Abstract
Background: Cognitive disorders are highly prevalent in individuals with alcohol use disorder. Treatments have so far mainly focused on the amelioration of the cognitive impairments, but interventions to prevent relapse tailored to people with alcohol-related cognitive disorders are lacking. Here we present a new intervention aimed at people with alcohol-related cognitive disorders. Methods: In total, 59 inpatients with alcohol-related cognitive impairments participated in this study. A total of 37 completed the Relapse Prevention for patients with Alcohol-related Cognitive Disorder (RP-ACD) intervention and 22 received treatment as usual (TAU). The RP-ACD is a tailored group intervention for substance use disorder consisting of 12 one-hour group sessions. Outcome measures were the Alcohol Abstinence Self-Efficacy Measure (AASE-12), the Alcohol Urge Questionnaire (AUQ) and the Social Support Questionnaire (SSQ6). The overall experience was explored using a short in-house developed questionnaire. Results: Post-treatment, patients reported an improved self-efficacy compared to the pre-treatment baseline, but no differences were found on the other measures. No significant changes were found in the TAU group. Overall experiences and acceptability were rated positively. Conclusions: The RP-ACD intervention is a feasible and promising group-based addiction treatment for patients with alcohol-related cognitive impairment. A randomized and controlled study in a larger sample is required to establish its efficacy and effectiveness.
1. Introduction
Cognitive impairments are highly prevalent in people with chronic, excessive alcohol use and alcohol use disorder (AUD), with estimates of their prevalence varying between 30 and 80% [1]. All cognitive domains may be affected, and their severity may range from mild or moderate to major neurocognitive disorder [2]. These cognitive impairments are an important source of individual differences that affect many aspects of the addiction treatment [3]. That is, a patient’s inability to receive, encode, integrate, and employ intervention-related information may affect motivational processes and adherence, complicate long-term abstinence, affect the severity of relapse, and consequently hamper full recovery of the alcohol addiction [3,4]. Also, in general, people with AUD and other mental comorbidities—including neurocognitive disorders—have a higher risk of discontinuing their treatment [5]. In turn, there is evidence that prolonged abstinence from alcohol may promote cognitive recovery. Compared to early abstinence (i.e., up to 1 year), meta-analyses have shown that prolonged abstinence (after more than a year) results in less profound cognitive deficits [6], even though widespread cognitive deficits may still persist in the domains of executive function, processing speed, working memory, language and learning and memory [7]. Thus, preventing relapse in individuals at risk of developing alcohol-related cognitive impairments (ARCIs) is key, as it not only reduces the negative effects of the addiction itself, but also promotes cognitive recovery over time.
Interestingly, relapse prevention in individuals with ARCI has to date received little attention. Previous intervention studies in patients with ARCI have predominantly focused on cognitive training or remediation, aimed at the amelioration of the cognitive deficits rather than the alcohol use itself [8]. However, strong evidence regarding the efficacy of such cognitive remediation programs in individuals with ARCI is lacking [9]. Also, most studies on cognitive (re)training in people with cognitive impairments due to acquired brain injury show that the beneficial effects are usually limited to near-transfer improvement on specifically trained tasks, with little or no far transfer to everyday abilities [10]. Optimizing interventions to prevent relapse and reduce craving may consequently result in more beneficial cognitive effects in the long-term compared to such a cognitive training approach and also reduce the risk of further cognitive decline in case abstinence is not realized. However, to date, there is little research on addiction interventions specifically aimed at individuals with alcohol-related cognitive disorders [3], even though tailored relapse prevention programs likely enhance long-term abstinence [11]. Such an intervention would ideally tailor therapeutic sessions to individuals with cognitive impairments, for instance, by reducing group sizes, employing external aids to support knowledge transfer, and by environmental adaptations [12]. In the current pilot study, we aim to provide first evidence on the feasibility of a newly developed intervention for relapse prevention, the Relapse Prevention for patients with Alcohol-related Cognitive Disorders (RP-ACD), with the aim to reduce craving, increase self-efficacy and promote social support as a coping strategy. This intervention is based on cognitive-behavioral therapy, with specific modifications to tailor the program to patients with mild-to-moderate alcohol-related cognitive disorders.
