Abstract
Background: Current clinical guidelines recommend 52 ng/L as the abnormal baseline cutoff in high-sensitivity cardiac troponin (hs-cTn) algorithms for the rapid diagnosis of acute myocardial infarction (AMI). Though abnormal, this threshold is not AMI-specific, leading to extensive workups for many non-AMI chest pain patients, overutilization of resources, and emergency department (ED) overcrowding. Hence, the performance of this baseline abnormal cutoff was compared against the refined new thresholds for rapid AMI diagnosis in ED chest pain patients. Methods: We included ED chest pain patients with hs-cTnT and hs-cTnI levels simultaneously measured and clinical outcomes adjudicated by cardiologists. We performed receiver operating characteristics (ROC) analyses across various thresholds for diagnostic performance, including sensitivity, specificity, negative and positive likelihood ratios, and predictive values. Statistical analysis was carried out using Graphpad Prism 10, with p < 0.05 considered as significant. Results: In our study, 17 patients were adjudicated as AMI, and 682 patients were ruled out for AMI. In 15/17 AMI cases, baseline hs-cTn values far exceeded 52 ng/L. Notably, among non-AMI individuals, 140 (hs-cTnT) and 91 (hs-cTnI) also exceeded this cutoff. ROC analyses identified optimal abnormal cutoffs of 82 ng/L for hs-cTnT and 122 ng/L for hs-cTnI, which improved specificity without compromising sensitivity. Post-discharge follow-up at 1, 3, and 12 months for cardiovascular events supported these revised thresholds. Conclusions: Increasing the baseline abnormal value from 52 ng/L to 82 ng/L for hs-cTnT and to 122 ng/L for hs-cTnI in care pathways could reduce false positives with the potential to decrease unnecessary testing and alleviate long stays in the ED and resource management. Larger, diverse cohort studies are warranted to validate these findings.
1. Introduction
Current guidelines from the American Heart Association, American College of Cardiology, and European Society of Cardiology endorse high-sensitivity cardiac troponins (hs-cTns) as the preferred biomarker in emergency department (ED) patients presenting with suspected myocardial infarction [,]. Several streamlined diagnostic algorithms utilizing baseline hs-cTn values and delta changes from serial measurements have been proposed for the rapid diagnosis of AMI [,,]. With the increasing adoption of hs-cTn testing in clinical practice by U.S. laboratories, there is an opportunity to evaluate the performance of various algorithms for AMI rule-in and rule-out [].
Given ED overcrowding and the need for rapid triaging of non-AMI patients, the clinical focus is on promptly excluding non-AMI cases. This is mainly accomplished through the baseline rule-out cutoff values and delta changes in timed serial samples. The FDA defines 6 ng/L (hs-cTnT) and 5 ng/L (hs-cTnI) as the baseline cutoffs for rapid rule-out of AMI, besides requiring numerical reporting of cTn levels at or above the limit of quantitation (LoQ) [,].
A baseline abnormal cutoff is incorporated into Tn algorithms for the early diagnosis of AMI cases so that immediate medical attention and timely intervention can be provided to those critical patients. The current AMI care pathway algorithms incorporate an early abnormal baseline value of 52 ng/L as the initial decision point in their flow charts [,]. Earlier studies and international clinical guidelines endorsed using the 99th percentile of hs-cTn assays as a rapid abnormal cutoff for identifying true AMI cases in their algorithm [,]. This threshold corresponds to the 99th percentile value derived from a healthy reference population. However, variability exists between different assays and laboratories in the exact numeric value for the 99th percentile, leading to differences in specific cutoffs commonly used in clinical pathways. Several research groups have shown 52 ng/L to be a better early abnormal cutoff than the 99th percentile of hs-cTns [,,]. Classification and regression tree (CART) analysis identified an optimal threshold value of 52 ng/L at presentation or absolute change in hs-cTnT within the first hour of 5 ng/L or higher to indicate a high probability of being a true AMI []. Based on these findings, current guidelines recommend 52 ng/L as the early abnormal cutoff value for both hs-cTnT and hs-cTnI tests. Our facility was one of the earliest facilities to implement the Roche fifth-generation TnT assay (hs-cTnT) in the U.S. Following the upgrade of our core laboratory automated chemistry system, we transitioned to the Abbott hs-cTnI assay. Throughout this changeover, the 52 ng/L cutoff continued to be used as an early abnormal cutoff for AMI rule-in across both assays.
Several studies have demonstrated that an elevated baseline abnormal hs-cTn value exceeding 52 ng/L is not uncommon in ED chest pain patients without MI, with such elevations often linked to sex, age, and kidney function differences [,,,]. Thus, although a 52 ng/L diagnostic threshold indicates abnormal troponin levels and may indicate high-risk, it is not specific enough to distinguish AMI exclusively from other causes of troponin elevation. Also, given the wide spectrum of patients encountered in the ED, with varying comorbidity burdens and age groups, an abnormal cutoff value derived from healthy individuals for hs-cTns may not adequately capture the normal variation seen in clinical practice. Therefore, it is critical to provide a clinically useful threshold where the assay ensures better alignment with patient heterogeneity and optimizes biomarker interpretation. Therefore, in the current study, we sought to evaluate whether the 52 ng/L cutoff remains the most effective diagnostic threshold at presentation for AMI diagnosis or whether refinements could enhance specificity without compromising sensitivity.
