Multiple Primary Cancers as an Independent Criterion for Germline Testing: Comparison with Guideline-Based Criteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. The Guideline and Non-Guideline Groups
2.3. Germline Genetic Testing and Variant Interpretation
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
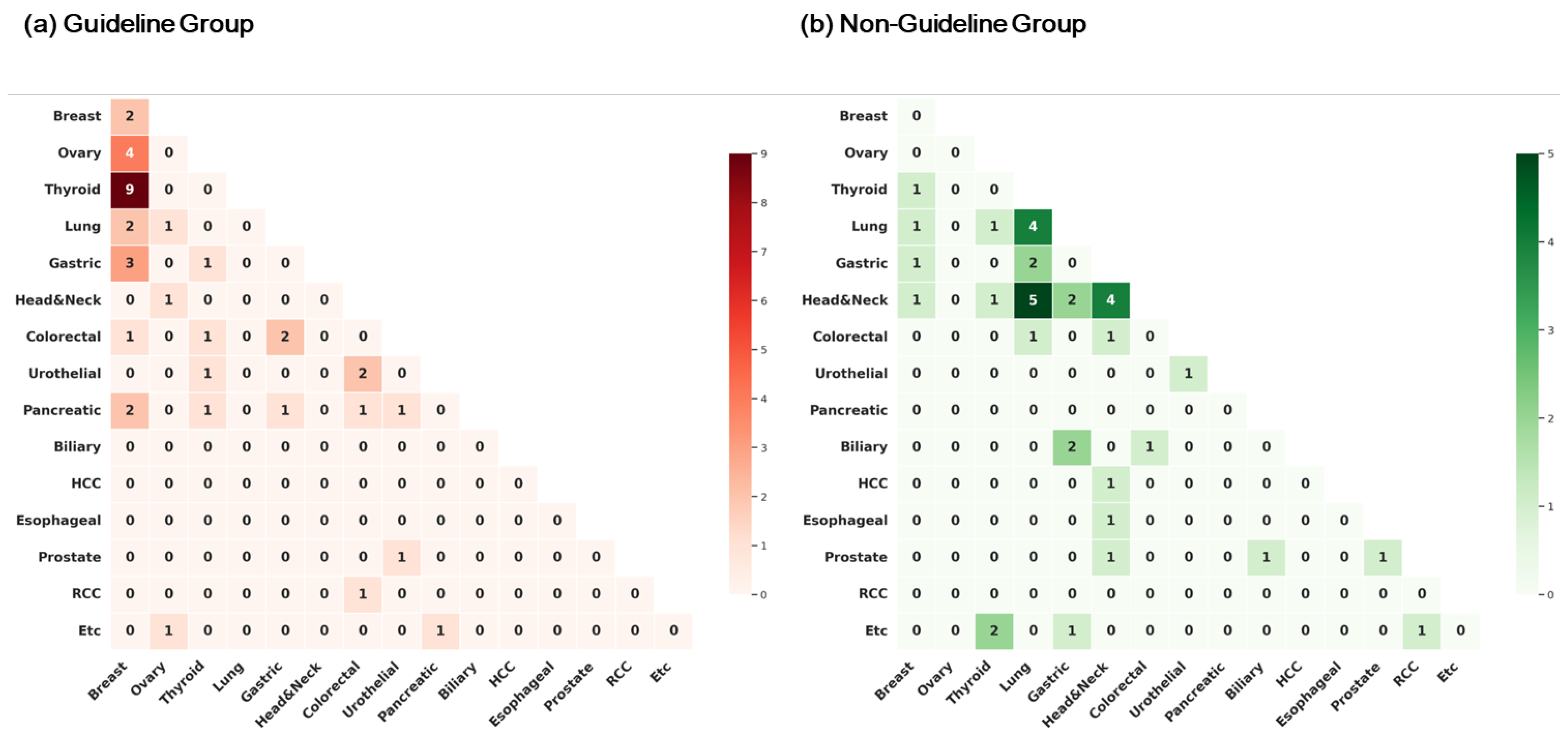
3.2. Types of Cancers
3.3. Genetic Variants Identified
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogt, A.; Schmid, S.; Heinimann, K.; Frick, H.; Herrmann, C.; Cerny, T.; Omlin, A. Multiple primary tumours: Challenges and approaches, a review. ESMO Open 2017, 2, e000172. [Google Scholar] [CrossRef]
- Coyte, A.; Morrison, D.S.; McLoone, P. Second primary cancer risk-the impact of applying different definitions of multiple primaries: Results from a retrospective population-based cancer registry study. BMC Cancer 2014, 14, 272. [Google Scholar] [CrossRef]
- Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Rhodes, D.; Wick, M.; Okuno, S.; Kunze, K.L.; Golafshar, M.; Uson, P.L.S., Jr.; Mountjoy, L.; et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients with Hereditary Cancer Syndrome. JAMA Oncol. 2021, 7, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Cadoo, K.A.; Mukherjee, S.; Khurram, A.; Tkachuk, K.; Kemel, Y.; Maio, A.; Belhadj, S.; Carlo, M.I.; Latham, A.; et al. Multiple Primary Cancers in Patients Undergoing Tumor-Normal Sequencing Define Novel Associations. Cancer Epidemiol. Biomark. Prev. 2022, 31, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.; Smith, P.S.; Martin, J.E.; West, H.; Luchetti, A.; Rodger, F.; Clark, G.; Carss, K.; Stephens, J.; Stirrups, K.; et al. Comprehensive Cancer-Predisposition Gene Testing in an Adult Multiple Primary Tumor Series Shows a Broad Range of Deleterious Variants and Atypical Tumor Phenotypes. Am. J. Hum. Genet. 2018, 103, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, S.E.; Nussbaum, R.L.; Kurian, A.W.; Nielsen, S.M.; Das, K.; Michalski, S.; Yang, S.; Ngo, N.; Blanco, A.; Esplin, E.D. Yield and Utility of Germline Testing Following Tumor Sequencing in Patients with Cancer. JAMA Netw. Open 2020, 3, e2019452. [Google Scholar] [CrossRef]
