens Placement or Rescue via a Sutureless Scleral Fixation Technique. Retina 2012, 32, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Scharioth, G.B.; Prasad, S.; Georgalas, I.; Tataru, C.; Pavlidis, M. Intermediate Results of Sutureless Intrascleral Posterior Chamber Intraocular Lens Fixation. J. Cataract. Refract. Surg. 2010, 36, 254–259. [Google Scholar] [CrossRef]
- Yamane, S.; Inoue, M.; Arakawa, A.; Kadonosono, K. Sutureless 27-Gauge Needle-Guided Intrascleral Intraocular Lens Implantation with Lamellar Scleral Dissection. Ophthalmology 2014, 121, 61–66. [Google Scholar] [CrossRef]
- Rossi, T.; Iannetta, D.; Romano, V.; Carlevale, C.; Forlini, M.; Telani, S.; Imburgia, A.; Mularoni, A.; Fontana, L.; Ripandelli, G. A Novel Intraocular Lens Designed for Sutureless Scleral Fixation: Surgical Series. Graefe Arch. Clin. Exp. Ophthalmol. 2021, 259, 257–262. [Google Scholar] [CrossRef]
- D’Agostino, I.; Parrulli, S.; De Angelis, S.; Invernizzi, A.; Bottoni, F.; Staurenghi, G.; Cereda, M.G. Sutureless Scleral Fixation: Comparison between 3-Piece IOL and New Single-Piece Foldable IOL. Graefe Arch. Clin. Exp. Ophthalmol. 2021, 259, 1365–1373. [Google Scholar] [CrossRef]
- Bellamy, J.P.; Queguiner, F.; Salamé, N.; Montard, M. Secondary Intraocular Lens Implantation: Methods and Complications. J. Fr. Opthalmol. 2020, 23, 73–80. [Google Scholar]
- Brunin, G.; Sajjad, A.; Kim, E.J.; Montes de Oca, I.; Weikert, M.P.; Wang, L.; Koch, D.D.; Al-Mohtaseb, Z. Secondary Intraocular Lens Implantation: Complication Rates, Visual Acuity, and Refractive Outcomes. J. Cataract. Refract. Surg. 2017, 43, 369–376. [Google Scholar] [CrossRef]
- Hahn, T.W.; Kim, M.S.; Kim, J.H. Secondary Intraocular Lens Implantation in Aphakia. J. Cataract. Refract. Surg. 1992, 18, 174–179. [Google Scholar] [CrossRef]
- Manousakis, E.; Gartaganis, P.S.; Manousakis, E.; Karmiris, E. A Novel Technique for Scleral Fixation of Carlevale Intraocular Lens Without Conjunctival Opening Utilizing Hoffman Pockets. Retin. Cases Brief. Rep. 2025, 19, 354–357. [Google Scholar] [CrossRef]
- Giannopoulos, T.; Panagiotou, E.S.; Giannoukaki, A.; Mikropoulos, D.G.; Konstas, A.G. A New Technique with Scleral Grooves for Sutureless Scleral Fixation of the Carlevale Intraocular Lens. Ophthalmol. Ther. 2024, 13, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Yamane, S.; Ito, A. Flanged Fixation: Yamane Technique and Its Application. Curr. Opin. Ophthalmol. 2021, 32, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Nowomiejska, K.; Haszcz, D.; Onyszkiewicz, M.; Choragiewicz, T.; Czarnek-chudzik, A.; Szpringer-wabicz, A.; Baltaziak, K.; Brzozowska, A.; Toro, M.D.; Rejdak, R. Double-Needle Yamane Technique Using Flanged Haptics in Ocular Trauma-A Retrospective Survey of Visual Outcomes and Safety. J. Clin. Med. 2021, 10, 2562. [Google Scholar] [CrossRef] [PubMed]
- Yavuzer, K.; Evcimen, Y. Sutureless Transconjunctival Intrascleral Intraocular Lens Fixation: The Modified Yamane Technique. Arq. Bras. Oftalmol. 2019, 82, 389–393. [Google Scholar] [CrossRef]
- Canabrava, S.; Canêdo Domingos Lima, A.C.; Ribeiro, G. Four-Flanged Intrascleral Intraocular Lens Fixation Technique: No Flaps, No Knots, No Glue. Cornea 2020, 39, 527–528. [Google Scholar] [CrossRef]