2. Materials and Methods
2.1. Patients
All participants were inpatients of the Center of Excellence for Korsakoff and Alcohol-Related Cognitive Disorders of the Vincent van Gogh Institute for Psychiatry in Venray, the Netherlands. Eligibility for admittance to our clinic was determined using the criteria outlined in Appendix A. At least 4 out of 12 had to be met to be indicative of alcohol-related cognitive impairment [13]. In addition, patients had to meet the criteria for severe alcohol use disorder, diagnosed in accordance with the DSM-5-TR criteria [14]. The Montreal Cognitive Assessment (MoCA) was administered for descriptive purposes [15]. Moreover, all patients underwent a thorough diagnostic work-up, consisting of an extensive neuropsychological assessment (consisting of intelligence testing, assessment of the cognitive domains of learning and memory, executive function, speed of information processing, social cognition, concentration, visuoconstruction, orientation, and language, and self-report and informant-based questionnaires, as well as performance validity testing), a neurological examination, standardized clinical observations, neuroimaging, and a review of the patients’ medical history by a multidisciplinary team to exclude other pathology.
A total of 59 patients participated in this study. The intervention was offered as part of the regular treatment program in the clinic and all patients gave permission for the use of the clinically obtained data for scientific research by written informed consent. Patients were at least 6 weeks abstinent from alcohol, verified via urinalysis, prior to the start of the intervention, as improvements in cognitive function have been reported to be most prominent in the first 6 weeks of abstinence [16]. A total of 37 patients enrolled in the RP-ACD group and 22 served as controls, receiving treatment as usual (TAU) in this period, as they were eligible for starting the intervention but could not enroll yet because of logistic reasons.
2.2. Intervention
The RP-ACD intervention is aimed to promote and increase appropriate coping skills, enhance self-efficacy in achieving alcohol abstinence, and reduce craving in patients with mild-to-moderate alcohol-related cognitive disorders. The program consists of 12 one-hour group sessions offered over a period of 6 weeks that encompassed psycho-education, coping skills training, and strategies to identify and avoid high-risk situations in daily life settings. The intervention is based on existing cognitive-behavioral therapy (CBT) guidelines for substance use disorders. CBT seeks to identify and modify the cognitive and behavioral processes that maintain addictive patterns. Interventions promote adaptive coping strategies and enhance self-regulatory capacities. Standard CBT protocols typically emphasize cognitive restructuring and behavioral change through functional analysis (i.e., examining the cause, context, and purpose of addiction), emotional regulation, and relapse prevention [17,18], while skill-based approaches prioritize behavioral modification and the development of alternative, intrinsically rewarding activities [19]. The current intervention integrates elements from both standard CBT protocols and skill-based approaches and is adjusted to account for neurocognitive impairments.
Modifications of the standard protocols include the selection of themes, adaptation of materials, exercises, and session structure. For instance, themed sessions are delivered with temporal separation rather than being consolidated into a single session. Information is presented concisely, utilizing simple language and concrete examples. Exercises are predominantly designed based on participants’ prior experiences. Both digital and physical visual aids (e.g., images, graphics) are incorporated, and participants are encouraged to actively engage throughout each session. Training sessions commence and conclude with a review of the session’s content. All sessions follow the same structure, which consists of psycho-education, role-playing exercises, exchange of personal experiences and homework assignments, and each session has a central topic that is discussed (see Appendix B for a detailed description). Groups consisted of 3 to 6 patients.
TAU consisted of a structured alcohol-free inpatient setting with interventions supporting a healthy lifestyle, exercise, and daily activities, various forms of occupational therapy, and individual sessions with one’s therapist. Allocation to the RP-ACD or control group was primarily based on availability and (care-)capacity.