- Hampel, H.; Bennett, R.L.; Buchanan, A.; Pearlman, R.; Wiesner, G.L. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: Referral indications for cancer predisposition assessment. Genet. Med. 2015, 17, 70–87. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Maxwell, K.N.; Churpek, J.; Kohlmann, W.; AlHilli, Z.; Arun, B.; Buys, S.S.; Cheng, H.; Domchek, S.M.; et al. NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2024. J. Natl. Compr. Cancer Netw. 2023, 21, 1000–1010. [Google Scholar] [CrossRef]
- Hodan, R.; Gupta, S.; Weiss, J.M.; Axell, L.; Burke, C.A.; Chen, L.M.; Chung, D.C.; Clayback, K.M.; Felder, S.; Foda, Z.; et al. Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric, Version 3.2024, NCCN Clinical Practice Guidelines In Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 695–711. [Google Scholar] [CrossRef]
- Mandelker, D.; Zhang, L.; Kemel, Y.; Stadler, Z.K.; Joseph, V.; Zehir, A.; Pradhan, N.; Arnold, A.; Walsh, M.F.; Li, Y.; et al. Mutation Detection in Patients with Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA 2017, 318, 825–835. [Google Scholar] [CrossRef]
- Tung, N.; Ricker, C.; Messersmith, H.; Balmaña, J.; Domchek, S.; Stoffel, E.M.; Almhanna, K.; Arun, B.; Chavarri-Guerra, Y.; Cohen, S.A.; et al. Selection of Germline Genetic Testing Panels in Patients with Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 2599–2615. [Google Scholar] [CrossRef]
- Esplin, E.D.; Nielsen, S.M.; Bristow, S.L.; Garber, J.E.; Hampel, H.; Rana, H.Q.; Samadder, N.J.; Shore, N.D.; Nussbaum, R.L. Universal Germline Genetic Testing for Hereditary Cancer Syndromes in Patients with Solid Tumor Cancer. JCO Precis. Oncol. 2022, 6, e2100516. [Google Scholar] [CrossRef]
- Subbiah, V.; Kurzrock, R. Universal Germline and Tumor Genomic Testing Needed to Win the War Against Cancer: Genomics Is the Diagnosis. J. Clin. Oncol. 