2. Materials and Methods
The institutional review board of the University of Texas Southwestern Medical Center (UTSW) approved this study, and it was performed in accordance with the Declaration of Helsinki. This retrospective cohort study was conducted in our care facility between May and September 2020 during our planned transition from Roche high-sensitivity cardiac troponin T (hs-cTnT) to Abbott high-sensitivity cardiac troponin I (hs-cTnI). Both markers are used in the detection of myocardial injury and the differential diagnosis of acute coronary syndromes. The hs-cTnT assay targets troponin subunit T and is manufactured by Roche Diagnostics, while the hs-cTnI assay targets troponin subunit I and is manufactured by other major vendors, such as Abbott and Siemens. Studies show comparable diagnostic performance for both assays, but hs-cTnT is elevated in renal dysfunction, while hs-cTnI remains less affected [,]. Adults > 18 years of age or older presenting to our university hospital ED and meeting the eligibility criteria were included in this study. The eligibility criteria included a. an initial encounter during the specified period, b. availability of the serial samples to complete the clinical decision pathway (CDP), c. ECG, and d. heart score diagnosis. Demographics, gender, and other clinical manifestations during the ED visit were obtained from the EPIC system. Patients missing hs-cTnT or hs-cTnI levels, ECG information, and repeated encounters were excluded from the analysis. Of the 1572 patients, only 699 patients were found to be eligible for the current study analysis, while the remaining samples were either missing a diagnosis or had repeated ED visits. A flow diagram of patients included in the study is shown in Figure 1. Simultaneous hs-TnT and hs-TnI testing was carried out on the initial presentation samples, with follow-up measurements at 1 h and 3 h, guided by clinical findings and clinician assessment []. Hs-cTnT measurements were performed on plasma samples collected in dipotassium EDTA, while the hs-cTnI assay used lithium heparin plasma [,].
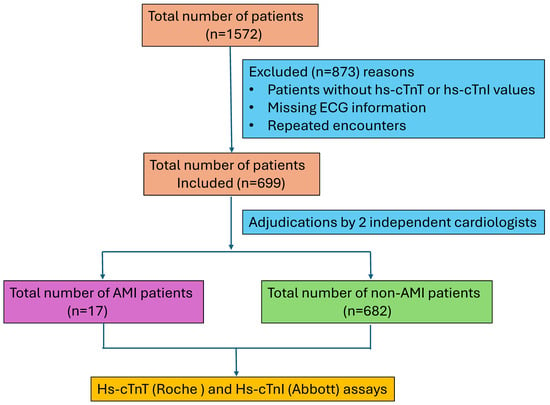
Figure 1.
Flow diagram of the patients included in this study.
Clinical Diagnosis
All cases were adjudicated by 2 cardiologists based on the hs-cTn protocol and the fourth universal definition of myocardial infarction. Adjudication categories included Type I and Type II MI and acute or chronic myocardial injury [,]. HEART score, nonischemic ECG, and clinical symptoms were considered in the adjudication. Non-AMI cases mostly had secondary complications, such as chronic kidney disease, sepsis, hypertension, respiratory syndrome, and coronary heart disease. Receiver operating characteristic (ROC) curve analysis was conducted using hs-cTnT and hs-cTnI levels measured at presentation to identify the optimal abnormal cutoff for AMI. Diagnostic performance parameters, such as clinical sensitivity, specificity, positive and negative predictive values, as well as likelihood ratios, were calculated for various threshold cutoff values. Clinical outcomes, including mortality, myocardial infarction, and revascularization, were tracked longitudinally over 30 days, 90 days, and up to one year through EPIC electronic medical records. Statistical analyses were performed using GraphPad Prism 10, considering p < 0.05 as statistically significant.
3. Results
At our university hospital ED, hs-cTn values of ≥52 ng/L are designated as abnormal for AMI in the care pathways. Among 699 patients presenting with chest pain, the majority (97.4%) were diagnosed as non-AMI cases, while 2.4% were found to be true positives for AMI (Figure 2a). Over half of the ED patients with acute chest pain had hs-cTn levels below 52 ng/L for both assays (Figure 2b,c). Although 26% of patients for hs-cTnT and 15% of patients for hs-cTnI exceeded the abnormal cutoff threshold of 52 ng/L, the majority of these patients were non-AMI patients. After adjudication, only 17 patients were confirmed for AMI. Of these patients, 15 had hs-cTn levels exceeding 52 ng/L at presentation, while 2 patients (patient numbers 6,7) initially tested below this threshold (Table 1). However, both these patients exhibited significant increases in troponin levels over time, with delta changes at 3 h post-presentation indicative of AMI or myocardial injury (Figure 3a,c).
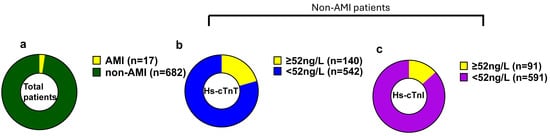
Figure 2.
Hs-cTnT and hs-cTnI baseline values in ED patients with chest pain. Hs-cTn baseline values in ED patients with chest pain. (a). Total number of patients. (b). Baseline hs-cTnT levels in non-AMI patients. (c). Baseline hs-cTnI levels in non-AMI patients.

Table 1.
hs-cTnT and hs-cTnI levels at 0,1, and 3 h in AMI patients.