2.3. Outcome Measures
A controlled pre–post pilot-study design was applied to evaluate the intervention’s feasibility and to provide first evidence supporting its efficacy with respect to self-efficacy, craving, and perceived social support.
Self-efficacy was measured with the 12-item version of the Alcohol Abstinence Self-Efficacy Measure (AASE-12) [20]. Here, patients had to rate how tempted they would be to use alcohol and how confident they would be to abstain from using alcohol in six situations on a 5-point Likert scale. The composite self-efficacy score is the difference score of confidence minus temptation, ranging from −30 to +30, with higher scores reflecting more self-efficacy).
Craving was assessed with the Alcohol Urge Questionnaire (AUQ) [21], which consists of 8 statements related to feelings and thoughts about drinking that have to be rated on a 7-point Likert scale. The AUQ total score is the sum of the individual item score (range 8–56) with higher scores reflecting more craving.
Perceived social support was measured with the Social Support Questionnaire—Short Form (SSQ6) [22]. This questionnaire consists of 6 items related to different aspects of social support (e.g., who can make you feel relaxed, on whom can you count when you need help, who comforts you when you are upset). For each item, the patient is asked to indicate the names of 1 to 9 supportive persons and to rate the overall satisfaction with that support on a 6-point Likert scale. The SSQ6 number score is the sum of the number of people to whom the patient can turn in various situations (range 0–54), the SSQ6 satisfaction score is the total score of the Likert ratings (range 6–36), expressing the level of satisfaction.
All self-report questionnaires were administered before start of the treatment and after treatment completion. A qualitative evaluation was performed using five open-ended questions (see Appendix C), administered in the last session of the RP-ACD intervention only.
The study design was pre-registered at Open Science Framework (OSF) (https://doi.org/10.17605/OSF.IO/NJM7A, accessed on 15 October 2025). The reporting of this study followed the Transparent Reporting of Evaluations with Non-randomized Designs (TRENDs) statement [23].
2.4. Analyses
Analyses were performed using IBM SPSS 29.0. Baseline characteristics were compared for the two groups using the appropriate parametric (Student’s t) or non-parametric test (Mann–Whitney U, χ2). Only complete pre–post pairs were analyzed using two-tailed non-parametric paired-sample Wilcoxon signed rank tests for the pre- and post-assessments to test the hypothesis that the intervention would reduce craving, improve self-efficacy, and enhance social support in the RP-ACD group but not in the TAU group. Missing data were not imputed given the small sample size, the pilot study design, and the high probability that these were not missing at random. Outcome data are presented as medians. Effect sizes (r) for the Wilcoxon test were computed using the following formula: . 95% Confidence intervals for the medians were computed using the bootstrapping procedure.
3. Results
Figure 1 shows the flowchart of the study, illustrating that all data points were not available for all participants (the main reason was that patients were already discharged before the T1 assessment was completed). The MoCA total score was slightly lower in the TAU group compared to the RP-ACD group (t(57) = 3.32, p = 0.002). The groups did not differ with respect to age (t(57) = 1.93, p = 0.06), education level (U = 306.0, Z = 1.40, p = 0.16), or sex distribution (χ2(1) = 0.028, p = 0.87). The mean time between T0 and T1 was shorter for the RP-ACD group than for the TAU group (t(12.8) = 2.58, p = 0.023) (Table 1).
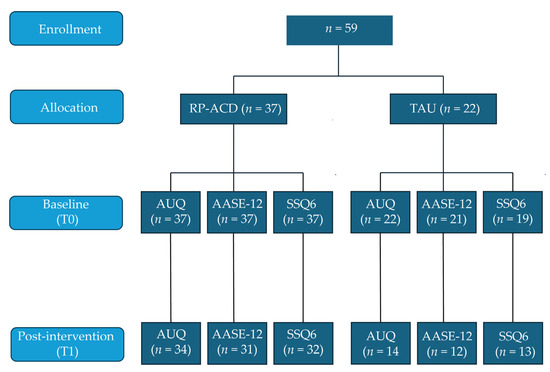
Figure 1.