2023, 41, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Bychkovsky, B.L.; Lo, M.T.; Yussuf, A.; Horton, C.; Richardson, M.; LaDuca, H.; Garber, J.E.; Rana, H.Q. Prevalence and spectrum of pathogenic variants among patients with multiple primary cancers evaluated by clinical characteristics. Cancer 2022, 128, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Kim, H.; Lee, J.K.; Hong, Y.J.; Kang, H.J.; Jang, Y.J. Incidence and Characteristics of Multiple Primary Cancers: A 20-Year Retrospective Study of a Single Cancer Center in Korea. Cancers 2024, 16, 2346. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Oh, S.J.; Song, J.Y.; Lee, H.B.; Park, M.H.; Jung, Y.; Park, W.C.; Lee, J.; Sun, W.Y. Clinical Characteristics and Prognosis Associated with Multiple Primary Cancers in Breast Cancer Patients. J. Breast Cancer 2018, 21, 62–69. [Google Scholar] [CrossRef]
- Yun, J.; Lee, D.S.; Lee, S.; Yun, H. Multiple Primary Cancers with Hematologic Malignancies and Germline Predisposition: A Case Series. Ann. Lab. Med. 2024, 44, 446–449. [Google Scholar] [CrossRef]
- Choi, Y.Y. Intrapatient genomic divergence across multiple primary tumors in young Korean patients. Korean J. Clin. Oncol. 2025, 21, 90–97. [Google Scholar] [CrossRef]
- Kang, M.C.; Lee, S.; Kim, H.; Kang, H.S.; Jung, S.Y.; Hwang, J.A.; Kwon, J.; Lee, K.S.; Lim, M.C.; Park, S.Y.; et al. PALB2 germline pathogenic variants: Frequency, clinical features, and functional analysis of c.3350+5G>A variant in 3987 Korean cancer patients. ESMO Open 2025, 10, 104132. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, G.D.; Kim, J.H.; Lee, S.N.; Chae, H.; Han, E.; Kim, Y.; Kim, M. Clinical Validity of Next-Generation Sequencing Multi-Gene Panel Testing for Detecting Pathogenic Variants in Patients with Hereditary Breast-Ovarian Cancer Syndrome. Ann. Lab. Med. 2020, 40, 148–154. [Google Scholar] [CrossRef]
- Han, E.; Yoo, J.; Chae, H.; Lee, S.; Kim, D.H.; Kim, K.J.; Kim, Y.; Kim, M. Detection of BRCA1/2 large genomic rearrangement including BRCA1 promoter-region deletions using next-generation sequencing. Clin. Chim. Acta 2020, 505, 49–54. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Fortuno, C.; Lee, K.; Olivier, M.; Pesaran, T.; Mai, P.L.; de Andrade, K.C.; Attardi, L.D.; Crowley, S.; Evans, D.G.; Feng, B.J.; et al. Specifications of the ACMG/AMP variant interpretation guidelines for germline TP53 variants. Hum. Mutat. 2021, 42, 223–236. [Google Scholar] [CrossRef]