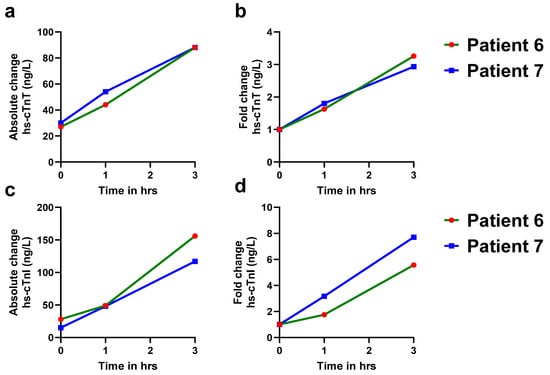
Figure 3.
Serial measurements of hs-cTnT and hs-cTnI levels in AMI patients with baseline values < 52 ng/L. A more than 20% increase in hs-cTn levels at 1 h and 3 h in both patients indicates dynamic troponin kinetics, which are more consistent with acute myocardial infarction (AMI). Baseline hs-cTnT and hs-cTnI levels in AMI patients. (a). Absolute change in hs-cTnT levels in patient #6 (green solid line) and patient #7 (blue solid line) at 0, 1, and 3 h. (b). Fold change in hs-cTnT levels in patient #6 (green solid line) and patient #7 (blue solid line) at 0, 1, and 3 h. (c). Absolute change in hs-cTnI levels in patient #6 (green solid line) and patient #7 (blue solid line) at 0, 1, and 3 h. (d). Fold change in hs-cTnI levels in patient #6 (green solid line) and patient #7 (blue solid line) at 0, 1, and 3 h.
A comparison of ROC curves for hs-cTnT and hs-cTnI demonstrated that both assays had a baseline sensitivity of 88% at the cutoff of 52 ng/L. For hs-cTnT, this sensitivity remained constant up to 82 ng/L (Figure 4a), and for hs-cTnI, it remained constant up to 122 ng/L (Figure 4b). Applying an 82 ng/L cutoff for hs-cTnT improved specificity from 79% (95% CI; 0.77, 0.82) to 87% (95% CI; 0.84,0.89). Similarly, raising the hs-cTnI cutoff to 122 ng/L increased specificity to 92% (95% CI: 0.90, 0.94). Notably, these increases in the cutoff improved specificity while maintaining similar sensitivity as that of 52 ng/L (Table 2). The negative predictive value (100%) and negative likelihood ratios were unchanged across all thresholds. Although sensitivities remained unchanged at the higher cutoffs, positive predictive value abnormal for AMI rose to approximately 1.6-fold for both hs-cTnT (0.09 (52 ng/L) vs. 0.15 (82 ng/L)) and hs-cTnI (0.14 (52 ng/L) vs. 0.22 (122 ng/L)) (Table 2). Importantly, we observed no corresponding change in positive likelihood ratios, suggesting the higher thresholds result in fewer false-positive cases at presentation in the ED. We also noticed that hs-cTnI has better sensitivity and specificity compared to hs-cTnT, consistent with reports from previous studies [,,].
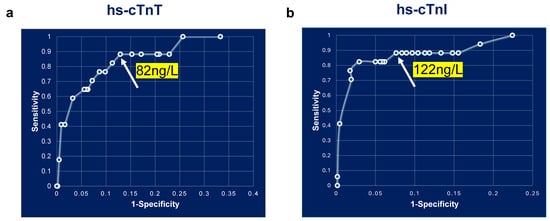
Figure 4.
ROC curve analysis of baseline hs-cTnT and hs-cTnI measurements. ROC curve analysis of baseline hs-cTn measurements. (a). hs-cTnT and (b). hs-cTnI.

Table 2.
Diagnostic performance of the baseline abnormal cutoff and newly derived abnormal cutoff of the hs-cTnT and hs-cTnI assays.
Next, we assessed whether applying the refined abnormal cutoffs of 82 ng/L for hs-cTnT and 122 ng/L for hs-cTnT could reduce the proportion of non-AMI chest pain patients flagged as abnormal for AMI in the ED. Compared to the existing threshold of 52 ng/L for AMI, these elevated cutoffs reduced false-positive cases by 37% for hs-cTnT and 43% for hs-cTnI (Figure 5a,c). We checked 0/1 h absolute changes in patients with baseline hs-cTn values between 52 and 82 ng/L (hs-cTnT) and 52 and 122 ng/L (hs-cTnI). (Figure 5b,d). We analyzed the delta change in patients who had 1 h hs-cTn values measured. No patients exhibited a delta change of more than a 20% increase in hs-cTnT levels (Figure 5b), while three patients exceeded a delta change of more than 20% in hs-cTnI levels (Figure 5d). Nevertheless, these cases were subsequently confirmed by cardiologists as nonischemic myocardial injury patients.
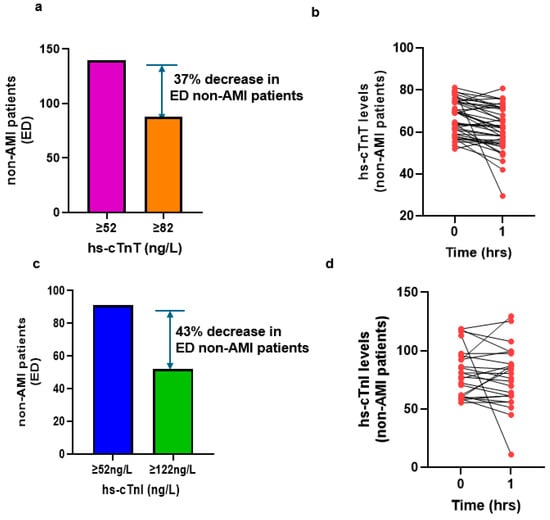
Figure 5.