Flowchart of the pilot study. RP-ACD = Relapse Prevention for patients with Alcohol-related Cognitive Disorders (RP-ACD); TAU = treatment as usual; AUQ = Alcohol Urge Questionnaire; AASE-12 = Alcohol Abstinence Self-Efficacy Measure; SSQ6 = Social Support Questionnaire—Short Form.

Table 1.
Baseline characteristics for the Relapse Prevention for Patients with Alcohol-related Cognitive Disorder (RP-ACD) intervention group and the treatment as usual (TAU) group.
Figure 2 and Table 2 show the results for the craving, self-efficacy, and social support outcome measures for the available data points for both groups. Analyses showed a significant improvement from baseline to post-intervention on the AASE-12 in the RP-ACD group (Z = −2.46, p = 0.014) but not the TAU group (Z = −0.26, p = 0.80). No statistically significant change was observed in craving (AUQ RP-ACD: Z = −0.71, p = 0.48; TAU: Z = −0.56, p = 0.57) or social support, neither in the number of people (RP-ACD Z = −0.09, p = 0.93; TAU: Z = −0.24, p = 0.81) nor in satisfaction (RP-ACD Z = −1.834, p = 0.18; TAU: Z = −0.18, p = 0.85). Visual qualitative inspection of the responses to the open-ended questions indicated that the intervention was evaluated positively: participants reported achieving their (personal) goals and were appreciative of the supporting workbook and SOS card as a means to maintain improvement in the nearby future.
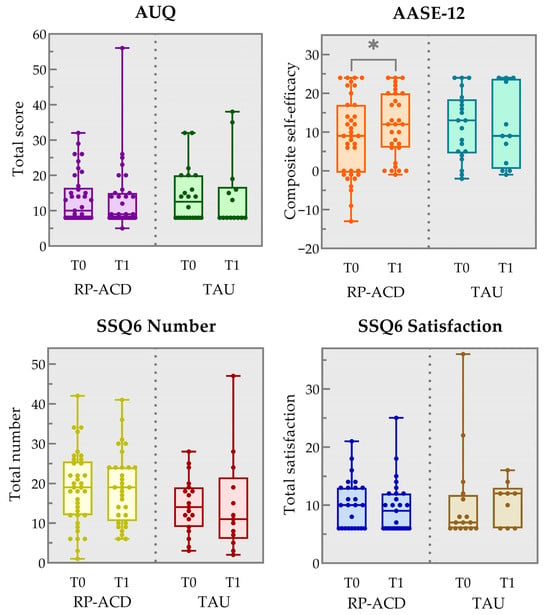
Figure 2.
Box and whisker plots showing the results for the pre-intervention (T0) and post-intervention (T1) timepoints on the Alcohol Urge Questionnaire (AUQ total item score), the Alcohol Abstinence Self-Efficacy Measure (AASE-12 composite self-efficacy score) and the Social Support Questionnaire (SSQ6 total number of people and total satisfaction score) for the Relapse Prevention for patients with Alcohol-related Cognitive Disorders (RP-ACD) group and the treatment as usual (TAU) group. The boxes indicate the 25th and 75th percentile, the center line represents the median and the whiskers the minimum and maximum values. * p = 0.014 (two-tailed).

Table 2.
Median pre- and post-intervention scores on the outcome measures for the Relapse Prevention for patients with Alcohol-related Cognitive Disorders (RP-ACD) group and the treatment as usual (TAU) group, and change scores, effect sizes, and levels of significance.