- Schranz, M.; Lisy, M.; Dimakopoulou, I.; Danzinger, V.; Schartmüller, D.; Abela-Formanek, C. Refractive Outcome, Lens Power Calculation, and Surgically Induced Astigmatism After Four-Flanged Intrascleral Intraocular Lens Fixation. J. Refract. Surg. 2024, 40, e985–e993. [Google Scholar] [CrossRef]
- Schranz, M.; Reumüller, A.; Kostolna, K.; Novotny, C.; Schartmüller, D.; Abela-Formanek, C. Refractive Outcome and Lens Power Calculation after Intrascleral Intraocular Lens Fixation: A Comparison of Three-Piece and One-Piece Intrascleral Fixation Technique. Eye Vis. 2023, 10, 29. [Google Scholar] [CrossRef]
- Sun, H.; Wang, C.; Wu, H. Recent Advances and Current Challenges in Suture and Sutureless Scleral Fixation Techniques for Intraocular Lens: A Comprehensive Review. Eye Vis. 2024, 11, 49. [Google Scholar] [CrossRef]
- Stewart, C.; Singh-Bhangu, J.; Dissanayake, S. New Surgical Technique for Scleral Fixation: A Novel Sutured Approach for Carlevale Lens Implantation. Am. J. Ophthalmol. Case Rep. 2025, 39, 102343. [Google Scholar] [CrossRef]
- Danese, C.; Lanzetta, P. Combined Transconjunctival Sutureless Three-Port Vitrectomy and Scleral Fixation of Intraocular Lens. Eur. J. Ophthalmol. 2021, 32, 1287–1290. [Google Scholar] [CrossRef]
- Danese, C.; Di Bin, F.; Lanzetta, P. A Mini-Invasive Surgical Technique for Carlevale IOL Implantation: Case Series Study and Description of Concomitant Surgery. Graefe Arch. Clin. Exp. Ophthalmol. 2023, 262, 487. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, G.; Siskou, E.; Koronis, S.; Tranos, P.; Gatzioufas, Z.; Balidis, M. Novel Sutureless Scleral Fixated IOL for Inadequate or Absent Capsular Support. J. Ophthalmol. 2022, 2022, 2161003. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, A.S.; Hoffer, K.J.; Greco, A.; Greco, A.; D’Amico, G.; Pasqualitto, V.; Carlevale, C.; Savini, G. Long-Term Outcomes and Complications of the New Carlevale Sutureless Scleral Fixation Posterior Chamber IOL. J. Refract. Surg. 2021, 37, 126–132. [Google Scholar] [CrossRef]
- Rouhette, H.; Meyer, F.; Pommier, S.; Benzerroug, M.; Denion, E.; Guigou, S.; Lorenzi, U.; Mazit, C.; Mérité, P.Y.; Rebollo, O. FIL-SSF Carlevale Intraocular Lens for Sutureless Scleral Fixation: 7 Recommendations from a Serie of 72 Cases. MICA Study (Multicentric Study of the Carlevale IOL). J. Fr. Ophtalmol. 2021, 42, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Georgalas, I.; Spyropoulos, D.; Gotzaridis, S.; Papakonstantinou, E.; Kandarakis, S.; Kanakis, M.; Karamaounas, A.; Petrou, P. Scleral Fixation of Carlevale Intraocular Lens: A New Tool in Correcting Aphakia with No Capsular Support. Eur. J. Ophthalmol. 2022, 32, 527–533. [Google Scholar] [CrossRef]
- Fiore, T.; Messina, M.; Muzi, A.; Lupidi, M.; Reibaldi, M.; Giansanti, F.; Cagini, C. A Novel Approach for Scleral Fixation Using Carlevale Lens. Eur. J. Ophthalmol. 2021, 31, 2947–2954. [Google Scholar] [CrossRef]
- Barca, F.; Caporossi, T.; de Angelis, L.; Giansanti, F.; Savastano, A.; Di Leo, L.; Rizzo, S. Trans-Scleral Plugs Fixated IOL: A New Paradigm for Sutureless Scleral Fixation. J. Cataract. Refract. Surg. 2020, 46, 716–720. [Google Scholar] [CrossRef]
- Gabai, A.; Zeppieri, M.; Toneatto, G.; Salati, C. Enhanced Surgical Technique for Sutureless Intrascleral Fixation of Intraocular Lenses. J. Cataract. Refract. Surg. 2021, 47, E75–E79. [Google Scholar] [CrossRef]
- Seknazi, D.; Colantuono, D.; Tahiri, R.; Amoroso, F.; Miere, A.; Souied, E.H. Secondary Sutureless Posterior Chamber Lens Implantation with Two Specifically Designed IOLs: Iris Claw Lens versus Sutureless Trans-Scleral Plugs Fixated Lens. J. Clin. Med. 2021, 10, 2216. [Google Scholar] [CrossRef]