- Hart, R.K.; Fokkema, I.; DiStefano, M.; Hastings, R.; Laros, J.F.J.; Taylor, R.; Wagner, A.H.; den Dunnen, J.T. HGVS Nomenclature 2024: Improvements to community engagement, usability, and computability. Genome Med. 2024, 16, 149. [Google Scholar] [CrossRef]
- Schneider, K.; Zelley, K.; Nichols, K.E.; Schwartz Levine, A.; Garber, J. Li-Fraumeni Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Kratz, C.P.; Freycon, C.; Maxwell, K.N.; Nichols, K.E.; Schiffman, J.D.; Evans, D.G.; Achatz, M.I.; Savage, S.A.; Weitzel, J.N.; Garber, J.E.; et al. Analysis of the Li-Fraumeni Spectrum Based on an International Germline TP53 Variant Data Set: An International Agency for Research on Cancer TP53 Database Analysis. JAMA Oncol. 2021, 7, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.; Chia, T.H.; Yuen, J.; Shaw, T.; Li, S.T.; Binte Ishak, N.D.; Chew, E.L.; Chong, S.T.; Chan, S.H.; Ngeow, J. Impact of Variant Reclassification in Cancer Predisposition Genes on Clinical Care. JCO Precis. Oncol. 2021, 5, 577–584. [Google Scholar] [CrossRef] [PubMed]
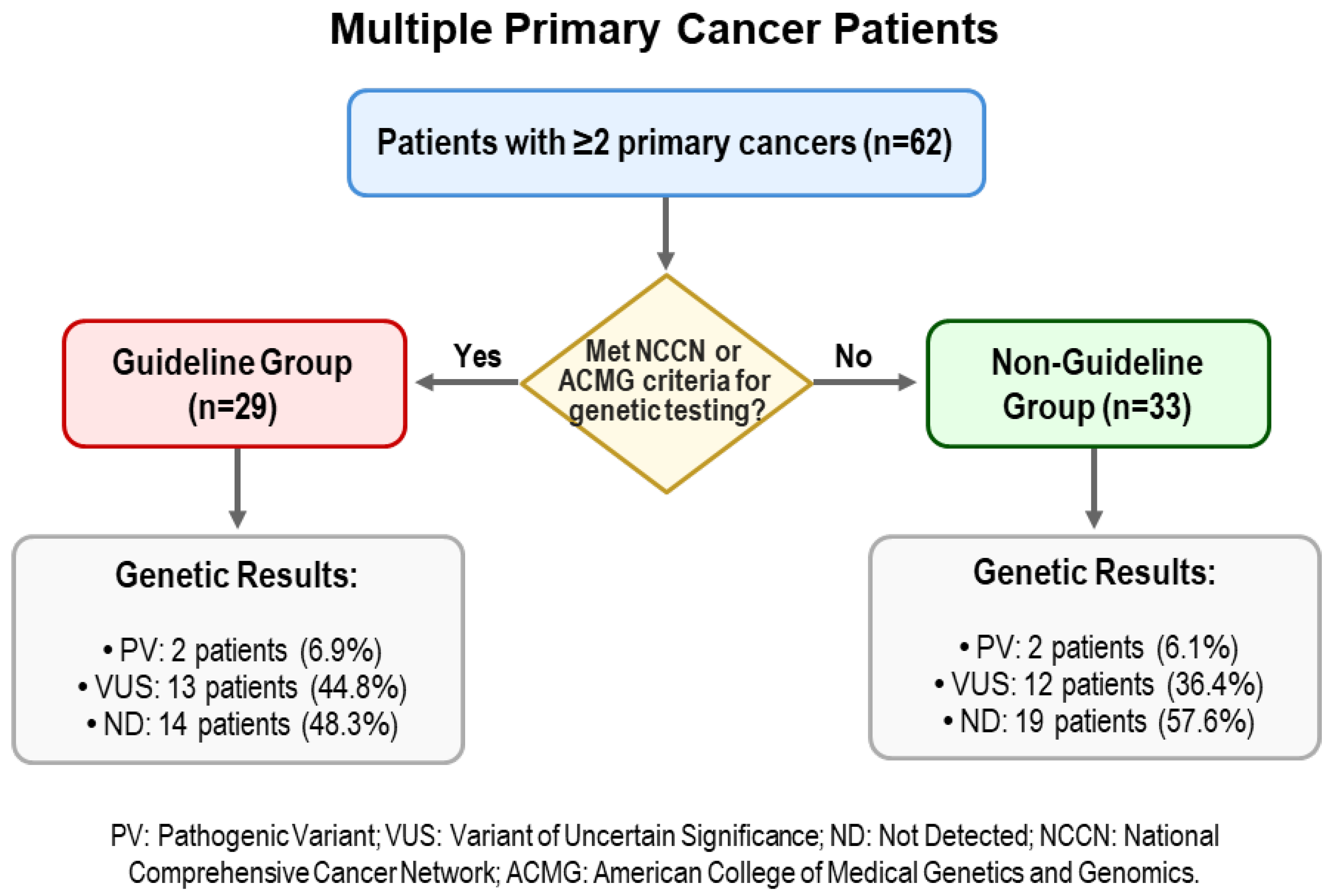
| Characteristics | Guideline Group n = 29 | Non-Guideline Group n = 33 | p-Value | ||
|---|---|---|---|---|---|
| Median age at testing (range, years) | 63 (39~84) | 70 (49~84) | 0.001 | ||
| Median age at 1st cancer diagnosis (years) | 53 (29~80) | 61 (46~82) | 0.002 | ||
| Median age at 2nd cancer diagnosis (years) | 59 (32~80) | 67 (48~82) | 0.006 | ||
| Sex, n (%) | 0.004 | ||||
| Male | 7 | 24.1% | 21 | 63.6% | |
| Female | 22 | 75.9% | 12 | 36.4% | |
| Number of primary cancers, n (%) | 0.565 | ||||
| Two primary cancers | 24 | 82.8% | 30 | 90.9% | |
| Three or more primary cancers | 5 | 17.2% | 3 | 9.1% | |
| Family history of cancer (1st degree), n (%) | 0.633 | ||||
| Yes | 15 | 51.7% | 14 | 42.4% | |
| No | 14 | 48.3% | 19 | 57.6% | |
| Germline results, n (%) | 0.763 | ||||
| PV/LPV | 2 | 6.9% | 2 | 6.1% | |
| VUS | 13 | 44.8% | 12 | 36.4% | |
| ND | 14 | 48.3% | 19 | 57.6% | |