4. Discussion
In this pilot study, we demonstrated that a newly developed intervention for relapse prevention in individuals with alcohol-related cognitive disorders (the Relapse Prevention for people with Alcohol-related Cognitive Disorders; RP-ACD), is feasible. Furthermore, using a controlled pre–post design, we showed that in the RP-ACD group, the patients’ abstinence self-efficacy improved (i.e., an increased confidence to abstain from using alcohol accompanied by a reduced temptation to use alcohol in specific situations), with a large effect size. This finding is very much in line with the content and aims of the intervention, which targets craving, peer pressure, slips, and relapses in every-day situations using goal setting, role-playing, and homework assignments. However, current craving and alcohol urge was not reduced after the intervention compared to the baseline assessment in the RP-ACD group. Visual inspection of the data shows that the pre-assessment alcohol urge was already relatively low, leaving little room for further reduction. However, it should be noted that while this assessment was conducted before the start of the RP-ACD intervention, patients were already abstinent from alcohol for at least 6 weeks, verified by urinalysis, and were inpatients of a ‘dry’ clinic with no access to alcohol, which may have substantially reduced physical craving symptoms compared to the levels pre-admission. Indeed, in untreated AUD populations, much higher AUQ scores have been reported (e.g., 28.8 in a community AUD population [25]). The number of people patients confided in did not change after the intervention compared to the baseline, neither did the level of social support satisfaction. It should, however, be noted that there was a substantial amount of missing data for the SSQ6 (especially on the satisfaction part), possibly reflecting the lack of a social support system, which is common in patients with AUD. Finally, the participants rated the intervention positively. No significant changes over time were observed in the TAU group.
Whilst these findings are promising, they are also preliminary in nature, as the present study should be regarded as a pilot study. That is, our study design comes with important limitations. First, it is not a randomized clinical trial. Allocation to either the RP-ACD or the TAU group was performed because of logistic reasons; as a result, the TAU group can be considered a waiting-list control group, as these patients would enroll in the RP-ACD treatment later on. Another limitation is the small sample size (especially for the TAU group) and the relatively large number of patients who did not complete all self-report measures post-intervention, notably in the TAU group, which may explain null-results. Moreover, the time between the baseline and post-interventions was longer for the TAU group than for the RP-ACD group and the lack of follow-up assessments on relapse is a limitation. Furthermore, we do not have detailed information on the patients’ somatic or psychiatric comorbidity or medication. A future trial could also include more context-sensitive outcome measures, such as coping skills, self-regulation tasks, or ecological momentary assessments of craving [26].
5. Conclusions
In conclusion, this controlled pilot study showed the feasibility of the RP-ACD intervention, a cognitive-behavioral-therapy-based addiction intervention tailored towards individuals with alcohol-related cognitive disorders. First results are promising: after the intervention, higher levels of self-efficacy were reported in the RP-ACD group but not in the TAU group. No reliable effects on drinking urge and social support were found. This null finding is possibly the result of our outcome measures not being sensitive to detect changes in a controlled inpatient setting with enforced abstinence. Participants rated the intervention and materials positively and found the intervention acceptable. The results of our non-randomized, small-sample pilot study suggest that the RP-ACD intervention is a promising group-based treatment to prevent relapse in alcohol use for patients with mild-to-moderate alcohol-related cognitive impairments, which to date is lacking [13]. A larger, adequately powered randomized and controlled study with longer-term follow-up assessments and relapse outcomes is required to establish the definitive efficacy and clinical effectiveness of the RP-ACD.
Author Contributions
Conceptualization, G.T.L.J.; methodology, G.T.L.J.; formal analysis, R.P.C.K.; investigation, G.T.L.J. and Y.C.M.R.; data curation, G.T.L.J.; writing—original draft preparation, G.T.L.J.; writing—review and editing, R.P.C.K. and Y.C.M.R.; visualization, R.P.C.K.; supervision, R.P.C.K.; project administration, G.T.L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and the design was approved by the Institutional Review Board of Vincent van Gogh Institute for Psychiatry CWOP (protocol code #11052020 and date of approval 1 November 2023).
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data underlying this article cannot be shared publicly as the informed consent forms did not include the possibility for public data sharing in a repository. The data can be obtained upon reasonable request from the corresponding author.