Number of non-AMI patients in the newly derived baseline abnormal cutoff and absolute changes in 1 h. Non-AMI patients in the ED with chest pain. (a). Baseline hs-cTnT levels ≥ 52 ng/L versus baseline ≥ 82 ng/L; (b). 1 h absolute change in hs-cTnT levels; (c). baseline hs-cTnI levels ≥ 52 ng/L versus baseline ≥ 122 ng/L; (d). 1 h absolute change in hs-cTnI levels.
To validate the effectiveness and assess the clinical utility of the revised abnormal cutoffs in non-AMI patients, we conducted a follow-up review of ED return visits in chest pain patients with baseline troponin levels between 52 and 82 ng/L for hs-cTnT and 52 and 122 ng/L for hs-cTnI. We documented reasons for the ED visits at 1-, 3-, and 12-month post-discharge (Table 3). There were 52 patients with baseline hs-cTnT values between 52 and 82 ng/L and 39 patients with baseline hs-cTnI values between 52 and 122 ng/L. No cardiac deaths were reported among those patients followed up after 1 month and 12 months (Table 3). However, one case was detected due to comorbidities of renal failure, pneumonia, and atrial fibrillation after 3 months of follow-up. The majority of the patients who re-visited the ED were due to non-cardiac-related issues, such as COPD, pneumonia, sepsis, hypertension, renal failure, end-stage renal disease, stroke, and cholecystitis. A very low incidence of cardiac-related events was observed after 1 month (0.2% for hs-cTnT; 0.5% for hs-cTnI), 3 months (0.1% for hs-cTnT; 0.3% for hs-cTnI), and 12 months (0.3% for both hs-cTnT and hs-cTnI).

Table 3.
Post-discharge clinical follow-up of non-AMI patients with baseline Tn values between 52 ng/L and newly derived cutoff ranges.
4. Discussion
In the current study, the optimal diagnostic abnormal thresholds for AMI were identified as 82 ng/L for the hs-cTnT assay and 122 ng/L for the hs-cTnI assay. Applying these revised diagnostic abnormal values at presentation in the ED decreased the detection of false-positive cases. Integrating these revised abnormal thresholds along with a 1 h delta troponin change criterion provided a good strategy for confirming true AMI cases in the ED.
Over the past few decades, high-sensitivity cardiac troponins have revolutionized the diagnosis of AMI. Numerous research groups have developed and validated diagnostic algorithms, defining rule-out/rule-in for AMI in ED patients with chest pain [,,,,]. The 52 ng/L rule-in/abnormal cutoff is part of a broader strategy that typically integrates serial measurements and evaluates dynamic changes in troponin levels over time. This was adopted from the European Society of Cardiology (ESC) rule-in criteria for acute coronary syndrome (ACS), which balanced the sensitivity and specificity—high enough to rule-in AMI in many cases, yet low enough to identify early cardiac injury. Within the ESC 0/1 h algorithm, 52 ng/L is applied to both hs-cTnT and hs-cTnI assays. For hs-cTnT, the abnormal criterion is either an initial value ≥ 52 ng/L or a 1 h rise of ≥ 5 ng/L []. Although this cutoff has been validated in multiple studies and incorporated into ESC guidelines, the hs-cTnT 0/1 h protocol allows for accurate early triage in only about 75% of patients, with 60% ruled out for MI and 15% ruled in []. In another study, the same cutoff of ≥52 ng/L (hs-cTnT) identified 26% of chest pain patients in a high-risk category, of which only 7% had an MI []. Adding to this complexity, in U.S. EDs, the troponin testing practices are inconsistent; in one large analysis of 97,085 ED visits from the National Hospital Ambulatory Medical Care Survey (NHAMCS), only 7% of patients had biomarker testing, and two-thirds of these patients lacked chest pain at presentation, with ACS prevalence below 2% [].
All AMI diagnostic algorithms share a central goal to ensure no true infarction case is overlooked. In Europe, these algorithms are well standardized with respect to diagnostic cutoff values and triage protocols. In contrast, many U.S. facilities remain in transition from contemporary troponin assays to hs-cTns as the preferred biomarker for ED chest pain patients. A multicenter pre–post cohort study of 17,384 patients revealed that using hs-cTns within the HEART pathway improved AMI detection and reduced healthcare utilization compared with conventional assays []. In the U.S. clinical setting, FDA-approved hs-cTn assays must report results only when values are at or above the limit of quantification (LoQ). For AMI rule-in, guidelines require the detection of a rise and fall pattern with at least one value above the 99th percentile URL [,]. The ESC recommends an absolute value of 52 ng/L as an abnormal cutoff for AMI indication [], consistent with CART analysis finding either 52 ng/L or a ≥ 5 ng/L change in 1 h []. This guideline recommends that the abnormal value of 52 ng/L be thoroughly evaluated in different U.S. populations [,]. Our ROC analysis identified 82 ng/L (hs-cTnT) and 122 ng/L (hs-cTnI) as optimal abnormal thresholds at presentation for diagnosing AMI. At this cutoff, the sensitivity remained comparable to that of 52 ng/L, while the specificity was improved. Importantly, these refined thresholds shifted patient triage patterns compared to 52 ng/L and have the potential to optimize ED resource use, cost, and workflow in chest pain management.