- Franco, F.; Serino, F.; Vicini, G.; Nicolosi, C.; Giansanti, F. Comparison of Visual and Aberrometric Outcomes in Suture-Free Scleral Fixation: Three-Piece Lenses versus Carlevale Lenses. J. Clin. Med. 2023, 12, 188. [Google Scholar] [CrossRef]
- Lundström, M.; Dickman, M.; Henry, Y.; Manning, S.; Rosen, P.; Tassignon, M.J.; Young, D.; Stenevi, U. Risk Factors for Refractive Error after Cataract Surgery: Analysis of 282 811 Cataract Extractions Reported to the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J. Cataract. Refract. Surg. 2018, 44, 447–452. [Google Scholar] [CrossRef]
- Raimondi, R.; Sorrentino, T.; Kilian, R.; Verma, Y.; De Rosa, F.P.; Cancian, G.; Tsoutsanis, P.; Fossati, G.; Allegrini, D.; Romano, M.R. Trans-Scleral Plugs Fixated FIL SSF IOL: A Review of the Literature and Comparison with Other Secondary IOL Implants. J. Clin. Med. 2023, 12, 1994. [Google Scholar] [CrossRef]
- Zemba, M.; Camburu, G. Uveitis-Glaucoma-Hyphaema Syndrome. General Review. Rom. J. Ophthalmol. 2017, 61, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Carlà, M.M.; Boselli, F.; Giannuzzi, F.; Caporossi, T.; Gambini, G.; Mosca, L.; Savastano, A.; Rizzo, S. Sutureless Scleral Fixation Carlevale IOL: A Review on the Novel Designed Lens. Int. Ophthalmol. 2023, 43, 2129. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Angunawela, R.; Motta, L. Scleral Fixation of Carlevale Intraocular Lens. Retina 2023, 43, 1750–1762. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Hengerer, F.; Eckardt, C. Retropupillary Fixation of the Iris Claw Lens in Aphakia. 1 Year Outcome of a New Implantation Techniques. Ophthalmologe 2002, 99, 580–583. [Google Scholar] [CrossRef]
- Forlini, M.; Soliman, W.; Bratu, A.; Rossini, P.; Cavallini, G.M.; Forlini, C. Long-Term Follow-up of Retropupillary Iris-Claw Intraocular Lens Implantation: A Retrospective Analysis. BMC Ophthalmol. 2015, 15, 143. [Google Scholar] [CrossRef]
- De Silva, S.R.; Arun, K.; Anandan, M.; Glover, N.; Patel, C.K.; Rosen, P. Iris-Claw Intraocular Lenses to Correct Aphakia in the Absence of Capsule Support. J. Cataract. Refract. Surg. 2011, 37, 1667–1672. [Google Scholar] [CrossRef]
- Long, C.; Wei, Y.; Yuan, Z.; Zhang, Z.; Lin, X.; Liu, B. Modified Technique for Transscleral Fixation of Posterior Chamber Intraocular Lenses. BMC Ophthalmol. 2015, 15, 127. [Google Scholar] [CrossRef]
- Evereklioglu, C.; Er, H.; Bekir, N.A.; Borazan, M.; Zorlu, F. Comparison of Secondary Implantation of Flexible Open-Loop Anterior Chamber and Scleral-Fixated Posterior Chamber Intraocular Lenses. J. Cataract. Refract. Surg. 2003, 29, 301–308. [Google Scholar] [CrossRef]
- Barbieri, F.; Maglionico, M.N.; Casini, G.; Guidi, G.; Figus, M.; Posarelli, C. Current Evidence for a New Surgical Technique for Scleral Fixation: The Implantation of a Carlevale Lens, a Systematic Review. J. Clin. Med. 2024, 13, 3287. [Google Scholar] [CrossRef]
- Schranz, M.; Dimakopoulou, I.; Lisy, M.; Danzinger, V.; Schartmüller, D.; Abela-Formanek, C. Long-term Results after Sutureless Intrascleral Fixation of the Carlevale Intraocular Lens: Changes in Scleral Pocket Thickness over Time. Acta Ophthalmol. 2025, 103, e290. [Google Scholar] [CrossRef]