Acknowledgments
The authors would like to thank Denice Verberkt and Kristin Kalesse for their assistance in the data collection and data management. No Generative AI tools were used during the preparation or editing of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AASE-12 | Alcohol Abstinence Self-Efficacy Measure |
| AUD | Alcohol Use Disorder |
| AUQ | Alcohol Urge Questionnaire |
| MoCA | Montreal Cognitive Assessment |
| RP-ACD | Relapse Prevention for patients with Alcohol-related Cognitive Disorders |
| SSQ6 | Social Support Questionnaire—Short Form |
| TAU | Treatment as usual |
Appendix A

Table A1.
(Severe) Alcohol-related cognitive impairments checklist (ACS) to be administered in case of (long-lasting) alcohol abuse (English translation from the original Dutch checklist [13]).
Table A1.
(Severe) Alcohol-related cognitive impairments checklist (ACS) to be administered in case of (long-lasting) alcohol abuse (English translation from the original Dutch checklist [13]).
| Item | Criterion | Response |
|---|---|---|
| 1 | Is there evidence for nutritional deficiency? | YES/NO |
| 2 | Are there any oculomotor abnormalities? | YES/NO |
| 3 | Are there any cerebellar dysfunctions (e.g., ataxia, instability, gait, disturbance) | YES/NO |
| 4 | Are there mental changes or is there a (mild) memory disorder? | YES/NO |
| 5 | Is there self-neglect, such as a loss of manners/decorum, etc.? | YES/NO |
| 6 | Is there disorientation in time and place? | YES/NO |
| 7 | Are there personality or behavioral changes compared to the pre-morbid level of functioning? | YES/NO |
| 8 | Are there any confabulations? | YES/NO |
| 9 | Are there objective deficits in the encoding of new information (inability to retain new information, for example, the inability to remember three objects after five minutes, or to remember what happened yesterday)? | YES/NO |
| 10 | Are there deficits in retrieving information from long-term memory (inability to remember events that were previously known or generally known facts)? | YES/NO |
| 11 | Is there a decrease in consciousness, accompanied by motor restlessness, reduced awareness of the environment, with or without visual sensations? | YES/NO/ PROBABLY |
| 12 | Are there one (or more) of the following cognitive impairments: aphasia (e.g., word-finding difficulties or inability to understand language), apraxia (inability to perform motor actions), visual agnosia (inability to recognize objects), executive dysfunction (i.e., planning, organizing, logical or abstract reasoning). | YES/NO/ PROBABLY |
Scoring: There is evidence of an alcohol-related cognitive disorder if more than four items were answered positively. There may be a Wernicke encephalopathy if two out of items 1 to 4 were answered positively. If item 11 is answered with ‘yes’ or ‘probably’, a delirium may be present. If item 12 is answered with ‘yes’ or ‘probably’, dementia may be present.
Appendix B

Table A2.
Overview of the content of the Relapse Prevention for Alcohol-related Cognitive Disorders intervention.
Table A2.
Overview of the content of the Relapse Prevention for Alcohol-related Cognitive Disorders intervention.
| Session | Topic | Content |
|---|---|---|
| 1 | Preparing for change I | Learning to cope with situations and factors that may provoke alcohol use |
| 2 | Preparing for change II | Discussion of the short-term and long-term positive and negative consequences of alcohol misuse |
| 3 | Personal goals and self-control I | Learning to avoid situations that are associated with (past) alcohol use and find alternatives for the future |
| 4 | Personal goals and self-control II | Stressing the importance of positive reinforcement by rewarding oneself and seeking social support and distraction in other activities |
| 5 | Slips and relapses I | Learning to identify self-doubt and high-risk situations and what to do in case of relapse |
| 6 | Slips and relapses II | Identification of personal high-risk situations and learning to apply self-control measures |
| 7 | Craving I | How to cope with triggers and craving in daily situations |
| 8 | Craving II | Engaging in pleasant activities to reduce craving |
| 9 | Refusing substances that are being offered I | Coping with peer pressure to start using alcohol or other substances |
| 10 | Refusing substances that are being offered II | Role-playing to practice situations in which peer pressure to start using again may take place |
| 11 | Evaluation and the SOS card I | Make a personalized ‘SOS card’ that contains information about one’s motivation to stop using, personal high-risk situations and what to do in case of craving and (possible) relapse |
| 12 | Evaluation and the SOS card II | Evaluate the sessions in relation to one’s goals and present one’s SOS card to the group |
Appendix C
Evaluation Questions
Congratulations! You successfully completed the Relapse Prevention for people with Alcohol-related Cognitive Disorders (RP-ACD) intervention. Please take some time to consider the following questions:
- Did I achieve my goals for this training?