ACC recommendations emphasize measuring hs-cTns serially to determine the change in concentration at 1 and 3 h after initial presentation. An increase of more than 20% is suggested to support a diagnosis of AMI, while changes below this threshold often indicate resolving AMI [,]. In our study, AMI patients consistently demonstrated more than 20% rises in hs-cTn levels, whereas most non-AMI patients with baseline values below the newly proposed thresholds (52–82 ng/L for hs-cTnT, 52–122 ng/L for hs-cTnI) showed <20% change or even a decrease after 1 h. Post-discharge follow-up data at 1, 3, and 12 months in patients within this intermediate range revealed minimal or negligible subsequent cardiac events. This suggests that a single hs-cTn test at presentation may be adequate for many non-AMI cases, though adding a 1 h delta measurement offers reassurance in excluding non-AMI cases and improving diagnostic confidence. However, the HEART score combined with the 0/1 h troponin algorithm is a strategy practiced in many EDs for the diagnosis of adverse cardiac events [].
Beyond AMI, elevated hs-cTn levels can occur in a variety of clinical settings, including, but not limited to, sepsis, stroke, CKD, left ventricular hypertrophy, hypertension, advanced age, and acute respiratory distress syndrome [,,,,,,]. Consistent with this, our findings indicate that 52 ng/L is not specific enough to rule out non-AMI, as many ED patients with chest pain have comorbidities, such as CKD, CHD, myopathies, and sepsis. Studies have shown a 2.5–2.8-fold change in delta values to perform relatively better in ruling in patients with comorbidities such as CKD []. Emerging evidence suggests that optimal abnormal cutoff values may vary depending on underlying disease states; for example, a threshold of 127 ng/L has been reported to offer good specificity for detecting obstructive coronary artery disease []. Elevated hs-cTn levels exceeding 100 ng/L are frequently observed in septic shock patients []. In the chronic renal insufficiency cohort study (CRIC), the 99th percentile for hs-cTnT was 126 ng/L []. Integrating troponin levels along with serial measurement, underlying disease conditions can differentiate acute from chronic elevations regardless of baseline troponin levels. The tailoring of diagnostic abnormal cutoffs should include patient-specific factors along with overall clinical status to enhance diagnostic accuracy.
5. Strengths and Limitations
Few reports exist comparing Roche hs-cTnT and Abbott hs-cTnI results from the same sample timed. Similarly, few studies have addressed the abnormal cutoff values for hs-cTn, as many U.S. facilities are still transitioning from conventional cTn assays to hs-cTn. In this report, we review the existing evidence for both hs-cTn assays, focusing on hs-cTn levels obtained at presentation combined with a 20% increase in 1 h delta change as abnormal criteria for AMI. Our analysis targets the baseline abnormal threshold specifically, given its critical importance in facilitating immediate intervention for true AMI cases. This approach supports rapid prioritization of true AMI patients while enabling timely triage of non-AMI individuals, helping to mitigate ED length of stay and optimizing resource management.
This study has important limitations to consider. Foremost, this is a monocentric study, which may limit the generalizability of our proposed AMI rule-in/abnormal diagnostic value to broader and more heterogeneous patient populations. Multicenter research across different regions and healthcare systems will be critical for confirming and standardizing these diagnostic criteria. Second, the study period coincided with the COVID pandemic, during which a considerable number of patients presented with comorbidities, such as shortness of breath, kidney disease, and sepsis, unrelated to AMI. Finally, the modest sample size reduces the statistical power, highlighting the need for larger studies to confirm diagnostic performance and support clinical adoption.
6. Conclusions
Given that hs-cTn levels are influenced by multiple conditions, our study identifies 82 ng/L for hs-cTnT and 122 ng/L for hs-cTnI as optimized baseline abnormal cutoffs for AMI diagnosis in the U.S. population. Large-scale studies within other U.S. facilities are essential to confirm and standardize these thresholds that can streamline ED triage and expedite definitive care for AMI patients.
Author Contributions
Conceptualization, K.S., M.N., R.V. and A.M.; methodology, K.S., M.N. and I.D.C.; software, K.S. and M.N.; validation, K.S., I.D.C. and R.V.; formal analysis, K.S., M.N. and I.D.C.; investigation, K.S., M.N., I.D.C., R.V. and A.M.; resources, J.B., R.Z., R.V. and A.M.; data curation, K.S., I.D.C., R.V. and A.M.; writing—original draft preparation, K.S.; writing—M.N., I.D.C., R.V. and A.M.; visualization, K.S., M.N., I.D.C., R.V. and A.M.; supervision, R.V. and A.M.; project administration, K.S., R.V. and A.M.; funding acquisition, NONE. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of UT Southwestern Medical Center, eIRB system (protocol code STU 052017-010 and date of approval 26 May 2017).