- Marolo, P.; Caselgrandi, P.; Gaidano, M.; Conte, F.; Parisi, G.; Borrelli, E.; Fallico, M.; Toro, M.D.; Ventre, L.; Vaiano, A.S.; et al. Long-Term Surgical Outcomes of Scleral Flap versus Scleral Pocket Technique for Sutureless Intrascleral One-Piece Lens Fixation. J. Clin. Med. 2024, 13, 4452. [Google Scholar] [CrossRef]
- Fiore, T.; Messina, M.; Muzi, A.; Tosi, G.; Lupidi, M.; Casini, G.; Marruso, V.; Cagini, C. Comparison of Two Different Scleral Fixation Techniques of Posterior Chamber Carlevale Lens. Medicine 2021, 100, E26728. [Google Scholar] [CrossRef]
| Result | Mean | Median | Standard Deviation |
|---|---|---|---|
| Preoperative visual acuity | 0.38 logMAR | 0.40 logMAR | 0.33 logMAR |
| Postoperative visual acuity | 0.11 logMAR | 0.05 logMAR | 0.11 logMAR |
| Refractive error | −0.83 D | −0.5 D | 1.05 D |
| Preoperative intraocular pressure | 16.81 mmHg | 16.00 mmHg | 4.17 mmHg |
| Postoperative intraocular pressure | 15.78 mmHg | 16.40 mmHg | 3.65 mmHg |
| Authors | Sample Size (Eyes) | BCVA Preoperatively [logMAR] | BCVA at the Last Follow-Up [logMAR] | Mean RE |
|---|---|---|---|---|
| Sidiropoulos et al. [33] | 27 | 0.85 ± 0.59 | 0.44 ± 0.30 at 1 month postoperatively 0.36 ± 0.34 at 6 months postoperatively | −0.5 ± 0.99 D |
| Vaiano et al. [34] | 54 | 0.93 ± 0.61 | 0.42 ± 0.34 at 3 months postoperatively 0.42 ± 0.37 at 6 months postoperatively 0.38 ± 0.38 at 12 months postoperatively | |
| Rouhette et al. [35] | 72 | 0.52 ± 0.5 | 0.15 ± 0.6 | |
| Georgalas et al. [36] | 169 | 0.58 ± 0.49 | 0.09 ± 0.1 | |
| Fiore et al. [37] | 18 | 0.41 ± 0.33 | −0.31 ± 0.71 D | |
| Barca et al. [38] | 32 | 0.46 ± 0.29 | 0.13 ± 0.12 | −0.24 ± 0.81 D |
| Gabai et al. [39] | 13 | 0.75 ± 0.5 | 0.28 ± 0.3 | |
| Rossi et al. [16] | 78 | 0.86 ± 0.56 | 0.38 ± 0.42 at 6 months postoperatively | |
| Seknazi et al. [40] | 44 | 0.14 ± 0.11 | ||
| Franco et al. [41] | 28 | 0.78 ± 0.37 | 0.23 | −0.33 D |
| Schranz [28] | 29 | 0.13 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słoka, A.; Chorągiewicz, T.; Urbańska, K.; Więsyk, P.; Woźniak, M.; Dolar-Szczasny, J.; Spyra, M.; Nowomiejska, K.; Toro, M.D.; Rejdak, R. Anatomical and Functional Outcomes of Sutureless Scleral-Fixated Carlevale Intraocular Lens Implantation: A Retrospective Study. J. Clin. Med. 2025, 14, 7309. https://doi.org/10.3390/jcm14207309
Słoka A, Chorągiewicz T, Urbańska K, Więsyk P, Woźniak M, Dolar-Szczasny J, Spyra M, Nowomiejska K, Toro MD, Rejdak R. Anatomical and Functional Outcomes of Sutureless Scleral-Fixated Carlevale Intraocular Lens Implantation: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(20):7309. https://doi.org/10.3390/jcm14207309
Chicago/Turabian StyleSłoka, Adam, Tomasz Chorągiewicz, Karolina Urbańska, Piotr Więsyk, Marcin Woźniak, Joanna Dolar-Szczasny, Mariusz Spyra, Katarzyna Nowomiejska, Mario Damiano Toro, and Robert Rejdak. 2025. "Anatomical and Functional Outcomes of Sutureless Scleral-Fixated Carlevale Intraocular Lens Implantation: A Retrospective Study" Journal of Clinical Medicine 14, no. 20: 7309. https://doi.org/10.3390/jcm14207309
APA StyleSłoka, A., Chorągiewicz, T., Urbańska, K., Więsyk, P., Woźniak, M., Dolar-Szczasny, J., Spyra, M., Nowomiejska, K., Toro, M. D., & Rejdak, R. (2025). Anatomical and Functional Outcomes of Sutureless Scleral-Fixated Carlevale Intraocular Lens Implantation: A Retrospective Study. Journal of Clinical Medicine, 14(20), 7309. https://doi.org/10.3390/jcm14207309