| Patients with family history (n = 29), n (%) | 0.544 | ||||
| PV/LPV | 2 | 13.3% | 2 | 14.3% | |
| VUS | 6 | 40.0% | 3 | 21.4% | |
| ND | 7 | 46.7% | 9 | 64.3% | |
| Group | Sex | Age | Gene | Variant | Site of Primary Malignancies (Age at Diagnosis) | Family History |
|---|---|---|---|---|---|---|
| Guideline group | F | 72 | CHEK2 (NM_007194.4) | c.846 + 1G > T | Breast (61) Ovary (61) | sister—breast cancer |
| F | 56 | BRCA2 (NM_000059.3) | c.5576_5579del, p.(Ile1859Lysfs) | Breast (36) Pancreas (54) | mother—breast cancer | |
| Non-Guideline group | F | 79 | ATM (NM_000051.4) | c.103C > T, p.(Arg35Ter) | Tongue (59) Breast (79) | brother—gastric cancer (70 s) brother—prostate cancer(early stage, 70 s) |
| M | 70 | TP53 (NM_000546.6) | 17p13.1del | Stomach (53) Nasal cavity (54) NSCLC (60) Tongue (64) | son—lung cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.; Kim, H.S.; Lee, H.Y.; Baek, J.M.; Lee, M.; Hong, S.H.; Lee, J.; Park, S.J.; Kim, M.; Woo, I.S. Multiple Primary Cancers as an Independent Criterion for Germline Testing: Comparison with Guideline-Based Criteria. J. Clin. Med. 2025, 14, 7310. https://doi.org/10.3390/jcm14207310
Shin K, Kim HS, Lee HY, Baek JM, Lee M, Hong SH, Lee J, Park SJ, Kim M, Woo IS. Multiple Primary Cancers as an Independent Criterion for Germline Testing: Comparison with Guideline-Based Criteria. Journal of Clinical Medicine. 2025; 14(20):7310. https://doi.org/10.3390/jcm14207310
Chicago/Turabian StyleShin, Kabsoo, Hoon Seok Kim, Hee Yeon Lee, Jong Min Baek, MyungAh Lee, Sook Hee Hong, Jieun Lee, Se Jun Park, Myungshin Kim, and In Sook Woo. 2025. "Multiple Primary Cancers as an Independent Criterion for Germline Testing: Comparison with Guideline-Based Criteria" Journal of Clinical Medicine 14, no. 20: 7310. https://doi.org/10.3390/jcm14207310
APA StyleShin, K., Kim, H. S., Lee, H. Y., Baek, J. M., Lee, M., Hong, S. H., Lee, J., Park, S. J., Kim, M., & Woo, I. S. (2025). Multiple Primary Cancers as an Independent Criterion for Germline Testing: Comparison with Guideline-Based Criteria. Journal of Clinical Medicine, 14(20), 7310. https://doi.org/10.3390/jcm14207310