Informed Consent Statement
Patient consent was waived as this is a retrospective study using the data from secured electronic data capture software and involves no patient recruitment. No individual patient information is identified.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sandoval, Y.; Apple, F.S.; Mahler, S.A.; Body, R.; Collinson, P.O.; Jaffe, A.S.; International Federation of Clinical Chemistry and Laboratory Medicine Committee on the Clinical Application of Cardiac Biomarkers. High-Sensitivity Cardiac Troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guidelines for the Evaluation and Diagnosis of Acute Chest Pain. Circulation 2022, 146, 569–581. [Google Scholar] [CrossRef]
- Giannitsis, E.; Blankenberg, S.; Christenson, R.H.; Frey, N.; von Haehling, S.; Hamm, C.W.; Inoue, K.; Katus, H.A.; Lee, C.-C.; McCord, J.; et al. Critical appraisal of the 2020 ESC guideline recommendations on diagnosis and risk assessment in patients with suspected non-ST-segment elevation acute coronary syndrome. Clin. Res. Cardiol. 2021, 110, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Boeddinghaus, J.; Nestelberger, T. Downstream Consequences of Implementing High-Sensitivity Cardiac Troponin: Why Indication and Education Matter. J. Am. Coll. Cardiol. 2021, 77, 3180–3183. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Li, S.; Wang, T.Y.; Raber, I.; Sandoval, Y.; Smilowitz, N.R.; Wasfy, J.H.; Pandey, A.; de Lemos, J.A.; Kontos, M.C.; et al. Implementation of High-Sensitivity Cardiac Troponin Assays in the United States. J. Am. Coll. Cardiol. 2023, 81, 207–219. [Google Scholar] [CrossRef]
- McRae, A.D.; Innes, G.; Graham, M.; Lang, E.; Andruchow, J.E.; Ji, Y.; Vatanpour, S.; Abedin, T.; Yang, H.; Southern, D.A.; et al. Undetectable Concentrations of a Food and Drug Administration-approved High-sensitivity Cardiac Troponin T Assay to Rule Out Acute Myocardial Infarction at Emergency Department Arrival. Acad. Emerg. Med. 2017, 24, 1267–1277. [Google Scholar] [CrossRef]
- Peacock, W.F.; Baumann, B.M.; Bruton, D.; Davis, T.E.; Handy, B.; Jones, C.W.; Hollander, J.E.; Limkakeng, A.T.; Mehrotra, A.; Than, M.; et al. Efficacy of High-Sensitivity Troponin T in Identifying Very-Low-Risk Patients With Possible Acute Coronary Syndrome. JAMA Cardiol. 2018, 3, 104–111. [Google Scholar] [CrossRef]
- Neumann, J.T.; Sorensen, N.A.; Ojeda, F.; Renne, T.; Schnabel, R.B.; Zeller, T.; Karakas, M.; Blankenberg, S.; Westermann, D. Early diagnosis of acute myocardial infarction using high-sensitivity troponin I. PLoS ONE 2017, 12, e0174288. [Google Scholar] [CrossRef]
- Reichlin, T.; Twerenbold, R.; Wildi, K.; Gimenez, M.R.; Bergsma, N.; Haaf, P.; Druey, S.; Puelacher, C.; Moehring, B.; Freese, M.; et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ 2015, 187, E243–E252. [Google Scholar] [CrossRef]
- Nseir, M.; Mokhtari, A.; Stanisic, M.; Ekstrom, U.; Labaf, A. Validation and correlation of high-sensitive troponin I and troponin T in the emergency department. BMC Cardiovasc. Disord. 2024, 24, 551. [Google Scholar] [CrossRef]
- Writing Committee; Kontos, M.C.; de Lemos, J.A.; Deitelzweig, S.B.; Diercks, D.B.; Gore, M.O.; Hess, E.P.; McCarthy, C.P.; McCord, J.K.; Musey, P.I., Jr.; et al. 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1925–1960. [Google Scholar] [CrossRef]
- Reichlin, T.; Cullen, L.; Parsonage, W.A.; Greenslade, J.; Twerenbold, R.; Moehring, B.; Wildi, K.; Mueller, S.; Zellweger, C.; Mosimann, T.; et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am. J. Med. 2015, 128, 369–379e4. [Google Scholar] [CrossRef]
- Pickering, J.W.; Greenslade, J.H.; Cullen, L.; Flaws, D.; Parsonage, W.; Aldous, S.; George, P.; Worster, A.; Kavsak, P.A.; Than, M.P. Assessment of the European Society of Cardiology 0-Hour/1-Hour Algorithm to Rule-Out and Rule-In Acute Myocardial Infarction. Circulation 2016, 134, 1532–1541. [Google Scholar] [CrossRef]
- Cullen, L.A.; Mills, N.L.; Mahler, S.; Body, R. Early Rule-Out and Rule-In Strategies for Myocardial Infarction. Clin. Chem. 2017, 63, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Schindler, C.; Drexler, B.; Twerenbold, R.; Reiter, M.; Zellweger, C.; Moehring, B.; Ziller, R.; Hoeller, R.; Gimenez, M.R.; et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012, 172, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Cyon, L.; Kadesjo, E.; Edgren, G.; Roos, A. Acute Kidney Injury and High-Sensitivity Cardiac Troponin T Levels in the Emergency Department. JAMA Netw. Open 2024, 7, e2419602. [Google Scholar] [CrossRef]