- What has changed and/or improved?
- What do I need to sustain this change and/or improvement?
- What goals have I not yet achieved?
- What more would be needed to achieve these goals?
References
- Bruijnen, C.J.W.H.; Dijkstra, B.A.G.; Walvoort, S.J.W.; Markus, W.; VanDerNagel, J.E.L.; Kessels, R.P.C.; DE Jong, C.A.J. Prevalence of cognitive impairment in patients with substance use disorder. Drug Alcohol Rev. 2019, 38, 435–442. [Google Scholar] [CrossRef]
- Jones, L.; Owens, L.; Thompson, A.; Gilmore, I.; Richardson, P. Informing the development of diagnostic criteria for differential diagnosis of alcohol-related cognitive impairment (ARCI) among heavy drinkers: A systematic scoping review. PLoS ONE 2023, 18, e0280749. [Google Scholar] [CrossRef]
- Cabé, N.; Laniepce, A.; Ritz, L.; Lannuzel, C.; Boudehent, C.; Vabret, F.; Eustache, F.; Beaunieux, H.; Pitel, A.-L. Troubles cognitifs dans l’alcoolodépendance: Intérêt du dépistage dans l’optimisation des prises en charge [Cognitive impairments in alcohol dependence: From screening to treatment improvements]. Encephale 2016, 42, 74–81. [Google Scholar] [CrossRef]
- Bates, M.E.; Pawlak, A.P. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychol. Addict. Behav. 2006, 20, 241–253. [Google Scholar] [CrossRef]
- Collin, A.; Ayuso-Muñoz, A.; Tejera-Nevado, P.; Prieto-Santamaría, L.; Verdejo-García, A.; Díaz-Batanero, C.; Fernández-Calderón, F.; Albein-Urios, N.; Lozano, Ó.M.; Rodríguez-González, A. Analyzing dropout in alcohol recovery programs: A machine learning approach. J. Clin. Med. 2024, 13, 4825. [Google Scholar] [CrossRef] [PubMed]
- Stavro, K.; Pelletier, J.; Potvin, S. Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addict. Biol. 2013, 18, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.F.; Cammisuli, D.M.; Stranks, E.K. Widespread cognitive deficits in alcoholism persistent following prolonged abstinence: An updated meta-analysis of studies that used standardised neuropsychological assessment tools. Arch. Clin. Neuropsychol. 2019, 35, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.E.; Buckman, J.F.; Nguyen, T.T. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol. Rev. 2013, 23, 27–47. [Google Scholar] [CrossRef]
- Nixon, S.J.; Lewis, B. Cognitive training as a component of treatment of alcohol use disorder: A review. Neuropsychology 2019, 33, 822–841. [Google Scholar] [CrossRef]
- Hallock, H.; Collins, D.; Lampit, A.; Deol, K.; Fleming, J.; Valenzuela, M. Cognitive training for post-acute traumatic brain injury: A systematic review and meta-analysis. Front. Hum. Neurosci. 2016, 10, 537. [Google Scholar] [CrossRef]
- Tabugan, D.C.; Bredicean, A.C.; Anghel, T.; Dumache, R.; Muresan, C.; Corsaro, L.; Hogea, L. Novel insights into addiction management: A meta-analysis on intervention for relapse prevention. Medicina 2025, 61, 619. [Google Scholar] [CrossRef]
- Bates, M.E.; Bowden, S.C.; Barry, D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Exp. Clin. Psychopharmacol. 2002, 10, 193–212. [Google Scholar] [CrossRef]