- Carlsson, A.C.; Bandstein, N.; Roos, A.; Hammarsten, O.; Holzmann, M.J. High-sensitivity cardiac troponin T levels in the emergency department in patients with chest pain but no myocardial infarction. Int. J. Cardiol. 2017, 228, 253–259. [Google Scholar] [CrossRef]
- Gartner, C.; Langhammer, R.; Schmidt, M.; Federbusch, M.; Wirkner, K.; Loffler, M.; Isermann, B.; Laufs, U.; Wachter, R.; Kaiser, T. Revisited Upper Reference Limits for Highly Sensitive Cardiac Troponin T in Relation to Age, Sex, and Renal Function. J. Clin. Med. 2021, 10, 5508. [Google Scholar] [CrossRef]
- Kong, N.; Chua, R.F.M.; Besser, S.A.; Heelan, L.; Nathan, S.; Spiegel, T.F.; van Wijk, X.M.R.; Tabit, C.E. A retrospective analysis of high sensitivity cardiac troponin-T ranges in non-myocardial infarction emergency department visits. BMC Cardiovasc. Disord. 2021, 21, 283. [Google Scholar] [CrossRef]
- Perrone, M.A.; Storti, S.; Salvadori, S.; Pecori, A.; Bernardini, S.; Romeo, F.; Guccione, P.; Clerico, A. Cardiac troponins: Are there any differences between T and I? J. Cardiovasc. Med. 2021, 22, 797–805. [Google Scholar] [CrossRef]
- Wan Nur Aimi, W.M.Z.; Noorazliyana, S.; Tuan Salwani, T.I.; Adlin Zafrulan, Z.; Najib Majdi, Y.; Noor Azlin Azraini, C.S. Elevation of Highly Sensitive Cardiac Troponin T Among End-Stage Renal Disease Patients Without Acute Coronary Syndrome. Malays J. Med. Sci. 2021, 28, 64–71. [Google Scholar]
- McDonald, S.; Furmaga, J.; Vigen, R.; Muthukumar, A.; Hall, H.M.; Turer, A.; Das, S.R.; de Lemos, A.A.; Diercks, D. Assessment of agreement of two high sensitivity troponin assays during an institutional transition. Vessel Plus 2021, 5, 38. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Roy, C.; Malinowski, P.; Clark, L.; Lamers, S.; Bamford, K.; Hill, S.; Worster, A.; Jaffe, A.S. Sample matrix and high-sensitivity cardiac troponin I assays. Clin. Chem. Lab. Med. 2019, 57, 745–751. [Google Scholar] [CrossRef]
- Lan, H.; Du, W.; Mo, Z.; Huang, H. The Influence of Blood Collection Tubes on Measurement of Cardiac Biomarkers. Clin. Lab. 2016, 62, 705–709. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Mahler, S.A.; Christenson, R.H.; Rymer, J.; Newby, L.K.; Body, R.; Morrow, D.A.; Jaffe, A.S. Recommendations for Institutions Transitioning to High-Sensitivity Troponin Testing: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 1059–1077. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, 20. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Anwari, E.; Kokabi, V.; Gyongyosi, M.; Bergler-Klein, J. Comparison of rapid test for high-sensitivity troponin I with laboratory high-sensitivity troponin T in patients with acute chest pain—A prospective monocentric study. Eur. Heart J. 2024, 45, ehae666.1597. [Google Scholar] [CrossRef]
- Vigen, R.; Kutscher, P.; Fernandez, F.; Yu, A.; Bertulfo, B.; Hashim, I.A.; Molberg, K.; Diercks, D.B.; Metzger, J.C.; Soto, J.; et al. Evaluation of a novel rule-out myocardial infarction protocol incorporating high-sensitivity troponin T in a US hospital. Circulation 2018, 138, 2061–2063. [Google Scholar] [CrossRef]
- Ola, O.; Akula, A.; De Michieli, L.; Dworak, M.; Crockford, E.; Lobo, R.; Rastas, N.; Knott, J.D.; Mehta, R.A.; Hodge, D.O.; et al. Clinical Impact of High-Sensitivity Cardiac Troponin T Implementation in the Community. J. Am. Coll. Cardiol. 2021, 77, 3160–3170. [Google Scholar] [CrossRef]
- Twerenbold, R.; Neumann, J.T.; Sorensen, N.A.; Ojeda, F.; Karakas, M.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; Giménez, M.R.; Puelacher, C.; et al. Prospective Validation of the 0/1-h Algorithm for Early Diagnosis of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 620–632. [Google Scholar] [CrossRef]
- Roos, A.; Edgren, G. Using historical cardiac troponins to identify patients at a high risk of myocardial infarction. Heart 2022, 109, 127–133. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Watson, N.W.; Wasfy, J.H.; Baugh, C.W.; Januzzi, J.L., Jr.; Secemsky, E.A. Cardiac Biomarker Testing in US Emergency Departments. JAMA Intern. Med. 2025, 185, 343–346. [Google Scholar] [CrossRef]
- Yore, M.; Sharp, A.; Wu, Y.L.; Kawatkar, A.; Lee, M.S.; Ferencik, M.; Redberg, R.; Shen, E.; Zheng, C.; Sun, B. Emergency Department Cardiac Risk Stratification with High-Sensitivity vs Conventional Troponin HEART Pathway. JAMA Netw. Open 2023, 6, e2348351. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef] [PubMed]
- Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; Wildi, K.; Rubini Gimenez, M.; Badertscher, P.; Mueller, C. Clinical Use of High-Sensitivity Cardiac Troponin in Patients with Suspected Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 70, 996–1012. [Google Scholar] [CrossRef] [PubMed]
- Knott, J.D.; Ola, O.; De Michieli, L.; Akula, A.; Yang, E.H.; Gharacholou, S.M.; Slusser, J.; Lewis, B.; Mehta, R.A.; Gulati, R.; et al. High Baseline High-Sensitivity Cardiac Troponin T Concentrations and Risk of Index Acute Myocardial Infarction. Mayo Clin. Proc. 2024, 99, 1435–1442. [Google Scholar] [CrossRef]
- Arslan, M.; Dedic, A.; Boersma, E.; Dubois, E.A. Serial high-sensitivity cardiac troponin T measurements to rule out acute myocardial infarction and a single high baseline measurement for swift rule-in: A systematic review and meta-analysis. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 14–22. [Google Scholar] [CrossRef]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J. Bio-Markers ITFoCAoC. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin. Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Lippi, G. How well do current cardiac troponin assays perform? Clin. Chem. Lab. Med. 2017, 55, 1817–1828. [Google Scholar]
- Allen, B.R.; Christenson, R.H.; Cohen, S.A.; Nowak, R.; Wilkerson, R.G.; Mumma, B.; Madsen, T.; McCord, J.; Veld, M.H.I.; Massoomi, M.; et al. Diagnostic Performance of High-Sensitivity Cardiac Troponin T Strategies and Clinical Variables in a Multisite US Cohort. Circulation 2021, 143, 1659–1672. [Google Scholar] [CrossRef]
- Gajardo, A.I.J.; Ferriere-Steinert, S.; Valenzuela Jimenez, J.; Heskia Araya, S.; Kouyoumdjian Carvajal, T.; Ramos-Rojas, J.; Medel, J.N. Early high-sensitivity troponin elevation and short-term mortality in sepsis: A systematic review with meta-analysis. Crit. Care 2025, 29, 76. [Google Scholar] [CrossRef]
- Broersen, L.H.A.; Stengl, H.; Nolte, C.H.; Westermann, D.; Endres, M.; Siegerink, B.; Scheitz, J.F. Association Between High-Sensitivity Cardiac Troponin and Risk of Stroke in 96 702 Individuals: A Meta-Analysis. Stroke 2020, 51, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Geladari, E.V.; Vallianou, N.G.; Evangelopoulos, A.; Koufopoulos, P.; Panagopoulos, F.; Margellou, E.; Dalamaga, M.; Sevastianos, V.; Geladari, C.V. Cardiac Troponin Levels in Patients with Chronic Kidney Disease: “Markers of High Risk or Just Noise”? Diagnostics 2024, 14, 2316. [Google Scholar] [CrossRef] [PubMed]
- Jayasimhan, D.; Foster, S.; Chang, C.L.; Hancox, R.J. Cardiac biomarkers in acute respiratory distress syndrome: A systematic review and meta-analysis. J. Intensive Care 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, X.; Chen, Z.; Xia, J.; Guan, F.; Li, Y.; Li, Y.; Chen, Y.; Zhao, Y.; Qiu, H.; et al. The use of high-sensitivity cardiac troponin T and creatinine kinase-MB as a prognostic markers in patients with acute myocardial infarction and chronic kidney disease. Ren. Fail. 2023, 45, 2220420. [Google Scholar] [CrossRef]
- McHugh, M.C.; Diercks, D.B. Interpreting High-Sensitive Troponins in Patients with Hypertension. Curr. Hypertens. Rep. 2022, 24, 349–352. [Google Scholar] [CrossRef]
- Hasselbalch, R.B.; Strandkjaer, N.; Kristensen, J.; Jorgensen, N.; Kock, T.O.; Lange, T.; Ostrowski, S.R.; Nissen, J.; Larsen, M.H.; Pedersen, O.B.V.; et al. Impact of age on cardiac troponin concentration among healthy individuals. Clin. Biochem. 2025, 138, 110956. [Google Scholar] [CrossRef]
- Hti Lar Seng, N.S.; Zeratsion, G.; Pena Zapata, O.Y.; Tufail, M.U.; Jim, B. Utility of Cardiac Troponins in Patients with Chronic Kidney Disease. Cardiol. Rev. 2024, 32, 62–70. [Google Scholar] [CrossRef]
- Twerenbold, R.; Wildi, K.; Jaeger, C.; Gimenez, M.R.; Taffe, P.; Reiter, M.; Nestelberger, T.; Rubini Gimenez, M.; Meissner, J.; Tanner, F.C.; et al. Optimal Cutoff Levels of High-Sensitivity Cardiac Troponin for the Diagnosis of Myocardial Infarction and Obstructive Coronary Artery Disease. Circulation 2015, 131, 2041–2050. [Google Scholar] [CrossRef]
- Jendoubi, A.; Jerbi, S.; Maamar, E.; Abbess, A.; Samoud, Z.; Kanzari, L.; Boutiba, I. Prognostic Value of High-sensitivity Troponin I in Patients with Septic Shock: A Prospective Observational Study. Indian J. Crit. Care Med. 2019, 23, 320–325. [Google Scholar] [CrossRef]
- Bansal, N.; Zelnick, L.R.; Ballantyne, C.M.; Chaves, P.H.M.; Christenson, R.H.; Coresh, J.; Defilippi, C.R.; de Lemos, J.A.; Daniels, L.B.; Go, A.S.; et al. Upper Reference Limits for High-Sensitivity Cardiac Troponin T and N-Terminal Fragment of the Prohormone Brain Natriuretic Peptide in Patients With CKD. Am. J. Kidney Dis. 2022, 79, 383–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).