- Walvoort, S.J.W.; van der Heijden, P.T.; Kessels, R.P.C.; Egger, J.I.M. Cognitieve stoornissen bij alcoholabusus [Cognitive impairments in alcohol use disorder]. Ins Ouds Tijdschr Geriatr. 2018, 6, 11–14. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text rev; APA: Arlington, VA, USA, 2022. [Google Scholar] [CrossRef]
- Bruijnen, C.J.W.H.; Jansen, M.; Dijkstra, B.A.G.; Walvoort, S.J.W.; Lugtmeijer, S.; Markus, W.; De Jong, C.A.J.; Kessels, R.P.C. The Montreal Cognitive Assessment as a cognitive screen in addiction health care: A validation study for clinical practice. J. Subst. Use 2018, 24, 47–54. [Google Scholar] [CrossRef]
- Walvoort, S.J.W.; Wester, A.J.; Egger, J.I.M. Neuropsychologische diagnostiek en cognitieve functies bij alcoholabstinentie [The neuropsychology of cognitive functions in alcohol abstinence]. Tijdschr. Psychiatr. 2013, 55, 101–111. [Google Scholar]
- Schippers, G.M.; Smeerdijk, M.; Merx, M.J.M. Handboek Cognitieve Gedragstherapie bij Middelengeberuik en Gokken [Handbook Cognitive Behavioural Therapy in Substance Use Disorders and Gambling]; Perspectief: Utrecht, The Netherlands, 2014. [Google Scholar]
- VanderNagel, J.E.L.; Kiewik, M. Handleiding CGT+: Cognitief Gedragstherapeutische Behandeling van Problematisch Middelengebruik bij Mensen met een Lichte Verstandelijke Beperking [Manual CBT+: Cognitive Behavioural Therapy in Substance Misuse in People with Mild Intellectual Disability]; Stichting Resultaten Scoren: Amersfoort, The Netherlands, 2016. [Google Scholar]
- Blankman, H. Liberman Module Omgaan met Verslaving [Liberman Module Coping with Addiction]; Trimbos: Utrecht, The Netherland, 2015. [Google Scholar]
- McKiernan, P.; Cloud, R.; Patterson, D.A.; Golder, S.; Besel, K. Development of a brief abstinence self-efficacy measure. J. Soc. Work Pract. Addict. 2011, 11, 245–253. [Google Scholar] [CrossRef]
- Bohn, M.J.; Krahn, D.D.; Staehler, B.A. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol. Clin. Exp. Res. 1995, 19, 600–606. [Google Scholar] [CrossRef]
- Sarason, I.G.; Sarason, B.R.; Shearin, E.N.; Pierce, G.R. A brief measure of social support: Practical and theoretical implications. J. Soc. Pers. Relat. 1987, 4, 497–510. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; The Trend Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef]
- Duits, A.; Kessels, R. Schatten van het premorbide functioneren [Estimating premorbid functioning]. In Neuropsychologische Diagnostiek: De Klinische praktijk; Hendriks, M., Kessels, R., Gorissen, M., Schmand, B., Duits, A., Eds.; Boom: Amsterdam, The Netherlands, 2012; pp. 173–186. [Google Scholar]
- Drummond, D.C.; Phillips, T.S. Alcohol urges in alcohol-dependent drinkers: Further validation of the Alcohol Urge Questionnaire in an untreated community clinical population. Addiction 2002, 97, 1465–1472. [Google Scholar] [CrossRef]
- Benvenuti, M.C.; Merkle, E.C.; McCarthy, D.M. Physical context of alcohol use and craving: An EMA exploratory study. Addict. Behav. 2025, 170, 108450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).